Abstract
Introduction
The variable disease course of autosomal dominant polycystic kidney disease (ADPKD) makes it important to develop biomarkers that can predict disease progression, from a patient perspective and to select patients for renoprotective treatment. We therefore investigated whether easy-to-measure urinary biomarkers are associated with disease progression and have additional value over that of conventional risk markers.
Methods
At baseline, inflammatory, glomerular, and tubular damage markers were measured in 24-hour urine collections (albumin, IgG, kidney injury molecule−1 (KIM-1), N-acetyl-β-d-glucosaminidase (NAG), β2 microglobulin (β2MG), heart-type fatty acid binding protein (HFABP), macrophage migration inhibitory factor (MIF), neutrophil gelatinase-associated lipocalin (NGAL), and monocyte chemotactic protein−1 (MCP-1). Disease progression was expressed as annual change in estimated glomerular filtration rate (eGFR, Chronic Kidney Disease EPIdemiology equation), measured glomerular filtation rate (mGFR, using 125I-iothalamate), or height-adjusted total kidney volume (htTKV). Multivariable linear regression was used to assess associations of these markers independent of conventional risk markers.
Results
A total of 104 ADPKD patients were included (40 ± 11 years, 39% female, eGFR 77 ± 30, mGFR 79 ± 30 ml/min per 1.73 m2 and htTKV 852 [510−1244] ml/m). In particular, β2MG and MCP-1 were associated with annual change in eGFR, and remained associated after adjustment for conventional risk markers (standardized β = −0.35, P = 0.001, and standardized β = −0.29, P = 0.009, respectively). Adding β2MG and MCP-1 to a model containing conventional risk markers that explained annual change in eGFR significantly increased the performance of the model (final R2 = 0.152 vs. 0.292, P = 0.001). Essentially similar results were obtained when only patients with an eGFR ≥ 60 ml/min per 1.73 m2 were selected, or when change in mGFR was studied. Associations with change in htTKV were less strong.
Conclusion
Urinary β2MG and MCP-1 excretion were both associated with GFR decline in ADPKD, and had added value beyond that of conventional risk markers. These markers therefore have the potential to serve as predictive tools for clinical practice.
Keywords: ADPKD, beta-2 microglobulin, kidney function decline, kidney volume, MCP-1, urinary biomarkers
The age at which patients with autosomal dominant polycystic kidney disease (ADPKD) will reach end-stage kidney disease (ESKD) shows large interindividual variability,1 even between family members that share the same mutation.2 Predicting the rate of disease progression has become important, now that the first therapeutic options for ADPKD have emerged.3, 4 Especially patients with a high likelihood of rapid disease progression should be selected for treatment, because in such patients the benefit-to-risk ratio of treatment is expected to be optimal.5, 6
Currently, several variables are available to predict disease progression in ADPKD. Glomerular filtration rate (GFR) indexed for age is a strong predictor but is less sensitive in early stages of this disease, when GFR can remain in the normal range due to compensatory hyperfiltration, while cysts are progressively formed.1 Therefore, much attention has been focused on total kidney volume (TKV) as a predictor.1, 7 Furthermore disease progression is influenced by the ADPKD genotype, with patients with a PKD1 mutation, especially truncating mutations, progressing faster toward ESKD compared to patients with a PKD2 mutation.2 However, assessment of TKV and genotype is laborious and expensive, and their associations with the rate of disease progression are limited at an individual patient level. Therefore, new risk markers need to be developed that, either alone or in combination with conventional risk markers, can predict the rate of disease progression in ADPKD.
Because ADPKD is a tubular disease with an inflammatory component, measurement of urinary tubular damage and inflammation markers is of interest, especially because these markers are relatively inexpensive and easy to measure. Several cross-sectional studies have shown that these markers are associated with ADPKD severity, assessed as GFR and TKV.8, 9, 10, 11 In this study, we aimed to determine, in a longitudinal setting, whether urinary tubular damage and inflammation markers are associated with rate of ADPKD progression assessed as annual change in GFR and TKV, and whether these markers have added value beyond that of currently used risk markers.
Methods
Setting and Subjects
From January 2007 until September 2012, a total of 133 ADPKD patients from the University Medical Center Groningen were included in an observational study. The diagnosis of APDKD was made based upon the revised Ravine criteria.12 Patients were considered ineligible if they received kidney replacement therapy, had undergone kidney surgery, were unable to undergo magnetic resonance imaging, or had other systemic diseases or used treatments or medications potentially affecting kidney function, such as calcineurin inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs).9, 10 For the present study, 29 patients were excluded because they had a follow up time < 1 year, leaving 104 patients for analysis. The study was performed in adherence to the Declaration of Helsinki, and all participants gave written informed consent. The review board of the University Medical Center Groningen deemed this study exempt from assessment because of its post hoc exploratory nature.
Measurements
At the baseline visit, a physical examination was performed, including blood pressure measurements. Fasting blood samples were drawn for the measurement of creatinine and PKD mutation analyses. The estimated GFR (eGFR) was calculated using the 2009 Chronic Kidney Disease EPIdemiology (CKD-EPI) equation.13 The PKD mutation analysis was performed with DNA isolation using PUREGENE nucleic acid purification chemistry on the AUTOPURE LS 98 platform (Qiagen, Venlo, the Netherlands), followed by sequencing of amplified coding exons directly (exons 34−46), or on long-range polymerase chain reaction products (exons 1−33).14 In addition, measured GFR (mGFR) was determined by a constant infusion method with 125I-iothalamate, and magnetic resonance imaging was performed to assess TKV, using a standardized abdominal magnetic resonance imaging protocol without the use of i.v. contrast. TKV was measured on T2-weighted coronal images using Analyze direct 9.0 (AnalyzeDirect, Inc., Overland Park, KS) by classical volumetry (i.e., manual tracing) and adjusted for height (htTKV).
The day before the baseline visit, patients collected a 24-hour urine, of which samples were stored frozen at −80°C that were used to measure albumin as a general kidney damage marker; IgG as a glomerular damage marker; and β2 microglobulin (β2MG), kidney injury molecule−1 (KIM-1), and N-acetyl-β-d-glucosaminidase (NAG) as proximal tubular damage markers; heart-type fatty acid binding protein (HFABP) as a distal tubular damage marker; and macrophage migration inhibitory factor (MIF), neutrophil gelatinase-associated lipocalin (NGAL), and monocyte chemotactic protein−1 (MCP-1) as inflammation markers.15, 16, 17, 18, 19, 20, 21, 22, 23
Urinary albumin was determined by immunonephelometry (BNII, Dade Behring Diagnostics, Deerfield, Illinois). Urinary IgG, HFABP (Hytest, Turku, Finland), β2MG (Anogen, Mississauga, Ontario, Canada), KIM-1, MIF, NGAL, and MCP-1 (R&D Systems, Minneapolis, Minnesota) were measured by enzyme-linked immunosorbent assay. NAG was measured with a modified enzyme assay according to Lockwood and corrected for nonspecific conversion (HaemoScan, Groningen, the Netherlands). Urine samples were diluted twice for KIM-1, β2MG, MCP-1, and MIF, 5 times for HFABP, and 100 times for NGAL and IgG. Detection limit for albumin was 0.003 mg/ml, for IgG 220 ng/ml, for β2MG 18 ng/ml, for KIM-1 0.087 ng/ml, for HFABP 0.38 ng/ml, for MIF 0.06 ng/ml, for NGAL 22 ng/ml, and for MCP-1 0.04 ng/ml. The intra- and interassay coefficients of variation were 2.2% and 2.6% for albumin, 6.3% and 8.5% for β2MG, 7.4% and 14.5% for KIM-1, 3.1% and 13.7% for NAG, 9.3% and 17.6% for H-FABP, 8.3% and 12.7% for MCP-1, 5.2% and 9.2% for MIF, and 6.8% and 19.6% for NGAL, respectively.
Statistical Analyses
Normally distributed data are expressed as mean ± SD, whereas non−normally distributed data are expressed as median with interquartile range (IQR). For cross-sectional comparison of baseline data, healthy controls (matched for sex and age) were asked to participate. These subjects had no medical history of cardiovascular and/or kidney disease, no medication use, normal blood pressure (< 140 systolic and < 90 diastolic) and preserved eGFR (> 60 ml/min per 1.73 m2).9 Differences between patients with ADPKD and healthy controls were tested using the 2-sample t test when normally distributed or a Mann−Whitney test when not normally distributed. A χ2 test was used in the case of categorical data.
Our primary endpoint was annual change in eGFR, and our secondary endpoints were annual change in mGFR and htTKV. These endpoints were calculated as follow-up minus baseline value divided by follow-up time in years, because there was only 1 follow-up value available for mGFR and htTKV. Annual change in eGFR was calculated in the same way so as to be in line with the analyses of the secondary endpoints. Annual change in eGFR was selected as our primary endpoint because disease progression is clinically assessed as eGFR decline and because more patients had data available for this endpoint than for change in mGFR. Multivariable linear regression analysis was used to investigate the associations of the various urinary biomarkers with annual change in eGFR, mGFR, and htTKV, with sequential adjustment for conventional risk markers (sex and baseline age, eGFR or mGFR, htTKV, and PKD mutation). All urinary biomarkers were log transformed to fulfill the requirement of normal distribution of the residuals except for albumin and MCP-1 excretion for annual change in htTKV. A subset of the included patients (27%) used tolvaptan between the baseline and follow-up assessment; therefore the associations of the biomarkers with all outcome measurements were additionally adjusted for tolvaptan use.
To investigate which variables had the strongest associations with annual change in eGFR, a stepwise backward linear regression analysis was performed for which the biomarkers with a univariate α ≤ 0.25 were selected, together with the conventional risk markers.
To investigate whether biomarkers had added prognostic value beyond that of conventional risk markers, we tested the difference in R squared (R2) for the various models. We first adjusted the R2 for optimism using bootstrapping.24 One thousand random samples of equal size were taken from the complete dataset using sampling with replacement. In these bootstrap samples, the coefficients of the final regression model were estimated (Mb,boot) and tested in the original sample (Mb,orig). Optimism was defined as R2(Mb,boot) – R2(Mb,orig). The R2 was adjusted for optimism by subtracting the optimism from the original R2 of the original dataset. We used the original data to select the final model from the stepwise backward analysis and subsequently adjusted this specific model for optimism. The for-optimism−adjusted R2 of the various models were compared with nested models using an F test. Akaike weight w(AIC) was used to compare the relative quality of the various unnested models.25
As sensitivity analyses, we tested the aforementioned associations, first in patients with a preserved kidney function only (eGFR ≥ 60 ml/min per 1.73 m2), and second in patients not using tolvaptan between the baseline and follow-up assessment. Third, we repeated the primary analysis with annual change in eGFR calculated as slope, with slope calculated by at least 3 eGFR measurements over > 1 year. This endpoint was chosen for the sensitivity analysis instead of the primary analysis because it could be performed only in a subset of patients. Fourth, we performed fractional polynomial regression analyses to test whether the associations between biomarkers and annual change in eGFR were linear.
Finally, we investigated how well urinary biomarker excretion was associated with annual change in eGFR compared to the Mayo htTKV classification.26 Therefore we transformed the urinary biomarker excretion in a urinary biomarker score (i.e., the combined ranking of the tertiles of the best-performing biomarkers) and bootstrapped the multivariable regression analyses with 1000 repetitions to obtain P values for the difference in the optimism-adjusted R2 between the models. The relative quality of the Mayo htTKV classification and the urinary biomarker score were compared by calculating the w(AIC).
Analyses were performed with SPSS version 22.0 (SPSS Inc., Chicago, IL) and R version 3.2.2. A 2-sided P < 0.05 was considered statistically significant.
Results
Subject Characteristics
Overall, our cohort was characterized by a relatively young population with a preserved kidney function. Healthy controls had a similar age and sex but had a higher eGFR (Table 1). As shown in Table 2, all urinary biomarker excretions were significantly higher in patients than in age- and sex-matched controls. The correlation amongst the various biomarkers is shown in Supplementary Table S1. All 104 patients had a follow-up eGFR, with a follow-up time of 3.82 ± 1.23 years and an annual change in eGFR of −3.22 ± 3.03 ml/min per 1.73 m2. Follow-up mGFR was available for 92 patients, with a follow-up time of 3.76 ± 1.23 years and an annual change in mGFR of −3.10 ± 2.97 ml/min per 1.73 m2. Follow-up htTKV was available for 81 patients, with a follow-up time of 3.78 ± 1.10 years and an annual change in htTKV of 6.17% ± 5.66%.
Table 1.
Baseline characteristics of ADPKD patients and control subjects
| Characteristic | ADPKD (n = 104) | Control (n = 102) | P value |
|---|---|---|---|
| Female sex, % | 39.4 | 42.2 | 0.69 |
| Age, yr | 40 ± 11 | 39 ± 12 | 0.66 |
| Weight, kg | 86 ± 18 | 74 ± 11 | <0.001 |
| Height, cm | 180 ± 10 | 178 ± 8 | 0.06 |
| BSA, m2 | 2.04 ± 0.24 | 1.91 ± 0.16 | <0.001 |
| SBP, mm Hg | 129 ± 12 | 122 ± 12 | <0.001 |
| DBP, mm Hg | 79 ± 9 | 72 ± 8 | <0.001 |
| AHT, % | 76.0 | 0.0 | <0.001 |
| RAASi, % | 69.2 | 0.0 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 77 ± 30 | 103 ± 12 | <0.001 |
| mGFR, ml/min per 1.73 m2 | 79 ± 30 | — | — |
| htTKV, ml/m | 852 (510−1244) | — | — |
| PKD mutation, % | |||
| PKD1 truncating | 44.2 | — | — |
| PKD1 nontruncating | 28.9 | — | — |
| PKD2 | 12.5 | — | — |
| Unknown | 1.9 | — | — |
| Missing | 12.5 | — | — |
ADPKD, autosomal dominant polycystic kidney disease; AHT, anti-hypertensive therapy; BSA, body surface area; DBP, diastolic blood pressure; GFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; mGFR, measured glomerular filtration rate; PKD, polycystic kidney disease; RAASi, renin−angiotensin−aldosterone system inhibitors; SBP, systolic blood pressure.
Variables are presented as mean ± SD, or as median (interquartile range) in case of nonnormal distribution.
Table 2.
Urinary biomarker excretions in ADPKD patients versus healthy control subjects
| Urinary biomarker | ADPKD | Control | P value |
|---|---|---|---|
| General | |||
| UAE (mg/24 h) | 37.8 (14.2–117.8) | 7.6 (6.2–12.8) | <0.001 |
| Glomerular | |||
| IgG (mg/24 h) | 13.7 (4.2–43.4) | 0.0 (0.0–0.0) | <0.001 |
| Proximal tubular | |||
| β2MG (μg/24 h) | 201.1 (81.2–579.3) | 78.4 (48.0–121.8) | <0.001 |
| KIM-1 (μg/24 h) | 1.5 (1.0–2.2) | 0.81 (0.4–1.3) | <0.001 |
| NAG (μg/24 h) | 3.3 (0.8–8.1) | 0.0 (0.0–2.4) | <0.001 |
| Distal tubular | |||
| HFABP (μg/24 h) | 2.0 (1.3–3.2) | 1.4 (1.0–2.2) | 0.001 |
| Inflammatory | |||
| MIF (ng/24 h) | 176.0 (106.5–258.0) | 129.5 (76.4–241.6) | 0.02 |
| NGAL (μg/24 h) | 73.0 (29.2–158.1) | 23.4 (16.3–30.9) | <0.001 |
| MCP-1 (ng/24 h) | 699.2 (533.6–1098.6) | 266.1 (175.3–396.9) | <0.001 |
β2MG, β2 microglobulin; HFABP, heart-type fatty acid binding protein; KIM-1, kidney injury molecule–1; MCP-1, monocyte chemotactic protein–1; MIF, macrophage migration inhibitory factor; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; UAE, urinary albumin excretion.
Variables are presented as median (interquartile range).
Associations of Urinary Biomarkers With Rate of Disease Progression
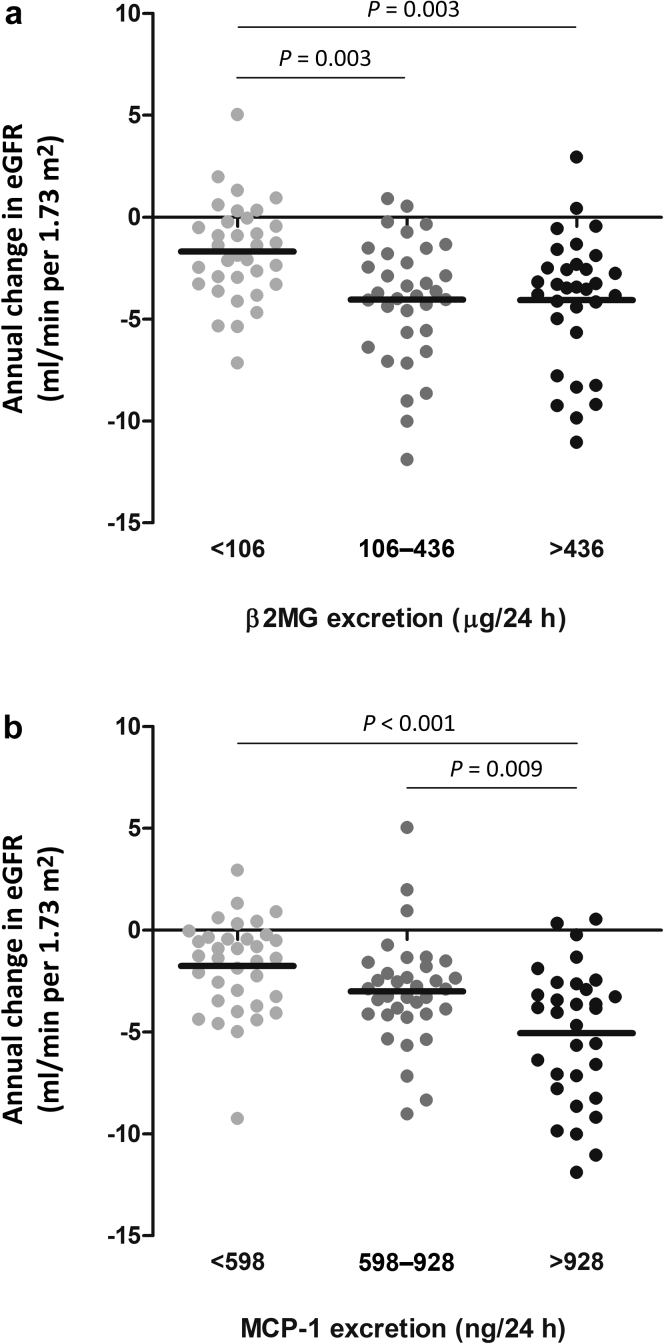
Both β2MG and MCP-1 were strongly associated with annual change in eGFR and remained significant after adjustment for conventional risk markers (standardized β = −0.35, P = 0.001, and standardized β = −0.29, P = 0.009 respectively). Less strong but also significant after adjustment for conventional risk markers was KIM-1 (standardized β = −0.24, P = 0.02) (Table 3). Essentially similar results were obtained with annual change in mGFR for β2MG, KIM-1, and MCP-1 (standardized β = −0.25, P = 0.03, standardized β = −0.25, P = 0.03, and standardized β = −0.21, P = 0.09, respectively) (Table 4), although these associations were less strong compared to the associations with annual change in eGFR. Figure 1 depicts the univariate associations of the 2 biomarkers with the strongest associations for annual change in eGFR in ADPKD patients, stratified according to tertiles of increasing levels of 24-hour urinary biomarker excretion. KIM-1 and MCP-1 excretion were both associated with annual change in htTKV in the crude analyses, but these associations lost significance after adjustment for sex, baseline age, mGFR, and htTKV (Table 5).
Table 3.
Associations of the urinary biomarkers with annual change in eGFR
| Urinary biomarker | Crude (n = 104) |
Model 1a (n = 104) |
Model 2b (n = 99) |
Model 3c (n = 89) |
||||
|---|---|---|---|---|---|---|---|---|
| St β | P | St β | P | St β | P | St β | P | |
| General | ||||||||
| UAE | –0.34 | 0.001 | –0.31 | 0.003 | –0.17 | 0.13 | –0.08 | 0.51 |
| Glomerular | ||||||||
| IgG | –0.30 | 0.003 | –0.28 | 0.004 | –0.17 | 0.09 | –0.12 | 0.27 |
| Proximal tubular | ||||||||
| β2MG | –0.28 | 0.006 | –0.29 | 0.004 | –0.23 | 0.02 | –0.35 | 0.001 |
| KIM-1 | –0.29 | 0.003 | –0.28 | 0.005 | –0.21 | 0.03 | –0.24 | 0.02 |
| NAG | –0.11 | 0.27 | –0.12 | 0.25 | 0.03 | 0.79 | 0.06 | 0.57 |
| Distal tubular | ||||||||
| HFABP | 0.04 | 0.68 | 0.03 | 0.77 | 0.16 | 0.15 | 0.08 | 0.51 |
| Inflammatory | ||||||||
| MIF | 0.10 | 0.35 | 0.10 | 0.34 | 0.12 | 0.19 | 0.07 | 0.48 |
| NGAL | –0.08 | 0.44 | –0.18 | 0.11 | 0.04 | 0.75 | 0.05 | 0.70 |
| MCP-1 | –0.51 | <0.001 | –0.49 | <0.001 | –0.38 | <0.001 | –0.29 | 0.009 |
β2MG, β2 microglobulin; eGFR, estimated glomerular filtration rate; HFABP, heart-type fatty acid binding protein; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemotactic protein–1; MIF, macrophage migration inhibitory factor; NAG, N-acetyl-β- d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; St β, standardized β; UAE, urinary albumin excretion.
Standardized β values and P values were calculated using multivariable linear regression. Dependent variable is annual change in eGFR. Independent variables are log transformed 24-h excretions of the various biomarkers.
Model 1: adjusted for age and sex.
Model 2: as for model 1 with additional adjustment for baseline eGFR and htTKV.
Model 3: as for model 2 with additional adjustment for PKD mutation.
Table 4.
Associations of the urinary biomarkers with annual change in mGFR
| Urinary biomarker | Crude (n = 92) |
Model 1a (n = 92) |
Model 2b (n = 88) |
Model 3c (n = 81) |
||||
|---|---|---|---|---|---|---|---|---|
| St β | P | St β | P | St β | P | St β | P | |
| General | ||||||||
| UAE | –0.37 | <0.001 | –0.37 | 0.001 | –0.27 | 0.02 | –0.16 | 0.20 |
| Glomerular | ||||||||
| IgG | –0.32 | 0.002 | –0.32 | 0.003 | –0.27 | 0.01 | –0.22 | 0.07 |
| Proximal tubular | ||||||||
| β2MG | –0.28 | 0.009 | –0.30 | 0.006 | –0.24 | 0.03 | –0.25 | 0.03 |
| KIM-1 | –0.25 | 0.02 | –0.24 | 0.03 | –0.20 | 0.06 | –0.25 | 0.03 |
| NAG | –0.13 | 0.23 | –0.15 | 0.20 | –0.03 | 0.82 | –0.02 | 0.89 |
| Distal tubular | ||||||||
| HFABP | 0.02 | 0.85 | 0.03 | 0.79 | 0.12 | 0.31 | 0.11 | 0.40 |
| Inflammatory | ||||||||
| MIF | 0.10 | 0.34 | 0.10 | 0.35 | 0.10 | 0.35 | 0.04 | 0.74 |
| NGAL | –0.34 | 0.001 | –0.46 | <0.001 | –0.38 | 0.002 | –0.34 | 0.01 |
| MCP-1 | –0.41 | <0.001 | –0.40 | <0.001 | –0.29 | 0.01 | –0.21 | 0.09 |
β2MG, β2 microglobulin; KIM-1, kidney injury molecule–1; mGFR, measured GFR; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; St β, standardized β; HFABP, heart-type fatty acid binding protein; MCP-1, monocyte chemotactic protein–1; MIF, macrophage migration inhibitory factor; UAE, urinary albumin excretion.
Standardized β values and P values were calculated using multivariable linear regression. Dependent variable is annual change in mGFR. Independent variables are log transformed 24-h excretions of the various biomarkers.
Model 1: adjusted for age and sex.
Model 2: as for model 1 with additional adjustment for baseline mGFR and htTKV.
Model 3: as for model 2 with additional adjustment for PKD mutation.
Figure 1.
Associations of urinary β2 microglobulin (β2MG) (a) and monocyte chemotactic protein−1 (MCP-1) excretion (b) with annual change in estimated glomerular filtration rate (eGFR). Patients are stratified according to tertiles of urinary biomarker excretion. P values were calculated using analysis of variance with a post hoc Bonferroni test.
Table 5.
Associations of the urinary biomarkers with annual change in htTKV
| Urinary biomarker | Crude (n = 81) |
Model 1a (n = 81) |
Model 2b (n = 81) |
Model 3c (n = 71) |
||||
|---|---|---|---|---|---|---|---|---|
| St β | P | St β | P | St β | P | St β | P | |
| General | ||||||||
| UAE | 0.20 | 0.06 | 0.13 | 0.22 | 0.06 | 0.60 | –0.01 | 0.94 |
| Glomerular | ||||||||
| IgG | 0.07 | 0.55 | 0.04 | 0.69 | –0.02 | 0.84 | –0.08 | 0.51 |
| Proximal tubular | ||||||||
| β2MG | 0.08 | 0.50 | 0.08 | 0.44 | 0.04 | 0.70 | 0.00 | 0.98 |
| KIM-1 | 0.21 | 0.05 | 0.21 | 0.05 | 0.18 | 0.09 | 0.16 | 0.20 |
| NAG | 0.09 | 0.43 | 0.10 | 0.35 | 0.04 | 0.76 | 0.01 | 0.95 |
| Distal tubular | ||||||||
| HFABP | –0.05 | 0.65 | –0.00 | 0.99 | –0.04 | 0.70 | –0.04 | 0.77 |
| Inflammatory | ||||||||
| MIF | 0.02 | 0.85 | 0.02 | 0.85 | 0.01 | 0.91 | –0.01 | 0.96 |
| NGAL | –0.04 | 0.73 | 0.09 | 0.47 | 0.01 | 0.97 | –0.03 | 0.82 |
| MCP-1 | 0.28 | 0.008 | 0.23 | 0.03 | 0.18 | 0.15 | 0.06 | 0.66 |
β2MG, β2 microglobulin; HFABP, heart-type fatty acid binding protein; htTKV, height-adjusted total kidney volume; KIM-1, kidney injury molecule–1; MCP-1, monocyte chemotactic protein–1; MIF, macrophage migration inhibitory factor; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; St. β, standardized β; UAE, urinary albumin excretion.
Standardized β values and P values were calculated using multivariable linear regression. Dependent variable is annual change in htTKV. Independent variables are log transformed 24-h excretions of the various biomarkers, except for UAE and MCP-1.
Model 1: adjusted for age and sex.
Model 2: as for model 1 with additional adjustment for baseline mGFR and htTKV.
Model 3: as for model 2 with additional adjustment for PKD mutation.
Added Value of Urinary Biomarkers Beyond That of Conventional Risk Markers
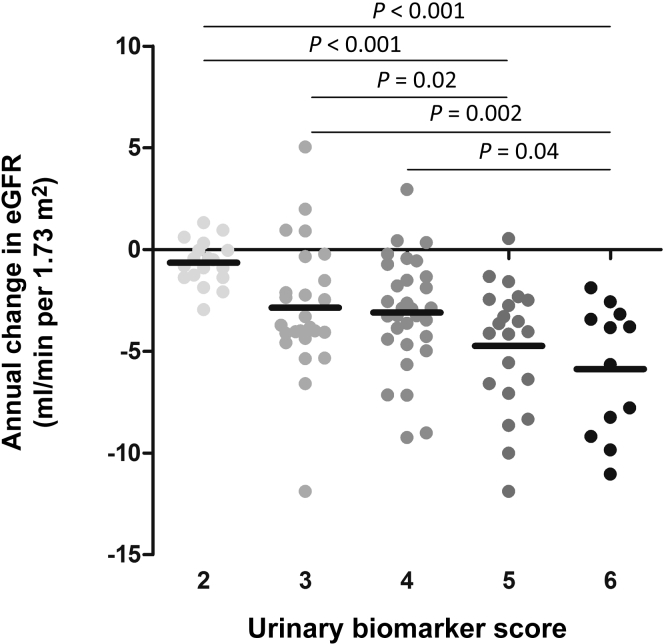
In Table 6, the strength of the associations of various models, including the 2 biomarkers with the strongest associations with annual change in eGFR, are compared. The R2 of the model with only the conventional risk markers (model 1) was compared with the model additionally including β2MG (model 2), MCP-1 (model 3), or both markers (model 4). Model 4 had the best fit for annual change in eGFR, with an R2 of 0.292 (P = 0.001, P = 0.03, and P = 0.006 compared to model 1, 2, and 3, respectively). Figure 2 displays the combined ranking of tertiles of urinary β2MG and MCP-1 excretion (urinary biomarker score) with annual change in eGFR.
Table 6.
Models explaining annual change in eGFR without and with urinary biomarkers (n = 83)
| Variable | Model 1a |
Model 2b |
Model 3c |
Model 4d |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St β | P | R2 | St β | P | R2 | St β | P | R2 | St β | P | R2 | |
| 0.152 | 0.247e | 0.216e | 0.292f | |||||||||
| Age | 0.20 | 0.17 | 0.11 | 0.44 | 0.13 | 0.37 | 0.05 | 0.69 | ||||
| Male sex | –0.07 | 0.51 | –0.05 | 0.63 | –0.08 | 0.41 | –0.06 | 0.51 | ||||
| eGFR | 0.13 | 0.34 | –0.04 | 0.79 | 0.05 | 0.73 | –0.09 | 0.51 | ||||
| htTKV | –0.44 | <0.001 | –0.43 | <0.001 | –0.30 | 0.009 | –0.31 | 0.004 | ||||
| PKD2 (ref)g | ||||||||||||
| PKD1 truncating | –0.44 | 0.008 | –0.51 | 0.001 | –0.32 | 0.05 | –0.41 | 0.009 | ||||
| PKD1 nontruncating | –0.45 | 0.004 | –0.49 | 0.001 | –0.35 | 0.02 | –0.40 | 0.005 | ||||
| β2MG | –0.35 | 0.001 | –0.31 | 0.002 | ||||||||
| MCP-1 | –0.33 | 0.003 | –0.28 | 0.008 | ||||||||
β2MG, β2 microglobulin; eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; MCP-1, monocyte chemotactic protein–1; PKD, polycystic kidney disease; St β, standardized β.
Standardized β and P values were calculated using multivariable linear regression. Dependent variable is annual change in eGFR. Independent variables are age, sex, baseline eGFR, baseline htTKV, PKD mutation, β2MG, and MCP-1.
Model 1: adjusted for age, sex, baseline eGFR, baseline htTKV and PKD mutation.
Model 2: as for model 1 plus β2MG.
Model 3: as for model 1 plus MCP-1.
Model 4: as for model 1 plus β2MG and MCP-1.
Significant compared to model 1 (P = 0.003 for model 2 and P = 0.02 for model 3).
Significant compared to models 1, 2, and 3 (P = 0.001, P = 0.03, and P = 0.006, respectively).
PKD mutation was used as dummy variable with PKD2 as reference group.
Figure 2.
Association of the combined ranking of tertiles of urinary β2 microglobulin (β2MG) and monocyte chemotactic protein−1 (MCP-1) excretion (urinary biomarker score) with annual change in estimated glomerular filtration rate (eGFR). P values were calculated using analysis of variance with a post hoc Bonferroni test.
Selection of Variables With the Strongest Association
Stepwise backward regression analysis was performed to select the variables with the strongest associations with annual change in eGFR. For this analysis age, sex, baseline eGFR, baseline htTKV, PKD mutation, UAE, β2MG, KIM-1, IgG, and MCP-1 were considered. The final model showed an R2 of 0.330 and included baseline htTKV, PKD mutation, β2MG, and MCP-1 excretion; KIM-1 excretion lost its significance (Table 7). UAE did also not remain significantly associated, from which we can conclude that these results were independent of albuminuria. Of note, this final model did not outperform model 4 in Table 6, where all conventional risk markers and both β2MG and MCP-1 were included (P = 0.43). The relative quality of each model was tested with the w(AIC). This analysis indicated that the model in Table 7 is the best model (Supplementary Table S2).
Table 7.
Results of the stepwise backward regression analysis with annual change in eGFR as dependent variable (n = 84)
| Variable | St β | P | R2 |
|---|---|---|---|
| 0.330 | |||
| htTKV | –0.29 | 0.005 | |
| PKD2 (ref)a | |||
| PKD1 truncating | –0.45 | 0.002 | |
| PKD1 nontruncating | –0.44 | 0.002 | |
| β2MG | –0.30 | 0.001 | |
| MCP-1 | –0.26 | 0.01 |
β2MG, β2 microglobulin; eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; MCP-1, monocyte chemotactic protein–1; PKD, polycystic kidney disease; St β, standardized β.
Standardized β and P values were calculated using multivariable linear regression. Dependent variable is annual change in eGFR. Independent variables are baseline htTKV, PKD mutation, β2MG, and MCP-1.
PKD mutation was used as dummy variable with PKD2 as reference group.
Sensitivity Analyses
Several sensitivity analyses were performed to test the robustness of our findings. First, we limited the analyses to patients with an eGFR ≥ 60 ml/min per 1.73 m2 (n = 73). Only β2MG excretion remained significantly associated after adjustment for conventional risk markers (standardized β = −0.37, P = 0.001). Second, we repeated the analyses in patients not using tolvaptan between the baseline and follow-up assessment (n = 76). β2MG, KIM-1, and MCP-1excretion remained significantly associated with annual change in eGFR after adjustment for conventional risk markers (standardized β = −0.45 P < 0.001, standardized β = −0.31, P = 0.009, and standardized β = −0.26, P = 0.05, respectively). Of note, no significant interactions were found between tolvaptan use and biomarker excretion with annual change in eGFR, mGFR, or htTKV. Repeating the primary analyses with annual change in eGFR calculated as slope instead of change in eGFR (n = 96) showed again that β2MG and MCP-1 remained significantly associated with annual change in eGFR after adjustment for conventional risk markers (Supplementary Table S3). Finally, Supplementary Figure S1 shows the distributions of individual data with respect to either β2MG or MCP-1 excretion versus annual change in eGFR and the corresponding fractional polynomial regression analyses. The regression lines are compatible with linear associations.
Comparison of Urinary Biomarkers With Risk Classification Based on htTKV Adjusted for Age
The performance of the urinary biomarker score was compared to the performance of the Mayo htTKV classification. According to this classification, 14.4% of the patients of our cohort were classified as “atypical.” From the patients with typical ADPKD, 3.5% were assigned to class A, 11.8% to class B, 35.3% to class C, 28.2% to class D, and 21.2% to class E. Differences between the subgroups in annual change in eGFR are shown in Supplementary Figure S2. When performing a multivariable linear regression analysis, the Mayo htTKV classification had a univariate R2 of 0.110 for annual change in eGFR. The urinary biomarker score had an R2 of 0.203 (P < 0.001). A comparison of the relative quality of the separate models [w(AIC)] is presented in Supplementary Table S4.
Discussion
In the present study, we investigated the association between urinary biomarker excretion and disease progression in ADPKD patients. Several cross-sectional studies have shown that urinary markers are associated with ADPKD severity, assessed as TKV and GFR.8, 9, 10, 11 To our knowledge, only 2 studies have previously investigated the association between urinary damage markers and disease progression in a longitudinal setting. Parikh et al. found no associations between urinary interleukin-18 and NGAL and annual change in eGFR and TKV in 107 ADPKD patients,11 whereas Park et al. found no association between urinary NAG and eGFR decline after 1 year in a cohort of 270 ADPKD patients.27 In these 2 studies, urinary interleukin-18, NGAL, and NAG concentrations were measured in urine samples that were stored frozen. We have previously shown that frozen storage decreases the measured concentration of urinary biomarkers and induces more variability.28 In particular, the increase in variability can reduce the strength of associations, as we have shown for urinary albumin concentration in non-ADPKD subjects.29 Given these considerations, we have cautioned against an overly skeptical view toward the utility of urinary biomarkers to predict disease progression in ADPKD.30 In the present study, we investigated additional urinary biomarkers, and our findings indicate that some of these markers are indeed useful despite the variability of marker concentrations induced by frozen storage.
It is assumed that cysts in ADPKD mainly originate from the distal tubule and collecting ducts.31 Remarkably, in our study, especially the proximal tubular marker β2MG and the inflammatory marker MCP-1 were associated with kidney function decline, suggesting that the proximal tubule and inflammation may be involved in the pathophysiology of ADPKD. We caution, however, against overinterpretation of our findings, because one should be aware of the strengths and limitations of the various assays. One assay is more reproducible than the other, and some markers are more subjected to degradation during long frozen storage than others.28, 32 Moreover, insights about the origin of certain markers may change, which, for example, is the case for urinary NGAL concentration. Although we consider it to be a general inflammatory marker of kidney damage, others have recently suggested that the collecting duct may be the main source of urinary NGAL.33 The fact that we did not find associations between some markers (such as the distal tubular damage marker HFABP) and disease progression should therefore not lead to conclusions as to which parts of the renal tubule are not involved with cystogenesis.
Surprisingly, the markers that we studied did not show associations with kidney growth. This may be due to insufficient power, as only 81 patients had a follow-up htTKV. On the other hand, it may also indicate that kidney growth represents a pathophysiological phenomenon other than kidney function decline in terms of urinary biomarkers. Of note, in our cohort there was considerable variability in annual change in htTKV and also in annual change in eGFR. However, the variability in these rates of disease progression are comparable to numbers that were found in other cohort studies, such as the control groups in the TEMPO (Tolvaptan Efficacy and safety in Management of autosomal dominant Polycystic kidney disease and Its Outcome) 3:4 trial and the HALT-PKD (Halt Progression of Polycystic Kidney Disease) trials.4, 34, 35, 36 This variability in rate of disease progression emphasizes furthermore the correctness of the practical rationale of our study, i.e., that because of high variability in rate of disease progression, markers are needed to predict prognosis and select patients for treatment.
Irazabal et al. recently developed a prognostic model based on htTKV and age (the Mayo htTKV classification).26 The overall value of this model to predict kidney function decline and incidence of ESKD is good; however, information on type of PKD mutation was not included in that study. In line with this predictive ability, we found that htTKV was strongly associated with annual change in eGFR. Of note, our results showed that type of PKD mutation remained associated with annual change in eGFR after adjustment for htTKV, indicating that type of PKD mutation has added value on top of baseline htTKV to predict kidney function decline. This is the first study to show such added value. Importantly, when we performed a stepwise backward analysis, urinary β2MG and MCP-1 excretion remained significantly associated with annual change in eGFR even after adjustment for htTKV and type of PKD mutation (Table 7).
The advantage of urinary biomarkers is especially that their measurement is relatively easy and inexpensive compared to measurement of TKV and PKD mutation analysis. Based on our results, one might therefore also consider using only urinary biomarkers for the prediction of kidney function decline when not all resources are available to measure TKV and to perform PKD mutation analysis. The regression models in this study show a relatively low R2, which has also been found in other studies investigating disease progression in ADPKD.37, 38, 39 This suggests that to reliably predict prognosis in ADPKD, multiple markers should probably be used together, to achieve adequate risk prediction. Our data suggest that, in this respect, including urinary excretion of tubular damage and inflammation markers on top of eGFR and htTKV should be considered a candidate approach.
Now, with vasopressin V2 receptor antagonists and possibly with somatostatin analogues the first therapeutic options for ADPKD have emerged, and it is important to be able to identify patients with a high likelihood of rapid disease progression at an early stage of their disease course. These patients especially can benefit from lifelong therapies with respect to absolute gain in dialysis-free years. For this reason, we performed a sensitivity analysis taking only patients into account with a relatively preserved kidney function (eGFR ≥ 60 ml/min per 1.73 m2). Even in this subgroup, urinary β2MG excretion was still associated with rate of kidney function decline, although the association with urinary MCP-1 excretion did not reach statistical significance, probably due to insufficient power.
Our data should be interpreted with caution, because our study has limitations. First, our cohort consisted of a relatively small number of ADPKD patients. For this reason, we adjusted our models for optimism by bootstrapping, which minimizes the risk of overfitting of data. In addition, we found similar associations with the various endpoints that were studied, including annual change in eGFR calculated as slope, suggesting that our results are robust. Second, we used data of some patients in our cohort who were taking the vasopressin V2 receptor antagonist tolvaptan between the baseline and follow-up visit, which could have influenced our results. To investigate this, a sensitivity analysis was performed, selecting only patients who had not used tolvaptan. Similar results were found with multivariable linear regression. In addition, no statistical interactions were found between biomarker excretions and tolvaptan use in their associations with each outcome variable. In the near future, the use of mixed populations for research, with some patients using tolvaptan and others not using it, will be everyday practice, as tolvaptan has been granted marketing authorization in Europe and other countries around the globe. It is reassuring that, in this study, tolvaptan use did not appear to influence our results. Third, we were not able to include early onset of clinical symptoms in our set of conventional risk markers, because such data were not routinely collected. Finally, some of these markers, such as KIM-1 and NGAL, are also found to be associated with acute kidney injury.40 Because our patients have CKD and were studied during a routine outpatient clinic, it is more likely that these markers reflect chronic rather than acute kidney injury in our study.
Strengths of this study are that we have information on multiple outcome measures, namely, annual change in eGFR, mGFR, and htTKV. In addition we have information on type of PKD mutation. This information has, to our knowledge, not previously been tested in conjunction with baseline htTKV and eGFR to predict the rate of disease progression in ADPKD. Importantly, we showed that urinary biomarkers were associated with annual change in eGFR even after adjustment for type of PKD mutation, baseline htTKV, and eGFR. Finally, all patients performed a 24-hour urine collection for biomarker assessment, which, because of the circadian rhythm in urinary excretion of these markers, may be better than the spot urines that are used in most other studies.
In conclusion, our study showed that urinary β2MG and MCP-1 excretion is associated with the rate of kidney function decline in patients with ADPKD independent of conventional risk markers. We demonstrated that these urinary biomarkers can be of value even beyond conventional risk markers in predicting kidney function decline. Measurement of urinary tubular damage and inflammation markers in ADPKD patients may therefore become an easy, rapid, and inexpensive tool to predict the rate of disease progression. Future studies should, however, corroborate our findings before measurement of urinary biomarkers can be used for this purpose.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The DIPAK Consortium is an interuniversity collaboration in the Netherlands established to study Autosomal Dominant Polycystic Kidney Disease and to develop treatment strategies for this disease. The DIPAK Consortium is sponsored by the Dutch Kidney Foundation (grants CP10.12 and CP15.01) and Dutch government (LSHM15018). For the present study, we acknowledge R.L. Kadijk for assistance at the outpatient clinic; R. Karsten-Barelds, D. Hesseling-Swaving and M. Vroom-Dallinga for their assistance during kidney function measurements; P. Kappert, J. Grozema and A. Sibeijn-Kuiper for assistance during MR imaging and M. Kaatee, M. de Jong, S.N. Voorrips, M.B. Wiertz and C. Plate for measuring TKVs.
Acknowledgments
Author Contributions
Each author contributed sufficiently to the intellectual content of the submission. RTG and EM had the idea for the research and study design, data acquisition was performed by ALM, EM, WEB, GEE, NFC, EMS, ML, DJMP. The data analysis was performed by ALM, EM, NFC, EMS, JGMB, RTG, and ALM drafted the manuscript; and EM, WEB, GEE, NFC, EMS and DJMP revised the manuscript. RGT approved the final version and was the supervisor of this research.
Footnotes
Supplementary Methods. Statistical analysis.
Table S1. Correlations among biomarker excretions. β2MG excretion was correlated with a general marker (albumin), a distal tubular marker (HFABP) and an inflammation marker (NGAL). MCP-1 excretion was correlated with a general marker (albumin), a glomerular marker (IgG), a proximal tubular marker (KIM-1) and an inflammation marker (NGAL).
Table S2. AICs and Akaike weights of the various models for annual change in eGFR. For annual change in eGFR, model 5 (the resultant of the stepwise backward analysis) had the lowest AIC. When comparing the w(AIC) of each model, model 5 had a normalized probability of 1.00 over model 1, 1.00 over model 2 and 1.00 over model 3, and 0.88 over model 4 indicating that model 5 comes closer to the truth and is the best model.
Table S3. Associations of the urinary biomarkers with annual change in eGFR calculated as slope through multiple (≥ 3) eGFR values. β2MG and MCP-1 were both associated with annual change in eGFR, and remained significant after adjustment for conventional risk markers (standardized β = −0.32, P = 0.002 and standardized β = −0.27, P = 0.02 respectively).
Table S4. AICs and Akaike weights for the Mayo htTKV classification and Urinary Biomarker Score. The urinary biomarker score had the lowest AIC. When comparing the w(AIC) of each model, the urinary biomarker score had a normalized probability of 1.00 over the Mayo htTKV classification, indicating that the biomarker score is preferred over the Mayo htTKV classification.
Figure S1. Scatter plot of urinary β2MG and MCP-1 excretion versus annual change in eGFR. This figure represents the value distributions of annual change in eGFR with either β2MG or MCP-1 excretion. The line represents the results of the fractional polynomial regression analysis. For β2MG excretion the association was linear, the association was non-linear for MCP-1 excretion < 200 ng/24 h.
Figure S2. Differences in annual change in eGFR between classes of the Mayo htTKV classification of ADPKD. This figure represents the differences in annual change in eGFR for the different Mayo htTKV classes. The annual change in eGFR was −1.2 ± 2.0 ml/min per 1.73 m2 for class A, −2.5 ± 2.2 ml/min per 1.73 m2 for class B, −2.7 ± 2.3 ml/min per 1.73 m2 for class C, −3.7 ± 5.5 ml/min per 1.73 m2 for class D, and −5.7 ± 2.8 ml/min per 1.73 m2 for class E.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Statistical analysis.
Correlations among biomarker excretions. β2MG excretion was correlated with a general marker (albumin), a distal tubular marker (HFABP) and an inflammation marker (NGAL). MCP-1 excretion was correlated with a general marker (albumin), a glomerular marker (IgG), a proximal tubular marker (KIM-1) and an inflammation marker (NGAL).
AICs and Akaike weights of the various models for annual change in eGFR. For annual change in eGFR, model 5 (the resultant of the stepwise backward analysis) had the lowest AIC. When comparing the w(AIC) of each model, model 5 had a normalized probability of 1.00 over model 1, 1.00 over model 2 and 1.00 over model 3, and 0.88 over model 4 indicating that model 5 comes closer to the truth and is the best model.
Associations of the urinary biomarkers with annual change in eGFR calculated as slope through multiple (≥ 3) eGFR values. β2MG and MCP-1 were both associated with annual change in eGFR, and remained significant after adjustment for conventional risk markers (standardized β = −0.32, P = 0.002 and standardized β = −0.27, P = 0.02 respectively).
AICs and Akaike weights for the Mayo htTKV classification and Urinary Biomarker Score. The urinary biomarker score had the lowest AIC. When comparing the w(AIC) of each model, the urinary biomarker score had a normalized probability of 1.00 over the Mayo htTKV classification, indicating that the biomarker score is preferred over the Mayo htTKV classification.
Scatter plot of urinary β2MG and MCP-1 excretion versus annual change in eGFR. This figure represents the value distributions of annual change in eGFR with either β2MG or MCP-1 excretion. The line represents the results of the fractional polynomial regression analysis. For β2MG excretion the association was linear, the association was non-linear for MCP-1 excretion < 200 ng/24 h.
Differences in annual change in eGFR between classes of the Mayo htTKV classification of ADPKD. This figure represents the differences in annual change in eGFR for the different Mayo htTKV classes. The annual change in eGFR was −1.2 ± 2.0 ml/min per 1.73 m2 for class A, −2.5 ± 2.2 ml/min per 1.73 m2 for class B, −2.7 ± 2.3 ml/min per 1.73 m2 for class C, −3.7 ± 5.5 ml/min per 1.73 m2 for class D, and −5.7 ± 2.8 ml/min per 1.73 m2 for class E.
References
- 1.Grantham J.J., Torres V.E., Chapman A.B. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E., Audrézet M.P., Chen J.M. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caroli A., Perico N., Perna A. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 4.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman A.B., Devuyst O., Eckardt K.U. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gansevoort R.T., Arici M., Benzing T. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and European renal best practice. Nephrol Dial Transplant. 2016;31:337–348. doi: 10.1093/ndt/gfv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutani H., Smith V., Rahbari-Oskoui F. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88:146–151. doi: 10.1038/ki.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petzold K., Poster D., Krauer F. Urinary biomarkers at early ADPKD disease stage. PLoS One. 2015;10:4. doi: 10.1371/journal.pone.0123555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer E., Boertien W.E., Nauta F.L. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis. 2010;56:883–895. doi: 10.1053/j.ajkd.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Boertien W.E., Meijer E., de Jong P.E. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65:833–841. doi: 10.1053/j.ajkd.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Parikh C.R., Dahl N.K., Chapman A.B. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int. 2012;81:784–790. doi: 10.1038/ki.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei Y., Obaji J., Dupuis A. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossetti S., Hopp K., Sikkink R.A. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23:915–933. doi: 10.1681/ASN.2011101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waanders F., van Timmeren M.M., Stegeman C.A. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220:7–16. doi: 10.1002/path.2642. [DOI] [PubMed] [Google Scholar]
- 16.Trof R.J., Di Maggio F., Leemreis J. Biomarkers of acute renal injury and renal failure. Shock. 2006;26:245–253. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 17.Parikh C.R., Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(4 suppl):S159–S165. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 18.Maatman R.G., Van Kuppevelt T.H., Veerkamp J.H. Two types of fatty acid-binding protein in human kidney: isolation, characterization and localization. Biochem J. 1991;273:759–766. doi: 10.1042/bj2730759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofstra J.M., Deegens J.K., Steenbergen E.J. Urinary excretion of fatty acid-binding proteins in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2008;23:3160–3165. doi: 10.1093/ndt/gfn190. [DOI] [PubMed] [Google Scholar]
- 20.Kuusniemi A.M., Lapatto R., Holmberg C. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005;68:121–132. doi: 10.1111/j.1523-1755.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K., Maruyama N., Maruyama T. Elevated macrophage migration inhibitory factor (MIF) levels in the urine of patients with focal glomerular sclerosis. Clin Exp Immunol. 2005;139:338–347. doi: 10.1111/j.1365-2249.2004.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan H.Y. Role of macrophage migration inhibition factor in kidney disease. Nephron Exp Nephrol. 2008;109:e79–e83. doi: 10.1159/000145463. [DOI] [PubMed] [Google Scholar]
- 23.Grandaliano G., Gesualdo L., Bartoli F. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58:182–192. doi: 10.1046/j.1523-1755.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 24.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Wagenmakers E.J., Farrell S. AIC model selection using akaike weights. Psychonom Bull Rev. 2004;11:192–196. doi: 10.3758/bf03206482. [DOI] [PubMed] [Google Scholar]
- 26.Irazabal M.V., Rangel L.J., Bergstralh E.J. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H.C., Hwang J.H., Kang A.Y. Urinary N-acetyl-beta-d glucosaminidase as a surrogate marker for renal function in autosomal dominant polycystic kidney disease: 1 year prospective cohort study. BMC Nephrol. 2012;13:93. doi: 10.1186/1471-2369-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauta F.L., Bakker S.J., Lambers Heerspink H. Effect of frozen storage on urinary concentration of kidney damage markers. Am J Kidney Dis. 2012;59:586–589. doi: 10.1053/j.ajkd.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman J.W., de Zeeuw D., Gansevoort R.T. Prolonged frozen storage of urine reduces the value of albuminuria for mortality prediction. Clin Chem. 2007;53:153–154. doi: 10.1373/clinchem.2006.081471. [DOI] [PubMed] [Google Scholar]
- 30.Boertien W.E., Meijer E., Gansevoort R.T. Urinary biomarkers in autosomal dominant polycystic kidney disease: is there no prognostic value? Kidney Int. 2012;82:361. doi: 10.1038/ki.2012.146. [DOI] [PubMed] [Google Scholar]
- 31.Devuyst O., Burrow C.R., Smith B.L. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol. 1996;271:F169–F183. doi: 10.1152/ajprenal.1996.271.1.F169. [DOI] [PubMed] [Google Scholar]
- 32.Nauta F.L., Scheven L., Meijer E. Glomerular and tubular damage markers in individuals with progressive albuminuria. Clin J Am Soc Nephrol. 2013;8:1106–1114. doi: 10.2215/CJN.04510512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao C, Zhang L, Zhang Y, et al. Insights into cellular and molecular basis for urinary tract infection in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. (in press). [DOI] [PMC free article] [PubMed]
- 34.Gansevoort R.T., Meijer E., Chapman A.B. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol Dial Transplant. 2016;31:1887–1894. doi: 10.1093/ndt/gfv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrier R.W., Abebe K.Z., Perrone R.D. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chonchol M., Gitomer B., Isakova T. Fibroblast growth factor 23 and kidney disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2017;12:1461–1469. doi: 10.2215/CJN.12821216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thong K.M., Ong A.C. The natural history of autosomal dominant polycystic kidney disease: 30-year experience from a single centre. Q J Med. 2013;106:639–646. doi: 10.1093/qjmed/hct082. [DOI] [PubMed] [Google Scholar]
- 38.Chen D., Ma Y., Wang X. Clinical characteristics and disease predictors of a large chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS One. 2014;9:e92232. doi: 10.1371/journal.pone.0092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashihara E., Horie S., Muto S. Renal disease progression in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2012;16:622–628. doi: 10.1007/s10157-012-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashani K., Cheungpasitporn W., Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55:1074–1089. doi: 10.1515/cclm-2016-0973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis.
Correlations among biomarker excretions. β2MG excretion was correlated with a general marker (albumin), a distal tubular marker (HFABP) and an inflammation marker (NGAL). MCP-1 excretion was correlated with a general marker (albumin), a glomerular marker (IgG), a proximal tubular marker (KIM-1) and an inflammation marker (NGAL).
AICs and Akaike weights of the various models for annual change in eGFR. For annual change in eGFR, model 5 (the resultant of the stepwise backward analysis) had the lowest AIC. When comparing the w(AIC) of each model, model 5 had a normalized probability of 1.00 over model 1, 1.00 over model 2 and 1.00 over model 3, and 0.88 over model 4 indicating that model 5 comes closer to the truth and is the best model.
Associations of the urinary biomarkers with annual change in eGFR calculated as slope through multiple (≥ 3) eGFR values. β2MG and MCP-1 were both associated with annual change in eGFR, and remained significant after adjustment for conventional risk markers (standardized β = −0.32, P = 0.002 and standardized β = −0.27, P = 0.02 respectively).
AICs and Akaike weights for the Mayo htTKV classification and Urinary Biomarker Score. The urinary biomarker score had the lowest AIC. When comparing the w(AIC) of each model, the urinary biomarker score had a normalized probability of 1.00 over the Mayo htTKV classification, indicating that the biomarker score is preferred over the Mayo htTKV classification.
Scatter plot of urinary β2MG and MCP-1 excretion versus annual change in eGFR. This figure represents the value distributions of annual change in eGFR with either β2MG or MCP-1 excretion. The line represents the results of the fractional polynomial regression analysis. For β2MG excretion the association was linear, the association was non-linear for MCP-1 excretion < 200 ng/24 h.
Differences in annual change in eGFR between classes of the Mayo htTKV classification of ADPKD. This figure represents the differences in annual change in eGFR for the different Mayo htTKV classes. The annual change in eGFR was −1.2 ± 2.0 ml/min per 1.73 m2 for class A, −2.5 ± 2.2 ml/min per 1.73 m2 for class B, −2.7 ± 2.3 ml/min per 1.73 m2 for class C, −3.7 ± 5.5 ml/min per 1.73 m2 for class D, and −5.7 ± 2.8 ml/min per 1.73 m2 for class E.