Abstract
Wolbachia, an intracellular endosymbiont present in up to 70% of all insect species, has been suggested as a sustainable strategy for the control of arboviruses such as Dengue, Zika and Chikungunya. As Mayaro virus outbreaks have also been reported in Latin American countries, the objective of this study was to evaluate the vector competence of Brazilian field-collected Ae. aegypti and the impact of Wolbachia (wMel strain) upon this virus. Our in vitro studies with Aag2 cells showed that Mayaro virus can rapidly multiply, whereas in wMel-infected Aag2 cells, viral growth was significantly impaired. In addition, C6/36 cells seem to have alterations when infected by Mayaro virus. In vivo experiments showed that field-collected Ae. aegypti mosquitoes are highly permissive to Mayaro virus infection, and high viral prevalence was observed in the saliva. On the other hand, Wolbachia-harboring mosquitoes showed significantly impaired capability to transmit Mayaro virus. Our results suggest that the use of Wolbachia-harboring mosquitoes may represent an effective mechanism for the reduction of Mayaro virus transmission throughout Latin America.
Introduction
Mosquitoes are effective at rapidly disseminating arboviral diseases for which outbreaks are common throughout the world. The mosquito Aedes aegypti is a species with nearly world-wide distribution that is highly anthropophilic and extremely opportunistic1,2. This insect vector severely increases the economic burden to public health, since they are involved in the transmission of disease agents such as Zika (ZIKV), Dengue (DENV), Chikungunya (CHIKV) and Yellow Fever (YFV)3–5 as well as others such as Mayaro virus (MAYV). MAYV is a member of the Togaviridae family in the genus Alphavirus and its transmission cycle involves mainly Haemagogus mosquitoes. However, laboratory studies have shown that Aedes spp. can be competent vectors for this virus6,7. MAYV was first identified in humans in 1954 in Mayaro, Trinidad and has subsequently been found in French Guiana, Suriname, Venezuela, Louisiana, Peru, Bolivia, and Brazil6,8–14. Sporadic cases of MAYV have been reported regularly across Brazil, including the Northern, Northeastern and Central West regions, with frequent occurrence in the states of Pará, Amazonas, Acre, and Mato Grosso11,13,15–18. MAYV infection symptoms are similar to those of DENV and CHIKV and are characterized by frontal headache, high fever, epigastric pain, myalgia, chronic arthralgia (more associated with CHIKV), maculopapular eruption, photophobia, and nausea. These symptoms may persist for several months, and can be incapacitating for infected persons19.
In recent years, several methods have been proposed for controlling arboviruses, including the use of Wolbachia as a biological control agent. This bacterium does not naturally occur in Ae. aegypti, but when introduced into this vector, has the ability to greatly reduce its capacity to harbor and transmit pathogens20–28. Wolbachia has been used in some parts of the world as a tool to control the transmission of DENV and other arboviruses (http://www.eliminatedengue.com/program). Mosquitoes harboring Wolbachia are released in the wild, and after establishment of the bacteria into native populations, pathogen infection and transmission can be reduced. More than five years after the introduction of Wolbachia-infected mosquitoes to natural populations in Australia, almost 100% of the Ae. aegypti populations still host the bacteria and have maintained the ability to block DENV29,30.
Taking into consideration the strength of Wolbachia as tool to reduce arboviral transmission and the relevance of MAYV as a potential human pathogen, it is important to explore the effect of this bacterium towards this virus. Here, we determined the vector competence of Ae. aegypti and the efficiency of Wolbachia (wMel strain) against MAYV infection as an alternative strategy to control the transmission of this virus.
Results
Morphological alterations in C6/36 cells caused by MAYV
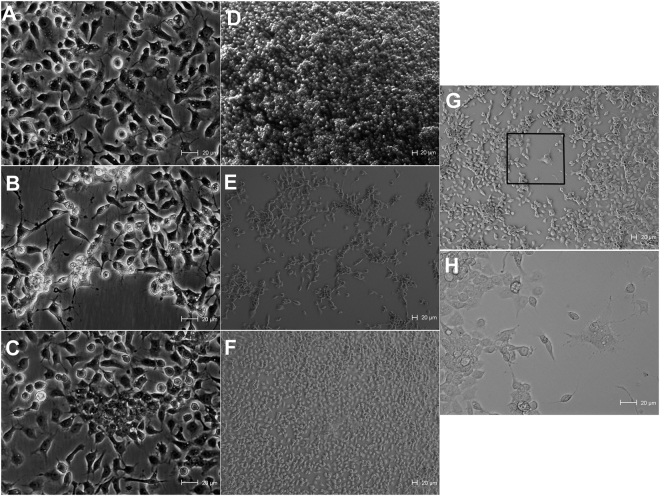
In order to verify whether MAYV would cause morphological alterations in C6/36 cells, we cultured MAYV and DENV-infected and uninfected cells (Fig. 1). MAYV seems to have caused some cell alterations, as well as a decrease in number of cells and a faster viral replication when compared to DENV. In addition, in this experiment an uncommonly cytopathic effect was observed only once time when MAYV was presented, which is similar what is caused by DENV, i.e. multinuclear giant cell formation due to the fusions of cytoplasmic membranes (Fig. 1G). We also observed a decreasing number of monolayer cells over time in the cells infected by MAYV (Fig. 1B and E). Cells were cultured uninfected (Fig. 1A and D) and infected DENV serotype 1 (Fig. 1C and F) to compare observed events.
Figure 1.
Optical microscopy of Ae. albopictus (C6/36) cell cultures infected by Mayaro or DENV. Uninfected C6/36 cells (A), MAYV-infected C6/36 cells (B), DENV-1-infected C6/36 cells (C). Cells were cultured in flasks and evaluated directly under optical microscopy without any preparation. These images were taken at 4 days post-infection (original magnification = 320x). Uninfected C6/36 cells (D), MAYV-infected C6/36 cells (E), DENV-1-infected C6/36 cells (F), these images were taken at 6 days post-infection (magnification = 100x). MAYV-infected C6/36 cells at 5 days post-infection (magnification = 100x) (G) the rare cytopathic effect caused by MAYV and (H) highlight for the cytopathic effect (magnification = 320x).
In vitro viral replication and Wolbachia blocking effect
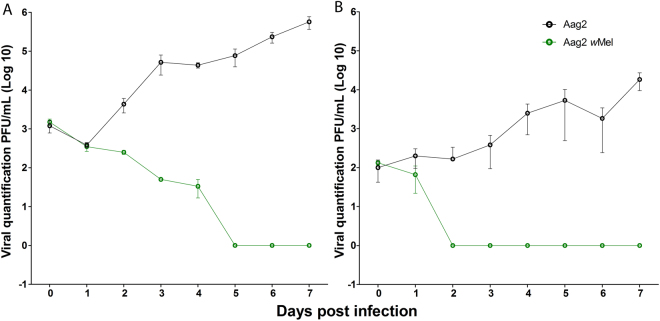
In order to check whether Wolbachia would exhibit any effect towards MAYV we firstly performed in vitro tests using Ae. aegypti cells (Aag2 with and without Wolbachia). Unfortunately, we did not have the C6/36 cells (also with Wolbachia) which is widely used for virus replication, to perform these experiments. In vitro tests in Aag2 cells showed that the kinetics of MAYV growth had a direct correlation with the different MOIs. Both MOIs in Aag2 cells without Wolbachia showed a similar growth pattern for MAYV (Fig. 2A and B). However, the MOI 0.1 produced higher viral titers compared to MOI 0.01. In the Aag2-wMel cells, we observed more rapid viral inhibition at MOI 0.01. The MOI 0.01 blocking effect started on the second day and was maintained until the end of the experiment (Fig. 2A). For the MOI 0.1, the blocking effect started between the third and fifth day and remained constant until the sixth day (Fig. 2B).
Figure 2.
Kinetics of MAYV viral growth and the Wolbachia blocking effect. (A,B) Aag2 cells were challenged with two different MOIs: (A) MOI 0.1 and (B) MOI 0.01. Aag2 without Wolbachia (black line) maintained steady growth for both MOIs. The Aag2-wMel cell line (green line) had significant effect on MAYV growth. MOI of 0.1 exhibited a later blocking effect. Viral titration of MAYV-containing supernatant was determined by plaque assay for 3 days after infection in Vero cells. Cells were infected in triplicate, and the values represent means ± SD.
MAYV infection in mosquitoes
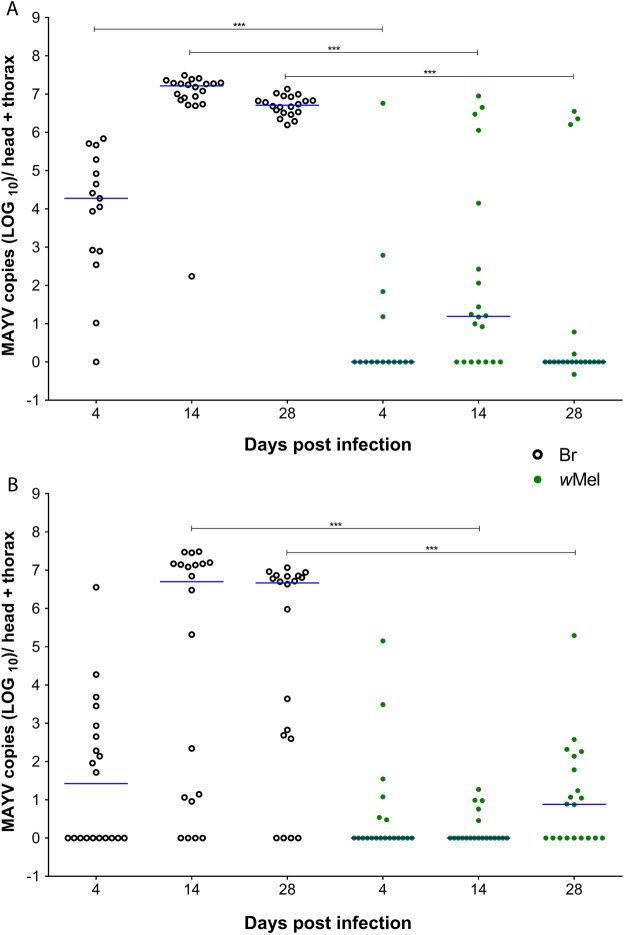
To evaluate mosquito competence for MAYV, we processed the head + thorax of individual mosquitoes at different time points post-infection. Among the 55 Br (control –Wolbachia uninfected) mosquitoes fed with fresh virus supernatant (MAYV), 49 (89.09%) had an infection level of >103 viral copies, 5 (9.09%) had an infection level of <103, and only 1 (1.82%) was uninfected. The median viral load of positive samples was 1.88 × 104, 1.65 × 107 and 5.13 × 106 copies/head + thorax for 4, 14 and 28 dpi, respectively.
For the wMel samples (Wolbachia-infected), among 55 mosquitoes, 34 (61.82%) were uninfected and of the remaining 21 mosquitoes, 12 (21.82%) had an infection level of <103, and 9 (16.36%) had an infection level of >103 viral copies. The median of the infected samples was 0, 1.55 × 101 and 0 copies/head + thorax for 4, 14 and 28 dpi (Fig. 3A). The Mann-Whitney test showed a significant difference between the groups (Br and wMel): P = 0.0003 for 4 dpi, P < 0.0001 for 14 dpi and P < 0.0001 for 28 dpi. The prevalence of MAYV infection for fresh virus was significantly reduced among Wolbachia-infected mosquitoes (Fisher’s exact test, P < 0.0001; Table 1). The infection prevalence (head + thorax) in the Br and wMel groups was 93.33% and 26.67% at 4 dpi (P = 0.005), 100% and 65% (P = 0.083) at 14 dpi and 100% and 25% (P < 0.0001) at 28 dpi, respectively.
Figure 3.
Susceptibility of Aedes aegypti and the ability of Wolbachia to block MAYV. (A,B) Mosquitoes were orally challenged with either (A) fresh virus or (B) frozen virus samples. Br mosquitoes (black circle) and wMel Wolbachia (green circle). Each circle represents a single adult female, and the blue lines indicate the median number of MAYV copies in each treatment. ∗∗∗P < 0.0001; Mann-Whitney U test.
Table 1.
Aedes aegypti were orally infected with fresh and frozen MAYV.
| MAYV | MAYV Titer (PFU/mL) | Days Post-infection | wMel | Br |
|---|---|---|---|---|
| Head/Thorax Infection Rate | ||||
| Fresh virus | >109 | 4 | 26.67(4/15) | 93.33 (14/15) |
| 14 | 65 (13/20) | 100 (20/20) | ||
| 28 | 25 (5/20) | 100 (20/20) | ||
| Frozen virus | >108 | 4 | 30 (6/20) | 50 (10/20) |
| 14 | 25 (5/20) | 80 (16/20) | ||
| 28 | 55 (11/20) | 80 (16/20) | ||
Infection rates are given as percentages. n = 15 or 20 per group unless specified; PFU, plaque-forming units; wMel: Wolbachia-infected; Br: Wolbachia-uninfected.
In the experiment where frozen virus was used, out of 60 BR mosquitoes, 29 (48.33%) had an infection level of >103 viral copies, 13 (21.67%) had an infection level of <103, and 18 (30%) were uninfected. The median of the infected samples was 2.59 × 101, 5.01 × 106 and 4.66 × 106 for 4, 14 and 28 dpi, respectively. Regarding the wMel samples, 38 (63.33%) out of the 60 mosquitoes were negative and of the remaining 22 mosquitoes, 19 (31.67%) had an infection level of <103, and 3 (5%) had an infection level of >103 viral copies (Fig. 3B). The median of the infected samples was 0, 5.5 × 101 and 0 for 4, 14 and 28 dpi, respectively. The Mann-Whitney U test showed a significant difference between the two groups (Br and wMel) at 14 dpi (P <0.0001) and 28 dpi (P< 0.0002), but not at 4 dpi (P = 0.1022). The infection prevalence for frozen MAYV was significantly different among Wolbachia-infected mosquitoes at 14 dpi (Fisher’s exact test, P < 0.0001) (Table 1). The infection prevalence for head + thorax in Br and wMel was 50% and 30% at 4 dpi (P = 0.3332), 80% and 25% (P = 0.0104) at 14 dpi, and 80% and 55% (P = 0.1760) at 28 dpi, respectively.
In order to check whether Wolbachia density would have influence on the amount of virus in mosquitoes, we have selected samples from both experiments that showed higher levels of virus in mosquito tissues as well as MAYV-negative samples. Our results show no significant difference of Wolbachia density between the two groups (Mann-Whitney U test, P = 0.6349). (Supplementary Figure S1).
Saliva injection and MAYV load in saliva
To verify whether infected mosquitoes were able to transmit the virus, we collected saliva from MAYV infected (Wolbachia-positive and negative) mosquitoes and injected it into naive Br mosquitoes. Of the 77 mosquitoes injected with Br saliva (Fig. 4A), 63 (81.81%) became infected with MAYV. In contrast, not a single mosquito out of the 75 injected with saliva from wMel-infected mosquitoes was positive for MAYV (Fig. 4B).
Figure 4.
Injection of saliva into naive mosquitoes. Saliva was collected from Br and wMel mosquitoes infected with fresh virus at 7 dpi. All of the Br saliva samples (A) were infectious, but no infections were observed when saliva samples originated from wMel mosquitoes (B). The color gradient indicates the infection level and varies according to the quantification cycle (Cq). The most infected are shown in black and there is a color gradient toward the uninfected in white. Values at the top of the graphs show the MAYV copy numbers in the head and thorax of the mosquito that the saliva was collected from (as determined by RT-qPCR).
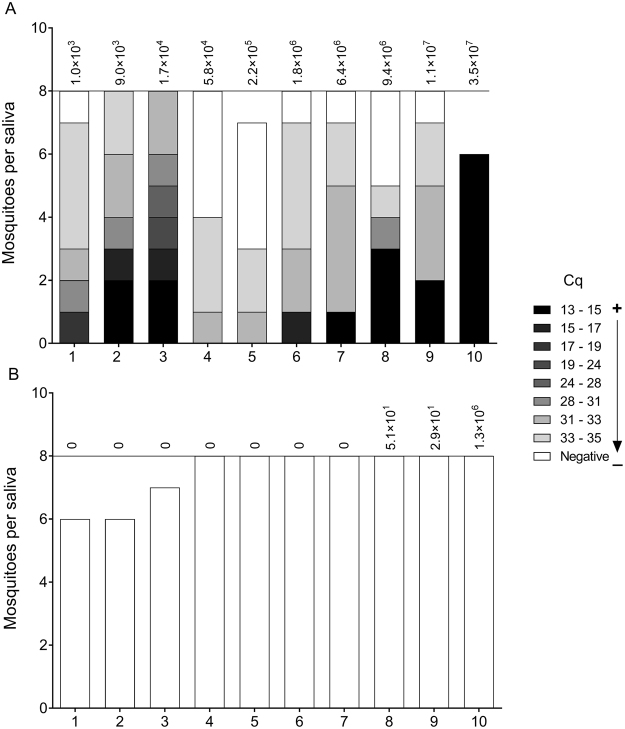
Additionally, we tried to detect MAYV directly in the saliva of both Br and wMel mosquitoes collected at 28 dpi after oral infection with either fresh or frozen virus (Fig. 5). We observed that 8/10 (80%) saliva samples from Br mosquitoes fed on fresh virus were positive, while 4/10 (40%) of the samples from Br mosquitoes fed on frozen MAYV had detectable virus. No MAYV was observed in the 20 wMel saliva samples tested (10 with fresh virus, 10 with frozen samples).
Figure 5.
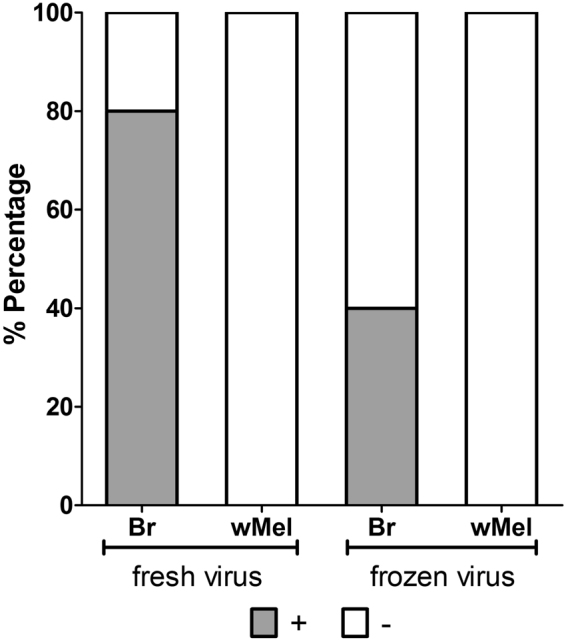
Quantification of MAYV directly from mosquito saliva through RT-PCR at 28 dpi. It was only possible to detect virus in Br mosquitoes (fresh and frozen). However, the numbers virus copies were lower when using frozen virus. It was not possible to detect virus in wMel mosquito saliva. The Fisher’s exact test showed no significant differences between fresh and frozen virus for Br mosquitoes (P = 0.1698).
Discussion
Our results indicate that C6/36 cells infected with MAYV may suffer some alterations such as reduction of cell numbers and an uncommonly cytopathic effect that seems to be limited and rare, promoting faster viral replication compared to DENV-1. In a previous report, the same pattern of growth (fast viral replication) was observed, but no cytopathic effects were reported31.
Ae. aegypti cells (Aag2) can efficiently sustain the growth of MAYV, exhibiting constant viral replication. The replication kinetics of MAYV in Aag2 were quite rapid. Wolbachia (wMel)-containing Aag2 cells seem to block MAYV regardless of the MOI. To our knowledge, this is the first report using Aag2 cells for MAYV growth and to evaluate the efficiency of Wolbachia against this virus. A previous report used C6/36 cells infected with another Wolbachia strain (wMelPop) and showed significant reduction of DENV virus replication when compared to Wolbachia-uninfected controls32.
We observed that Brazilian field populations (Br) were highly permissible to MAYV. Br mosquitoes showed a greater number of viral particles at 14 dpi, with a median of 1.65 × 107 for fresh virus and 5.01 × 106 for frozen virus. Ae. aegypti is a competent vector for MAYV, as is Aedes albopictus and Aedes scapularis6,33. The percentage of Ae. aegypti infected with MAYV in the laboratory increased with dosage above a certain threshold7. In addition to laboratory studies, Brazilian Ae. aegypti and Culex quinquefasciatus populations have been found to be infected with MAYV in their natural habitats34. The transmission of MAYV in an urban cycle has been proposed in Manaus13 and Cuiabá18. The rapid viral replication (both in vitro and in vivo) shown here combined with the global distribution of Ae. aegypti35 indicates that this virus may spread through different areas of the world in a short period of time.
Ae. aegypti mosquitoes harboring Wolbachia (wMel) showed a drastic reduction in MAYV infection. Previous studies have shown the ability of different Wolbachia strains to block pathogens and reduce the ability of mosquitoes to transmit viruses such as ZIKV, DENV, CHIKV, and YFV, as well as malaria parasites20,21,23–27,36–39. Furthermore, several different strains of Wolbachia bacterium can cause inhibition, for example the wMelPop which is able to block different DENV serotypes as well as other arboviruses20,27. Other Wolbachia infections, particularly wAlbB, wMel and wMelPop-CLA, into Ae. aegypti has been shown to significantly reduce the vector competence of this mosquito in the laboratory21,24,40. The list of pathogens that Wolbachia exerts an effect upon may possibly be extended as further studies become available.
To determine the transmission of MAYV, saliva originating from Br mosquitoes was injected into naive Br mosquitoes and resulted in high infection rates, confirming that Ae. aegypti are potential vectors of MAYV. A previous study has shown that MAYV was efficiently transmitted by Ae. aegypti to suckling mice, showing its potential as a vector for this arbovirus33. In contrast, Wolbachia significantly inhibited MAYV transmission in Ae. aegypti. When wMel-mosquito saliva was injected into naive Br mosquitoes, not one of the 75 injected mosquitoes became infected. The same methodology was previously used for ZIKV, and no mosquitoes injected with wMel-originated saliva became infected26. Our data show that in addition to becoming infected, Ae. aegypti mosquitoes can also transmit MAYV; moreover, we show that Wolbachia has a strong impact on the transmission of MAYV.
The use of frozen supernatant was shown to limit viral infection in mosquitoes and produced a lower rate of detectable viral particles in saliva. Infection rates and vector competence can be significantly lower for mosquitoes fed with frozen virus41,42. In addition, experiments showed that freezing and thawing ZIKV significantly impaired mosquito infection43. Therefore, we believe that the use of fresh virus should be the preferred choice, as it can better simulate natural conditions.
Overall, the results presented here suggest that if Ae. aegypti becomes a vector of MAYV in urban areas, the wMel strain may be used to reduce the prevalence and severity of this arbovirus. Ongoing field trials of Ae. aegypti mosquitoes harboring wMel are already in place in several countries as part of a global initiative.
Materials and Methods
Cell culture
C6/36 Aedes albopictus cells were maintained in Leibowitz L-15 medium supplemented with 10% fetal bovine serum (Gibco) and maintained at 28 °C, whereas the Aag2 cells (Ae. aegypti cell line) were grown on Schneider’s insect medium with L-glutamine (Gibco) supplemented with 10% fetal bovine serum (Gibco) at 28 °C as previously described by Hamel44.
Virus culture
MAYV and DENV stocks were maintained on the C6/36 Aedes albopictus cell line previously described by Hamel44. The C6/36 cells were grown in adherent flasks (25 cm2) to produce large quantities of infected supernatant.
The MAYV was part of a virus collection of the Federal University of Rio de Janeiro and DENV serotype 1 (DENV-1) was isolated during an outbreak in 2015 in Contagem, MG, Brazil.
Mosquito rearing
Two Ae. aegypti mosquito lines were used: the F2 generation of a (Br) Brazilian field population (Wolbachia-uninfected) collected from ovitraps in the suburb of Urca, RJ, Brazil in the beginning of 2017, and mosquitoes harboring the Wolbachia strain (wMel) backcrossed with field-collected male mosquitoes from suburb of Urca, RJ, Brazil every five generations to maintain a similar genetic background between the two lines. The methodology used to homogenize the genetic background of the mosquito lines was the same shown by Dutra45.
The insects were reared under a 12:12 h photoperiod at 28 °C ± 2 °C with a relative humidity of 60 ± 10%. Larvae were grown in plastic trays containing 300 larvae in 3 liters of water and fed with ½ ground Tetramin tropical fish food tablet each day. Sucrose solution (10%) was continuously provided to adults as a sugar source for feeding.
Assays
Morphological alterations in MAYV-infected C6/36 cells
Cellular morphology was compared between MAYV-infected and uninfected C6/36 cells with a parallel infection with DENV-1 used as a point of comparison. Three different groups were grown in flasks (25 cm2) and maintained under the same conditions: C6/36 only, C6/36 + MAYV, and C6/36 + DENV-1. Cell growth was observed under light microscopy and photographed every day for 7 days.
In vitro viral replication and the Wolbachia blocking effect
For this experiment, we used an uninfected cell line and a line in which the wMel Wolbachia strain had previously been stably introduced (Aag2- wMel cell line). The in vitro blocking assay was performed in a 96-well plate containing 2 × 105 cells per well. The multiplicities of infection (MOIs) tested were 0.1 and 0.01. The viral replication kinetics were examined by collecting supernatant from cells daily up to 7 days.
The supernatant was then frozen at −80 °C and used to infect Vero cells in a semi-solid medium using the carboxymethylcellulose system46. The total plaque forming units per milliliter (PFU/mL) were counted three days after the viral infection of the cells. This experiment was repeated three times.
MAYV mosquito infection
Five-day-old adult female mosquitoes (Br and wMel) were starved for 24 hours prior to oral infection. A mixture of 2:1 virus/blood was offered through glass feeders using pig intestine as the membrane and a water jacket system with the temperature maintained at 37 °C. Mosquitoes were allowed to feed on the blood-virus mixture for 30–60 minutes. Immediately after feeding, fully engorged females were screened and maintained on 10% sucrose for the duration of the experiment.
Mosquitoes were collected from both groups on different days post-infection and stored at −80 °C before processing. In the first experiment, we used fresh supernatant from infected C6/36 cells harvested five days after viral adsorption with a viral titer of >109 PFU/mL. In the second experiment, we used frozen supernatant from infected C6/36 cells with a corresponding viral titer of >108 PFU/mL.
The most important region for virus transmission in the mosquito is the head, where the salivary glands are located47; thus, in this experiment, we used only mosquito heads and thoraces. To facilitate analysis, we categorized the number of viral copies found in mosquito head + thorax into 3 groups: those with no viral copies (0), those with less than 1,000 viral copies (<103), and those with more than 1,000 viral copies (>103).
The human blood used in these experiments was obtained as an expired component from a blood bank (Hemominas), and was donated to our group for research purposes, according to the terms of an agreement with René Rachou Institute (OF.GPO/CCO - Nr 224/16).
Saliva collection and injection
Individual mosquito saliva samples were collected at 7 days post-infection with MAYV. Mosquitoes were anesthetized with CO2 and kept on an ice plate while the legs and wings were removed. Each mosquito proboscis was inserted into a 10 µL pipette tip containing a 1:1 solution of 5 µL of sterile fetal bovine serum and 30% sucrose solution. After 30 minutes, the contents of the tips were collected in 0.6 mL tubes and stored at −80 °C until processing. RNA from all samples was extracted using the High Pure Viral Nucleic Acid Kit (Roche) following the manufacturer’s instructions.
Ten undiluted saliva samples from each group (Br and wMel) collected at 7 dpi were injected into 6–8 naive Br mosquitoes using a Nanoject II handheld injector (Drummond) as described by Dutra26. Each mosquito was injected intrathoracically with 207 nL of saliva. Injected mosquitoes were collected at 5 days post-injection and stored at −80 °C.
Direct detection of MAYV in saliva
For direct detection of MAYV in saliva samples, we used samples collected from Br and wMel mosquitoes at 28 days post-infection (dpi) according to the methodology described above. MAYV levels in mosquito saliva (fresh and frozen virus) were quantified via Real Time qPCR (RT-qPCR) and primers specific for MAYV were used in a multiplex assay (see below). To improve detection, saliva samples were grouped into pools of two, forming 10 pairs for each group.
MAYV quantification and Wolbachia detection
MAYV levels in orally infected Ae. aegypti mosquitoes were quantified using Real Time qPCR (RT-qPCR) using a LightCycler® 96 (Roche). A multiplex assay was performed with previously developed primers specific for MAYV: MayV-F 5′/GTGGTCGCACAGTGAATCTTTC/3′/MayV-R 5′/CAAATGTCCACCAGGCGAAG/3 and May-Probe 5′/FAM/ATG GTG GTA GGC TAT CCG ACA GGT C/3lABkFQ/3′7. The Ae. aegypti ribosomal S17 (RPS17) primers are 17S-F 5′/TCC GTG GTA TCT CCA TCA AGC T/3′/17S-R 5′/CAC TTC CGG CAC GTA GTT GTC/3′ and probe 5′/HEX/CAG GAG GAG GAA CGT GAG CGC AG/3BHQ2/3′20. The primers for Wolbachia detection in cells and mosquito samples were WSP-TM2 F: 5′-CAT TGG TGT TGG TGT TGG TG-3′/WSP-TM2 R: 5′-ACA CCA GCT TTT ACT TGA CCA G-3′ and probe 5′-/56-FAM/TCC TTT GGA/ZEN/ACC CGC TGT GAA TGA/3lAbRQSp/-3′30. All fluorophores were modified from those presented in the original publications for use in our multiplex assay.
Total RNA from the mosquito heads + thoraces was extracted with the High Pure Viral Nucleic Acid Kit (Roche) following the manufacturer’s instructions. RNA samples were quantified using a Thermo Scientific™ NanoDrop 2000, diluted to 50 ng/µL in nuclease-free water, and stored at −80 °C.
Thermocycling conditions were as follows: an initial reverse transcription step at 50 °C for 10 min; RT inactivation/initial denaturation at 95 °C for 30 s, and 40 cycles of 95 °C for 5 s and 60 °C for 30 s, followed by cooling at 37 °C for 30 s. The total reaction volume contained 10 µL (5 × LightCycler® Multiplex RNA Virus Master (Roche), 1 µM primers and probe, and 125 ng of RNA template).
All samples were tested in duplicate for MAYV, WSP-TM2 and RPS17 and were analyzed using absolute quantification through serial dilutions of cloned target gene product into pGEMT-Easy plasmid (Promega) according to the manufacturer’s instructions. A negative control sample was normalized and used to determine a minimum threshold for positive samples. Absolute MAYV and WSP-TM2 copy numbers were calculated as the total number of copies per tissue or saliva sample.
Data analysis
The data were first analyzed with the D’Agostino and Person omnibus normality test. Fisher’s exact test was then used to assess differences in viral prevalence. Viral load data were compared using a Mann-Whitney U test. Comparisons were considered to be significant for P values lower than 0.05. All analyses were performed using Prism V6 (Graphpad).
Electronic supplementary material
Acknowledgements
We wish to thank Prof. Scott L. O’Neill for the donation of the original wMel infected mosquito line and Dra. Ana Maria Bispo de Filippis for donating the MAYV strain. We also acknowledge Dr. Heverton Leandro Carneiro Dutra, Simone Brutman Elias Mansur for technical assistance and Dr. Eric Pearce Caragata for reviewing the manuscript. We are in debt to Dr. Marco Antônio Silva Campos and Dr. Alexandre de Magalhães Vieira Machado and your team, who provided viral culture infrastructure in the laboratory of Imunologia de Doenças Virais (IRR - FIOCRUZ). We are grateful to all members of the Mosquitos Vetores Group (MV - IRR/FIOCRUZ and the team of Eliminar a Dengue: Desafio Brasil, particularly the Entomology team for providing field mosquito eggs. This work was supported by FAPEMIG, CNPq (LAM), CAPES (TNP and PHFS), the Brazilian Ministry of Health and a grant to Monash University from the Bill and Melinda Gates Foundation.
Author Contributions
T.N.P., M.N.R., and L.A.M. contributed to conceive, perform and design all the experiments. T.N.P., M.N.R., P.H.F.S. and F.D.C. were involved in experiments with mosquitoes. M.N.R. provided the viral strains and cell culture. T.N.P., M.N.R. and F.D.C., analyzed the data. T.N.P. and L.A.M. drafted the manuscript. L.A.M. supervised the research. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Thiago Nunes Pereira and Marcele Neves Rocha contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25236-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lourenço de Oliveira, R. Biologia e comportamento do vetor. in DengueTeorias e práticas (ed. Valle, D.)75–92 (2015).
- 2.Carvalho FD, Moreira LA. Why is Aedes aegypti Linnaeus so Successful as a Species? Neotrop. Entomol. 2017;46:243–255. doi: 10.1007/s13744-017-0520-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CR, Wilbur GD, George HW, Norman WA, Alick AR. Mayaro Virus: A New Human Disease Agent II. Isolation from Blood of Patients in Trinidad, B.W.I.1. Am. Soc. Trop. Med. Hyg. 1957;6:1012–1016. doi: 10.4269/ajtmh.1957.6.1012. [DOI] [PubMed] [Google Scholar]
- 4.Tchankouo-Nguetcheu S, et al. Differential protein modulation in midguts of Aedes aegypti infected with chikungunya and dengue 2 viruses. PLoS One. 2010;5:e13149. doi: 10.1371/journal.pone.0013149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staples EJ, Fischer M. Chikungunya Virus in the Americas - What a Vectorborne Pathogen Can Do. N. Engl. J. Med. 2014;371:885–887. doi: 10.1056/NEJMp1407698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz M, Navarro JC. Virus Mayaro: un arbovirus reemergente en Venezuela y Latinoamérica. Biomédica. 2012;32:288–302. doi: 10.1590/S0120-41572012000300017. [DOI] [PubMed] [Google Scholar]
- 7.Long KC, et al. Experimental transmission of Mayaro virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011;85:750–757. doi: 10.4269/ajtmh.2011.11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karbaat J, Jonkers AH, Spence L. Arbovirus infections in Sutch military personnel in Suriname: a preliminary study. Trop. Geogr. Med. 1964;16:370–376. [PubMed] [Google Scholar]
- 9.Halsey ES, et al. Mayaro Virus. Emerg. Infect. Dis. 2013;19:2010–2013. doi: 10.3201/eid1911.130777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forshey BM, et al. Arboviral etiologies of acute febrile illnesses in western south America, 2000–2007. PLoS Negl. Trop. Dis. 2010;4:2000–2007. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinheiro FP, et al. An outbreak of Mayaro vírus disease in Belterra, Brazil. I. Clinical and virological findings. Am. J. Trop. Med. Hyg. 1981;30:674–681. doi: 10.4269/ajtmh.1981.30.674. [DOI] [PubMed] [Google Scholar]
- 12.Causey OR, Maroja OM. Mayaro virus: a new human disease agente. III. Investigation of an epidemic of acute febrile illnes on the River Guama in Pará, Brazil, and isolation of Mayaro virus as a causative agent. Am J Trop Med Hyg. 1957;6:1017–1023. doi: 10.4269/ajtmh.1957.6.1017. [DOI] [PubMed] [Google Scholar]
- 13.Mourão MPG, et al. Mayaro Fever in the City of Manaus, Brazil, 2007–2008. Vector-Borne Zoonotic Dis. 2012;12:42–46. doi: 10.1089/vbz.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calisher CH, Ernesto GV, Kathryn SC, Maness BS, D.L. R. Isolation of Mayaro Virus From a Migrating Bird Captured in Louisiana in 1967’. Paho Bull. 1974;8:243–248. [PubMed] [Google Scholar]
- 15.Figueiredo RP, et al. Doenças exantemáticas e primeira epidemia de dengue ocorrida em Manaus, Amazonas, no periodo de 1998-1999. Rev. Soc. Bras. Med. Trop. 2004;37:476–479. doi: 10.1590/S0037-86822004000600009. [DOI] [PubMed] [Google Scholar]
- 16.Tavares-Neto J, et al. Pesquisa de anticorpos contra arbovírus e o vírus vacinal da febre amarela em uma amostra da população de Rio Branco, antes e três meses após a vacina 17D. Rev. Soc. Bras. Med. Trop. 2004;37:1–6. doi: 10.1590/S0037-86822004000100001. [DOI] [PubMed] [Google Scholar]
- 17.Silva NM, Malafronte RS, Luz BA, Souza EA. TheAcre Project: the epidemiology of malaria and arthropodborne virus infections in a rural Amazonian population. Cad Saude Pub. 2006;22:1325–1334. doi: 10.1590/S0102-311X2006000600021. [DOI] [PubMed] [Google Scholar]
- 18.Zuchi N, Silva LB, Santos MAM, Pereira FC, Slhessarenko RD. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem. Inst. Oswaldo Cruz. 2014;109:820–823. doi: 10.1590/0074-0276140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azevedo RS, et al. Mayaro fever virus, Brazilian amazon. Emerg. Infect. Dis. 2009;15:1830–1832. doi: 10.3201/eid1511.090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira LA, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;7:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 22.Aliota MT, et al. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016;10:1–13. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian G, et al. Wolbachia Invades Anopheles stephensi. Science (80-.). 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 24.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blagrove MC, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachiastrain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. 2012;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutra HLC, et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Den Hurk AF, et al. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye YH, et al. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis. 2015;6:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann AA, et al. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frentiu FD, et al. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014;8:1–11. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueiredo LT. Uso de células de Aedes albopictus C6/36 na propagação e classificação de arbovírus das famílias Togaviridae, Flaviviridae, Bunyaviridae e Rhabdoviridae. Rev. Soc. Bras. Med. Trop. 1990;23:13–18. doi: 10.1590/S0037-86821990000100003. [DOI] [PubMed] [Google Scholar]
- 32.Frentiu FD, Robinson J, Young PR, McGraw EA, O’Neill SL. Wolbachia-Mediated resistance to dengue virus infection and death at the cellular level. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long KC, et al. Experimental transmission of Mayaro virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011;85:750–757. doi: 10.4269/ajtmh.2011.11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira SO, Fernandes CB, Ribeiro AL, Santos FAL, Dezengrini S. R. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz. 2016;111:20–29. doi: 10.1590/0074-02760150390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraemer UG, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aliota MT, et al. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016;10:1–7. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science (80-.). 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye YH, et al. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joubert DA, et al. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016;12:1–19. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards SL, Pesko K, Alto BW, Mores CN. Reduced infection in mosquitoes exposed to blood meals containing previously frozen flaviviruses. Virus Res. 2007;129:224–227. doi: 10.1016/j.virusres.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller B. Increased yellow fever virus infection and dissemination rates in Aedes aegypti mosquitoes orally exposed to freshly grown virus. Trans. R. Soc. Trop. Med. Hyg. 1987;81:1011–1012. doi: 10.1016/0035-9203(87)90381-6. [DOI] [PubMed] [Google Scholar]
- 43.Ciota AT, et al. Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence -Volume 23, Number 7—July 2017 - Emerging Infectious Disease journal - CDC. Emerg. Infect. Dis. 2017;23:1110–1117. doi: 10.3201/eid2307.161633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamel R, et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutra HLC, et al. From Lab to Field: The Influence of Urban Landscapes on the Invasive Potential of Wolbachia in Brazilian Aedes aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2015;9:1–22. doi: 10.1371/journal.pntd.0003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dulbeccc R, Vogt M. Some problems of animal virology as studied by the plaque technique. Cold Spring Harb. Symp. Quant. Biol. 1953;18:273–279. doi: 10.1101/SQB.1953.018.01.039. [DOI] [PubMed] [Google Scholar]
- 47.Clements, A. The Physiology of Mosquitoes. (1963).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.