Abstract
The biogeochemical cycle of iron is intricately linked to numerous element cycles. Although biological processes that catalyze the reductive side of the iron cycle are established, little is known about microbial oxidative processes on iron cycling in sedimentary environments—resulting in the formation of iron oxides. Here we show that a potential source of sedimentary iron oxides originates from the metabolic activity of iron-oxidizing bacteria from the class Zetaproteobacteria, presumably enhanced by burrowing animals in coastal sediments. Zetaproteobacteria were estimated to be a global total of 1026 cells in coastal, bioturbated sediments, and predicted to annually produce 8 × 1015 g of Fe in sedimentary iron oxides—55 times larger than the annual flux of iron oxides deposited by rivers. These data suggest that iron-oxidizing Zetaproteobacteria are keystone organisms in marine sedimentary environments—despite their low numerical abundance—yet exert a disproportionate impact via the rejuvenation of iron oxides.
Main
Large amounts of iron oxides are released by continental weathering (~1.4 × 1014 g per year) that are transported by rivers, and deposited in coastal and continental shelf sediments ([1] and references therein). Poorly crystalline, highly reactive iron oxides (i.e., ferrihydrite and lepidocrocite) comprise a major component of fine-grained riverine sediments [1] that are rapidly transformed between reduced and oxidized phases in marine sediments upon deposition by numerous chemical and biological redox reactions. The biological and chemical reduction of iron oxides is estimated to be 100–300 times faster than riverine input [2]. These high rates of reduction would quickly exhaust the sedimentary pool of iron oxides and thus require a rapid re-oxidation of ferrous iron [Fe(II)] in sediments to produce fresh, amorphous, authigenic iron oxides [1]. Conventionally, the aerobic re-oxidation of Fe(II) in pore waters is primarily thought to be a chemical process, especially in areas with significant sediment mixing and irrigation by animals—bioturbation and bioirrigation—where oxygen is transported deep into sediments by burrow flushing [1, 3, 4]. Although sedimentary chemical oxidation of iron is important under saturated oxygen conditions at neutral pH [4], bioirrigated sediments contain microenvironments that have oxygen levels well below air saturation [5, 6] where the biological contribution to iron oxidation is quantitatively more significant [7]. Collectively, these findings suggest that biology may contribute significantly to aerobic iron oxidation in marine sediments where bioturbation by macrofauna stimulates iron cycling [8, 9].
The Zetaproteobacteria represent a class of iron-oxidizing bacteria (FeOB) that are exclusively found in marine and saline-influenced environments that contain high ferrous iron [Fe(II)] concentrations [10–13]. Coastal marine sediments can have pore water Fe(II) concentrations ranging from below detection up to 2000 µmol L−1 that are capable of supporting lithoautotrophic populations of Zetaproteobacteria [14]. Recent studies have identified Zetaproteobacteria in surface openings of benthic macrofauna in the Mediterranean Sea [15], worm burrows in submarine groundwater discharge into sands in Delaware [16], coastal sediments in Denmark [17], and Baltic and North Sea sediments [18]. Zetaproteobacteria appeared to be the dominant iron-oxidizing microorganisms in coastal sediments of Denmark demonstrated by microaerobic and anaerobic culture-dependent techniques [17]. Moreover, quantitative PCR with Zetaproteobacteria-specific 16S rRNA gene primers revealed abundances of ~106 cells per gram sediment [17]. These recent studies suggest that Zetaproteobacteria may play a significant role in iron oxidation in marine sediments—a quantitative estimate of their abundance and distribution is necessary to determine their potential biogeochemical role on a global scale.
We analyzed coastal sediment microbial communities to determine the presence and abundance of Zetaproteobacteria from geographically diverse sites (n = 90; Supplementary Table 1) utilizing 16S rRNA gene sequencing. Aerobic iron oxidation appears ubiquitous in the Zetaproteobacteria [12, 19, 20], thus 16S rRNA gene homology can be used to infer this specific metabolism. We also enriched for environmentally relevant FeOB from coastal sediments in Maine, which provided further metabolic evidence of the importance of iron oxidation in marine sediments. A meta-analysis of 16S rRNA gene studies revealed the potential importance of Zetaproteobacteria in the global sedimentary iron biogeochemical cycle.
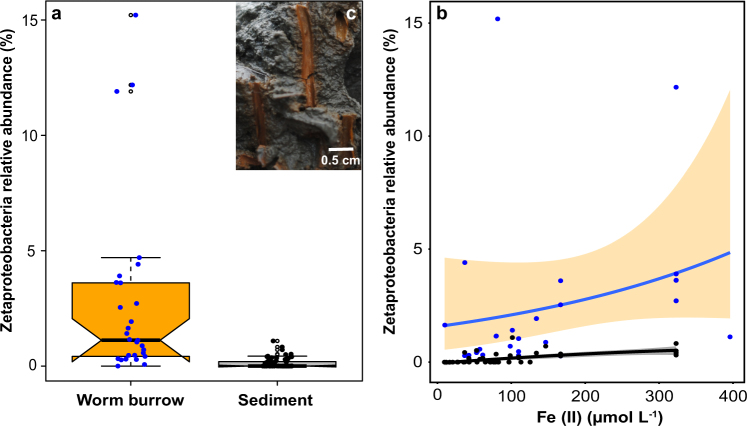
We identified Zetaproteobacteria in 60% of our samples from coastal sediments (Supplementary Table S1; Supplementary Figure S1). The median relative abundance of Zetaproteobacteria was 1.1% of the total microbial community (range = 0.04–15 %) in worm (e.g., polychaete) burrows in coastal sediments (Fig. 1a). Zetaproteobacteria were 10 times less abundant in bulk sediments (Fig. 1a) with a median of 0% (range = 0.0–1.0 %), and were significantly different from worm burrows (p-value = 9.2 × 10−7, Wilcoxon test). The large variability (0.04–15%) and non-normal distribution of Zetaproteobacteria relative abundance in worm burrows (Supplementary Materials and Methods) was most likely a combination of differences in burrow ventilation rates and efficiencies [5, 6], differences in sediment physicochemical conditions (Supplementary Table S1), and sampling bias (for example, residual sediment on burrow walls).
Fig. 1.
Boxplots of the relative abundance of Zetaproteobacteria a in iron oxide-lined worm burrow walls (n = 29) and surrounding sediments (n = 61). Notches are representative of 95% confidence interval and the medians (solid black lines) between worm burrows and sediments (1.1% and 0%, respectively) are statistically different (p-value = 9.2 × 10−7, Wilcoxon test). Filled circles represent individual data points and open circles indicate outliers. Zetaproteobacteria relative abundance (%) as a function of pore water ferrous iron [Fe(II)] concentration (µmol L−1) b from worm burrows (blue circles, fitted blue line, orange fill = 95% confidence interval) and sediments (black circles, fitted black line, gray fill = 95% confidence interval; see Methods for details on line fits). Characteristic iron oxide-lined worm burrow walls (c) from “The Eddy”, Sheepscot River, Maine, USA (image from 27 August 2015). Burrow walls are likely created by the polychaete, Nereis diversicolor, or hemichordate, Saccoglossus kowalevskii, which are both common to these intertidal sediments in Maine
Zetaproteobacteria were not the only iron-oxidizing taxa that were positively correlated with the worm burrow environment (Supplementary Figure S1). Freshwater iron-oxidizing Gallionella spp. were also found at some sites that had lower salinity values, potentially indicating significant freshwater mixing (Supplementary Figure S1; Supplementary Table S1). Similar findings have been reported from brackish estuaries in Maine [11] and from coastal sediments in the North and Baltic Seas [18]; there is no definitive evidence of iron-oxidizing members of Gallionella in marine environments. Although there may be other microorganisms capable of aerobic iron oxidation in marine sediments [21], they could not be solely determined by 16S rRNA gene sequences.
Bioirrigation by benthic animals increases the extent of oxidative processes in these sediments, thus biological iron oxidation can occur at greater depths (10 s of centimeters) than typical oxygen penetration of a few millimeters into coastal surface sediments [22]. The abundance of Zetaproteobacteria at the burrow walls correlated with the concentration of pore water ferrous iron [Fe(II)] (Fig. 1b), which is their main energy source, and could result in the production of solid-phase iron oxides around worm burrows (Fig. 1c). Quantitatively, highly reactive, iron oxides—operationally extractable by sodium dithionite [23]—were three times higher at burrow walls compared to the surrounding sediment, and accounted for 20–40% of the iron oxides with depth (Supplementary Figure S2 and S3). These freshly produced iron oxides are important substrates for iron-reducing microorganisms that release Fe(II) into pore waters, thus creating a tight cycling of iron between reduced and oxidized phases near burrow walls [8]. Eventually, dissolved iron (dFe) is transported out of the sediment, supplying a critical nutrient for phytoplankton primary production in coastal and continental shelf waters [24]. These iron oxides play a key role in various sedimentary processes: mineralization of organic matter in marine sediments by iron-reducing bacteria [4]; substrates for early diagenesis of pyrite [25]; enhancement of organic carbon burial [26]; and inhibition of pore water hydrogen sulfide, preventing euxinic conditions detrimental to benthic animals [5, 27].
The relative abundance of Zetaproteobacteria in worm burrows resembles the Fe(II) concentration profile (Supplementary Figure S4), and both were at their maximum values around 2–3 cm. The high Fe(II) (~40–140 µM) and low oxygen (~20–60 µM) conditions present in bioturbated sediment pore waters (Supplementary Figure S3) are suitable habitats for microaerophilic Zetaproteobacteria to thrive [7]. The relative abundance of Zetaproteobacteria decreased with depth in both burrows and sediments (Supplementary Fig. S4), possibly due to the decrease in Fe(II) with depth and increase in hydrogen sulfide production by sulfate-reducing bacteria [28]. Although there is oxygen in these sediments at depth (Supplementary Figure S4), hydrogen sulfide may inhibit oxygen respiration in Zetaproteobacteria under these conditions. The formation of iron sulfide minerals with increasing depth by biogenic sulfide may also compete with Zetaproteobacteria for access to Fe(II). Under these iron-rich settings in bioturbated sediments (1–10 cm deep), biotic rates of Fe(II) oxidation could exceed abiotic chemical oxidation [7].
Two Zetaproteobacteria operational taxonomic units (herein, referred to as ZetaOTUs) dominated the Zetaproteobacterial diversity in worm burrows (Supplementary Table S2). The dominant ZetaOTU across all samples was ZetaOTU14, which comprised 32% of all ZetaOTUs (Supplementary Table S2), and is represented by four single-cell amplified genomes (SAGs) from diffuse flow vent systems [12, 13, 19]. We isolated the first member of ZetaOTU14 (strain CSS-1) from iron oxide surface flocculent in a laboratory bioturbation microcosm (Supplementary Figure S5). This strain grew best under low oxygen (~60 µM O2) and high Fe(II) concentrations similar to those measured from sediment pore waters (Supplementary Figure S3). Strain CSS-1 produced fine stalks (<1 µm thick) encrusted with poorly crystalline iron oxides under laboratory conditions (Supplementary Figure S4). These iron oxides are similar to those produced by other Zetaproteobacteria [29], as well as in naturally occurring iron mats associated with diffuse flow hydrothermal vents, which are highly reactive [30]. Single-cell genomes from ZetaOTU14 representatives contained genes essential for growth on iron and low oxygen conditions (Supplementary Table S2). The second most abundant OTU was ZetaOTU9 (22%) and is represented by two cultured isolates (Ghiorsea bivora strains TAG-1 and SV108) [31], as well as five SAGs from deep-sea vents (Supplementary Table S2). ZetaOTU9 isolates also had genes necessary for growth on iron and low oxygen (Supplementary Table S2), and have also been shown to grow via hydrogen oxidation, which may explain the ubiquity of this OTU in sediments and other environments (see below). There was no clear distribution of ZetaOTUs 14 and 9 with respect to depth (Supplementary Figure S4) in worm burrows and sediments, although it is likely that they are adapted for specific Fe(II) and O2 concentrations [19].
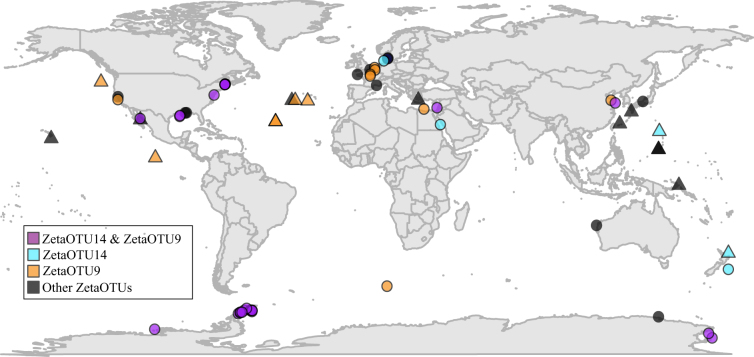
We searched for Zetaproteobacterial 16S rRNA gene sequences in marine sediment data sets (Supplementary Table S3), and identified them in numerous sediments on a global scale (Fig. 2). We found a pattern consistent with our samples—ZetaOTUs 14 and 9 were present and generally the most abundant ZetaOTUs in coastal and shelf sediments (Fig. 2). Zetaproteobacteria relative abundance was not found to exceed 1% in other studies, as microenvironments were not considered, which are abundant in bioturbated sediments [5, 6]. Accordingly, we hypothesize that when the abundance of Zetaproteobacteria exceeds ~0.5% in sediments, there is active growth and iron oxidation associated with bioturbating and bioirrigating animals.
Fig. 2.
Global distribution of Zetaproteobacteria in marine sediments (circles) and non-sediment sites (triangles) such as hydrothermal vents. The relative abundance of Zetaproteobacteria in sediments from other 16S rRNA gene studies was never above 1% and was typically within the range measured from bulk marine sediments from Maine. Sequences are from numerous studies (Supplementary Table S3) that include Sanger, 454, and Illumina sequencing technologies
We can estimate the potential contribution of iron-oxidizing Zetaproteobacteria to the production of iron oxides in the upper 10 cm of bioturbated shelf sediments [32] utilizing these data. Prior research has shown good correlation between relative abundance measurements from quantitative PCR and high-throughput 16S rRNA gene amplicon sequencing from sediments [16]. The total abundance of cells in worm burrows and sediments (10 cm depth) was estimated to a median of 2.0 × 109 (range = 8.0 × 106–7.2 × 109) and 4.1 × 108 (range = 1.2 × 107–5.4 × 109) cells per gram sediment, respectively (Supplementary Figure S6). Coastal environments comprise 7% of the total area of ocean sediments and harbor 33% of the total sedimentary microbial biomass [33], which contain 2.1 × 1019 cm3 of sediment ([34, 35]. From our estimations, this would equate to median values of 4.2 × 1028 and 8.2 × 1027 total cells in worm burrows and sediments, respectively, which are in agreement with other estimates based on direct cell counts [6, 33]. Zetaproteobacteria relative abundance ranged from 0.04 to 15% (median = 1.1%) of the total cells in coastal bioturbated sediments (Fig. 1a), and this yields a median value of 1.1 × 1026 total cells (range = 3.8 × 1024−1.4 × 1027 cells). The global Zetaproteobacteria abundance estimate was then used with recent iron oxide production rate measurements from diffuse flow vents (~1.3 × 10−16 mol Fe per cell per h) [37], which could result in the production of ~8 Pg of Fe in iron oxides per year (range = 0.1–70 Pg Fe per year; see Supplemental Materials and Methods for more details on calculations). Recent two-dimensional, sub-millimeter Fe(II) measurements in bioturbated sediments revealed extensive Fe(II) oxidation occurring within the immediate vicinity of worm burrows and a rapid re-oxidation rate of 3.8 ± 1.4 mmol Fe per m2 per day [8]. These chemical rate measurements combined with an estimate of the global volume of bioturbated coastal sediments 10 cm deep (~2.1 × 1013 m3) [34] would equate to an annual production of 1.6 ± 1.1 Pg of Fe in iron oxides. These two independent estimations of iron oxide production rates are well within the range of one another by less than a factor of 5. There are many assumptions and unknowns in these estimates such as the spatiotemporal heterogeneity that likely exist in these sedimentary environments, but do provide a first glimpse of the possible contribution of iron-oxidizing Zetaproteobacteria on iron oxide rejuvenation in coastal sediments. Based on these estimates, the annual biological oxidation of iron in sediments—forming iron oxides—could exceed the annual flux of iron oxides from rivers to coastal sediments [38] by up to a factor of 55.
Zetaproteobacteria may exert a profound impact on global sedimentary biogeochemistry via the production of biogenic, highly reactive iron oxides despite their low estimated global abundance (~0.11 %)—effectively functioning as keystone microorganisms (for example, [39]) in bioturbated coastal sediments. Zetaproteobacteria could contribute significantly to the rapid rates of Fe(II) re-oxidation measured and observed in coastal sediments [8]. Climate change outcomes such as coastal hypoxia may have positive or negative impacts on the sedimentary iron biogeochemical cycle—either stimulating microaerobic bacterial iron oxidation resulting in an increase in iron oxide production, thus enhancing dFe release or inhibiting oxidation by the increase in hydrogen sulfide production, precipitating Fe as iron sulfides. The result of an increase or decrease in the dFe efflux would be enhanced or reduced primary productivity by phytoplankton, respectively. Thus, sedimentary iron oxide formation by Zetaproteobacteria may have a direct impact on important water column processes such as carbon fixation. Lithoautotrophic Zetaproteobacteria also contribute directly to dark carbon fixation in sediments, and may provide important organic substrates for heterotrophic microorganisms. The iron-cycling microbial communities in combination with ecosystem engineering macrofauna [40] in coastal sediments likely act in concert to recharge riverine iron oxides by intense redox recycling and result in the formation of an enriched highly reactive pool of biogenic iron oxides [30]. The increase in bioturbation intensity and depth that occurred 400 millions years ago [32] has resulted in an interdependent, ecologically complex interaction with sediment-dwelling microorganisms and burrowing macrofauna that are essential to the modern global biogeochemical cycle of iron.
Electronic supplementary material
Acknowledgements
This project was funded by a National Science Foundation Biological Oceanography Award number OCE-1459600 (D.E.). Sample collection for the Oregon margin and Gulf of Mexico was funded by National Science Foundation grants OCE-1029889 and OCE-1147407, and written contributions by OCE-1715106 (to J.M.). F.J.R.M. was supported through ERC Grant 306933 under the European Union’s Seventh Framework Program. C.S.C was supported by a National Science Foundation Biological Oceanography award (OCE-1155290). We appreciate Sarabeth George for field and laboratory assistance, Peter Larsen for marine polychaete and hemichordate identification, Anton Tramper for sampling worm burrow and sediments from Netherlands, Peter Girguis and David Johnston for helpful discussions, Megan Harder for assistance with iron oxide and DNA extractions, and Matthew Wade for logistical support. We appreciate the comments from two anonymous reviewers that greatly improved the quality of the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41396-017-0032-6) contains supplementary material, which is available to authorized users.
References
- 1.Raiswell R, Canfield DE. The iron biogeochemical cycle past and present. Geochemical Perspectives. 2012;1:1–220. doi: 10.7185/geochempersp.1.1. [DOI] [Google Scholar]
- 2.Canfield DE, Thamdrup B, Hansen JW. The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim Cosmochim Acta. 1993;57:3867–83. doi: 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- 3.Aller RC. The Effects of Macrobenthos on Chemical Properties of Marine Sediment and Overlying Water. In: McCall PL, Tevesz MJS, editors. Animal-sediment relations: the biogenic alteration of sediments. Boston, MA: Springer US; 1982. pp. 53–102. [Google Scholar]
- 4.Canfield DE. Reactive iron in maine sediments. Geochim Cosmochim Acta. 1989;53:619–32. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen E, Kostka JE. Macrofaunal burrows and irrigation in marine sediment: microbiological and biogeochemical interactions. In: Kristensen E, editor. Interactions between macro- microorganisms in marine sediments. Washington, DC: American Geophysical Union; 2005. pp. 125–57. [Google Scholar]
- 6.Bertics VJ, Ziebis E. Biodiversity of benthic microbial communities in bioturbated coastal sediments is controlled by geochemical microniches. ISME J. 2009;3:1269–85. doi: 10.1038/ismej.2009.62. [DOI] [PubMed] [Google Scholar]
- 7.Emerson D, Fleming EJ, McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol. 2010;64:561–83. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- 8.de Chanvalon AT, Metzger E, Mouret A, Knoery J, Geslin E, Meysman FJR. Two dimensional mapping of iron release in marine sediments at submillimetre scale. Mar Chem. 2017;191:34–49. doi: 10.1016/j.marchem.2016.04.003. [DOI] [Google Scholar]
- 9.van de Velde S, Meysman FJR. The influence of bioturbation on iron and sulphur cycling in marine sediments: a model analysis. Aquat Geochem. 2016;22:469–504. doi: 10.1007/s10498-016-9301-7. [DOI] [Google Scholar]
- 10.McAllister SM, Davis RE, McBeth JM, Tebo BM, Emerson D, Moyer CL. Biodiversity and emerging biogeography of the neutrophilic iron-oxidizing Zetaproteobacteria. Appl Environ Microbiol. 2011;77:5445–57. doi: 10.1128/AEM.00533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBeth JM, Fleming EJ, Emerson D. The transition from freshwater to marine iron-oxidizing bacterial lineages along a salinity gradient on the Sheepscot River, Maine, USA. Environ Microbiol Rep. 2013;5:453–63. doi: 10.1111/1758-2229.12033. [DOI] [PubMed] [Google Scholar]
- 12.Scott JJ, Breier JA, Luther GW, Emerson D. Microbial iron mats at the mid-atlantic ridge and evidence that Zetaproteobacteria may be restricted to iron-oxidizing marine systems. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott JJ, Glazer BT, Emerson D. Bringing microbial diversity into focus: high-resolution analysis of iron mats from the Lō‘ihi Seamount. Environ Microbiol. 2017;19:301–16. doi: 10.1111/1462-2920.13607. [DOI] [PubMed] [Google Scholar]
- 14.Emerson D. The irony of iron—biogenic iron oxides as an iron source to the ocean. Front Microbiol. 2016;6:1–6. doi: 10.3389/fmicb.2015.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin-Blum M, Antler G, Tsadok R, Shemesh E, Austin JA, Coleman DF, et al. First evidence for the presence of iron oxidizing zetaproteobacteria at the levantine continental margins. PLoS ONE. 2014;9:1–10. doi: 10.1371/journal.pone.0091456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAllister SM, Barnett JM, Heiss JW, Findlay AJ, MacDonald DJ, Dow CL, et al. Dynamic hydrologic and biogeochemical processes drive microbially enhanced iron and sulfur cycling within the intertidal mixing zone of a beach aquifer. Limnol Oceanogr. 2015;60:329–45. doi: 10.1002/lno.10029. [DOI] [Google Scholar]
- 17.Laufer K, Nordhoff M, Schmidt C, Behrens S, Jørgensen BB, Kappler A. Co-existence of microaerophilic, nitrate-reducing, and phototrophic Fe(II)-oxidizers and Fe(III)-reducers in coastal marine sediment. Appl Environ Microbiol. 2016;82:1433–47. doi: 10.1128/AEM.03527-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes C, Dellwig O, Dahnke K, Gehre M, Noriega-Ortega BE, Bottcher ME, et al. Bacterial communities potentially involved in iron-cycling in Baltic Sea and North Sea sediments revealed by pyrosequencing. FEMS Microbiol Ecol. 2016;92:fiw054. doi: 10.1093/femsec/fiw054. [DOI] [PubMed] [Google Scholar]
- 19.Field EK, Sczyrba A, Lyman AE, Harris CC, Woyke T, Stepanauskas R, et al. Genomic insights into the uncultivated marine Zetaproteobacteria at Loihi Seamount. ISME J. 2015;9:857–70. doi: 10.1038/ismej.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barco RA, Emerson D, Sylvan JB, Orcutt BN, Jacobson Meyers ME, Ramírez GA, et al. New insight into microbial iron oxidation as revealed by the proteomic profile of an obligate iron-oxidizing chemolithoautotroph. Appl Environ Microbiol. 2015;81:5927–37. doi: 10.1128/AEM.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barco RA, Hoffman CL, Ramírez GA, Toner BM, Edwards KJ, Sylvan JB. In-situ incubation of iron-sulfur minerals reveals a divese chemolithoautotrophic community and a new biogeochemical role for Thiomicrospira. Environ Microbiol. 2017;19:1322–37. doi: 10.1111/1462-2920.13666. [DOI] [PubMed] [Google Scholar]
- 22.Glud RN. Oxygen dynamics of marine sediments. Mar Biol Res. 2008;4:243–89. doi: 10.1080/17451000801888726. [DOI] [Google Scholar]
- 23.Poulton SW, Canfield DE. Development of a sequential extraction procedure for iron: Implications for iron partitioning in continentally derived particulates. Chem Geol. 2005;214:209–21. doi: 10.1016/j.chemgeo.2004.09.003. [DOI] [Google Scholar]
- 24.Severmann S, McManus J, Berelson WM, Hammond DE. The continental shelf benthic iron flux and its isotope composition. Geochim Cosmochim Acta. 2010;74:3984–4004. doi: 10.1016/j.gca.2010.04.022. [DOI] [Google Scholar]
- 25.Berner RA. Sedimentary pyrite formation: an update. Geochim Coschim Acta. 1984;48:605–15. doi: 10.1016/0016-7037(84)90089-9. [DOI] [Google Scholar]
- 26.Lalonde K, Mucci A, Ouellet A, Gelinas Y. Preservation of organic matter in sediments promoted by iron. Nature. 2012;483:198–200. doi: 10.1038/nature10855. [DOI] [PubMed] [Google Scholar]
- 27.Seitaj D, Schauer R, Sulu-Gambari F, Hidalgo-Martinez S, Malkin SY, Burdorf LDW, et al. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc Natl Acad Sci USA. 2015;112:13278–83. doi: 10.1073/pnas.1510152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jørgensen BB. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature. 1982;296:634–5. doi: 10.1038/296643a0. [DOI] [Google Scholar]
- 29.Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J. 2011;5:717–27. doi: 10.1038/ismej.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard A, Kappler A, Schmid G, Quaroni L, Obst M. Experimental diagenesis of organo-mineral structures formed by microaerophilic Fe(II)-oxidizing bacteria. Nat Commun. 2015;6:6277. doi: 10.1038/ncomms7277. [DOI] [PubMed] [Google Scholar]
- 31.Mori JF, Scott JJ, Hager KW, Moyer CL, Küsel K, Emerson D. Physiological and ecological implications of an iron- and hydrogen-oxidizing member of the Zetaproteobacteria, Ghiorsea bivora, gen. nov. sp. nov. ISME J. 2017;11:2624–36. doi: 10.1038/ismej.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudreau BP. Mean mixed depth of sediments: the wherefore and the why. Limnol Oceanogr. 1998;43:524–6. doi: 10.4319/lo.1998.43.3.0524. [DOI] [Google Scholar]
- 33.Kallmeyer J, Pockalny R, Adhikari RR, Smith DC, D’Hondt S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc Natl Acad Sci USA. 2012;109:16213–6. doi: 10.1073/pnas.1203849109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teal LR, Bulling MT, Parker ER, Solan M. Global patterns of bioturbation intensity and mixed depth of marine soft sediments. Aquat Biol. 2008;2:207–18. doi: 10.3354/ab00052. [DOI] [Google Scholar]
- 35.Emerson D, Scott JJ, Leavitt AH, Fleming E, Moyer CL. In situ estimates of iron-oxidation and accretion rates for iron-oxidizing bacterial mats at Loihi Seamount. Deep Sea Research Part I. 2017;126:31–9. doi: 10.1016/j.dsr.2017.05.011. [DOI] [Google Scholar]
- 36.LaRowe DE, Burwicz E, Arndt S, Dale AW, Amend JP. Temperature and volume of global marine sediments. Geology. 2017;45:275–278. doi: 10.1130/G38601.1. [DOI] [Google Scholar]
- 37.Poulton SW, Raiswell R. The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition. Am J Sci. 2002;302:774–805. doi: 10.2475/ajs.302.9.774. [DOI] [Google Scholar]
- 38.Lynch MDJ, Neufeld JD. Ecology and exploration of the rare biosphere. Nat Rev Microbiol. 2015;13:217–29. doi: 10.1038/nrmicro3400. [DOI] [PubMed] [Google Scholar]
- 39.Meysman FJR, Middelburg JJ, Heip CHR. Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol Evol. 2006;21:688–95. doi: 10.1016/j.tree.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Tarhan LG, Droser ML, Planavsky NJ, Johnston DT. Protracted development of bioturbation through the early Palaeozoic era. Nat Geosci. 2015;8:865–9. doi: 10.1038/ngeo2537. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.