Abstract
Introduction
A recent study suggested that orally dosed ferric citrate hydrate (FC) corrects renal anemia in patients on hemodialysis (HD), suggesting biological differences in effects of iron supplementation using different routes of administration. To address this issue, the present study compared oral FC with i.v. saccharated ferric oxide (FO) in stable HD patients.
Methods
Participants comprised 6 patients administered 3 consecutive protocols in the first HD session of the week in a fasting state: nothing given, as control (C); oral load of FC (480 mg iron), and 5 minutes of i.v. FO (40 mg iron). Iron dynamics in the body and biological impact on redox-inflammation status during the study (6 hours) were examined.
Results
Significant increases in serum iron and transferrin saturation were seen with both FC and FO. Regarding total iron-binding capacity as the sum of serum iron and unsaturated iron-binding capacity, no changes were found in FC, whereas significant increases were seen in FO (appearance of non–transferrin-binding iron [NTBI]), despite the lower serum iron levels in FO. Compared with C, increases were seen in serum myeloperoxidase (oxidative marker) with accompanying significant decreases in thioredoxin (antioxidant) in FO, whereas no changes were found in FC.
Conclusion
Oral FC differs from i.v. FO in areas such as less NTBI generation and less induction of oxidative stress. The result indicates potential clinical benefits of oral FC in terms of iron supplementation for renal anemia in HD patients.
Keywords: ferric citrate hydrate, FGF23, hemodialysis, iron, oxidative stress, renal anemia
Renal anemia in patients with chronic kidney disease (CKD) and those on chronic dialysis treatment increases the risks of cardiovascular events, progression of CKD, and mortality, and decreases quality of life, mental health, and cognitive function.1 Adequate management of renal anemia is thus important for these patients.
Primary pathologic mechanisms of renal anemia include relatively suppressed production of erythropoietin and impaired utilization of iron in the body. And enhanced oxidative stress, and micro-inflammation by uremia could be involved with the pathology of poor response to erythropoietin-stimulating agent, and underutilization of iron in the body.2, 3 In addition, iron loss associated with blood loss in patients on hemodialysis (HD) reportedly reaches up to 2000 to 6000 mg annually.4 Given this pathologic background, adequate iron supplementation should be a mainstay for renal anemia management in HD patients.
At present, i.v. iron administration is a common practice in HD treatment, because the superior effectiveness of i.v. iron has been confirmed as compared with oral iron intake, as recently reviewed by Albaramki et al.5 and Shepshelovich et al.6 However, meta-analysis of randomized clinical trials in a wide range of clinical backgrounds (a total of 75 studies including 19 of renal anemia) have revealed that i.v. iron administration increases the risks of infectious diseases, as compared with oral iron and no iron intake.7 Furthermore, a recent randomized clinical trial conducted in patients with pre-dialysis CKD have suggested increased risks for cardiovascular disease and infection8 with i.v. iron administration, suggesting that i.v. iron may damage endothelial and immune cells to disturb those functions.
Ferric citrate hydrate (FC) is used as a phosphate binder for patients with CKD,9, 10, 11, 12, 13 and has recently gained attention with respect to iron supplementation for CKD.11, 14, 15 Patients who have been receiving FC as a phosphate binder have recently been reported to show significantly decreased prevalences of i.v. iron administration, decreased dose of erythropoietin-stimulating agents, and increased level of hemoglobin during a 12-month observation period,15 suggesting that FC benefits patients with renal anemia. Notably, a recent randomized clinical trial of renal anemia revealed the effectiveness of FC for increasing hemoglobin in patients with pre-dialysis CKD.16 Taking these findings together, orally administered FC may offer clinical advantages with respect to the iron load to patients with CKD. The biological characteristics of this agent thus need to be clarified.
The present study examined differences between orally administered FC and i.v.-administered saccharated ferric oxide (FO), in terms of iron kinetics and biological impact on redox-inflammation status in the body during the acute phase of iron loading.
Patients and Methods
Patients
Six patients on maintenance HD were recruited for the study from Jikyukai Tani Hospital (Motomiya, Japan). They had been receiving HD 3 times a week at 4 hours per session. Patient characteristics are shown in Table 1. Patients who had acute infection, malignancy or gastrointestinal disease, or who were current smokers were excluded from the study. Informed consent was obtained from all participants, and the study protocol was fully approved by the ethics committee at Fukushima Medical University (No. 2605).
Table 1.
Patient demographics
| n | 6 |
| Age, yr | 70.3 ± 6.2 |
| Sex, male | 3 |
| Dialysis vintage, yr | 2.4 (1.6 to 12.0) |
| Body weight, kg | 58.1 ± 7.4 |
| Height, cm | 155 ± 5 |
| Underlying kidney disease | |
| Nephrosclerosis | 3 |
| Diabetic nephropathy | 3 |
| ESA | |
| Darbepoetin (15–40)/wk | 5 |
| None | 1 |
| WBC (× 102/μl) | 5117 ± 1060 |
| Neutrophils, % | 70.5 ± 9.5 |
| Hemoglobin, g/dl | 11.4 ± 1.6 |
| Total protein, g/dl | 6.5 ± 0.1 |
| Albumin, g/dl | 3.7 ± 0.3 |
| Creatinine, mg/dl | 7.7 ± 1.0 |
| CRP, mg/dl | 0.12 ± 0.18 |
| Iron, μg/ml | 56 ± 23 |
| Ferritin, ng/ml | 27.0 (17.1 to 244.0) |
| TIBC, μg/dl | 293 ± 28 |
| Transferrin saturation, % | 19.2 ± 7.6 |
CRP, C-reactive protein; ESA, erythropoiesis-stimulating agents; TIBC, total iron-binding capacity; WBC, white blood cells.
Values are mean ± SD or median (minimum to maximum).
Patients participating in the study received 3 consecutive protocols. Under the first protocol (phase I), nothing was given. Second (phase II), 8 tablets of FC (Riona 250 mg; Japan Tobacco Inc., and Torii Pharmaceutical Company, Ltd., Tokyo, Japan) containing a total of 480 mg of iron were given orally. In the study, the dose of FC was decided according to the allowance of the Japanese medical insurance system. Permissible daily maximum dose of FC (Riona 250 mg) is 6 g (24 tablets: oral 3 times a day) in Japan. Based on this, we designed to give the maximum amount of 1 time of FC to patients (e.g., 8 tablets at once). Third (phase III), saccharated FO (Fesin; Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) containing 40 mg of iron was infused during the first 5 minutes of HD. The dose of infused FO was decided according to the standard clinical practice in Japan. Fesin is the only injectable iron drug commercially available in Japan, and it is recommended to inject once a week if needed from Japanese Society Dialysis Treatment (Guideline for Renal Anemia in Chronic Kidney Disease, J Jpn Soc Dial Ther 2016; 49(2):89–158, in Japanese).
The study was performed in all patients during the first HD session of the week in a fasting state in the morning over the course of 3 weeks. Blood samples were taken from an arterial access site at 0 hour (T0) just before starting HD, and at 0.5, 1, 2, and 4 hours (at the end of HD), and 6 hours (2 hours after the end of HD), respectively.
Measurements
Sample Stock
Blood samples were immediately centrifuged with ethylenediamine tetraacetic acid, and the plasma was stored at −80°C until measurements were obtained. For RNA extraction and leukocyte analysis, fresh samples were treated within 24 hours.
Enzyme-Linked Immunosorbent Assay (ELISA)
The following parameters were measured using ELISA. Myeloperoxidase (MPO) was measured using a Human Myeloperoxidase ELISA kit (Abcam, Cambridge, UK). Concentrations of 8-hydroxy-2′-deoxyguanosine were measured using highly sensitive 8-hydroxy-2′-deoxyguanosine Check (Japan Institute for the Control of Aging: JaICA, Shizuoka, Japan). Thioredoxin (TRX) was measured using Human Thioredoxin Assay Kit (IBL, Shizuoka, Japan). Highly sensitive C-reactive protein was measured by serum (cardio phase highly sensitive C-reactive protein II; Siemens Healthcare Diagnostic, Erlangen, Germany). Serum 2-thiobarbituric acid-reactive substances were measured by the lipid peroxidation assay method using the 2-thiobarbituric acid-reactive substances Assay Kit (Cayman Chemical, Ann Arbor, MI). Concentrations of tumor necrosis factor α; interleukin-1, -6, and -10; interferon; and vascular endothelial growth factor were measured using BD CBA Flex Sets (BD Biosciences, Piscataway, NJ). Fibroblast growth factor (FGF)-23 was measured using an FGF23 ELISA Kit (KAINOS Laboratories, Tokyo, Japan). FGF23 c-terminal was measured using an FGF23 (C-terminal) Multi-Matrix ELISA Kit (Biomedica Immunoassays, Vienna, Austria).
RNA Preparation and Quantitative Reverse-Transcriptase–Mediated Polymerase Chain Reaction
Total RNA was isolated from the blood using the TRIzol (Thermo Fisher Scientific, Yokohama, Japan) according to the instruction manual.
Real-time polymerase chain reaction analysis was performed using probe sets from the Bio-Rad CFX96 system (Bio-Rad Laboratories, Hercules, CA). Gene-specific primers were as follows: glyceraldehyde-3-phosphate dehydrogenase: forward, GAAGGTGAAGGTCGGAGTC; reverse, GAAGATGGTGATGGGATTTC; B-cell lymphoma 2: forward, CCTGTGGATGACTGAGTACCTGAAC; reverse, CAGAGTCTTCAGAGACAGCCAGGA; B-cell lymphoma 2 Associated Athanogene 1: forward, AGAGTCGTTGAAGTCCCAGGAA; reverse, GCCTTGTCCACAAGTCTACCTCTAC; Bcl-2–associated death promoter: forward, CAGTGATCTGCTCCACATTC; reverse, TCCAGCTAGGATGATAGGAC; and FAS: forward, AGGAGTACACAGACAAAGCCCATT; reverse, GGTGCAAGGGTCACAGTGTTC; These primers were used for the amplification of specific cDNA with the iScript one-step reverse-transcriptase polymerase chain reaction kit (Bio-Rad). Relative expression levels of each mRNA were normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA levels.
Analysis of Apoptotic Leukocytes
A suspension of leukocytes was made by lysing whole-blood with Pierce IP Lysis Buffer (Thermo Fisher Scientific, Yokohama, Japan). One microliter of annexinV (fluorescein isothiocyanate labeled) (200 μg/ml) and 5 μl of propidium iodide (200 μg/ml) were added (Annexin V-FITC kit; Becton, Dickinson and Company, Franklin Lakes, NJ) to a final volume of 100 μl and cells were incubated for 15 minutes in the dark. Cytofluorometric analysis was performed immediately after staining using an SH800 Cell Sorter (Sony, Tokyo, Japan). A total of 50,000 cells were analyzed in each sample. Apoptosis was defined as annexinV-positive and propidium iodide–negative, whereas secondary necrosis was defined as annexinV-positive and propidium iodide–positive.
Hepcidin 25
Levels of hepcidin 25 were measured by high-performance liquid chromatography tandem mass spectrometry (Medical Care Proteomics Biotechnology, Kanazawa, Japan).
Reactive Oxygen Metabolites and Biological Antioxidant Potential
Values for Reactive Oxygen Metabolites Test and Biological Antioxidant Potential were ascertained using the Free Radical Analytical System 4 (FRAS4; H&D SRL, Parma, Italy), as reported elsewhere. Briefly, the Reactive Oxygen Metabolites level is proportional to the serum hydroperoxide concentration. The results are expressed in conventional units of Carratelli units (U.CARR), with 1 U.CARR corresponding to 0.8 mg/l of H2O2. Biological Antioxidant Potential measurement is based on the ability of a colored solution, containing a source of ferric (Fe3+) ions bound to a chromogenic substrate (thiocyanate derivative), to decolor when Fe3+ ions are reduced to ferrous ions (Fe2+) by the reductive activity of blood samples. Preliminary data from nonuremic healthy individuals indicate that a normal Biological Antioxidant Potential value is greater than 2200 μmol/l.
Serum Iron, Ferritin, and Unsaturated Iron-binding Capacity
Ferritin was measured by lumi pulse presto II (Fujirebio, Tokyo, Japan), serum iron was measured by Quick auto-neo Fe (Shino-test, Tokyo, Japan), and unsaturated iron-binding capacity was measured by Quick auto-neo unsaturated iron-binding capacity (Shino-test).
Transferrin saturation was calculated from the following formula: serum iron at respective time points / [serum iron (0 h) + unsaturated iron-binding capacity (0 h)]. Non–transferrin-binding iron (NTBI) was defined as the difference between the level of total iron-binding capacity (TIBC: sum of serum iron and unsaturated iron-binding capacity) at respective time and that of 0 hour, and calculated by the following formula as estimated NTBI: (TIBC [Time point] – TIBC [0 h])(phase II or III) – (TIBC [Time point] –TIBC[0 hour])(phase I). NTIB(%) in phase II and III among serum iron was calculated by the following formula: 100*estimated NTBI (Time point)/(serum iron [Time point] – serum iron [Time point – 0 hour] in phase I).
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM, Chicago, IL). All values are expressed as the mean ± SD, or median as appropriate. For comparisons between 2 groups, the paired t-test or Wilcoxon test was used for continuous variables, as appropriate. A within-group comparison among values from T0 to T5, and comparisons of the groups at each time point, were analyzed by analysis of variance for repeated-measures or Friedman test, as appropriate, with values of P < 0.05 considered statistically significant.
Results
Iron Kinetics
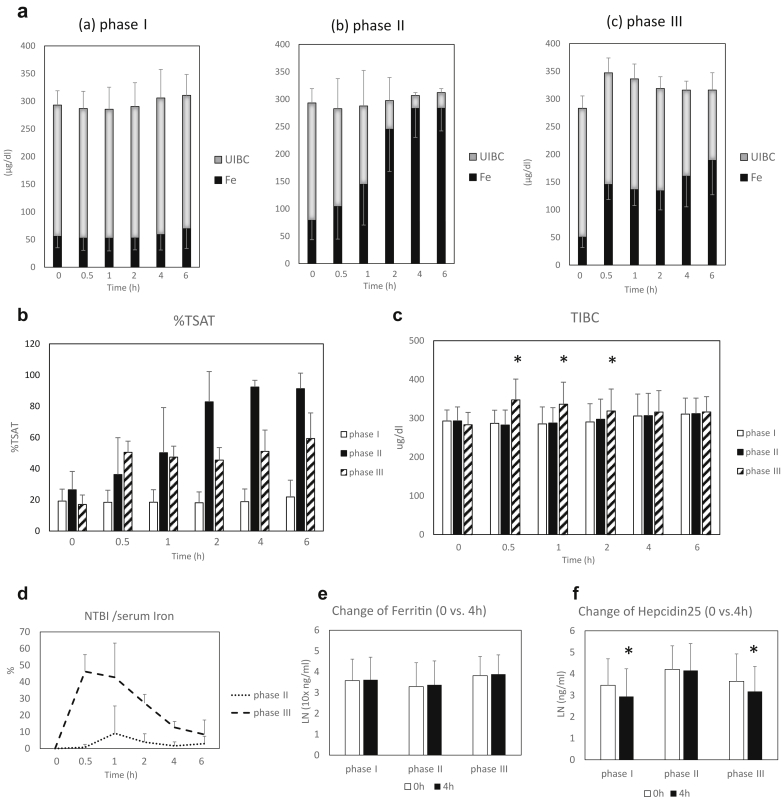
The temporal courses of changes in serum iron and unsaturated iron-binding capacity levels are shown in Figure 1a. Serum iron levels were significantly increased in both phase II and III as compared with 0 hour, respectively. Reflecting these increases in serum iron levels in phases II and III, transferrin saturation was increased during the course of study in both phases, as compared to 0 hour, respectively (Figure 1b). Regarding changes of TIBC, no significant changes were found in phases I and II, but significant increases were seen in phase III at 0.5 to 2 hours after dosing (appearance of NTBI), as compared with 0 hour, respectively (Figure 1c).
Figure 1.
Serial changes in serum iron and related parameters during the course of the study; phase I: control, phase II: oral intake of ferric citrate hydrate, and phase III: i.v. administration of saccharated ferric oxide. (a) Temporal course of changes in serum iron levels and unsaturated iron-binding capacity (UIBC), phase I (a), phase II (b), and phase III (c). (b) Temporal course of changes in transferrin saturation (TSAT; serum iron/TIBC [0 h]). (c) Temporal course of changes in total iron-binding capacity (TIBC; sum of serum iron levels and UIBC). P < 0.05, among the 3 phases; *P < 0.05 versus 0 hour in phase III (2-way repeated-measures analysis of variance). (d) Temporal course of changes in ratio of non–transferrin binding iron (NTBI) per serum iron in phases II and III. (e) Comparisons of serum ferritin levels at 0 and 4 hours in respective phases. (f) Comparisons of serum Hepcidin25 levels at 0 and 4 hours in respective phases. *P < 0.05 versus 0 hour (paired t-test; log-transformed data were used for analysis).
The temporal course of NTBI(%) of phases II and III is shown in Figure 1d. No significant changes were found in phase II, but significant increases were seen in phase III. In comparison of 0 and 4 hours, no differences were seen for serum ferritin levels among the 3 phases (Figure 1e). Significant decreases were found in serum hepcidin 25 levels in phases I and III, but no changes were seen in phase II (Figure 1f).
Redox-Inflammation Status
Changes to redox-inflammation markers with the respective interventions are shown in Table 2. Significant changes in MPO, highly sensitive C-reactive protein, and TRX levels were identified in phase III (Table 2). Otherwise no significant changes were found for other groups or markers.
Table 2.
Comparisons of surrogate markers in respective phases: 0 hour versus 4 hours
| Phase I, 0 h | Phase I, 4 h | P | Phase II, 0 h | Phase II, 4 h | P | Phase III, 0 h | Phase III, 4 h | P | |
|---|---|---|---|---|---|---|---|---|---|
| Oxidation/Inflammation | |||||||||
| 8-OHdG, ng/ml | 2.06 ± 1.04 | 3.01 ± 1.43 | NS | 2.57 ± 1.31 | 3.98 ± 1.08 | NS | 4.16 ± 1.26 | 3.45 ± 1.72 | NS |
| TBARS, μM | 75.9 ± 21.7 | 82.9 ± 8.0 | NS | 77.4 ± 5.0 | 75.4 ± 7.4 | NS | 82.4 ± 36.7 | 80.0 ± 13.2 | NS |
| Ln (MPO, pg/ml) | 6.16 ± 0.40 | 6.19 ± 0.60 | NS | 6.31 ± 0.60 | 7.18 ± 0.54 | NS | 7.55 ± 0.99 | 8.74 ± 0.73 | < 0.05 |
| d-ROM, Unit | 169 ± 62 | 183 ± 64 | NS | 165 ± 30 | 191 ± 42 | NS | 174 ± 17 | 191 ± 42 | NS |
| Ln (hsCRP, ng/ml) | 5.61 ± 1.12 | 5.74 ± 1.23 | NS | 6.19 ± 1.51 | 6.33 ± 1.48 | NS | 7.07 ± 1.43 | 7.22 ± 1.46 | < 0.01 |
| IL-6, pg/ml | 14.8 ± 5.5 | 17.4 ± 8.9 | NS | 12.0 ± 10.9 | 15.1 ± 15.0 | NS | 11.5 ± 5.6 | 10.2 ± 4.9 | NS |
| TNFα, pg/ml | 0.0 ± 0.0 | 0.6 ± 1.2 | NS | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.5 ± 1.0 | 0.0 ± 0.0 | NS |
| IL-10, pg/ml | 0.79 ± 0.51 | 0.93 ± 0.98 | NS | 0.79 ± 0.78 | 0.90 ± 1.18 | NS | 0.52 ± 0.65 | 0.23 ± 0.26 | NS |
| Antioxidation | |||||||||
| BAP, μmol/l | 2434 ± 90 | 2124 ± 179 | NS | 2265 ± 204 | 2117 ± 231 | NS | 2174 ± 238 | 2024 ± 237 | NS |
| TRX, ng/ml | 0.267 ± 0.102 | 0.684 ± 0.515 | NS | 0.268 ± 0.112 | 0.401 ± 0.398 | NS | 0.274 ± 0.059 | 0.170 ± 0.088 | < 0.05 |
Phase I: control, Phase II: oral intake of ferric citrate hydrate, Phase III: i.v. administration of saccharated ferric oxide.
8-OHdG, 8-hydroxy-2′-deoxyguanosine; BAP, Biological Antioxidant Potential; d-ROM, Reactive Oxygen Metabolites; hsCRP, highly sensitive C-reactive protein; IL-6, 10, interleukin -6, -10; Ln, natural logarithm; MPO; myeloperoxidase; NS, not significant; TBARS, 2-thiobarbituric acid-reactive substances; TNFα, tumor necrosis factor α; TRX, Thioredoxin.
Changes in Serum MPO and TRX Levels
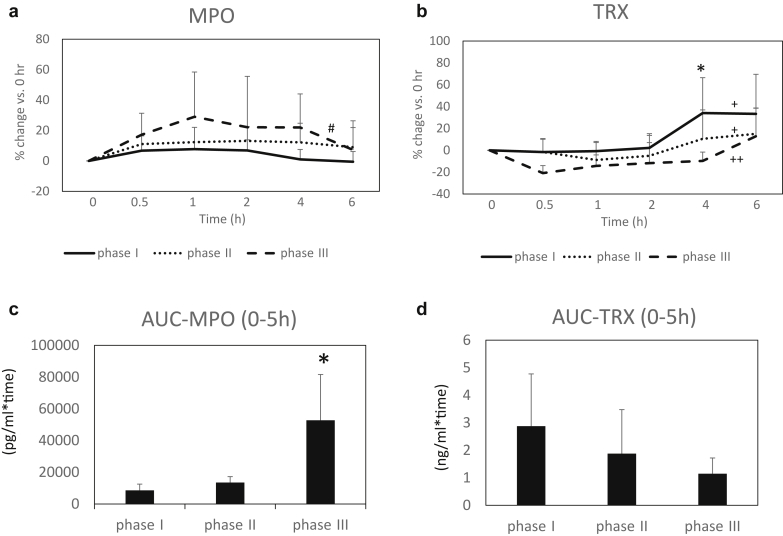
The temporal course of percentage changes in MPO and TRX levels are shown in Figure 2a and b. For within-group comparisons, there was a significant change of %MPO during the course in phase III, whereas no significant changes were found in phases I and II (Figure 2a). However, no significant differences were found at respective time points (0.5 to 6 hours) among the 3 groups. Regarding %TRX, there were significant changes in 3 phases, respectively. However, there was a significant difference at 4 hours among the 3 phases, with phase III being significantly higher than the others (Figure 2b). Comparisons of areas under the curve for MPO and TRX of serum levels during the study periods are shown in Figure 2c and d, with significantly larger in MPO, in phase III as compared with phase I (Figure 2c), but no significant differences were seen in TRX among the 3 phases (Figure 2d).
Figure 2.
Serial changes in serum myeloperoxidase (MPO) and thioredoxin (TRX) levels and area under the curve (AUC) during the course of the study. Phase I: control (a), phase II: oral intake of ferric citrate hydrate (b), and phase III: i.v. administration of saccharated ferric oxide (c). (a) Temporal course of changes in MPO levels (% change vs. basal level [0 hour]). Within-group comparisons: #P < 0.01 in phase III, and NS in phase I and II, respectively (Friedman test). (b) Temporal course of changes in TRX levels (% change vs. basal level [0 hour]). Within-group comparisons: +P < 0.05 in phase I, and II, ++P < 0.001 in III, respectively (Friedman test). Comparisons of the groups: *P < 0.05 at 4 hours (repeated-measures analysis of variance). (c) AUC of serum MPO levels. *P < 0.05, versus control (repeated-measures analysis of variance). (d) AUC of serum TRX levels.
Leukocyte Apoptosis
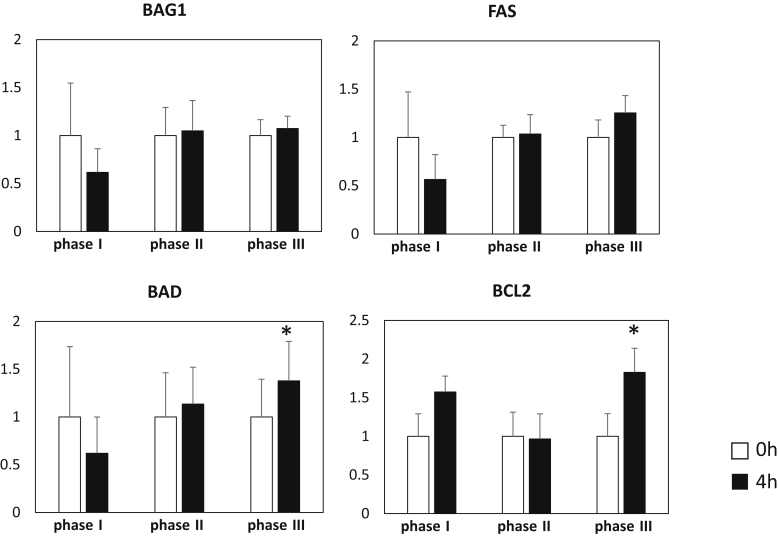
No significant differences or changes were found in the presence of leukocyte apoptosis among the 3 phases: 0.19% ± 0.17% (0 hour) versus 0.11% ± 0.09% (4 hours) in phase I, 0.62% ± 0.08% (0 hour) versus 0.62% ± 0.77% (4 hours) in phase II, and 0.51% ± 0.27% (0 hour) versus 0.46% ± 0.26% (4 hours) in phase III. In real-time PCR of apoptosis-related genes, expressions of B-cell lymphoma 2 and Bcl-2–associated death promoter (0 vs. 4 hours) were significantly enhanced in phase III (Figure 3).
Figure 3.
Apoptosis-related gene expression of peripheral leukocytes. BAD, Bcl-2–associated death promoter; BAG1, Bcl-2–associated athanogene 1; BCL2, B-cell lymphoma 2. *P < 0.05, versus 0 hour (paired t-test).
Influence on FGF23
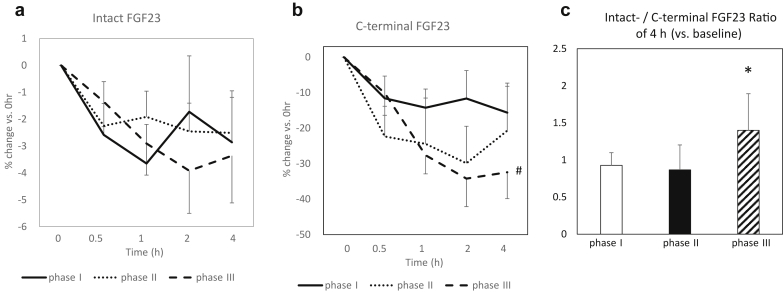
Temporal courses of % changes in serum intact-FGF23 and c-terminal FGF23 are shown in Figure 4a and b.
Figure 4.
Serial changes in serum intact and C-terminal fibroblast growth factor-23 (FGF23) during the course of the study. Phase I: control (a), phase II: oral intake of ferric citrate hydrate (b), and phase III: i.v. administration of saccharated ferric oxide (c). (a) Temporal course of changes in intact-FGF23 levels (% change vs. basal level [0 hour]). Within-group comparisons: NS in phase I, II, III, respectively (Friedman test). (b) Temporal course of changes in c-terminal FGF23 levels (% change vs. basal level [0 hour]). Within-group comparisons: NS in phase I, II, respectively, #P < 0.01 in phase III (Friedman test). (c) Change (×1) of intact-/c-terminal FGF23 ratio of 4 hours versus basal level. *P < 0.05, versus phase I (paired t-test; log-transformed values of measured data were used for the analyses).
No significant changes were found in % intact-FGF23 during the course in all 3 phases (Figure 4a). Regarding %c-terminal FGF23, there were no significant changes in phases I and II, but a significant decrease was found in phase III (Figure 4b). In the comparisons of the groups, there were no significant differences at each time point.
Regarding ratio of intact-FGF23 and c-terminal FGF23 (0 vs. 4 hours), the ratio of phase III was significantly higher than that of phase I (Figure 4c).
Discussion
With the recent findings of the effectiveness of FC for renal anemia, the present study aimed to explore biological differences in orally dosed FC as compared with i.v.-administered FO in HD patients.
We compared the iron kinetic profiles of oral FC containing 480 mg of iron and 5 minutes of intravenous FO containing 40 mg of iron in 6 patients. The main findings of the study were as follows: (i) significant generation of NTBI was seen with FO but not with FC, despite the fact that serum iron levels were lower with FO during the course of the study; (ii) increases in serum oxidative-inflammation markers such as MPO and highly sensitive C-reactive protein, with accompanying reductions in antioxidant molecule such as TRX, were seen with FO, but not with FC; and (iii) ratio of intact-FGF23 to c-terminal FGF23 at 4 hours after starting HD were significantly higher with FO, as compared with FC.
Significant increases in serum iron and transferrin saturation were identified in FC and FO, and TIBC levels were unchanged in FC as compared with the control condition. Conversely, TIBC were significantly increased in FO, indicating the appearance of NTBI in blood, 2 hours after administration. In the present study, we calculated estimated NTBI. As expected in the changes of TIBC, NTBI (%) in serum iron were significantly higher in FO, despite the fact that serum iron levels were significantly lower in FO as compared with FC.
As a physiological process, FC is absorbed as Fe2+ through DMT-1 in the intestine, and binds to transferrin as Fe3+. On the other hand, part of FO ([Fe(OH)3]m[C12H22O11]n) administered i.v. binds to transferrin as Fe3+, and the rest of the FO could become NTBI, a potential source of free iron. Zanen et al.17 reported that the generation of NTBI depends on the total administered dose of iron, as well as the speed of administration. Based on that report, in the case of glucuronated iron, the administered dose must be less than 15 mg/h to prevent the generation of NTBI. Thus, in terms of the generation of NTBI with FO containing 40 mg of iron, we speculated that 5 minutes of FO administration was too short to allow completion of Fe3+ binding to transferrin.
To examine the biological impact of iron administration to the body, we measured oxidative and antioxidative products. Among these, increases were seen in serum MPO (an oxidative marker) with accompanying significant decreases in TRX (an antioxidant) with FO, whereas no changes were found with FC (Table 2, Figure 2a–c). Our observations may support the notion from previous studies that i.v. iron single administration acutely enhances oxidative stress in HD patients.18, 19, 20, 21, 22, 23 Furthermore, although no significant changes in apoptosis cell ratio were found during the course of study in the different phases, expressions of apoptosis (Bcl-2–associated death promoter) and anti-apoptosis (B-cell lymphoma 2) genes in peripheral leukocytes were significantly influenced by FO. We speculate that NTBI generated in blood, and free iron (Fe2+) contained in the sample,24 may trigger the Fenton reaction to induce oxidative cellular injury of peripheral leukocytes. Where this pathologic reaction is repeated or exaggerated, the NTBI or injury to neutrophils could potentially play a role on infection risk.
Although there were short-time but significant changes in surrogate markers (e.g., MPO) and NTBI, by the single administration of FO, but it remains unclear if those changes were clinically relevant. However, it has been reported that serum MPO level correlates with levels of markers of inflammation, and is a significant independent factor for increased cardiovascular risk and mortality in patients on chronic dialysis treatment.25, 26 There are reports that a cumulative dose of i.v. administered iron was associated with an adverse cardiovascular outcome, and a higher mortality,27 or risk of hospitalization28 as compared with patients without iron administration among chronic HD patients. Taking these data together, we suppose that accumulation of the single impact by i.v. iron administration cannot be clinically underestimated, and this issue needs to be addressed in the future.
In addition to the possible induction of oxidative stress and micro-inflammation by i.v. FO, the present study found various points of difference between oral FC and i.v. FO. One of them was the response in hepcidin25 by iron load, and the data indicate the activation of physiological feedback in FC, whereas lack of feedback in FO. The increase of intact- to c-terminal FGF23 ratio with FO was also the difference observed. It has been hypothesized that i.v. iron infusion could reduce FGF23 transcription, but, on the other hand, carbohydrate moieties of i.v. iron formula could inhibit FGF23 degradation.29 Thus, the net effect of i.v. iron infusion on FGF23 metabolism may be greatly influenced by the type of iron agents. In Japan, FO is the only commercially available iron for i.v. use. Interestingly, Takeda et al.30 reported that repeated i.v. administration of 40 mg FO resulted in increased levels of intact-FGF23. The present observation of the relative increase of intact FGF23 by single FO administration may support the finding.
Several limitations to this study must be considered. First, we did not examine dose-response profiles of orally dosed FC. In the present study, FC with 480 mg of iron was examined, and showed no changes in TIBC as compared with control. However, whether oral intake of a larger dose of FC could generate NTBI remains unclear. Second, we examined the temporal course of changes in study parameters for 6 hours; however, changes after 6 hours remain unknown. It would be valuable to look at the iron profile for the extended time over 6 hours. However, we could not extend the time because of ethical reasons; the study actually forced patients to keep the fasting state for more than 8 hours from wake-up time to exclude the potential influence of iron absorption from food, or iron binding to phosphate in food. Third, we do not know whether the present data for FC can be generalized as a profile of orally dosed iron, because the FC used in this study has a unique property of showing a higher dissolution rate as compared with that used in food additives, and the intestinal absorption of iron via DMT-1 pathway may be primarily impaired in patients on HD3 due to elevation of hepcidin 25. For these reasons, further studies are needed to determine the difference in iron absorption between FC and standard oral ion preparations. And last, the data of this study were obtained from only 6 patients, and, therefore, further studies on more patients, with data collected at longer time points, should be done to confirm the observations made on this limited patient population.
In conclusion, oral FC differs from i.v. FO in terms of points such as TIBC, NTBI generation, induction of oxidative stress, and FGF23 metabolism. Oral FC may have benefits in iron supplementation for patients with CKD anemia. Clinical significance of this pathology needs to be addressed.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was conducted by the research fund from Japan Tobacco Inc. (Tokyo, Japan) and Torii Pharmaceutical Co., Ltd. (Tokyo, Japan).
References
- 1.Mimura I., Tanaka T., Nangaku M. How the target hemoglobin of renal anemia should be. Nephron. 2015;131:202–209. doi: 10.1159/000440849. [DOI] [PubMed] [Google Scholar]
- 2.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaritsky J., Young B., Wang H.J. Hepcidin—a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rostoker G., Vaziri N.D., Fishbane S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs. 2016;76:741–757. doi: 10.1007/s40265-016-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albaramki J., Hodson E.M., Craig J.C. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1:CD007857. doi: 10.1002/14651858.CD007857.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Shepshelovich D., Rozen-Zvi B., Avni T. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68:677–690. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Litton E., Xiao J., Ho K.M. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R., Vasavada N., Sachs N.G. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama K., Akiba T., Fukagawa M. JTT-751 for treatment of patients with hyperphosphatemia on peritoneal dialysis. Nephron Clin Pract. 2014;128:135–140. doi: 10.1159/000366482. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama K., Akiba T., Fukagawa M. A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant. 2014;29:1053–1060. doi: 10.1093/ndt/gft483. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K., Akiba T., Fukagawa M. Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr. 2014;24:261–267. doi: 10.1053/j.jrn.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama K., Hirakata H., Akiba T. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol. 2014;9:543–552. doi: 10.2215/CJN.05170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama K., Hirakata H., Akiba T. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36:478–487. doi: 10.1159/000344008. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama K., Fukagawa M., Akiba T. Ferritin elevation and improved responsiveness to erythropoiesis-stimulating agents in patients on ferric citrate hydrate. Kidney Int Rep. 2017;2:359–365. doi: 10.1016/j.ekir.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umanath K., Jalal D.I., Greco B.A. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26:2578–2587. doi: 10.1681/ASN.2014080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block G.A., Fishbane S., Rodriguez M. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3–5. Am J Kidney Dis. 2015;65:728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Zanen A.L., Adriaansen H.J., van Bommel E.F. 'Oversaturation' of transferrin after intravenous ferric gluconate (Ferrlecit(R)) in haemodialysis patients. Nephrol Dial Transplant. 1996;11:820–824. doi: 10.1093/oxfordjournals.ndt.a027405. [DOI] [PubMed] [Google Scholar]
- 18.Tovbin D., Mazor D., Vorobiov M. Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am J Kidney Dis. 2002;40:1005–1012. doi: 10.1053/ajkd.2002.36334. [DOI] [PubMed] [Google Scholar]
- 19.Bishu K., Agarwal R. Acute injury with intravenous iron and concerns regarding long-term safety. Clin J Am Soc Nephrol. 2006;1:S19–S23. doi: 10.2215/CJN.01420406. [DOI] [PubMed] [Google Scholar]
- 20.Kuo K.L., Hung S.C., Wei Y.H. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol. 2008;19:1817–1826. doi: 10.1681/ASN.2007101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Fernandez N., Echeverria A., Sanchez-Ibarrola A. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrology (Carlton) 2010;15:178–183. doi: 10.1111/j.1440-1797.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 22.Shema-Didi L., Kristal B., Ore L. Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during hemodialysis. Nutr Res. 2013;33:442–446. doi: 10.1016/j.nutres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Showkat A., Bastnagel W.R., Hudson J.Q. Effect of alpha-lipoic acid on oxidative stress in end-stage renal disease patients receiving intravenous iron. ISRN Nephrol. 2014;2014:634515. doi: 10.1155/2014/634515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A., Pratt R.D., Crumbliss A.L. Ferrous iron content of intravenous iron formulations. Biometals. 2016;29:411–415. doi: 10.1007/s10534-016-9923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K., Brennan M.L., Hazen S.L. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Wang A.Y., Lam C.W., Chan I.H. Prognostic value of plasma myeloperoxidase in ESRD patients. Am J Kidney Dis. 2010;56:937–946. doi: 10.1053/j.ajkd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Kuo K.L., Hung S.C., Lin Y.P. Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS One. 2012;7:e50295. doi: 10.1371/journal.pone.0050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailie G.R., Larkina M., Goodkin D.A. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87:162–168. doi: 10.1038/ki.2014.275. [DOI] [PubMed] [Google Scholar]
- 29.Wolf M., Koch T.A., Bregman D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y., Komaba H., Goto S. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol. 2011;33:421–426. doi: 10.1159/000327019. [DOI] [PubMed] [Google Scholar]