Abstract
Mixed adenoneuroendocrine carcinoma (MANEC) is a rare disease that was first defined in the 2010 World Health Organization classification of endocrine tumors. We present the case of a 65-year-old man with an ulcerative depressed lesion measuring 3 cm in diameter and found in the lower gastric body. It was diagnosed as a MANEC, and we performed subtotal gastrectomy with D2 lymph node dissection. The patient is still alive, and no recurrence was observed at a 12-month follow-up. Most MANECs tend to have a poor prognosis. Curative resection, including an adequate lymph node dissection, should be considered, and intense follow-up is needed.
Keywords: Adenoneuroendocrine, Stomach, Stomach neoplasms
INTRODUCTION
The term mixed adenoneuroendocrine carcinoma (MANEC) was introduced by the World Health Organization (WHO) in 2010, referring to a neoplasm with dual adenocarcinomatous and neuroendocrine differentiation, each component representing at least 30% of the tumor [1].
MANEC is a rare tumor that has been presented only as a case report, with only 40 cases reported to date in the English literature. Owing to its rarity, the treatment of MANEC is unclear. In this report, we present a rare case of a gastric MANEC treated with distal gastrectomy.
CASE REPORT
A 65-year-old man was admitted to the hospital under the diagnosis of neuroendocrine tumor. An ulcerative depressed lesion measuring 3 cm in diameter was found in the lower gastric body on a routine endoscopic checkup. Endoscopic biopsy revealed a neuroendocrine tumor, grade I, and immunohistochemistry was positive for panCK, synaptophysin, CD56, physin, and CD56. Laboratory data were normal, including the tumor markers CA 19-9 and CEA. Computed tomography revealed a highly enhanced mass 2.1 cm in diameter at the anterior wall of the gastric body, with multiple highly enhanced lymph node enlargements. The patient underwent total laparoscopic distal gastrectomy with Billroth II gastrojejunostomy with Braun anastomosis. No tumor was found in the frozen sections from the proximal and distal margins. The pathology report showed a well-defined ovoid submucosal mass (mass 1) with mucosal ulceration, measuring 2.5 cm × 1.7 cm in size, at the lower body of the anterior wall. It showed a well-defined multinodular submucosal mass (mass 2) at the lesser curvature of the lower body, measuring 4.7 cm × 2.8 cm in size. Masses 1 and 2 showed a yellow-white homogenous cut surface without necrosis. Mass 2 grossly involved the mucosa, extending to the subserosa (Figs. 1, 2). A final diagnosis of MANEC with neuroendocrine carcinoma involving the muscularis propria (pT2) was made, without metastasis to 28 regional lymph nodes (pN0). Immunohistochemistry was negative for CD56 but positive for synaptophysin, chromogranin, and p53 (Fig. 3). The patient recovered well and was discharged on postoperative day 10. We recommended chemotherapy for the patient, but he did not want to be treated with adjuvant chemotherapy. Nevertheless, no recurrence was observed at the 12-month follow-up after the surgery.
Fig. 1. Gross specimen opened at greater curvature. It shows a well-defined ovoid submucosal mass (mass 1) with mucosal ulceration, measuring 2.5 cm × 1.7 cm in size lower body of anterior wall. And it shows a well-defined multinodular submucosal mass (mass 2) at lesser curvature of lower body, measuring 4.7 cm × 2.8 cm in size. The masses 1 and 2 show yellow white homogenous cut surface without necrosis. The mass 2 is involving mucosa and extend to subserosa, grossly.

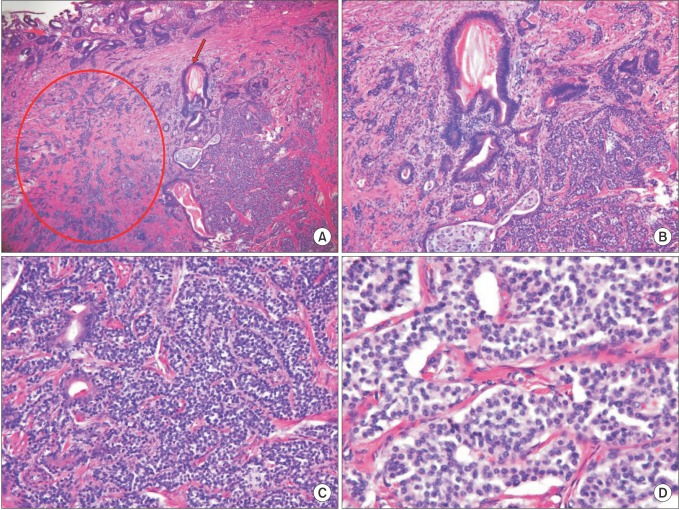
Fig. 2. (A) Mass 1 is composed of adenocarcinoma and grade 2 neuroendocrine tumor (H&E, ×40) Adenocarcinoma component (arrowed), Neuroendocrine tumor component (encircled). (B) An enlarged view of adenocarcinoma component (30%) with tubular, moderately differentiated (H&E, ×100). (C) An enlarged view of neuroendocrine tumor component (70%) with grade 2 neuroendocrine neoplasm by World Health Organization 2010 (H&E, ×200). (D) Mitosis is present: 2/10 HPFs (H&E, ×400).
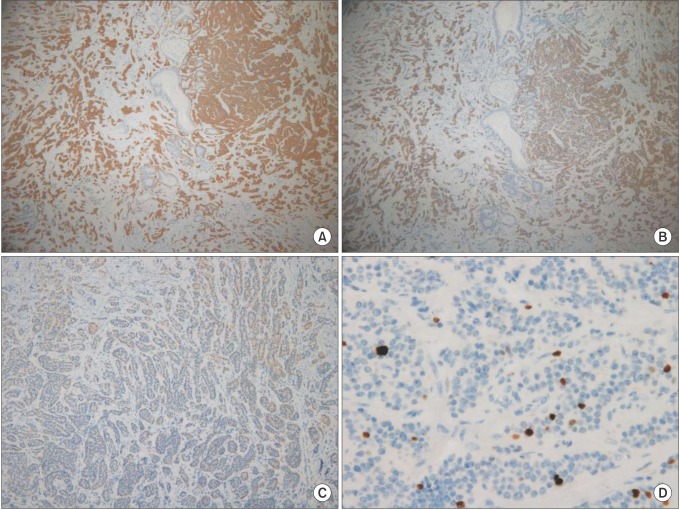
Fig. 3. Immunohistochemistry of neuroendocrine component. (A) Synaptophysin (×40), (B) CD56 (×40), (C) chromogranin (×100), (D) Ki67. Neuroendocrine component is positive for synaptophysin, CD56, focal positive chromogranin, Ki67 proliferation index is 4.5%, otherwise adenocarcinoma component is negative for immunoreactivity (×400).
Written informed consent was obtained from the patients before the publication of this case report and accompanying images.
DISCUSSION
Cases of gastrointestinal tumors, including exocrine and neuroendocrine components, have been reported. The first description of a gastrointestinal tumor with an exocrine and a neuroendocrine component was published by Cardier in 1924. Since then, several cases have been reported using many different names, including composite carcinoid and mucin-producing carcinoid. These different names have confused physicians, pathologists, and surgeons. To avoid this confusion, mixed adenoneuroendocrine carcinoma, defined as when an exocrine and a neuroendocrine component represents at least 30% of the lesion according to the 2010 WHO classification [1]. MANEC tumors rarely occur in the gastrointestinal tract. A few cases have been reported, most often from Eastern countries because of the higher incidence of gastric cancer.
In our patient, 70% of the neuroendocrine tumor component was grade 2 according to the 2010 WHO classification, with a 30% adenocarcinoma component. No data are available that summarize MANEC. However, the available data summarize the neuroendocrine features of gastric cancer. According to the results of the previous study, gastric carcinoma that contains neuroendocrine components is an intestinal type rather than a diffuse type. The mean patient age is 58 years, and the polypoid lesions are usually located in the lower third of the stomach [2]. Although some authors sustain that clinical behavior depends on the grade of the neuroendocrine component, others reveal that the characteristics of the adenocarcinoma component influence the outcome in well-differentiated neuroendocrine components [2,3].
Even though good prognosis of MANEC was reported [4], most cases are believed to be rapidly growing lesions, with most patients showing a poor prognosis [5,6]. The assessment of MANEC is still incomplete because the results of the studies conducted before 2010 did not reflect the exact definition. Neuroendocrine carcinoma is known to frequently invade the lymphatic and vascular lumens and subsequently metastasize to the lymph nodes and liver, even during the early stages, owing to its aggressive biological behavior. The biological behavior of MANEC is similar to that of NEC.
In the literature review, recent reports seem to suggest that lymph node metastasis found during surgery is an important prognostic factor (Table 1). Cases with lymph node metastasis tended to be malignant. In our patient, we found no lymph node metastasis coexisting with any other distal metastasis. We assumed that our patient underwent curative surgery before malignant features of the disease were observed. A substantial number of cases should be studied to prove this hypothesis. Nevertheless, intense follow-up for possible recurrence is necessary, as the disease can lead to poor prognosis.
Table 1. Review of the recent mixed adenoneuroendocrine carcinoma case reports.
The optimal treatment of MANEC is unclear because of the rarity of the tumor. However, MANECs containing a poorly differentiated NEC component should be treated as NECs [7]. Even though the MANEC in our patient contained a well-differentiated NEC component, we considered applying platinum-based chemotherapy because of the associated poor prognosis. However, our patient refused to receive any adjuvant chemotherapy after the operation. Nevertheless, the patient had no signs of recurrence after the operation.
Most MANECs tend to have a poor prognosis. Thus, curative resection should be considered, and adjuvant chemotherapy with intense follow-up is needed.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. p. 417. [Google Scholar]
- 2.Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011;11:195–199. doi: 10.5230/jgc.2011.11.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Kim HW, Kang DH, Choi CW, Park SB, Kim SH. A gastric composite tumor with an adenocarcinoma and a neuroendocrine carcinoma: a case report. Clin Endosc. 2013;46:280–283. doi: 10.5946/ce.2013.46.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuba N, Yuki T, Ishihara S, Sonoyama H, Tada Y, Kusunoki R, et al. Gastric mixed adenoneuroendocrine carcinoma with a good prognosis. Intern Med. 2014;53:2585–2588. doi: 10.2169/internalmedicine.53.3328. [DOI] [PubMed] [Google Scholar]
- 5.Sugishita K, Tanno M, Kijima M, Kamoshita H, Mitamura T, Hayakawa K. Malignant hypercalcemia due to gastric endocrine cell carcinoma. Intern Med. 1995;34:104–107. doi: 10.2169/internalmedicine.34.104. [DOI] [PubMed] [Google Scholar]
- 6.Volante M, Righi L, Berruti A, Rindi G, Papotti M. The pathological diagnosis of neuroendocrine tumors: common questions and tentative answers. Virchows Arch. 2011;458:393–402. doi: 10.1007/s00428-011-1060-7. [DOI] [PubMed] [Google Scholar]
- 7.Hervieu V, Scoazec JY. Mixed endocrine tumors. Ann Pathol. 2005;25:511–528. doi: 10.1016/s0242-6498(05)86164-4. [DOI] [PubMed] [Google Scholar]
- 8.Levi Sandri GB, Carboni F, Valle M, Visca P, Garofalo A. Mixed adenoneuroendocrine gastric carcinoma: a case report and review of the literature. J Gastric Cancer. 2014;14:63–66. doi: 10.5230/jgc.2014.14.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurzu S, Kadar Z, Bara T, Bara T, Jr, Tamasi A, Azamfirei L, et al. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J Gastroenterol. 2015;21:1329–1333. doi: 10.3748/wjg.v21.i4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok CM. Mixed Adenoneuroendocrine Carcinoma of the Stomach. Case Rep Gastroenterol. 2015;9:241–245. doi: 10.1159/000437293. [DOI] [PMC free article] [PubMed] [Google Scholar]