Abstract
Purpose
The incidence of papillary thyroid carcinoma (PTC) arising from the isthmus is low; however, these tumors have aggressive clinical and pathological features. Moreover, the existing guidelines regarding the extent of surgery for this type of tumor are unclear.
Methods
This study enrolled 282 patients who underwent total thyroidectomy with bilateral central lymph node dissection. The patients were divided into 2 groups based on the location of the median line of the PTC. Group I included patients in whom the median line was located between the lateral margins of the trachea; group II included all others. We compared the 2 groups and conducted a multivariate analysis to assess risk factors for contralateral node metastasis from a PTC arising from the isthmus.
Results
Patients in group I had significantly higher frequencies of extrathyroidal extension and central lymph node metastasis. Group I also had a higher frequency of contralateral node metastasis, and a tumor size >1.0 cm was identified as an independent risk factor for contralateral node metastasis among patients in this group.
Conclusion
Bilateral central lymph node dissection could be considered for patients with isthmic PTCs >1.0 cm in size who have clinically suspicious node metastasis.
Keywords: Papillary thyroid carcinoma, Papillary thyroid microcarcinoma, Lymph node excision
INTRODUCTION
The thyroid isthmus is a small tissue segment that connects both thyroid lobes and is adjacent to the trachea anteriorly and strap muscles dorsally. Papillary thyroid carcinomas (PTCs) rarely arise from the isthmus; however, these tumors exhibit relatively more aggressive features, such as multifocality, extrathyroidal extension, and lymphovascular invasion, compared to those arising from the thyroid lobes [1,2,3].
Central lymph node metastasis is frequently observed in patients with PTC, with reported incidences ranging from approximately 20% to 39% [4,5,6,7,8]. Furthermore, PTC in the isthmus, or isthmic PTC, tends to more strongly associate with central lymph node metastasis, compared to lobar tumors [9]. However, the 2015 American Thyroid Association guidelines and 2017 National Comprehensive Cancer Network guidelines lack recommendations regarding central lymph node dissection for patients with isthmic PTC [10,11].
To our best knowledge, although some studies have addressed the extent of thyroidectomy for patients with PTC arising in the isthmus, few have discussed the extent of central lymph node dissection. Therefore, in this study we compared clinicopathological findings by PTC location and investigated the risk factors for contralateral node metastasis in patients with isthmic PTC.
METHODS
Study population
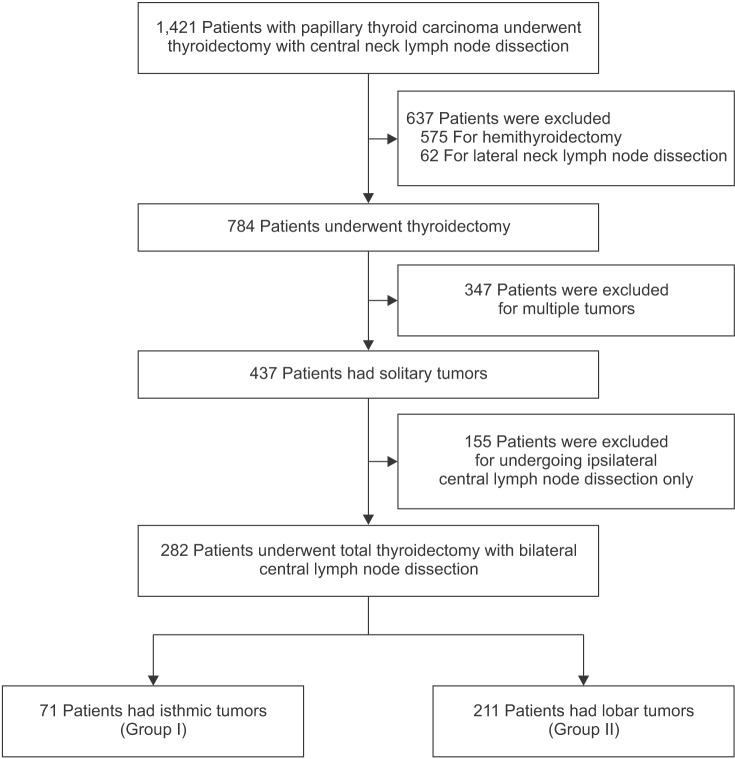
Between January 2008 and December 2016, we retrieved the records of 1,421 patients with PTC who underwent thyroidectomy with central neck lymph node. Among these, 575 patients who underwent hemi-thyroidectomy and 62 patients who underwent lateral neck lymph node dissection were excluded. To avoid the influence of lymph node metastases from multifocal thyroid tumors and focus the analysis only on contralateral lymph node metastasis, 347 patients with multiple tumors and 155 patients who underwent only ipsilateral central lymph node dissection were also excluded. Finally, 282 patients who underwent total thyroidectomy with bilateral central lymph node dissection remained eligible and were divided into 2 groups as described below (Fig. 1). At out institute, bilateral central lymph node dissection was performed in patients with clinically suspicious node metastasis in preoperative radiologic findings or intraoperative suspicious node metastasis. This retrospective study was approved by the Institutional Review Board of Korea University Medical Center, Ansan (approval number: AS17032). A waiver of informed consent was requested, and approval was obtained.
Fig. 1. Flowchart of patients with papillary thyroid cancer in this study. Group I, patients had isthmic tumors; group II, patients had lobar tumors.
Definitions
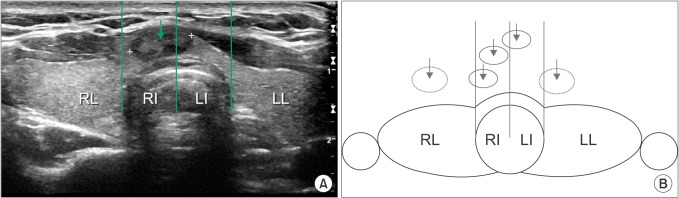
The preoperative ultrasonography findings of all 282 patients in this study were reviewed; the patients were divided into 2 groups based on the location of the median line of the PTC. Group I included patients in whom the median line of the PTC was located between the lateral margins of the trachea; group II was defined as all other eligible patients with PTC. Each group was further subdivided as follows for the contralateral central lymph node analysis: right lobe, left lobe, right isthmus, and left isthmus (Fig. 2).
Fig. 2. (A) Ultrasonogram of a papillary thyroid carcinoma (arrow) in the isthmus. (B) Schematic illustration of the structures used to define groups in this study. Tumors (solid circle) for which the median line (arrow) was located between the lateral margins of trachea were classified as group I; all others (dotted circle) were classified as group II. RL, right lobe; RI, right isthmus; LI, left isthmus; LL, left lobe.
Surgery and pathologic examination
Central lymph node dissection was performed with the following boundaries: the hyoid bone superiorly, the carotid sheath laterally, the manubrium inferiorly, and the prevertebral fascia dorsally. The central lymph nodes were divided into right and left using the median line of the trachea, and the Delphian nodes were included as ipsilateral lymph nodes. The entire surgical thyroid specimen and central lymph nodes were sent for pathologic examination, and the histologic findings were recorded and analyzed. TNM staging was performed according to the American Joint Committee on Cancer staging manual (8th edition).
Statistical analysis
Continuous data are presented as medians with ranges, and Student t-test was used to compare 2 groups. For both groups, the number of metastatic lymph nodes is presented as a mean value with a range because many patients had no lymph node metastases. Categorical data are presented as numbers with percentages and were compared using the chi-square test or Fisher exact test. A binary logistic regression was used to conduct the multivariate analysis. Differences were considered statistically significant at a P-value of <0.05. All data were analyzed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
RESULTS
Patient and tumor characteristics
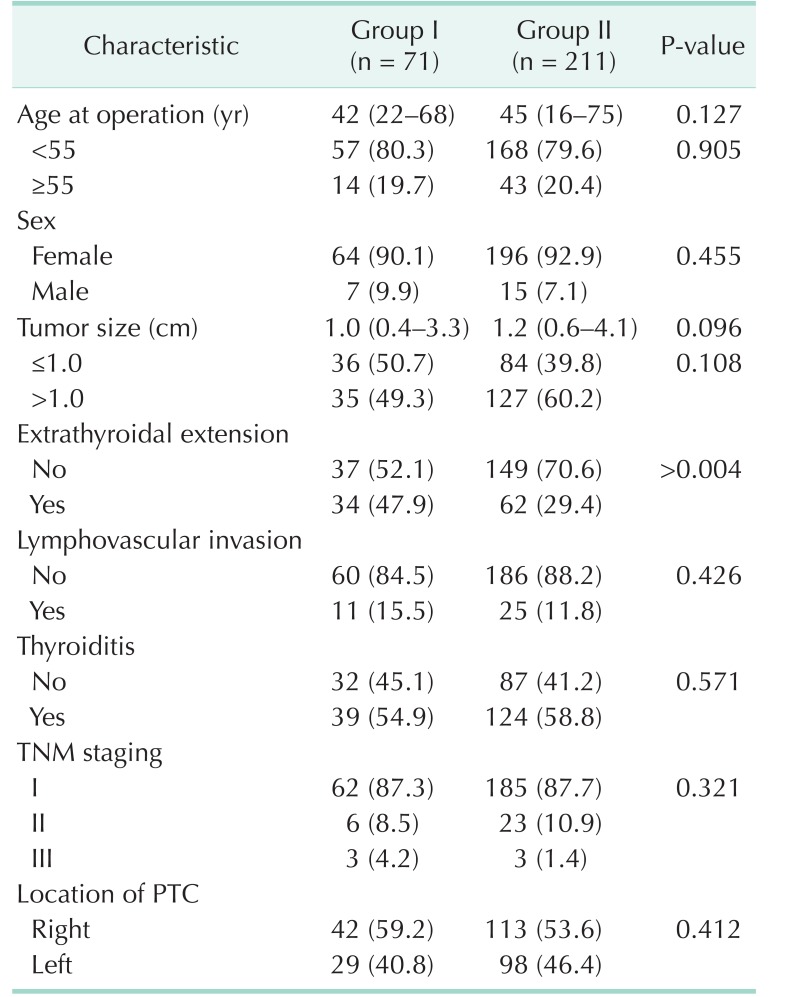
The patient and tumor characteristics are listed and compared in Table 1. The study population included 71 patients assigned to group I and 211 assigned to group II. The mean age at the time of surgery (43.2 ± 12.0 vs. 45.6 ± 11.5, respectively, P = 0.13) and the proportion of patients older than 55 years did not differ significantly between the groups. Similarly, no significant differences were observed in the sex ratio, mean tumor size (1.2 ± 0.7 vs. 1.4 ± 0.6, respectively, P = 0.10), and the proportion of patients with tumors >1.0 cm in size. Patients in group I had a significantly higher frequency of extrathyroidal extension, compared to those in group II (47.9% vs. 29.4%, respectively, P < 0.01). The 2 groups had similar frequencies of lymphovascular invasion and thyroiditis. TNM staging did not differ between the 2 groups (Table 1).
Table 1. Clinicopathological characteristics of all patients with PTC in this study (n = 282).
Values are presented as median (range) or number (%).
Group I, patients had isthmic tumors; group II, patients had lobar tumors; PTC, papillary thyroid carcinoma.
Detailed comparison of the aspects of central lymph node metastasis
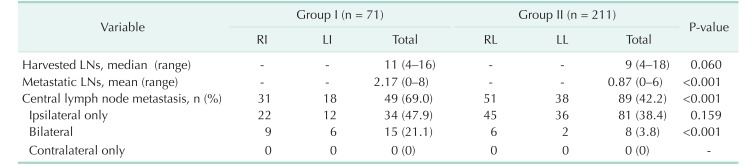
The mean number of harvested lymph nodes was comparable between the 2 groups (10.42 ± 2.98 vs. 9.60 ± 3.26, respectively, P = 0.06); however, the mean number of metastatic lymph nodes was significantly higher in group I (2.17 ± 2.16 vs. 0.87 ± 1.33, respectively, P < 0.01). Patients in group I also had a higher frequency of central lymph node metastasis, compared with group II (69.0% vs. 42.2%, respectively, P < 0.01). Regarding the location of lymph node metastasis, no significant differences were observed with respect to ipsilateral node metastasis. However, the frequency of bilateral node metastasis was significantly higher among patients in group I, compared to group II (21.1% vs. 3.8%, respectively, P < 0.01). No patients in this study had only contralateral node metastasis (Table 2).
Table 2. Incidence of central lymph node metastasis in patients with PTC in this study (n = 282).
Group I, patients had isthmic tumors; group II, patients had lobar tumors; PTC, papillary thyroid carcinoma; LN, lymph node; RI, right isthmus; LI, left isthmus; RL, right lobe; LL, left lobe.
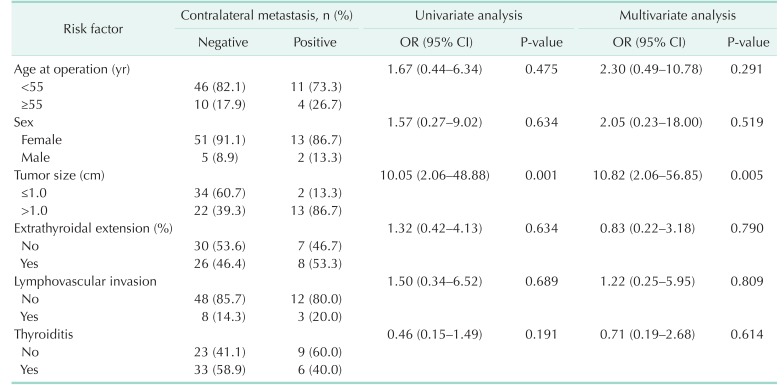
Risk factors for contralateral node metastasis
Table 3 lists the evaluated risk factors for contralateral node metastasis in patients with isthmic PTC. The univariate analysis revealed that a tumor size > 1.0 cm was significantly associated with contralateral node metastasis (P < 0.01). No other risk factors were found to associate with contralateral node metastasis. After all variables were included in the binary logistic regression model for the multivariate analysis, a tumor size > 1.0 cm remained an independent risk factor for contralateral node metastasis.
Table 3. Risk factors for contralateral central lymph node metastasis in patients with PTC arising from the isthmus (n = 71).
PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval.
DISCUSSION
The reported incidence of PTC arising from the isthmus has ranged from 2.5% to 12.3% in recent studies [2,12,13,14]. At our institute, the incidence of PTC arising from the isthmus was 10.2% (145 of 1421 patients) during the study period, regardless of the type of surgery, and was comparable with that of previous studies.
Despite its low incidence, isthmic PTC is associated with more aggressive clinical and pathological features. In our study, the frequencies of extrathyroidal extension and central lymph node metastasis were higher in group I, even though the groups did not differ with respect to other clinicopathological characteristics. Previous studies also reported that patients with PTCs located in the isthmus had poorer prognoses, compared to patients with lobar PTCs [2,3,13,14]. The aggressive clinicopathological features of isthmic PTC might be attributable to the thin shape of the isthmus, which could facilitate the invasion of adjacent tissues.
In this study, the incidences of central lymph node metastasis in groups 1 and 2 were 69.0% and 42.2%, respectively, slightly higher than in other studies of central lymph node metastasis from PTC [4,5,6,7,8]. We could attribute this difference to the determination of total thyroidectomy with bilateral central lymph node dissection, which was based on the surgeon's decision in cases with clinically suspected node metastasis on preoperative imaging studies [15,16]. However, our findings were agreed with similar reports stating that patients with isthmic PTC tend to have higher frequencies of central lymph node metastasis.
The definitions of the isthmus used in previous studies were discordant or unclear. We defined a PTC of the isthmus as a tumor in which the median line was located between the lateral margins of the trachea [17]. Because if most of the tumor is located in the trachea despite the tumor margin beyond the boundaries of the trachea, the tumor would have characteristics of a PTC arising from the isthmus rather than from the lobes. Therefore, we reviewed the preoperative ultrasonographic findings of all patients who underwent total thyroidectomy with bilateral central lymph node dissection in this study to facilitate subgroup classification.
Lymphatic drainage travels more frequently from the isthmus, compared to the lobes, to the prelaryngeal and pretracheal nodes and then spreads to the paratracheal node [18,19,20]. Therefore, we hypothesized that an isthmic PTC would confer a stronger risk of contralateral node metastasis than a lobar PTC, and accordingly analyzed the correlation between contralateral node metastasis and tumor location. The rate of contralateral node metastasis was indeed significantly higher in group I. Although no patients had only contralateral node metastasis, the frequencies of bilateral node metastasis in groups 1 and 2 were 21.1% and 3.8%, respectively. A previous study with a similar concept also reported an increased frequency of contralateral node metastasis among patients with isthmic PTC and suggested bilateral central lymph node dissection for this population [21]. Although we agreed with that suggestion, only 21.1% (15 of 71) of patients in our group 1 presented with contralateral node metastasis; therefore, an analysis of risk factors for contralateral node metastasis of isthmic PTC was needed.
A binary logistic regression modal was used to conduct a multivariate analysis of risk factors for contralateral node metastasis in group I; here, we identified a tumor size > 1.0 cm as an independent risk factor for contralateral node metastasis. Isthmic PTC is believed to more frequently exhibit extrathyroidal extension, which is associated with total central lymph node metastasis; however, in contrast to our expectations, extrathyroidal extension was not found to be an independent risk factor in this study. Most patients with contralateral node metastasis had a tumor size > 1.0 cm, whereas only 2 cases (2 of 36, 5.6%) involving tumors ≤ 1 cm in size had contralateral node metastases, although the tumors were isthmic. In summary, larger isthmic PTCs might have more opportunities to spread through contralateral lymphatic channels. Hence, our results suggest that bilateral central lymph node dissection could be considered for patients with isthmic PTCs > 1.0 cm in size who have clinically suspicious node metastasis.
The limitations of this study include its retrospective design, which introduces the risk of bias. In this study, the number of patients with PTCs arising from the isthmus and central lymph node metastases was relatively small. Moreover, the central lymph nodes were classified only into right and left subgroups, but not into subsite groups, given our lack of data. Therefore, we could not conduct analyses based on central lymph node subsites and predict spreading patterns of lymph node metastases from isthmic PTCs. Our study did not present the greatest dimension of metastatic lymph nodes. We have started measuring the size of metastatic lymph node in recent years and data based on its size could not be analyzed. Accordingly, our results must be interpreted cautiously, and a randomized controlled study is needed to overcome these limitations.
In conclusion, central lymph node metastasis occurred more frequently in patients with isthmic PTC than in those with lobar PTC. In the former group, contralateral node metastasis was more commonly observed in patients with tumors > 1.0 cm in size. Therefore, bilateral central lymph node dissection could be considered for patients with isthmic PTCs that exceed this size threshold when node metastasis is suspicious clinically.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Nixon IJ, Palmer FL, Whitcher MM, Shaha AR, Shah JP, Patel SG, et al. Thyroid isthmusectomy for well-differentiated thyroid cancer. Ann Surg Oncol. 2011;18:767–770. doi: 10.1245/s10434-010-1358-8. [DOI] [PubMed] [Google Scholar]
- 2.Lei J, Zhu J, Li Z, Gong R, Wei T. Surgical procedures for papillary thyroid carcinoma located in the thyroid isthmus: an intention-to-treat analysis. Onco Targets Ther. 2016;9:5209–5216. doi: 10.2147/OTT.S106837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Sun H, Gao L, Xie L, Cai X. Evaluation of thyroid isthmusectomy as a potential treatment for papillary thyroid carcinoma limited to the isthmus: a clinical study of 73 patients. Head Neck. 2016;(38 Suppl 1):E1510–E1514. doi: 10.1002/hed.24270. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–689. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 5.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 6.Choi SJ, Kim TY, Lee JC, Shong YK, Cho KJ, Ryu JS, et al. Is routine central neck dissection necessary for the treatment of papillary thyroid microcarcinoma? Clin Exp Otorhinolaryngol. 2008;1:41–45. doi: 10.3342/ceo.2008.1.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–1278. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 8.Chang YW, Kim HS, Kim HY, Lee JB, Bae JW, Son GS. Should central lymph node dissection be considered for all papillary thyroid microcarcinoma. Asian J Surg. 2016;39:197–201. doi: 10.1016/j.asjsur.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Karatzas T, Charitoudis G, Vasileiadis D, Kapetanakis S, Vasileiadis I. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: a case control study. Int J Surg. 2015;18:64–68. doi: 10.1016/j.ijsu.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN guidelines for patients [Internet] Fort Wathington (PA): National Comprehensive Cancer Network; 2017. [cited 2017 Apr 19]. Available from: http://www.klinikum.uni-muenchen.de/Schilddruesenzentrum/download/inhalt/Leitlinien/NCCN/thyroid_2017.pdf. [Google Scholar]
- 12.Sugenoya A, Shingu K, Kobayashi S, Masuda H, Takahashi S, Shimizu T, et al. Surgical strategies for differentiated carcinoma of the thyroid isthmus. Head Neck. 1993;15:158–160. doi: 10.1002/hed.2880150212. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Jeong JJ, Nam KH, Chung WY, Chang HS, Park CS. Papillary carcinoma located in the thyroid isthmus. World J Surg. 2010;34:36–39. doi: 10.1007/s00268-009-0298-6. [DOI] [PubMed] [Google Scholar]
- 14.Lim ST, Jeon YW, Suh YJ. Correlation between surgical extent and prognosis in node-negative, early-stage papillary thyroid carcinoma originating in the isthmus. World J Surg. 2016;40:344–349. doi: 10.1007/s00268-015-3259-2. [DOI] [PubMed] [Google Scholar]
- 15.Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407. doi: 10.1097/01.SLA.0000055273.58908.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245:604–610. doi: 10.1097/01.sla.0000250451.59685.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn SY, Han BK, Ko EY, Shin JH, Ko ES. Ultrasound findings of papillary thyroid carcinoma originating in the isthmus: comparison with lobe-originating papillary thyroid carcinoma. AJR Am J Roentgenol. 2014;203:637–642. doi: 10.2214/AJR.13.10746. [DOI] [PubMed] [Google Scholar]
- 18.Chai YJ, Kim SJ, Choi JY, Koo do H, Lee KE, Youn YK. Papillary thyroid carcinoma located in the isthmus or upper third is associated with Delphian lymph node metastasis. World J Surg. 2014;38:1306–1311. doi: 10.1007/s00268-013-2406-x. [DOI] [PubMed] [Google Scholar]
- 19.Santrac N, Besic N, Buta M, Oruci M, Djurisic I, Pupic G, et al. Lymphatic drainage, regional metastases and surgical management of papillary thyroid carcinoma arising in pyramidal lobe--a single institution experience. Endocr J. 2014;61:55–59. doi: 10.1507/endocrj.ej13-0316. [DOI] [PubMed] [Google Scholar]
- 20.Moore KL, Dalley AF, Agur AM. Clinically oriented anatomy. 7th ed. Philadelphia(PA): Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. [Google Scholar]
- 21.Song CM, Lee DW, Ji YB, Jeong JH, Park JH, Tae K. Frequency and pattern of central lymph node metastasis in papillary carcinoma of the thyroid isthmus. Head Neck. 2016;(38 Suppl 1):E412–E416. doi: 10.1002/hed.24009. [DOI] [PubMed] [Google Scholar]