Evolution teaches cichlids to outwit baby-killing cuckoo catfish.
Abstract
Obligate brood parasites manipulate other species into raising their offspring. Avian and insect brood parasitic systems demonstrate how interacting species engage in reciprocal coevolutionary arms races through behavioral and morphological adaptations and counteradaptations. Mouthbrooding cichlid fishes are renowned for their remarkable evolutionary radiations and complex behaviors. In Lake Tanganyika, mouthbrooding cichlids are exploited by the only obligate nonavian vertebrate brood parasite, the cuckoo catfish Synodontis multipunctatus. We show that coevolutionary history and individual learning both have a major impact on the success of cuckoo catfish parasitism between coevolved sympatric and evolutionarily naïve allopatric cichlid species. The rate of cuckoo catfish parasitism in coevolved Tanganyikan hosts was 3 to 11 times lower than in evolutionarily naïve cichlids. Moreover, using experimental infections, we demonstrate that parasite egg rejection in sympatric hosts was much higher, leading to seven times greater parasite survival in evolutionarily naïve than sympatric hosts. However, a high rejection frequency of parasitic catfish eggs by coevolved sympatric hosts came at a cost of increased rejection of their own eggs. A significant cost of catfish parasitism was universal, except for coevolved sympatric cichlid species with previous experience of catfish parasitism, demonstrating that learning and individual experience both contribute to a successful host response.
INTRODUCTION
Providing parental care not only can greatly increase offspring survival but also exposes parents to the risk of exploitation by brood parasites, which manipulate parents to transfer the care of their own young to their hosts. Brood parasitism can occur within or among species and potentially imposes severe fitness costs on foster parents (1–7). At an interspecific level, brood parasitism has been reported in the Hymenoptera (ants, bees, and wasps; termed social parasitism), Coleoptera (beetles), and birds. In birds, interspecific parasitism has evolved independently in seven different clades, with over 100 brood parasitic species that are distributed worldwide (3). Studies on brood parasitism in birds and social insects have demonstrated complex evolutionary arms races involving the evolution of defenses in hosts and counteradaptations in brood parasites (8–12), revealing striking parallels, such as exploitation of general behavioral rules in the host, as well as fundamental differences, such as the preponderance of facultative intraspecific brood parasitism in birds (2, 6, 13). Given the limited taxonomic range of obligate brood parasites, a better understanding of brood parasite–host interactions can be derived only from the investigations of additional brood parasite systems with different evolutionary origins (1).
The cuckoo catfish (Synodontis multipunctatus) is an obligate brood parasite of mouthbrooding cichlid fishes in Lake Tanganyika (14). It is the only known example of nonavian obligate brood parasitism in vertebrates. In many African cichlids, females care for their offspring in their buccal cavity, a care system termed mouthbrooding (15). During mating, which involves an elaborate sequence of complex male and female behaviors, the eggs are laid on a prepared sand nest and quickly collected by the female in her mouth. The eggs are fertilized inside the female’s mouth when she collects sperm from a male by nipping at “egg spots” on his anal fin (movie S1) (16). This specialized mode of spawning and brood care protects offspring from predation and is found in all >1800 species of haplochromine cichlids that have given rise to spectacular radiations in the East African lakes (17). Eggs hatch inside the female buccal cavity within days and are retained there for 2 to 3 weeks until depletion of the embryonic yolk sac and commencement of independent exogenous feeding (18).
Cuckoo catfish are unique to be able to exploit cichlid mouthbrooding by interrupting their spawning and quickly laying their own, nonmimetic eggs that are mistakenly picked up by the female cichlid, who subsequently cares for them in her mouth. Catfish offspring hatch earlier than cichlids and prey on the developing cichlid embryos inside their mother’s buccal cavity (15, 19). The host female eventually releases a mixture of her own offspring and parasitic catfish, though the host’s offspring are frequently entirely eliminated by the parasite before completing embryo development (14, 19). Aspects of this system closely resemble the relationship between avian brood parasites and their hosts, but the cuckoo catfish system is particularly amenable to experimental manipulation in captivity (19), something that is more difficult to accomplish with avian brood parasites (20).
Here, we present the results of the first experimental study on the cuckoo catfish, designed to partition the effects of coevolutionary history and individual experience on the success of catfish parasitism. In the case of avian systems, hosts that have coevolved with brood parasites have repeatedly been shown to have adaptations that limit the risks and costs of parasitism (7), whereas individual experience has received relatively little attention and is typically treated separately from coevolutionary responses [(21, but see the study of Strausberger and Rothstein 22)].
First, conspecific groups of African mouthbrooding cichlids were maintained with groups of cuckoo catfish, and the prevalence of brood parasitism was compared between coevolved and evolutionarily naïve host species. We then experimentally investigated the capacity of host cichlids to reject parasite eggs to examine whether the lower prevalence of catfish parasitism that we observed in sympatric coevolved hosts arose from an ability to discriminate against parasite eggs and reject them, including the role of individual experience. Using in vitro fertilized cuckoo catfish eggs, we infected broods of naturally spawned sympatric and allopatric host cichlids that either were individually naïve or had been previously exposed to catfish parasitism. We tested whether the ability to reject parasite eggs involved a potential cost to females through higher rates of rejection of their own eggs. Finally, to examine the overall success and fitness costs of parasitism, we also tested how short-term egg rejections were reflected in the survival of the parasite and host broods until independence at the age of 2 to 3 weeks. Although brood success was primarily measured as survival of at least a single juvenile to independence as a conservative approximation of reproductive success, we also report numbers of individual hosts and parasites surviving to independence and quantify the rates of mixed brood survival.
RESULTS
Natural prevalence of brood parasitism in sympatric and allopatric hosts
The prevalence of natural cuckoo catfish parasitism was significantly higher in allopatric, evolutionarily naïve host species from other African lakes than Tanganyika (Table 1), supporting the role of coevolutionarily evolved defenses. The prevalence of brood parasitism in sympatric Simochromis diagramma corresponded with the prevalence of catfish infections in Lake Tanganyika (5.5% in captive and 7.7% in wild S. diagramma) (14), indicating that experimental laboratory conditions replicated critical features of the natural environment well.
Table 1. Prevalence of cuckoo catfish parasitism in host cichlid species during natural spawning in species-specific experimental aquaria.
| Species | Lake of origin | Evolutionary history with cuckoo catfish | Prevalence | Broods (n) |
| Haplochromis sp. 44 | Victoria | Allopatric | 63.0% | 59 |
| Haplochromis aeneocolor | George | Allopatric | 22.0% | 235 |
| Copadichromis borleyi | Malawi | Allopatric | 17.0% | 6 |
| Simochromis diagramma | Tanganyika | Sympatric | 5.5% | 54 |
Cichlid host ability to reject parasitic catfish eggs
Using in vitro fertilized cuckoo catfish eggs, we experimentally infected broods of naturally spawned sympatric and allopatric host cichlids that either were individually naïve or had previously experienced catfish parasitism. The rejection of cuckoo catfish eggs over the first 24 hours by sympatric Tanganyikan S. diagramma females (90%; Fig. 1A) was dramatically higher than rejections by Haplochromis aeneocolor from Lake George (evolutionarily naïve to cuckoo catfish) [7%; generalized linear model (GLM) with Bernoulli distribution and logit-link function: χ2 = 50.4, n = 60, P < 0.001] (Fig. 1C). Previous individual experience by hosts with catfish brood parasitism had no effect on early rejection of parasite eggs (GLM: χ2 = 0.6, P = 0.438; interaction: χ2 = 1.2, P = 0.275) (Fig. 1, black versus white bars, and table S1). Sympatric S. diagramma was equally effective in rejecting the eggs of heterospecific cichlid eggs used as a control (Fig. 1B).
Fig. 1. Short-term rejection rate of parasite eggs.
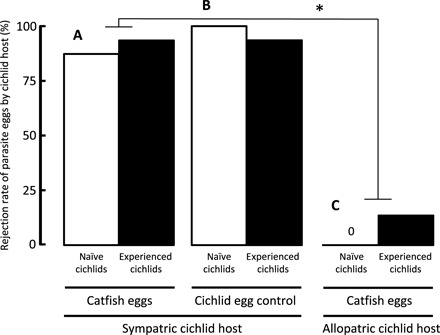
The proportion (in percentage) of clutches with parasite egg rejection 24 hours after experimental infection with foreign eggs in sympatric hosts parasitized by cuckoo catfish (A) or heterospecific cichlid (B) and in allopatric hosts parasitized by the cuckoo catfish (C). Females individually naïve to brood parasitism (white bars) and experienced host females (black bars) are distinguished. Asterisk denotes statistically significant (P < 0.05) difference between rejection rates of cuckoo catfish eggs after experimental parasitism of sympatric and allopatric host females.
Cost to host cichlid of parasite egg rejection
The rejection of parasite eggs came at a cost to female cichlids through the rejection of their own eggs. The rejection of their own eggs following experimental parasite infection tended to be higher in sympatric host females (GLM with Bernoulli distribution and logit-link function: χ2 = 3.83, n = 60, P = 0.0503; Fig. 2, A versus D) and was even further elevated in individuals with previous experience of catfish parasitism (GLM: χ2 = 6.26, P = 0.012) (Fig. 2, A and B, black versus white bars). Host females also rejected their own eggs following sham controls (handling only), at a rate that was similar to females exposed to experimental parasitism (Fig. 2, C and E), but with no difference between naïve and experienced control females (GLM: χ2 =0.34, n = 60, P = 0.561; interaction: χ2 = 1.97, P = 0.161) (Fig. 2, C and E, black and white bars). The same rejection rate of their own eggs was recorded during experimental parasitism of sympatric hosts with the eggs of a heterospecific cichlid (GLM, egg source: χ2 = 1.2, n = 61, P = 0.276; individual experience: χ2 < 0.1, P = 0.973; interaction: χ2 = 1.2, P = 0.172) (Fig. 2B). Sympatric hosts had, therefore, higher overall rejection rates of their own eggs compared to allopatric hosts, irrespective of parasitism treatment (GLM, controls: χ2 =17.8, P < 0.001) (Fig. 2, A to C versus D and E), demonstrating a significant fitness cost of evolving parasite resistance.
Fig. 2. Short-term rejection rate of own eggs following experimental parasitism.
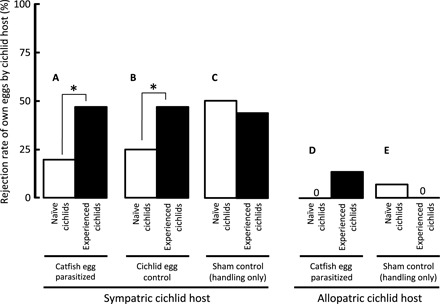
The proportion (in percentage) of clutches with own egg rejection 24 hours after experimental infection with foreign eggs in sympatric hosts artificially parasitized by cuckoo catfish (A), heterospecific cichlid (B), and in sham controls (handling only) (C), and in allopatric hosts parasitized by cuckoo catfish (D) and in sham controls (E). Females individually naïve to brood parasitism (white bars) and experienced host females (black bars) are distinguished. Asterisks denote statistically significant (P < 0.05) difference in the pairwise contrasts between naïve and experienced host females.
Host and parasite brood survival until independence
Parasite offspring showed poor survival to independence in sympatric hosts (13%; Fig. 3A) but good survival in allopatric hosts (86%; Fig. 3B) (GLM with Bernoulli distribution and logit-link function: χ2 = 36.0, n = 60, P < 0.001), irrespective of previous host experience at the individual level (χ2 = 0.0, P = 0.997; interaction: χ2 = 0.0, P = 0.919) (Fig. 3, black versus white bars, and table S2). Experimental parasitism by cuckoo catfish decreased host cichlid reproductive success substantially (χ2 = 32.8, n = 120, P < 0.001) (Fig. 4, black versus white bars), and the decrease was stronger in allopatric hosts (evolutionary contrast by parasitism treatment interaction: χ2 = 7.46, P = 0.006) (Fig. 4 and table S3).
Fig. 3. The success of parasite broods over the duration of brood care.
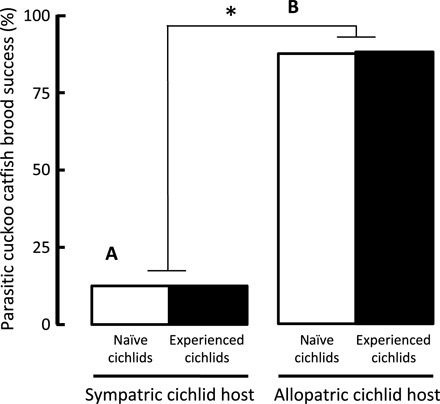
The proportion of clutches with at least a single juvenile catfish surviving to independence following experimental parasitism in sympatric (A) and allopatric (B) hosts. Females individually naïve to brood parasitism (white bars) and experienced host females (black bars) are distinguished. Asterisk denotes statistically significant (P < 0.05) difference in the contrast between sympatric and allopatric hosts.
Fig. 4. The success of host broods over the duration of brood care.
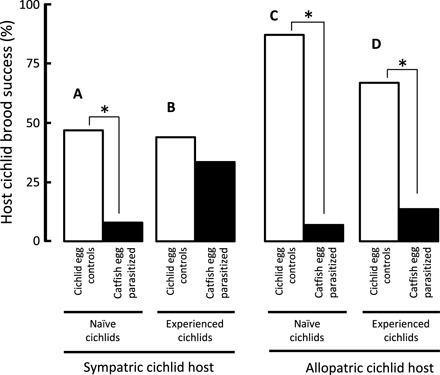
The proportion of clutches with at least a single host juvenile surviving to independence following experimental parasitism (black bars) and in control broods (white bars) in sympatric hosts that were individually naïve to cuckoo catfish parasitism (A) or experienced host females (B), and in allopatric naïve (C) and experienced (D) hosts. Asterisks denote statistically significant (P < 0.05) difference in the pairwise contrasts between host brood success after experimental parasitism and the control treatment.
In sympatric hosts, 19% of 31 females raised their own brood to independence after exposure to experimental parasitism, compared to 44% of 32 control females. This pattern was driven by females that were naïve to parasitism, with only 1 of 16 naïve parasitized sympatric females (6%) successfully raising its own brood to independence (significantly lower than in naïve control broods: χ2 = 6.58, n = 32, P = 0.010; Fig. 4A), compared to 44% of 16 experienced females (no difference from control broods: χ2 = 0.36, n = 31, P = 0.551; Fig. 4B). Individual experience of catfish parasitism did not affect host brood success in parasitized allopatric females [naïve allopatric: χ2 = 18.03, n = 30, P <0.001 (Fig. 4C); experienced allopatric: χ2 = 18.96, n = 33, P < 0.001 (Fig. 4D)], with a low host success overall (10% of 29; Fig. 4, C and D, black bars). In contrast, control, nonparasitized females had high brood success (82% of 34; Fig. 4, C and D, white bars), demonstrating a substantial cost of a lack of defense against brood parasites.
Quantitative estimates and mixed brood survival
We used six parasite eggs (the typical parasite number in nature) (14) for experimental infections. In cases where parasitism was successful, 3 ± 2.4 (mean ± SD, n = 4) catfish per brood survived in sympatric hosts and 5 ± 2.2 (n = 27) in allopatric hosts. An analysis using the number of juveniles surviving to independence as the response variable gave a qualitatively identical outcome as the conservative approach (survival of at least a single juvenile to independence), confirming the presence of a major cost of parasitism in all host treatments, except in the case of experienced sympatric hosts (fig. S1). Parasite offspring typically ate the entire brood of the host. Occasionally, however, host and parasite offspring survived together to independence in the same brood. In one brood raised by the sympatric host S. diagramma (of 4 only that raised parasite offspring to independence, 25%), a single catfish survived along with 19 host cichlid offspring. Three cases of mixed broods were recorded in allopatric females (12% of 25 clutches with surviving parasite offspring), with five to six parasite offspring surviving along with three to seven host cichlid offspring.
DISCUSSION
Hosts at risk of brood parasitism adopt a range of strategies to cope with this threat, depending on evolutionary history, individual experience, encounter rate, and the virulence of the parasite (1–12). Using experimental infections, we demonstrate that host cichlids suffer major costs to their reproductive success through cuckoo catfish brood parasitism. In addition to nest defense against parasite intrusions (movie S2), sympatric host cichlids have also evolved effective rejection behavior. However, we show that they experience a high fitness cost of parasite resistance through an elevated propensity to reject their own offspring (Fig. 2, A to C). In contrast, allopatric, coevolutionarily naïve hosts lack protection from parasitism beyond generalized nest defense and suffer a substantial fitness cost when exposed to catfish parasitism (Fig. 4, C and D), a possible example of an evolutionary lag (8, 23, 24).
We find that sympatric hosts also demonstrate a significant learned component in their response against brood parasitism, with females previously exposed to cuckoo catfish capable of raising their own offspring following repeated parasitism more successfully than females with no prior experience of parasitism (Fig. 4, A and B). In hosts of avian brood parasites, a learned component in host responses to parasitism has also been reported, although it has been described in only a limited number of species (21, 25), even at high rates of brood parasitism (26). In the Eurasian magpie (Pica pica), parents can learn to discriminate and reject the great spotted cuckoo (Clamator glandarius) eggs (27), and fairy wrens (Malurus cyaneus) improve their ability to recognize their own chicks (9, 28) over successive breeding seasons. In the American coot (Fulica americana), parents use first-hatched chicks to learn to recognize their own offspring and are thereby able to avoid intraspecific brood parasitism (29). However, in some avian host species, a high density of brood parasites can lead to imprinting on parasite eggs by first-time breeders, thereby resulting in an elevated incidence of brood parasitism in species that typically reject parasite eggs (22). A form of imprinting on the appearance of their own eggs was also reported in great reed warblers, Acrocephalus arundinaceus, which suffer parasitism by the common cuckoo, Cuculus canorus. In this case, learning is potentially hampered by intraclutch variability in egg visual appearance, which tends to be greater for less-experienced females (30). Although age and experience can potentially be conflated in experiments (25), this was not the case in the present study, and breeding experience rather than age itself played a role of in the learned response of sympatric hosts. In addition to individual experience with breeding, social learning can play a role in host responses to avian brood parasites (10, 31) and remains to be tested in the catfish-cichlid system. Social learning will likely play a greater role as the first line of host defense (avoidance of cuckoo catfish intrusions and egg dumping) than in response to the presence of brood parasitic eggs in the buccal cavity, the investigation of which was the primary focus of the present study. Overall, we demonstrate that learning plays a role in sympatric cichlid host responses to cuckoo catfish, a finding comparable to that shown for some avian systems.
The response of sympatric hosts to parasitic eggs in the form of egg rejections appears to be a generalized response to foreign eggs. Sympatric cichlid hosts (S. diagramma) rejected the eggs of a heterospecific cichlid (H. aeneocolor) at the same rate as the eggs of the cuckoo catfish (Fig. 1, A and B). Cuckoo catfish eggs are nonmimetic and are spherical and smaller (2 mm in diameter) than the oval-shaped eggs of its natural hosts (S. diagramma: 4.5 mm) and experimental allopatric hosts (Copadichromis borleyi: 3.5 mm; Haplochromis sp. 44: 3.8 mm; and H. aeneocolor: 2.5 mm) (32, 33). This finding suggests that hosts do not tune their response to specific characteristics that are unique to cuckoo catfish eggs (for example, egg shape, size, or olfactory cues). The trade-off between rejection and acceptance by cuckoo catfish hosts seemingly arises from a compromise between parasite-imposed costs and the cost associated with parasite recognition errors. In the case of avian brood parasite systems, this trade-off similarly results in adaptive acceptance of brood parasite eggs, particularly at low rates of parasitism, and is associated with sophisticated learning mechanisms (30).
A caveat to our study is the limited number of host species used. Only a single allopatric host species, along with three allopatric species, was used for tests of natural parasitism rates (Table 1). Cohen (34) used a similar approach to ours to assess the prevalence of catfish parasitism in species-specific spawnings in aquaria but with different allopatric and sympatric host species. Ctenochromis horei, another sympatric cichlid species confirmed as the host of the cuckoo catfish (14), was parasitized at a rate of 17% (n = 100 broods), whereas all four allopatric species were parasitized at a higher rate (Haplochromis nubilus from the Greater Lake Victoria region: 24%; Haplochromis latifasciatus from Lakes Kyoga and Nawampasa: 33%; Metriaclima estherae from Lake Malawi: 25%; and Metriaclima zebra (albino strain) from Lake Malawi 46%; n = 100 broods in all cases) (34). Although parasitism rates of sympatric C. horei in the study by Cohen (34) are three times higher than that of the sympatric S. diagramma in our own, the results match the naturally higher prevalence of C. horei recorded from Lake Tanganyika [15% of 20 examined broods in (14)]. Together, these findings show that sympatric hosts are parasitized at substantially lower rates than allopatric hosts. It would be interesting to broaden experimental parasite infections to include more sympatric and allopatric species to identify the effects of egg characteristics, clutch size, and other species-specific traits on host responses and the success of host and parasite brood survival. It is notable, however, that the egg and clutch traits expressed by the six sympatric hosts reported to be parasitized by the cuckoo catfish in the wild (14) broadly overlap with those found in the set of allopatric hosts used in the present study and by Cohen (34).
We found that experimentally parasitized sympatric females that had previously experienced catfish parasitism were more successful in raising their own offspring to independence than experimentally parasitized naïve sympatric females (Fig. 4, A and B, black bars), despite their numerically higher rate of rejection of their own eggs during the first 24 hours of incubation (Fig. 2, A and B, black bars). This seeming mismatch between the strength of the initial host response and overall host reproductive success over the whole period of incubation is interesting. However, an increase in the rejection rate of their own eggs in experienced females was associated with a high rate of rejections of parasitic eggs (80%; Fig. 1A) and was observed after experimental parasitism but not in the case of the sham control treatment (Fig. 2, A to C).
In addition, egg rejections likely continue throughout brood development. This possibility could not be monitored as the offspring reside inside the buccal cavity of brooding females, and our estimates of the immediate response to parasitism (rejection rates within the first 24 hours) and overall outcome of the parasitism (survival to independence after 12 to 18 days) were based on a different set of replicates. In this context, it should be acknowledged that sham (handling only) control females also rejected their own eggs. This outcome implies that handling stress resulted in some egg rejection, irrespective of parasitism. However, all our inferences on the effect of parasitism are based on the contrasts between experimentally parasitized and control females rather than absolute rates of rejections and brood survival, and thus, our conclusions are not biased by handling stress. Comparable experimental artifacts similarly influence the outcome of other ecological studies, including the effect of nest visitation by the experimenters on nest desertion in avian brood parasites (35).
Cuckoo catfish brood parasitism has important differences to that in avian systems. In cichlids, clutch sizes are larger, gametes are released externally, male catfish participate in parasitism, and the temporal window for brood parasitism is extremely short—oviposition occurs in a few seconds before the eggs are taken into the female’s buccal cavity (15). Unlike avian brood parasites, developing cuckoo catfish prey directly on host eggs and young (14), a strategy more effective than simple host brood elimination that has evolved in many avian brood parasites (1, 3, 4, 36, 37). The cuckoo catfish–cichlid system offers unique avenues for future research on brood parasitism. Coupled with its amenability to experimental manipulation in controlled captive conditions, it represents an exceptional opportunity to understand the evolutionary benefits and constraints of brood parasitism.
MATERIALS AND METHODS
Study species
The cuckoo catfish is widespread in Lake Tanganyika (38), the oldest of the three African Great Lakes that support diverse radiations of cichlid fishes (39). The cichlid species flock in Lake Tanganyika (9 to 12 million years old) is composed of approximately 250 species and is older than radiations in Lakes Malawi and Victoria (0.2 to 5 million years old) (40). Other fish and nonfish lineages have also radiated in Lake Tanganyika (38, 39). Synodontis catfishes (family Mochokidae), to which the cuckoo catfish belongs, represent another large fish radiation in Lake Tanganyika with at least 10 species, but with only a single species (lineage) evolving obligate brood parasitism (38, 41). Mouthbrooding cichlids in Lake Tanganyika belong to the tribe Tropheini, a diverse lineage that is endemic to Lake Tanganyika (42). The cuckoo catfish is a generalist brood parasite, reported to use at least six host species of the Tropheini in the wild (16).
Host cichlids are all maternal mouthbrooders. Their spawning consists of a male digging and cleaning a breeding site (a flat rock or sand/gravel pit), which is the focus of male courtship displays. During mating, a pair circles over the breeding site, with the female depositing batches of one to three eggs on the substrate, collecting them in her buccal cavity when they are fertilized by sperm she collects when pecking at colored spots on the male’s anal fin (movie S1) (16, 18). The process is repeated until all the female’s ovulated eggs have been spawned. In the experimental sympatric host, S. diagramma, 10 to 35 eggs are laid during a complete spawning event, which can last 1 to 3 hours. In C. borleyi from Lake Malawi, 15 to 40 eggs are laid. In the cases of H. aeneocolor from Lake George and Haplochromis sp. 44 from Lake Victoria, the number of eggs can be higher (25 to 120 eggs), but the duration of spawning is comparable with that in S. diagramma. In all experimental species, once spawning is completed, the female incubates the brood for 2 to 3 weeks (32, 33).
The cuckoo catfish parasitizes its hosts by invading the breeding site of a pair of spawning cichlids, disrupting spawning, and releasing its own eggs among those of the spawning pair (movies S2 and S3). Spawning interruptions are often performed by a group of catfish and can be accompanied by consumption of host eggs in conjunction with the catfish scattering their own eggs. Catfish eggs, along with the host’s eggs, are collected by the host female. The spawning process is rapid, lasting only 2 to 3 s, and chaotic, with the host male attacking the intruding catfish and the host female quickly attempting to collect displaced eggs (movies S2 and S3). Catfish eggs are nonmimetic and are spherical and smaller (2 mm in diameter) than the oval-shaped eggs of its natural hosts (S. diagramma: 4.5 mm) and experimental allopatric hosts (C. borleyi: 3.5 mm; Haplochromis sp. 44: 3.8 mm; and H. aeneocolor: 2.5 mm).
Our experimental stock consisted of 100 cuckoo catfish—20 fish imported from the wild as subadults and 80 individuals of their progeny (F1 generation). The sex of each individual was assessed from the shape of the urogenital papilla. Sexual maturity was confirmed by the expression of eggs or sperm under light pressure to the abdomen. Host cichlids (Table 1) were obtained from a commercial breeder (DH Cichlid) as young adults. Groups of 4 males and 12 to 16 females were housed together with five pairs of cuckoo catfish. We targeted host species that represented geographically diverse groups of potential hosts (Table 1).
Experimental setting
All experiments were conducted between February 2014 and March 2017. Experimental aquaria were equipped with internal filters and continuous aeration. The bottom was covered by fine gravel (3 to 10 mm) and enriched with shelters (sections of plastic tubing and ceramic pots). Water temperature was held at 26° to 28°C, and water conductivity was 550 μS/cm2. Fish experienced a 13:11 (light:dark) photoperiod.
Estimates of parasite prevalence were obtained from conspecific groups of host fish, each housed with five pairs of adult cuckoo catfish in a 350-liter aquaria (C. borleyi: 6 broods from a total of 12 females distributed between two aquaria; H. aeneocolor: 235 broods from 85 females in five groups; Haplochromis sp. 44: 59 broods from 34 females in two groups; and S. diagramma: 54 broods from 44 females in four groups). Brooding host females were identified during daily inspections by an extended buccal cavity. They were captured, and the number of their own eggs and catfish eggs was recorded by gently opening the mouth and flushing the entire contents of their buccal cavity into a container. All brooding females were checked within 12 hours of spawning.
Artificial infections were achieved using in vitro fertilized cuckoo catfish eggs. We selected reproductively active male and female cuckoo catfish by visual inspection of their genital papillae. Mature eggs were stripped from females by gently squeezing their abdomen, with the eggs collected in a dry Petri dish. Sperm was similarly stripped from males and collected directly from the genital opening with a pipette. Sperm and eggs were mixed on a Petri dish in 2 ml of aquarium water. Fertilized eggs were incubated for 24 hours in an artificial brooding chamber to ensure that unfertilized or damaged eggs were not used for experimental infections. After 24 hours, the cuckoo catfish eggs were used to parasitize host females that were brooding eggs of the same age (12 to 24 hours). Six catfish eggs were collected in a plastic pipette and released into the mouth of the host female when she opened her mouth during normal ventilatory movements. Host females were restrained underwater, and the entire process of egg introduction took <60 s. Sham control females received exactly the same handling with the exception that the pipette contained no eggs. An additional control was performed using the naturally spawned eggs of an allopatric cichlid host (H. aeneocolor) to infect sympatric host females (S. diagramma). The eggs of H. aeneocolor (diameter, 2.5 mm) are comparable in size to those of the cuckoo catfish (2 mm). Recently fertilized eggs were washed from the buccal cavity of female H. aeneocolor and used for experimental infections. Apart from the source of eggs, the procedure was identical to experimental infection with cuckoo catfish eggs. A full cross-fostering design was not possible because large S. diagramma eggs (4.5 mm) could not be successfully placed into the buccal cavity of allopatric female H. aeneocolor.
Experimentally infected host females (and sham controls) were individually housed in a separate 54-liter aquaria with a false floor constructed from stainless steel (4-mm mesh) and equipped with filtration and refuges. Any rejected offspring were retained below the mesh, preventing their potential subsequent consumption by the female and permitting unambiguous identification of their number and identity. Aquaria were visually isolated to minimize disturbance and were checked for rejected eggs 2 and 24 hours after experimental infection. After 24 hours, each female was captured, the contents of her buccal cavity were gently washed into a dish, and the number of host and parasite eggs was recorded. The removal of eggs from the buccal cavity always terminated the experimental trial; the eggs were never returned or replaced.
Parasite-naïve fish had never been exposed to cuckoo catfish before experimental parasitism. Parasite-experienced fish had all spawned in the presence of cuckoo catfish at least 2 months before their use in experiments. To retain a stable host density and sex ratio, we returned experienced females used in the experiment to their original home aquarium. New females were recruited as naïve hosts. Fish were not marked, and some host females may have been used more than once during the experiment. Groups of 45 naïve and 40 experienced H. aeneocolor and 34 naïve and 24 experienced S. diagramma females were used as experimental hosts.
Individual experience with the cuckoo catfish parasitism was not conflated with age. At the start of the experiment, naïve sympatric fish were 8 to 10 months old, and experienced sympatric fish were 8 to 12 months old. At the end of the experiment, naïve sympatric fish were 17 to 19 months old, whereas experienced sympatric fish were 17 to 21 months old. Both naïve and experienced fish reproduced before their use in the experiment. Therefore, overall reproductive experience (in contrast to the experience of parasitism) was not confounded with individual exposure to parasitism (parasite-naïve versus parasite-experienced females).
To measure the success of cuckoo catfish parasitism in terms of parasite and host brood survival until the completion of incubation, we used a procedure identical to the rejection rate experiment but with females housed in separate aquaria for 12 and 18 days for H. aeneocolor and S. diagramma, respectively. The duration of the experimental period corresponded with the natural brooding cycle of each host species. Sham controls were performed to provide estimates of brooding success and clutch size in the absence of parasitism. At the end of the brood care period, the entire contents of the female buccal cavity were flushed into a container, and the number of parasite and host juveniles was counted to ensure that all had been released by the female. Estimates of brood survival were obtained with a separate set of replicates; they do not represent an extension of the experiment on immediate rejections.
A caveat to our study is that experienced fish were housed with a group of catfish over the experiment (completed over 12 months) to control for any potential decay in their experience with catfish parasitism (19). A negative consequence of this setting was the possibility that experimentally infected (and control) experienced females could have been naturally parasitized during spawnings prior to experimental treatments, increasing the number of catfish eggs to which treatment females were exposed and introducing parasite eggs into sham (handling only) controls. This effect was likely marginal in sympatric S. diagramma females, where the natural prevalence of catfish parasitism was only 5.5% (Table 1), and we recorded no catfish eggs in any controls. However, allopatric H. aeneocolor females, with a parasitism prevalence of 22% (Table 1), did have catfish eggs in sham controls with experienced females (8 of 42 cases, 19%), and more parasite offspring than were experimentally introduced were subsequently retrieved from host females in 2 of 31 cases (5.5%). Consequently, these replicates (comprising 14% of the total) were discarded, although their inclusion did not alter the qualitative outcome of the analysis. It is conceivable that more than two experimentally infected broods were already naturally parasitized but not detected as such (up to seven parasitized broods were expected with a natural prevalence of 22% for 31 experimental infections, but only two were detected). Given the direction of bias and the outcome of our study, this experimental limitation makes our results more conservative (that is, the success of parasitized females was greater in experienced sympatric hosts). There was no difference in the number of parasite eggs or host eggs (including sham controls) between naïve and experienced females (tables S2 and S3).
Statistical analysis
For rates of natural parasitism in experimental aquaria, we recorded the occurrence of parasite eggs in host broods to estimate the prevalence of parasitism as a proportion of broods that contained at least one parasite egg.
To analyze rejection rates in experimentally parasitized broods, we recorded the number of parasite and host eggs that were found below the false mesh floor of aquaria after 2 and 24 hours and the number of parasite and host eggs that were recovered from the buccal cavities of brooding females after 24 hours. All analyses were conducted with data on egg rejections after 24 hours. Data on rejections after 2 hours provided identical results (tables S4 and S5), although rejection rates were generally lower (table S6), indicating that recorded rejections were not an immediate response to the presence of a foreign object in the buccal cavity or handling stress, but continued beyond 2 hours.
We initially coded parasite eggs data as a bivariate vector (ratio of inoculated to retained parasitic eggs) and fitted a GLM with binomial error structure with a log-link function in the lmer package in the R statistical environment. The rejected eggs were those collected from the aquarium floor. Retained eggs were those collected from the host buccal cavity. Evolutionary (sympatry and allopatry) and individual experience (naïve and experienced) with brood parasitism were fully crossed fixed factors. Given a potential bias arising from the presence of nonexperimental parasite eggs with parasite-experienced females and the potential role of handling on partial rejections, we presented conservative results for a Bernoulli GLM (table S1) as the main result. In this analysis, females were binary-coded as rejectors (rejected all parasite eggs) or acceptors (retained at least one parasite eggs). Note that the outcome for the original bivariate GLM (table S7) provided an identical interpretation and conclusion.
The success of both parasite and host broods over the incubation period were similarly analyzed by fitting a Bernoulli distribution (presence/absence of at least a single parasite or host offspring, respectively) to provide a conservative estimate. Given a complex, fully crossed three-way design in the analysis of host brood success, we additionally directly compared host brood success between experimentally parasitized and control broods for each of the four main treatments (sympatric naïve, sympatric experienced, allopatric naïve, and allopatric experienced) using a Bernoulli GLM with log-link function and single fixed factor (parasitism: experimentally infected, sham control).
In addition to presence/absence data, we also provided results for the size of own brood for each experimental scenario, with the mean number of offspring calculated over all broods (that is, including unsuccessful broods with 0 offspring) (fig. S1). This GLM was fitted with a quasi-Poisson error distribution (number of eggs) and log-link function. Separate models on the number of own eggs in broods were fitted for each host species (evolutionary contrast) because the natural size of their clutches was known to differ a priori, and that difference was not related to the study question. Host brood size declined after experimental parasitism except for experienced sympatric females (fig. S1).
Supplementary Material
Acknowledgments
We thank C. Tyler, R. Spence, and four anonymous referees for comments on the manuscript. National and institutional animal care and use guidelines were followed. Funding: This study was financially supported by the Czech Science Foundation (P505/12/G112 and 18-00682S). Author contributions: The study was conceived by M.R., R.B., M.P., C.S., and M.H. and designed by M.R., M.P., and R.B. Data were collected by R.B. and M.P. and analyzed by M.R. The manuscript was drafted by M.R., with important contributions made by all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are deposited at Figshare (doi: 10.6084/m9.figshare.5789349). Additional information related to this paper may be requested from M.R. (reichard@ivb.cz).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar4380/DC1
fig. S1. The cost of experimental parasitism in terms of the size of host own brood.
table S1. Full analysis of variance (ANOVA) table for a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 24 hours.
table S2. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on parasite brood survival over the incubation period.
table S3. Full ANOVA table of a Bernoulli GLM testing the roles of parasite treatment, evolutionary experience, and individual experience on host brood survival over the incubation period.
table S4. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 2 hours.
table S5. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of own eggs following experimental parasitism over the first 2 hours.
table S6. The number of eggs rejected over 2 and 24 hours by experimental females.
table S7. The results of a bivariate GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 24 hours.
movie S1. Undisturbed spawning of sympatric cichlid hosts, Simochromis diagramma.
movie S2. Spawning of sympatric host Simochromis diagramma with repeated cuckoo catfish intrusions and spawning.
movie S3. Undisturbed spawning of allopatric host Haplochromis aeneaocolor followed by cuckoo catfish intrusions and spawning.
REFERENCES AND NOTES
- 1.C. N. Spottiswoode, R. M. Kilner, N. B. Davies, Blood parasitism, in The Evolution of Parental Care, N. J. Royle, P. T. Smiseth, M. Kölliker, Eds. (Oxford Univ. Press, 2012), pp. 226–243. [Google Scholar]
- 2.Davies N. B., Bourke A. F. G., de L Brooke M., Cuckoos and parasitic ants: Interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278 (1989). [DOI] [PubMed] [Google Scholar]
- 3.S. I. Rothstein, S. K. Robinson, Parasitic Birds and Their Hosts: Studies in Coevolution (Oxford Univ. Press, 1998). [Google Scholar]
- 4.N. B. Davies, Cuckoos, Cowbirds, and Other Cheats (Poyser, 2000). [Google Scholar]
- 5.Brandt M., Foitzik S., Fischer-Blass B., Heinze J., The coevolutionary dynamics of obligate social parasite systems—Between prudence and antagonism. Biol. Rev. 80, 251–267 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Kilner R. M., Langmore N. E., Cuckoos versus hosts in insects and birds: Adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Medina I., Langmore N. E., The evolution of acceptance and tolerance in hosts of avian brood parasites. Biol. Rev. 91, 569–577 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Soler M., Møller A. P., Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature 343, 748 (1990). [Google Scholar]
- 9.Langmore N. E., Hunt S., Kilner R. M., Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Davies N. B., Welbergen J. A., Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Canestrari D., Bolopo D., Turlings T. C., Röder G., Marcos J. M., Baglione V., From parasitism to mutualism: Unexpected interactions between a cuckoo and its host. Science 343, 1350–1352 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Feeney W. E., Medina I., Somveille M., Heinsohn R., Hall M. L., Mulder R. A., Stein J. A., Kilner R. M., Langmore N. E., Brood parasitism and the evolution of cooperative breeding in birds. Science 342, 1506–1508 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Lyon B. E., Eadie J. M., Conspecific brood parasitism in birds: A life-history perspective. Annu. Rev. Ecol. Evol. Syst. 39, 343–363 (2008). [Google Scholar]
- 14.Sato T., A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58–59 (1986). [DOI] [PubMed] [Google Scholar]
- 15.R. J. Wootton, C. Smith, Reproductive Biology of Teleost Fishes (Wiley-Blackwell, 2015). [Google Scholar]
- 16.Wickler W., ‘Egg-dummies’ as natural releasers in mouth-breeding cichlids. Nature 194, 1092–1093 (1962). [Google Scholar]
- 17.Wagner C. E., Harmon L. J., Seehausen O., Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366 (2012). [DOI] [PubMed] [Google Scholar]
- 18.A. Konings, Back to Nature: Guide to Tanganyika Cichlids (Cichlid Press, ed. 3, 2005). [Google Scholar]
- 19.Wisenden B. D., Alloparental care in fishes. Rev. Fish Biol. Fish. 9, 45–70 (1999). [Google Scholar]
- 20.Payne R. B., Woods J. L., Payne L. L., Parental care in estrildid finches: Experimental tests of a model of Vidua brood parasitism. Anim. Behav. 62, 473–483 (2001). [Google Scholar]
- 21.Grim T., Samaš P., Hauber M. E., The repeatability of avian egg ejection behaviors across different temporal scales, breeding stages, female ages and experiences. Behav. Ecol. Sociobiol. 68, 749–759 (2014). [Google Scholar]
- 22.Strausberger B. M., Rothstein S. I., Parasitic cowbirds may defeat host defense by causing rejecters to misimprint on cowbird eggs. Behav. Ecol. 20, 691–699 (2009). [Google Scholar]
- 23.Hosoi S. A., Rothstein S. I., Nest desertion and cowbird parasitism: Evidence for evolved responses and evolutionary lag. Anim. Behav. 59, 823–840 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Reichard M., Polačik M., Tarkan A. S., Spence R., Gaygusuz Ö., Ercan E., Ondračková M., Smith C., The bitterling–mussel coevolutionary relationship in areas of recent and ancient sympatry. Evolution 64, 3047–3056 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Procházka P., Konvičková-Patzenhauerová H., Požgayová M., Trnka A., Jelínek V., Honza M., Host genotype and age have no effect on rejection of parasitic eggs. Naturwissenschaften 101, 417–426 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Hauber M. E., Yeh P. J., Roberts J. O. L., Patterns and coevolutionary consequences of repeated brood parasitism. Proc. R. Soc. Lond. B Biol. Sci. 271 (suppl. 5), S317–S320 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina-Morales M., Martínez J. G., Martín-Gálvez D., Dawson D. A., Burke T., Avilés J. M., Cuckoo hosts shift from accepting to rejecting parasitic eggs across their lifetime. Evolution 68, 3020–3029 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Langmore N. E., Kilner R. M., Why do Horsfield’s bronze-cuckoo Chalcites basalis eggs mimic those of their hosts? Behav. Ecol. Sociobiol. 63, 1127–1131 (2009). [Google Scholar]
- 29.Shizuka D., Lyon B. E., Coots use hatch order to learn to recognize and reject conspecific brood parasitic chicks. Nature 463, 223–226 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Lotem A., Nakamura H., Zahavi A., Constraints on egg discrimination and cuckoo-host co-evolution. Anim. Behav. 49, 1185–1209 (1995). [Google Scholar]
- 31.Thorogood R., Davies N. B., Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337, 578–580 (2012). [DOI] [PubMed] [Google Scholar]
- 32.M. H. A. Keenleyside, Parental care, in Cichlid Fishes: Behavior, Ecology and Evolution, M. H. A. Keenleyside, Ed. (Chapman and Hall, 1991), pp. 191–208. [Google Scholar]
- 33.R. Coleman, Cichlid egg size summary (1991); http://cichlidresearch.com/eggtabt.html.
- 34.M. S. Cohen, “Host-parasite interactions of the African cuckoo catfish (Synodontis multipunctatus),” thesis, University of Colorado at Boulder (2015). [Google Scholar]
- 35.Šulc M., Procházka P., Capek M., Honza M., Common cuckoo females are not choosy when removing an egg during parasitism. Behav. Ecol. 27, 1642–1649 (2016). [Google Scholar]
- 36.Kilner R. M., The evolution of virulence in brood parasites. Ornithol. Sci. 4, 55–64 (2005). [Google Scholar]
- 37.Spottiswoode C. N., Koorevaar J. A., A stab in the dark: Chick killing by brood parasitic honeyguides. Biol. Lett. 8, 241–244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day J. J., Wilkinson M., On the origin of the Synodontis catfish species flock from Lake Tanganyika. Biol. Lett. 2, 548–552 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salzburger W., Van Bocxlaer B., Cohen A. S., Ecology and evolution of the African Great Lakes and their faunas. Annu. Rev. Ecol. Evol. Syst. 45, 519–545 (2014). [Google Scholar]
- 40.Seehausen O., African cichlid fish: A model system in adaptive radiation research. Proc. R. Soc. Lond. B Biol. Sci. 273, 1987–1998 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koblmüller S., Sturmbauer C., Verheyen E., Meyer A., Salzburger W., Mitochondrial phylogeny and phylogeography of East African squeaker catfishes (Siluriformes: Synodontis). BMC Evol. Biol. 6, 49 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koblmüller S., Egger B., Sturmbauer C., Sefc K. M., Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Mol. Phylogenet. Evol. 55, 318–334 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar4380/DC1
fig. S1. The cost of experimental parasitism in terms of the size of host own brood.
table S1. Full analysis of variance (ANOVA) table for a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 24 hours.
table S2. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on parasite brood survival over the incubation period.
table S3. Full ANOVA table of a Bernoulli GLM testing the roles of parasite treatment, evolutionary experience, and individual experience on host brood survival over the incubation period.
table S4. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 2 hours.
table S5. The results of a Bernoulli GLM to test the roles of evolutionary experience and individual experience on rejection of own eggs following experimental parasitism over the first 2 hours.
table S6. The number of eggs rejected over 2 and 24 hours by experimental females.
table S7. The results of a bivariate GLM to test the roles of evolutionary experience and individual experience on rejection of parasite eggs over the first 24 hours.
movie S1. Undisturbed spawning of sympatric cichlid hosts, Simochromis diagramma.
movie S2. Spawning of sympatric host Simochromis diagramma with repeated cuckoo catfish intrusions and spawning.
movie S3. Undisturbed spawning of allopatric host Haplochromis aeneaocolor followed by cuckoo catfish intrusions and spawning.


