Abstract
The retinal pigment epithelium (RPE) is a highly polarized multi-functional epithelium that is located between the neural retina and the choroid of the eye. It is a single sheet of pigmented cells that are hexagonally packed and connected by tight junctions. The main functions of the RPE include absorption of light, phagocytosis of the shed photoreceptor outer segments, spatial buffering of ions, transport of nutrients, ions and water as well as active involvement in the visual cycle. With such important and diverse functions, it is critically important to study the biology of RPE cells. A number of RPE cell lines have been established; however, passaged and immortalized cells are known to quickly lose some of the morphological and physiological characteristics of natural RPE cells. Thus, primary cells are more suitable for studying different aspects of RPE cell biology and function. Mouse primary RPE cell culture is very useful to researchers since mouse models are widely used in biological studies, however collecting RPE cells from mouse is also very challenging due to their small size. Here, we present a protocol for establishing primary mouse RPE cell cultures which includes enucleation and dissection of the eyes and isolation of the RPE sheets to yield the cells for culturing. This method enables efficient cell recovery. The RPE cells obtained from two mice can reach confluency on one 12 mm polyester membrane insert pre-loaded in culture plate after one week of culture and display some of the original properties of bona fide RPE cells such as hexagonal shape and pigmentation after two weeks of culture.
Keywords: Biology, Issue 133, Retinal pigment epithelium, primary cell culture, ocular cell lines, eye dissection, isolation of eye tissues, ophthalmology, mouse
Introduction
The retinal pigment epithelium (RPE) is a single layer of polarized epithelial cells that is located between the neural retina and the choroid of the eye. The functionality of the RPE cells and the integrity of the RPE monolayer are critical for vision because the RPE plays a major role in multiple processes such as maintaining the outer blood-retinal barrier, transport of water and ions between the retina and the choroid, light absorption, protection from oxidative stress, control of retinoid metabolism, and phagocytosis of the outer segments of the photoreceptors1,2. The location of the RPE at the back of the eye, as well as its barrier function preventing drugs administered systemically from passing from the blood to the vitreous humor, make it difficult to study the complexity of the RPE function in vivo. Thus, there is a great need for the establishment of RPE cell cultures to study the RPE cells in a flexible, controlled environment3,4.
A number of established RPE cell lines exist, providing an easy and convenient way of obtaining and storing the cells; however, passaged cells have some disadvantages compared to primary cells2,3,4. First, they are often characterized by changes in cell morphology. For example, none of the existing cell lines were found to be suitable for a reliable study of the RPE barrier properties due to the loss of cell polarity phenotype and partial disappearance of tight junctions4. In addition to the loss of polarity and proper cell-to-cell connections, the RPE cell lines quickly lose their pigmentation due to the absence of the key melanogenesis enzymes in the adult RPE5. The pigmentation can be restored, but the comprehensive analysis of the mechanism of re-pigmentation which would include a combination of transmission electron microscopy, gene expression analysis and chemical assays to confirm the presence of melanin has never been performed6. One more limitation is that the RPE cell lines have extended cell life (sometimes - immortality) and under certain conditions can transform into self-renewing multipotent stem cells that detach from the substrate and form floating colonies7,8. This limitation makes it impossible to use the cell lines for transplantation experiments3.
Considering the disadvantages of the established RPE cell lines, primary RPE cell cultures obtained from fresh tissues might serve as a more biologically relevant model to study the RPE. Primary RPE cells have been used not only to study RPE-specific functions such as vitamin A metabolism9, phagocytosis of the photoreceptor outer segments10 and ion transport11, but also to study basic cell biology such as epithelial cell polarity2 , lysosomal homeostasis, and autophagy12,13.
In the last couple of years there have been a number of publications on establishing primary RPE cultures, indicating a growing interest in this area of research3,14,15. Numerous protocols for human RPE cells and non-human RPE cells such as bovine and porcine RPE cells were published16,17,18,19. However, it is more difficult to handle mouse RPE cells because of their much smaller size. Even though quite a number of publications have described protocols to isolate RPE cells from the mouse14,20,21, there are still many researchers struggling to isolate RPE cells without contamination of choroid cells or cells from neural retina debris. Here we present the protocol for establishing primary mouse RPE cell culture, including obtaining the eyes from the mouse, dissection of the eyes and isolation of the RPE sheets to yield the cells for culturing. This video protocol would be especially useful for researchers who are starting to work with mouse primary RPE cultures and need guidance on the dissection techniques.
Protocol
Procedures involving animal subjects have been approved by the Institutional Animal Care and Use Committee (IACUC) at University of Pittsburgh
1. Prepare solutions
Prepare growth medium by supplementing Dulbecco Modified Eagle Medium (DMEM), high glucose with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 2.5 mM L-glutamine, and 1x MEM nonessential amino acids. Pre-warm the media in 37 °C incubator before use.
- Prepare 2% (wt/vol) dispase II working solution:
- Prepare a stock solution of 100 mg/mL dispase II by dissolving 30 mg of dispase II in 300 µL HEPES-buffered saline (50 mM HEPES/KOH pH 7.4, 150 mM NaCl). This volume is for 3 - 4 mice, it can be scaled up or down based on the number of mice for the experiment). This stock can be stored for at least 1 week at 4 °C.
- Prepare the working solution of 2% dispase by adding 300 µL of the 100 mg/mL dispase II stock to 1.2 mL of sterile DMEM, high glucose and filtering the solution through a 0.22 µm filter. This step should be performed in the laminar flow cabinet under sterile conditions. Pre-warm the prepared dispase II in 37 °C incubator before use.
2. Obtaining the mouse eyes
Euthanize the mouse using any approved method and place it on an absorbent pad.
Wearing gloves, put the thumb and index finger around the eye and gently push the skin to proptose the eyeball.
Insert the tip of angled scissors between the skin and the eyeball and carefully cut out the eye. To avoid contamination, briefly dip the eye in 70% ethanol.
Immediately place the eye in the Phosphate-buffered Saline (PBS) in a Petri dish.
Repeat steps 2.2 - 2.4 to remove the second eye.
3. Eye dissection
- Carefully remove connective tissue from the eye under the dissecting microscope. This can be done either in PBS or on the dry Petri dish.
- Use suturing forceps to lift the connective tissues from the eyeball and cut them out using Vannas scissors. Do not cut the sclera because it is practically impossible to obtain the intact posterior eyecups if the sclera is cut.
- After cleaning the connective tissue, keep the resulting eyeballs in PBS for the next washing step. NOTE: All the following steps should be performed in the laminar flow cabinet under sterile conditions.
Wash the eyes twice in pre-warmed 37 °C DMEM (high glucose). Carefully transfer the eyeballs into the pre-warmed 37 °C DMEM (high glucose) using forceps. Aspirate the medium and transfer eyeballs to fresh medium in a new dish.
Aspirate DMEM (high glucose) medium and incubate the eyes in the pre-warmed 2% (wt/vol) dispase II working solution for 45 min in the 37 °C incubator. NOTE: In all the following steps, place and manipulate eyes or eyecups in the growth medium, which will protect the tissue and provide a good medium for the dissection.
Remove the dispase II solution and wash the eyes twice in growth medium by aspirating the old medium and adding the new pre-warmed 37 °C growth medium (Figure 1a). NOTE: After dissociation by dispase II, the eyeballs become soft.
- Hold the eye with forceps and make an incision around the ora serrata of each eye using Vannas scissors under the dissecting microscope placed in the laminar flow cabinet.
- Keeping the eyeball in the solution, carefully remove the anterior cornea by extending the cut around the ora serrata circumference and pulling the anterior cornea away after the cut has been complete.
- Remove the lens capsule and associated iris pigmented epithelium by gently pulling them out of the eyecup with teethed forceps (Figure 1b, 1c).
Incubate the resulting posterior eyecups in the growth medium for 20 min at 37 °C to facilitate separation of the neural retina from the RPE.
- Cut the posterior eyecups into 4 petals under the dissecting microscope placed in the laminar flow cabinet. The cuts should be long enough to flatten the eyecup, but short enough to keep the petals connected (Figure 1d).
- Flatten the eyecup and remove the neural retina by very gently pulling it with forceps while holding the remaining RPE-choroid-sclera complex with another forceps (Figure 1e). Start pulling from the edges rather than from the center.
Transfer all the quartered tissues into a new sterile culture dish filled with pre-warmed 37 °C growth medium.
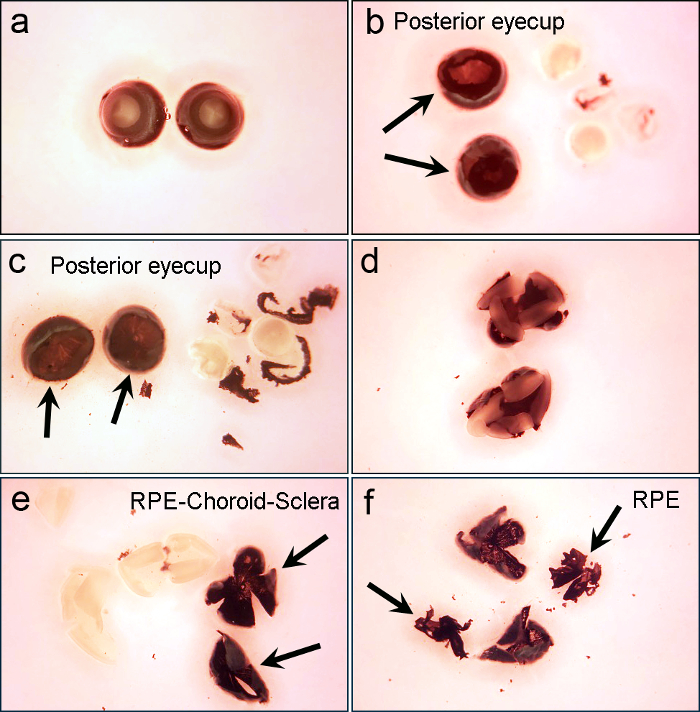
4. Isolation of primary RPE cells
- Holding one petal of the quartered RPE-choroid-sclera complex with super fine forceps, gently peel off the intact sheets of RPE from the underlying basement membrane (Bruch's membrane) by using a microsurgical crescent knife under the dissecting microscope placed in the laminar flow cabinet (Figure 1f).
- Transfer the RPE sheets into the new sterile culture dish containing growth medium by using a P200 micropipette. When transferring RPE sheets, wet the pipette tips with growth medium by pipetting several times to prevent tissue from sticking inside the tip. RPE will be clearly distinguishable from the choroid: RPE looks more brownish, while choroid is darker and looks sticky and fluffy.
- Alternatively, use enzymatic digestion and gentle dissociation without forceps to detach the RPE from the choroid14.
Wash the RPE sheets 2 - 3 times using pre-warmed 37 °C growth medium in the sterile culture dish. After each washing step, carefully collect the RPE sheets while avoiding other tissues such as retina debris or choroid. At the end of the last wash collect the RPE sheets with a P200 micropipette and place them in a new 1.5 mL microcentrifuge tube. NOTE: There is no need to centrifuge the RPE sheets: they will sediment by gravity within 1 min.
Remove the extra growth medium using a P200 micropipette. Resuspend the cells in fresh pre-warmed growth medium and gently triturate them using a P200 micropipette. Avoid formation of bubbles. A single-cell suspension is ideal, but also avoid too much trituration, otherwise RPE cells are not able to survive. We recommend very gently trituration for 40 - 50 times.
5. Culturing RPE cells
- Plate the cells on a culture plate.
- If downstream applications of the RPE cells require retaining of their polarity, plate the RPE cells from 2 mice in one 12 mm polyester membrane insert pre-loaded in 12-well culture plates. Plating the cells at high density is desired because in that case RPE cells are able to keep the original properties, such as hexagonal shape and pigmentation.
Do not move the plate or change the growth medium for the first 72 h of culturing. After 3 days, replace the old medium with the fresh pre-warmed growth medium. After that change the culture medium every other day. Once RPE cells get confluency, reduce the FBS in growth medium to 2%. NOTE: The cells can be split using 0.25% trypsin. However, after splitting the cells may lose their hexagonal shape and pigmentation in the subsequent passages. We do not recommend splitting the cells, but if it is required by the downstream applications of the culture, keep in mind that the primary cells cannot be passaged indefinitely: they are known to de-differentiate after 5 - 7 passages.
Representative Results
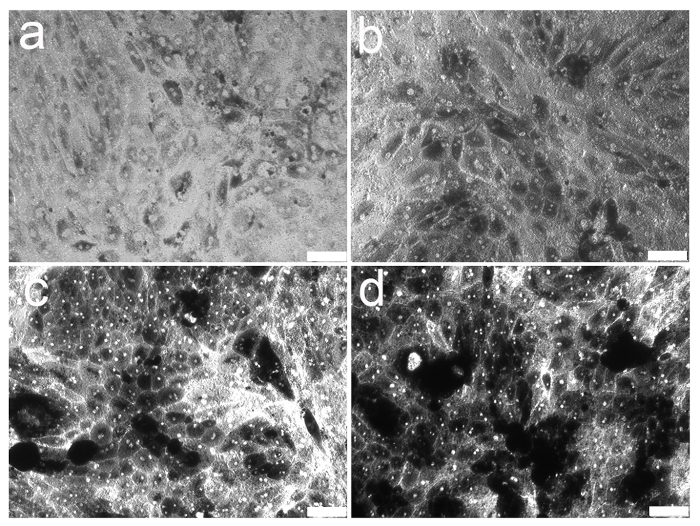
The mouse primary RPE culture established from 2 mice in 12 mm polyester membrane insert reaches 90 - 95% confluency after 1 week in culture. After 2 weeks of culture, the cells reached 100% confluency and started to form a mosaic of hexagonal, pigmented, and bi-nucleated cells. By 3 weeks of culture, the cells continued to form the shape and pigmentation, however, after 4 weeks a portion of cells got hyperpigmented (Figure 2).
The purity of the primary RPE cell culture can be assessed by immunostaining using the RPE65 antibody (retinoid isomerohydrolase, a critical enzyme in the vertebrate visual cycle that enables the conversion of all-trans-retinyl esters to 11-cis-retinol during phototransduction) (Figure 3, red). The integrity of the cell-to-cell junctions and the presence of hexagonal shape can be demonstrated using phalloinin staining (Figure 3, green). In addition, expression of ZO-1 protein, an important component of tight junctions, can be validated using Western blotting (Figure 4) or immunostaining.
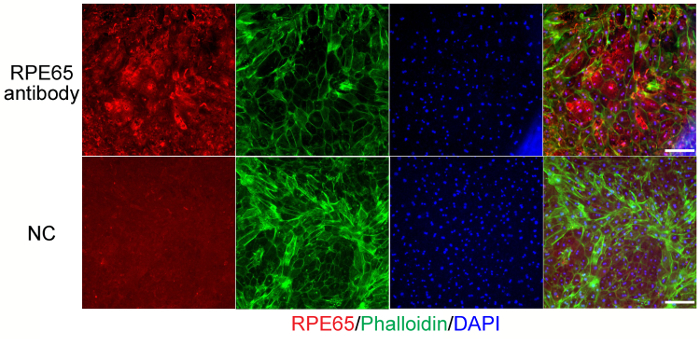
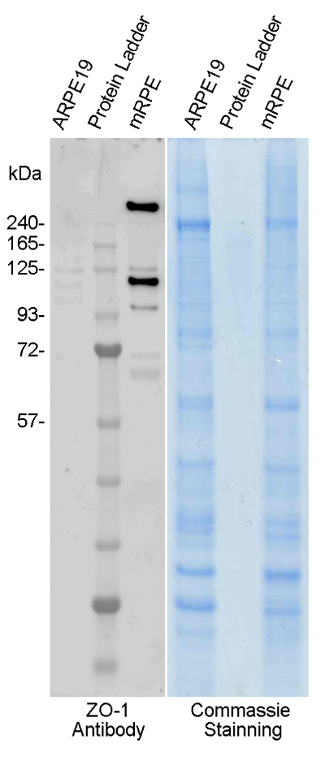
Our results demonstrate that the obtained mouse primary RPE cultures are able to proliferate, retain pigmentation, hexagonal shape and tight junctions, and express functional markers such as RPE65.
Figure 1. Different steps of eye dissection to obtain primary RPE cell cultures. (a) After dissociation in 2% dispase, the eye is washed in growth medium. (b) The posterior eyecup is obtained by removing the cornea and lens. (c) The resulting eyecup is further cleaned by removing the associated iris pigmented epithelium. (d) After further incubation in growth medium, the eyecup is cut radially to generate quadrants. (e)The neural retina is peeled off from the remaining RPE-choroid-sclera complex. (f) The RPE sheets are gently separated from the Bruch's membrane. Note that the RPE looks more brownish (arrows), while choroid is darker and very sticky. Please click here to view a larger version of this figure.
Figure 2. Primary RPE cells from a 3-week old mouse cultured on a 12 mm polyester membrane insert pre-loaded in 12-well culture plates. The mouse primary RPE culture established from 2 mice in 12 mm polyester membrane insert reaches 90 - 95% confluency after 1 week in culture (a). After 2 weeks of culture, the cells reached 100% confluency and started to form a mosaic of hexagonal, pigmented and bi-nucleated cells (b). By 3 weeks of culture the cells continued to form the shape and pigmentation (c), however, after 4 weeks a portion of cells got hyperpigmented (d). Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 3. The RPE cell marker, RPE65, is detectable in primary mouse RPE cells. Primary mouse RPE cells after 4 weeks of culture were fixed in 4% paraformaldehyde (PFA) and incubated with either RPE65 antibody or blocking buffer as the negative control (NC). Phalloidin (green) and DAPI (blue) were also added to stain the cell membranes and nuclei. Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 4. The marker of tight junctions, ZO-1 is expressed in the primary RPE cells. Primary mouse RPE cells (mRPE) were harvested after 3 weeks of culture and 8 µg of the cell lysate was run on a Western blot. The equivalent amount of the ARPE-19 cells (commercially available stable RPE cell line) were used as a negative control. ZO-1 was highly expressed in the primary RPE cells while being absent from the ARPE-19 cells. Please click here to view a larger version of this figure.
Discussion
The presented detailed protocol enables reliable establishment of mouse primary RPE cultures that reach confluency after 1 week and present the main RPE characteristics such as hexagonal shape and pigmentation after 2 weeks. The obtained RPE cells can be used for a number of downstream applications such as vitamin A metabolism9, phagocytosis of the photoreceptor outer segments10 and ion transport11, epithelial cell polarity2, lysosomal homeostasis, and autophagy12,13. Depending on the downstream application, the morphology of the derived cultures can be validated by transmission and scanning electron microscopy as described in detail elsewhere14. Additional assays to demonstrate maturation of the cultures might include transepithelial electrical resistance (TEER) assay to demonstrate polarity of the RPE cells, permeability assays4,22, functional tests (phagocytosis23) and dome formation.
In our experience, the more cells that are plated in a well, the better the confluency and the phenotype. In this protocol, we seeded the RPE cells from 2 mice on one 12 mm inserts. However, it is also feasible to put RPE cells from 2 mice into one 6.5 mm insert to get better confluency and TEER. In many studies, the plates for RPE cell cultures are coated with Matrigel or laminin. We have not seen a significant difference in RPE cell attachment to these vessels with or without coating. In addition, the percentage of FBS in the culture medium might be modified to achieve better results. 10% FBS is usually good for reaching confluency, but dropping to less FBS seems to be beneficial for RPE differentiation/maturation14,17.
The main limitation of this method is the need for precise training of the person performing the eye dissections to ensure maximum efficiency of RPE cell recovery without cross-contamination with cells derived from other ocular tissues. To avoid cross-contamination, the researcher should master the critical step of this procedure: careful peeling off the retina followed by precise separation of the RPE from the choroid. The purity of the obtained cultures can be validated using RPE-specific immunostaining. A number of RPE markers are available for characterization of the RPE cultures, including genes involved in the visual cycle, barrier and transport function, metabolism, phagocytosis, and melanogenesis15.
Another limitation is the number of eyes that can be processed simultaneously to obtain the cultures. In general, we do not recommend working with more than 2 mice at a time. To ensure viability of the RPE cells, the eyes should be dissected as quickly as possible. After the user become more proficient, it is possible to handle more mice by alternating incubation times.
Overall, a number of methods have been published on the establishing the primary mouse RPE cultures3,14,15, however, for the first time, we present a video version of the complete protocol. This protocol can be used as guidance on the eye dissection techniques. However, the optimal balance between the high speed and precision required for the dissection can be achieved only with practice.
Disclosures
DS has received research funding from Bayer HealthCare, Germany and F. Hoffmann-La Roche, Switzerland. The remaining authors declare no competing financial interests. This work is supported by Research to Prevent Blindness (unrestricted grants to the Wilmer Eye Institute and University of Pittsburgh). This work is also supported by start-up funds to DS from Ophthalmology, University of Pittsburgh.
Acknowledgments
This study was partly funded by The BrightFocus Foundation (to DS).
References
- Martínez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–777. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- Lehmann GL, Benedicto I, Philp NJ, Rodriguez-Boulan E. Plasma membrane protein polarity and trafficking in RPE cells: past, present and future. Exp Eye Res. 2014;126:5–15. doi: 10.1016/j.exer.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronk AH, Vargis E. Methods for culturing retinal pigment epithelial cells: a review of current protocols and future recommendations. J Tissue Eng. 2016;7 doi: 10.1177/2041731416650838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ. Barrier properties of cultured retinal pigment epithelium. Exp Eye Res. 2014;126:16–26. doi: 10.1016/j.exer.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Lu F, Yan D, Zhou X, Hu DN, Qu J. Expression of melanin-related genes in cultured adult human retinal pigment epithelium and uveal melanoma cells. Mol Vis. 2007;13:2066–2072. [PubMed] [Google Scholar]
- Boulton ME. Studying melanin and lipofuscin in RPE cell culture models. Exp Eye Res. 2014;126:61–67. doi: 10.1016/j.exer.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini JS, Temple S, Stern JH. Human Retinal Pigment Epithelium Stem Cell (RPESC) Adv Exp Med Biol. 2016;854:557–562. doi: 10.1007/978-3-319-17121-0_74. [DOI] [PubMed] [Google Scholar]
- Akrami H, et al. Retinal pigment epithelium culture;a potential source of retinal stem cells. J Ophthalmic Vis Res. 2009;4:134–141. [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bok D. The use of cultured human fetal retinal pigment epithelium in studies of the classical retinoid visual cycle and retinoid-based disease processes. Exp Eye Res. 2014. pp. 46–50. [DOI] [PMC free article] [PubMed]
- Mazzoni F, Safa H, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res. 2014;126:51–60. doi: 10.1016/j.exer.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart N, Strauss O. Ion channels and transporters of the retinal pigment epithelium. Exp Eye Res. 2014;126:27–37. doi: 10.1016/j.exer.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Valapala M, et al. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang P, et al. The amino acid transporter SLC36A4 regulates the amino acid pool in retinal pigmented epithelial cells and mediates the mechanistic target of rapamycin, complex 1 signaling. Aging Cell. 2017. [DOI] [PMC free article] [PubMed]
- Fernandez-Godino R, Garland DL, Pierce EA. Isolation, culture and characterization of primary mouse RPE cells. Nat Protoc. 2016;11:1206–1218. doi: 10.1038/nprot.2016.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer BA, Philp NJ. Cell culture of retinal pigment epithelium: Special Issue. Exp Eye Res. 2014;126:1–4. doi: 10.1016/j.exer.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Singh S, Woerly S, McLaughlin BJ. Natural and artificial substrates for retinal pigment epithelial monolayer transplantation. Biomaterials. 2001;22:3337–3343. doi: 10.1016/s0142-9612(01)00171-5. [DOI] [PubMed] [Google Scholar]
- Sonoda S, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Del Priore LV, Kaplan HJ. Tissue culture of retinal pigment epithelium following isolation with a gelatin matrix technique. Experimental eye research. 1997;64:133–139. doi: 10.1006/exer.1996.0199. [DOI] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Lakkaraju A. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Exp Eye Res. 2014. pp. 74–85. [DOI] [PMC free article] [PubMed]
- Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci USA. 2003;100:6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Williams DS. Isolation and culture of primary mouse retinal pigmented epithelial cells. Adv Exp Med Biol. 2003;533:347–352. doi: 10.1007/978-1-4615-0067-4_44. [DOI] [PubMed] [Google Scholar]
- Pitkänen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC. Analysis of photoreceptor outer segment phagocytosis by RPE cells in culture. Methods Mol Biol. 2013;935:285–295. doi: 10.1007/978-1-62703-080-9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]


