Abstract
Purpose
Noninvasive precursor lesions for pancreatic adenocarcinoma include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm, and mucinous cystic neoplasm. PanIN is often found synchronously adjacent to resected pancreatic ductal adenocarcinoma (PDAC) tumors. However, its prognostic significance on outcome after PDAC resection is unknown. The purpose of the current study was to determine if the presence of PanIN has a prognostic or predictive effect on survival after resection for PDAC with curative intent.
Methods
We retrospectively reviewed the clinicopathologic data of patients who underwent pancreatectomy for PDAC from January 2002 to January 2013. Intraductal papillary mucinous lesions and mucinous cystic neoplasms were excluded. All available postoperative imaging and clinical follow-up data were reviewed.
Results
There were 95 patients who underwent pancreatectomy. Tumors were most commonly located in the pancreas head and as such pancreaticoduodenectomy was the most commonly performed operation. The median tumor size was 3.2 cm. An absence of PanIN lesions was identified in 39 patients (41%). Of the patients with PanIN lesions, high-grade PanIN (grade 3) was the most common type (64.3%) followed by grade 2 (28.6%). There was no significant difference in overall survival or disease-free survival between the non-PanIN and PanIN groups.
Conclusion
The presence or absence of PanIN lesions did not affect survival in patients undergoing resection for pancreatic cancer. However, patients with high-grade PanINs tended to have better overall survival. Larger studies with longer follow up are needed to accurately determine its clinical significance.
Keywords: Intraepithelial neoplasm, Pancreatic neoplasms, Survival
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive types of human malignancy, with a 5-year survival rate of 5% [1]. Only 10%–15% of patients are resectable at diagnosis [1]. However, prognostic effects of presence of precursor lesion in pancreatic cancer has not been regarded as an important factor so far. There exist a few studies about survival benefits on the presence of precursor lesions in ovarian cancer, cervical cancer, and colorectal cancer. However, studies in pancreatic cancer are scant [2,3].
Distinct noninvasive precursor lesions for pancreatic adenocarcinoma include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN). The term PanIN was proposed in an effort to develop a model of progression of precursor lesions to pancreatic adenocarcinoma [4]. This lesion is described as a small (generally <5 mm) intraductal noninvasive lesion that is formed by proliferation and metaplasia of ductal epithelium.
The published literature suggests that the presence of PanIN seems to correlate with the risk of pancreatic malignancy. Andea et al. [5] described progressively increased PanIN frequency and grade from normal pancreas to pancreatitis and pancreatic adenocarcinoma specimens. At the same time, it is known that PanIN has increased in prevalence and grade in cases of familial pancreatic cancer. Shi et al. [6] described increased frequency of PanIN and IPMN and higher-grade PanIN lesions in familial pancreatic cancer patients compared to sporadic pancreatic cancer patients.
However, PanIN has been poorly studied compared with other precursor lesions and the exact mechanism through which PanIN lesions lead to invasive adenocarcinoma is largely unknown. Also, the relationship between the presence of PanIN and prognosis is not known. One reason may be is that PanIN lesions cannot be accurately identified preoperatively by existing imaging modalities and can only be identified histologically postoperatively in contrast to IPMNs and MCNs, which can readily be seen radiographically and diagnosed preoperatively [7]. When we examined pathologically invasive ductal carcinoma of the pancreas, PanINs were often observed in the pancreatic duct around the invasive carcinoma component. Because the presence of PanIN has not so far been regarded as important for clinical follow-up and pathological diagnosis, little is known about the relationship between PanINs and clinicopathological findings, including prognosis [8].
The purpose of our study is to identify whether the presence of PanIN has a prognostic or predictive effect on survival in the resectable PDAC.
METHODS
This study was approved by the Institutional Review Board of Korea University Anam Hospital (approval number: 2014AN0371, ED14215). We retrospectively reviewed the clinicopathologic data of patients who underwent pancreatectomy for PDAC from January 2002 to August 2013 at our center. A total of 95 patients were included in this study and their demographics and pathologic characteristics were reviewed. The patients were subgrouped based on the presence of PanIN.
For preoperative evaluation, all patients received abdominal CT scan and magnetic resonance cholangiopancreatography. Only patients who underwent curative surgery for PDAC were included. Patients diagnosed with other malignant or premalignant lesions such as MCN, IPMN, solid pseudopapillary tumor, or neuroendocrine neoplasms were excluded. Patients who underwent surgery for benign disease or receiving surgery for the other concomitant malignancies were also excluded. All available postoperative imaging and clinical follow-up data were reviewed. The pathology reports were reviewed and categorized using the highest degree of PanIN found. All PanIN lesions were found around the main mass. There were no PanIN lesions at the resection margin. Experienced pathologists who were blinded to the clinical information (BHK, JYK) reviewed all relevant histological slides for this study. In cases where no PanIN was identified in the pathology report, the histology slides were rereviewed by pathologists to confirm the absence of PanIN.
Continuous variables were compared using the Student t-test and categorical variables were compared using the chi-square or Fisher exact tests. Kaplan-Meier method was used to calculate and compare survivals by the log-rank test. Cox proportional hazards model was used for multivariate analysis of overall survival (OS). P-value below 0.05 was considered statistically significant.
RESULTS
Clinical demographics
There were 56 and 39 patients in the PanIN group and non-PanIN group (Fig. 1A), respectively (Table 1). The mean age in the PanIN group and non-PanIN group was 64.14 ± 10.66 and 64.53 ± 9.82 years old, respectively. Most patients were male in each group and the most common presenting symptom was abdominal pain followed by jaundice, dyspepsia, weight loss and nausea, vomiting. In both groups, among various procedures, pancreaticoduodenectomy was the predominantly performed procedure (60.0% and 48.7%, PanIN and non-PanIN group) (Table 1). The mean hospital stay was 22.42 days in the PanIN group and 19.1 days in the non-PanIN group. The mean follow-up in the PanIN group and non-PanIN group was 17.90 and 14.52 months, respectively. Most patients received postoperative adjuvant chemotherapy in both groups. There was only 1 patient receiving neoadjuvant therapy for pancreatic body cancer. The patient received concomitant chemoradiation consisting of gemcitabine. All other patients received adjuvant chemotherapy consisting of gemcitabine and erlotinib 1 month after surgery if N stage was positive (Table 1).
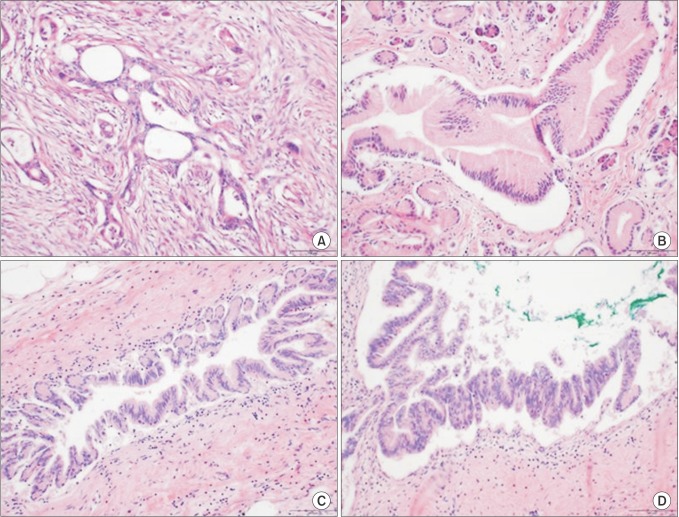
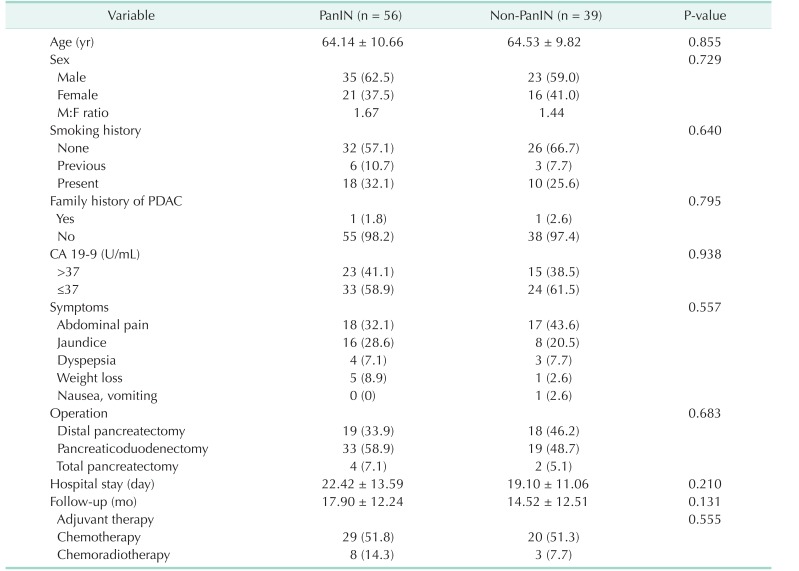
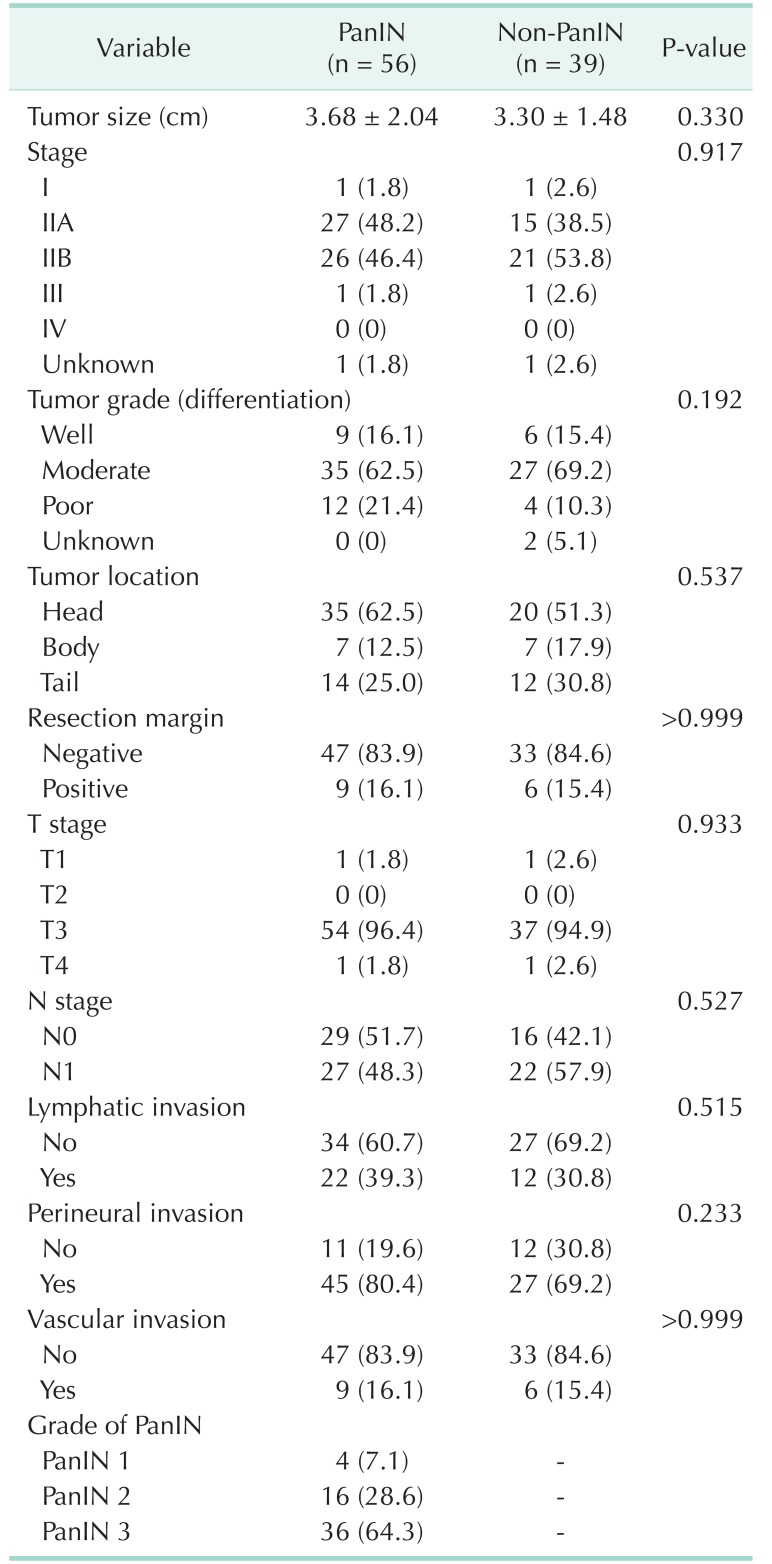
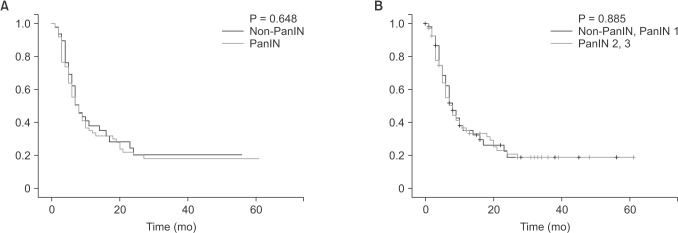
Fig. 1. Histological features of pancreatic adenocarcinoma and pancreatic intraepithelial neoplasias (PanINs). PanINs are graded according to degrees of cellular atypia. Pancreatic adenocarcinoma without PanIN (non-PanIN) (A), PanIN 1 (B), PanIN 2 (C), and PanIN 3 (D). H&E stain, ×500.
Table 1. Clinical demographics of the study cohort.
Values are presented as mean ± standard deviation or number (%).
PanIN, pancreatic intraepithelial neoplasia; PDAC, Pancreatic ductal adenocarcinoma.
Pathologic characteristics
There were no significant differences in tumor size, grade, margin status, and PDAC stage between the 2 groups (Table 2). The mean tumor size was 3.68 cm in PanIN group and 3.30 cm in non-PanIN group. The tumor grade was mostly moderately differentiated in both groups. The majority of the PDAC were located on the head of the pancreas (62.5% and 51.3%, PanIN and non-PanIN group). The pathologic T stage of most lesions was T3 (96.4% and 94.9%, PanIN and non-PanIN group) (Table 2). There was no significant difference in frequency of positive lymph nodes, lymphatic invasion, perineural invasion, and vascular invasion (Table 2). Of the PanIN group, there were 4 patients (7.1%) with PanIN grade 1 lesions (Fig. 1B), 16 patients (28.6%) had PanIN grade 2 (Fig. 1C) and 36 patients (64.3%) had PanIN grade 3 lesions (Fig. 1D, Table 2).
Table 2. Pathological characteristics of the study cohort.
Values are presented as mean ± standard deviation or number (%).
PanIN, pancreatic intraepithelial neoplasia.
Survival analysis
Disease-free survival (DFS) and OS were analyzed between the PanIN group and non-PanIN group. We also compared the DFS of patients with higher-grade PanIN lesions (PanIN 2 and PanIN 3) to lower-grade PanIN lesions (non-PanIN and PanIN 1) to better clarify the prognostic potential of PanIN lesions.
There were no significant differences in DFS between the PanIN group and non-PanIN group (P = 0.648, Fig. 2A). In addition, there were no significant differences in DFS between patients with higher-grade PanIN lesions and lower-grade PanIN lesions (P = 0.885, Fig. 2B). Mean DFS of PanIN group was 18.28 months (range, 12.83–23.72 months) and of non-PanIN group was 17.34 months (range, 11.34–23.44 months). Mean DFS of the higher-grade PanIN group is 18.0 months (range, 12.83–23.72 months) and of the lower-grade PanIN group is 17.34 months (range, 11.34–23.44 months).
Fig. 2. (A) Comparison of disease-free survival between the PanIN and non-PanIN group. Mean disease-free survival of PanIN group was 18.28 months (12.83–23.72 months) and of non-PanIN group was 17.34 months (11.34–23.44 months) (P = 0.648). (B) Comparison of disease-free survival between the higher-grade PanIN group (PanIN 2 and PanIN 3) and the lower-grade PanIN group (non-PanIN and PanIN 1). Mean disease-free survival of the higher-grade PanIN group was 18.0 months (range, 12.83–23.72 months) and of the lower grade PanIN group was 17.34 months (range, 11.34–23.44 months) (P = 0.885). PanIN, pancreatic intraepithelial neoplasia.
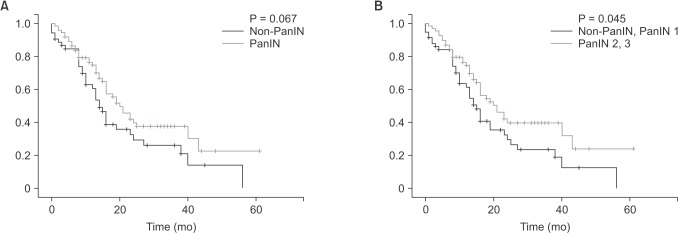
When comparing OS, although the PanIN group tended to have better OS than the non-PanIN group (Fig. 3A), there was no statistical significance (P = 0.067). However, patients with higher-grade PanIN lesions had better OS than lower-grade PanIN lesions (P = 0.045) (Fig. 3B). Mean OS of PanIN group was 28.33 months (range, 22.33–34.32 months) and of non-PanIN group was 21.04 months (range, 15.34–26.75 months). Mean OS of the higher-grade PanIN group was 29.01 months (range, 22.75–35.26 months) and of the lower-grade PanIN group was 20.62 months (range, 15.29–25.94 months).
Fig. 3. (A) Comparison of overall survival between PanIN and non-PanIN group. Mean overall survival of PanIN group was 28.33 months (range, 22.33–34.32 months) and of non-PanIN group was 21.04 months (range, 15.34–26.75 months) (P = 0.067). (B) Comparison of overall survival between the higher-grade PanIN group (PanIN 2 and PanIN 3) and the lower-grade PanIN group (non-PanIN and PanIN 1). Mean overall survival of the higher-grade PanIN group was 29.01 months (range, 22.75–35.26 months) and of the lower-grade PanIN group was 20.62 months (range, 15.29–25.94 months) (P = 0.045). PanIN, pancreatic intraepithelial neoplasia.
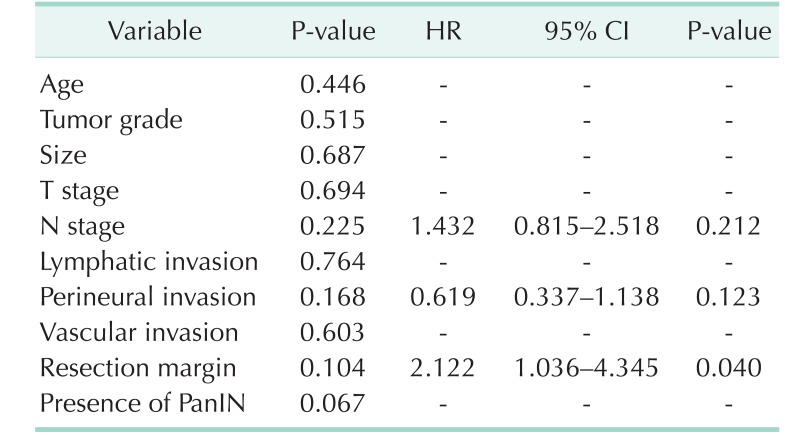
On multivariate analysis, however, although status of the resection margin was significantly associated with poor OS (95% confidence interval [CI], 1.036–4.345; P = 0.040) (Table 3), the presence of PanIN lesion was not associated with OS (95% CI, 0.619–1.984; P = 0.729) (Table 3).
Table 3. Univariate and multivariate analysis of the study cohort with regard to overall survival.
HR, hazard ratio; CI, confidence interval; PanIN, pancreatic intraepithelial neoplasia.
DISCUSSION
PDAC is one of the most aggressive types of human malignancy. There exist a few validated prognostic factors predicting survival after PDAC resection but the prognostic effect of presence of precursor lesion has not been regarded as an important factor so far. To determine whether the presence of PanIN, one of the known premalignant lesions, has a prognostic or predictive effect on survival after resection for PDAC or not, 95 patients who underwent resection of PDAC were reviewed in this study.
The patients were subgrouped based on the presence of the PanIN lesion, and DFS and OS were analyzed between the 2 groups. There were no significant differences in DFS and OS between PanIN and non-PanIN group. On the other hand, when comparing higher-grade PanIN lesions (PanIN 2 and PanIN 3) and lower-grade PanIN lesions (non-PanIN and PanIN 1), higher-grade PanIN lesions showed better OS than lower-grade PanIN lesions, although there was no significant difference in DFS.
It is clear from genetic studies that significant heterogeneity exists between individual tumors, and patterns have been identified to allow pathologists to subclassify PDAC tumors with respect to both morphology and molecular genetics [6,9]. Therefore, subtypes of PDAC tumors may exist, which develop via independent pathways. Consistent with this concept that PDAC represents a collection of disease subtypes, PDAC is known to arise from a heterogeneous group of precursor lesions, including not only PanIN but also IPMNs and MCNs [10].
Evidence suggests that PDAC may arise from precursor lesions through a series of genetic events. PanIN is the most widely recognized described precursor pathway [11]. Recently, some aspects of this pathway have been identified, including the role of notch signaling pathway for the progression of PanIN to PDAC. K-ras oncogene mutations appear as a universal and early phenomenon in this process [11,12].
However, since the exact mechanism through which PanIN lesions lead to invasive adenocarcinoma is largely unknown, this study was designed to determine if the presence of PanIN has a prognostic or predictive effect on survival after resection for PDAC. Konstantinidis et al. [13] reported that the presence of PanIN of any grade did not result in an appreciable cancer risk in the pancreatic remnant after short-term follow-up. Hassid et al. [7] reported that an absence of PanIN in the pancreatic tissue adjacent to the resected PDAC tumor had shorter postresection survival. Similarly in our study, the PanIN group tended to have better OS than the non-PanIN group (P = 0.067) and patients with higher-grade PanIN lesions (PanIN 2 and PanIN 3) had better OS than lower-grade PanIN lesions (non-PanIN and PanIN 1) (P = 0.045). This may be explained by the fact that the subset of tumors lacking PanIN may represent such a biologically distinct group, populated with more locally aggressive and poorly differentiated cancers arising independently from the usual PanIN route in an as yet undefined pathway [7]. Pancreatic carcinogenesis may therefore mirror colon carcinogenesis, in that although most sporadic colon cancers arise via the progression from adenoma to carcinoma, there exist other independent pathways to carcinoma [14]. For example, defects in DNA mismatch repair give rise to colon cancers in an adenoma-independent route in patients with the hereditary nonpolyposis colorectal cancer syndrome. Given the understanding that PDAC can arise from various precursor lesions, it is possible that subtypes of tumors are biologically distinct and arise from a PanIN independent pathway [7]. These tumors were found to be more poorly differentiated and patients with this subtype of cancer may have a worse prognosis that would suggest the need for more aggressive treatment with the goal of prolonging survival [7,13]. Further investigation is warranted to elucidate the tumor biology of PDAC that is associated with high-grade PanIN lesions and those that do not demonstrate PanIN lesions.
However, there are limitations to this study. First, the retrospective nature in addition to the small sample size and short-term follow-up may have limited the results of this study. Second, the number of PanIN lesions in relation to survival was not analyzed. Future studies with a larger number of patients and longer follow-up period in addition to detailed genetic analysis of tumors are needed to determine whether the presence of the PanIN has prognostic effects in PDAC patients.
In conclusion, although patients with higher-grade PanINs tended to have better OS than patients with lower-grade PanINs, the presence or absence of PanIN lesions did not affect survival in patients undergoing resection for pancreatic cancer. Larger studies with longer follow-up are needed to accurately determine its clinical significance.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George SM, Makinen MJ, Jernvall P, Makela J, Vihko P, Karttunen TJ. Classification of advanced colorectal carcinomas by tumor edge morphology: evidence for different pathogenesis and significance of polypoid and nonpolypoid tumors. Cancer. 2000;89:1901–1909. doi: 10.1002/1097-0142(20001101)89:9<1901::aid-cncr5>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 6.Shi C, Daniels JA, Hruban RH. Molecular characterization of pancreatic neoplasms. Adv Anat Pathol. 2008;15:185–195. doi: 10.1097/PAP.0b013e31817bf57d. [DOI] [PubMed] [Google Scholar]
- 7.Hassid BG, Lucas AL, Salomao M, Weng C, Liu F, Khanna LG, et al. Absence of pancreatic intraepithelial neoplasia predicts poor survival after resection of pancreatic cancer. Pancreas. 2014;43:1073–1077. doi: 10.1097/MPA.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda Y, Aishima S, Morimatsu K, Shindo K, Fujino M, Mizuuchi Y, et al. Pancreatic intraepithelial neoplasia in the background of invasive ductal carcinoma of the pancreas as a prognostic factor. Histopathology. 2014;65:389–397. doi: 10.1111/his.12397. [DOI] [PubMed] [Google Scholar]
- 9.Lucas AL, Shakya R, Lipsyc MD, Mitchel EB, Kumar S, Hwang C, et al. High prevalence of BRCA1 and BRCA2 germline mutations with loss of heterozygosity in a series of resected pancreatic adenocarcinoma and other neoplastic lesions. Clin Cancer Res. 2013;19:3396–3403. doi: 10.1158/1078-0432.CCR-12-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology. 2011;43:183–200. doi: 10.1097/PAT.0b013e3283445e3a. [DOI] [PubMed] [Google Scholar]
- 11.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Park CY, Lee JH, Kim JC, Cho CK, Kim HJ. Ki-67 and p53 expression as a predictive marker for early postoperative recurrence in pancreatic head cancer. Ann Surg Treat Res. 2015;88:200–207. doi: 10.4174/astr.2015.88.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinidis IT, Vinuela EF, Tang LH, Klimstra DS, D'Angelica MI, Dematteo RP, et al. Incidentally discovered pancreatic intraepithelial neoplasia: what is its clinical significance? Ann Surg Oncol. 2013;20:3643–3647. doi: 10.1245/s10434-013-3042-2. [DOI] [PubMed] [Google Scholar]
- 14.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009;4:343–364. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]