Abstract
Introduction
There are limited data on the cost of hyperkalemia.
Methods
This retrospective analysis of the Truven MarketScan claims database assessed the economic burden of hyperkalemia among selected adult patients with hyperkalemia and matched controls.
Results
A total of 39,626 cases (patients with hyperkalemia) were matched to 39,626 controls (patients without hyperkalemia) based on age, dialysis, chronic kidney disease (CKD) stage, heart failure, and renin-angiotensin aldosterone system inhibitor use. Compared with controls, cases incurred $4128 (95% confidence interval [CI] $3893–$4363) higher 30-day total health care costs ($5994 vs. $1865) and $15,983 (95% CI $15,026–$16,940) higher 1-year costs ($31,844 vs. $15,861). Among 11,221 matched pairs of patients with CKD and/or heart failure, cases incurred $5553 (95% CI $5059–$6047) higher 30-day total health care costs ($8165 vs. $2612) and $24,133 (95% CI $21,748–$26,518) higher 1-year costs ($48,994 vs. $24,861) than controls. The multivariable adjusted 1-year total health care cost difference was $15,606 (95% CI $14,648–$16,576) among all patients and $25,156 (95% CI $23,529–$26,757) among patients with CKD and/or heart failure. Cases had higher resource utilization rates including inpatient admissions (30-day: 0.14 vs. 0.03; 1-year: 0.44 vs. 0.19), outpatient visits (30-day: 3.33 vs. 2.28; 1-year: 26.58 vs. 18.53), and emergency department visits (30-day: 0.16 vs. 0.06; 1-year: 0.86 vs. 0.50) (all P < 0.001). When hospitalized, cases stayed 1.51 days (95% CI 1.22–1.80) longer and were 40% more likely to be readmitted.
Conclusion
These data indicate that hyperkalemia is associated with a significant economic burden on afflicted patients and the health care system.
Keywords: costs, health care resource utilization, hospital readmission, hyperkalemia
Hyperkalemia, defined as abnormally high serum potassium (>5.0 mEq/l), is a potentially life-threatening acute electrolyte abnormality.1, 2, 3 Although hyperkalemia is often asymptomatic, high serum potassium concentrations are associated with muscle cramps and weakness, muscle hypotonia, dyspnea, and cardiac arrhythmias.1, 2, 4, 5, 6
Hyperkalemia is mainly caused by reduced renal function and hence reduced excretion of potassium and increased serum potassium.7, 8 In addition to renal insufficiency, heart failure, diabetes mellitus, hypertension, and medication use are key risk factors for hyperkalemia.9 Use of renin-angiotensin-aldosterone system inhibitors (RAASi), such as aldosterone receptor antagonists, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and direct renin inhibitors, have been shown to both increase the frequency as well as the severity of hyperkalemia.10 Other potential mechanisms resulting in hyperkalemia include high dietary potassium intake, acidosis, and alterations in renal potassium handling.11, 12
Elevations in serum potassium levels have been associated with increased mortality, especially among elderly patients and patients with comorbidities.5, 13, 14, 15, 16, 17 A variety of studies have shown that in patients with cardiovascular diseases and/or chronic kidney disease (CKD), hyperkalemia is a significant risk factor for all-cause mortality.5, 13, 15, 17
There are limited published studies characterizing the economic burden of hyperkalemia. One study found an increased rate of hospital admissions associated with hyperkalemia among patients with cardiovascular diseases, such as heart failure and CKD.5 A study by Dunn et al.18 in 2011 identified a mean inpatient cost of $24,178 per episode and an average length of stay of 3.2 days among patients admitted from emergency departments to hospitals for elevated potassium levels. However, this study analyzed only those hospitalizations in which hyperkalemia was the primary diagnosis; this was only a small fraction of the total number of hospitalizations in which hyperkalemia was diagnosed. Furthermore, this study did not assess how hyperkalemia affected the frequency of hospitalizations, the resource intensity of the hospitalizations, or the effect on outpatient costs. A study by Chazard et al.19 analyzed a sample of hospitalizations in France and concluded that hyperkalemia led to an increase in lengths of stay by 2.3 to 4.6 days.
The objectives of the present study were to assess the health care costs and resource utilization for patients with hyperkalemia in comparison with those of patients without hyperkalemia, while matching (and adjusting for) a wide range of factors, including comorbidities, treatments, and demographics. These analyses were conducted for the overall patient population as well as for specific patient subgroups defined by hyperkalemia-related comorbidities, such as patients with heart failure and/or CKD.
Methods
Data Source
This study was a retrospective analysis of the Truven MarketScan claims and encounters research database with the MarketScan laboratory database (January 1, 2010, to December 31, 2014). The MarketScan claims and encounters research database captures the medical experience of insured persons and their dependents for active employees, early retirees, consolidated omnibus budget reconciliation act (COBRA) beneficiaries, and Medicare-eligible retirees with employer-provided Medicare Supplemental plans. The database includes enrollment history and claims for medical (provider and institutional) and pharmacy services. The MarketScan laboratory database clinically enriches the medical and prescription drug data for a subset of patients in the MarketScan databases by linking patients’ claims data with predominately outpatient laboratory test results.
Sample Selection
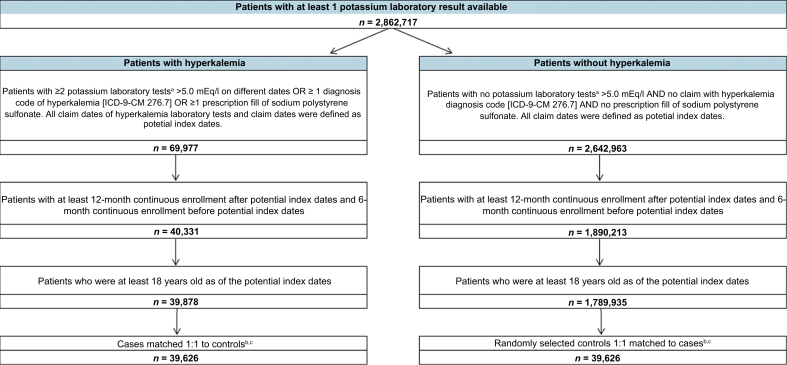
Adult patients with hyperkalemia (cases) and patients without hyperkalemia (controls) were selected. Hyperkalemia cases were identified as adult patients with at least 2 laboratory tests (a second positive test was required to avoid inclusion of patients with elevated potassium due to testing errors, e.g., hemolysis) indicating hyperkalemia (serum potassium >5.0 mEq/l) or at least 1 diagnosis code corresponding to hyperkalemia (276.7) or at least 1 prescription fill of sodium polystyrene sulfonate (the only treatment indicted for hyperkalemia at the time of the study). Controls were identified as adult patients with at least 1 potassium laboratory test available and without any laboratory tests >5.0 mEq/l, any diagnosis codes corresponding to hyperkalemia, or any prescription fills of sodium polystyrene sulfonate. Both cases and controls were required to be continuously enrolled in their health care plan for at least 12 months after the index date (study period) and at least 6 months before the index date (baseline period). For cases, all claim dates indicating hyperkalemia were defined as potential index dates; for controls, all claims dates were defined as potential index dates. If a patient had multiple potential index dates that met all the inclusion criteria, the index date used for the purposes of the study was randomly selected from the eligible potential index dates. This method is superior to just selecting the first such potential index event and date because use of the first event/date would lead to a sample overly composed of early-stage disease events rather than the full disease process. The objective of this study was to describe costs through all stages of the disease, so events were selected from the entire disease spectrum.
Among all eligible potential controls, controls were randomly selected to exactly match one-to-one to cases on age group (18–64 or 65+ years), dialysis treatment, CKD stage (stage 3, stage 4, stage 5, and unspecified CKD stage), heart failure, and RAASi use.
Identification of Comorbidities
Comorbidities, including dialysis treatment, CKD, heart failure, type 2 diabetes, and hypertension were identified. CKD was identified by diagnosis code or estimated glomerular filtration rate (eGFR) results indicating stage 3 to 5 CKD categorized as follows: stage 3 CKD was defined as having a CKD stage 3 diagnosis code (585.3) or an eGFR between 30 and 59 ml/min; stage 4 CKD was defined as having a CKD stage 4 diagnosis code (585.4) or an eGFR between 15 and 29 ml/min; stage 5 CKD was defined as having a CKD stage 5 or end-stage diagnosis code (403 except for 403.x0, 404 except for 404.x1, 585.5, and 585.6), or an eGFR below 15 ml/min. The unspecified CKD stage group included patients with an International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code for unspecified CKD (585.9, 403.x0, 404.x0, and 404.x1), without International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code for CKD stage, without eGFR results, and without dialysis. Where there was inconsistency regarding CKD stage across different indicators, the most severe stage among these 3 indicators was used to define the CKD stage of a patient. Dialysis patients and patients with CKD were mutually exclusive, and dialysis treatment was identified using procedure codes. Heart failure, diabetes, and hypertension were identified via International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes.20
Statistical Analyses
Patient Characteristics
Patient characteristics were measured during the 6-month baseline period. The characteristics included (i) patient demographic characteristics reported on the index date, including age, gender, region, insurance type, and place of service for the index event; (ii) comorbidity profile, including hyperkalemia-related comorbidities, such as CKD, diabetes, heart failure, and hypertension, and the Charlson comorbidity index (CCI)21; and (iii) medication use, including the use of RAASi and sodium polystyrene sulfonate. Patient characteristics were compared between cases and controls using unadjusted generalized estimating equation models, which was used to account for the correlation between cases and controls due to matching.
Health Care Resource Utilization
Health care resource utilization was calculated within 30 days and within 1 year of the index date and compared between cases and controls. Among all patients, number of all-cause inpatient admissions, outpatient visits, and emergency department visits; presence of at least 1 inpatient admission, outpatient visit, and emergency department visit; and number of inpatient days were evaluated.
Among patients who had at least 1 inpatient admission, the length of stay per inpatient admission and the number of inpatient days per patient were assessed. In addition, 30-, 60-, and 90-day inpatient readmission rates were assessed for hospitalizations within 275 days of the index date (primary hospitalizations). The 275-day cutoff was chosen so that readmission within 90 days could be fully observed for all primary hospitalizations. Both the average number of inpatient re-admissions within 30, 60, and 90 days of the discharge date of each primary hospitalization and the number of hospitalizations with at least 1 inpatient readmission within 30, 60, and 90 days were calculated by averaging across all primary hospitalizations.
Health care resource utilization was compared between cases and controls using paired t-tests for continuous variables and McNemar tests for categorical variables; 95% confidence intervals (CIs) were calculated for the differences in mean health care resource utilization measures between cases and controls.
Health Care Costs
During the 1-year study period, all-cause health care costs were described both within 30 days and 1 year of the index date and compared between cases and controls. All-cause health care costs included medical costs (inpatient, outpatient, and emergency department) as well as pharmacy costs.
For the primary analysis, paired t-tests were used to compare all-cause health care costs between cases and controls; 95% CIs were calculated for the cost differences between cases and controls.
To assess the robustness of the results of the primary analyses, secondary analyses based on multivariable regression analysis using generalized linear models with gamma distribution was performed.22, 23, 24 The covariates included in the generalized linear models were diabetes, hypertension, CKD stage (stage 3, stage 4, stage 5, and unspecified stage), dialysis treatment, heart failure, RAASi use, age groups (<45, 45–54, 55–64, 65–74, and ≥75), gender, and CCI. Generalized estimating equations were used to account for the correlation between cases and controls due to matching; 95% CIs and P values were obtained using a bootstrap approach with 1000 replications.
Health Care Costs in Patient Subgroups
All-cause health care costs were also described and compared between cases and controls among patient subgroups defined by hyperkalemia-related comorbidities. The subgroups considered included patients with heart failure or CKD, patients with CKD stage 5, patients with CKD stage 4, patients with CKD stage 3, patients with unspecified CKD stage, patients on dialysis, patients with heart failure, patients with diabetes, patients with hypertension, and patients without the aforementioned comorbidities.
All-cause health care costs were compared between cases and controls within each patient subgroup using paired t-tests; 95% CIs were calculated for the cost differences between cases and controls within each patient subgroup. Again, as secondary analyses, adjusted costs were described and compared between cases and controls in each patient subgroup. Similarly, generalized estimating equations were used to account for the correlation between cases and controls due to matching; 95% CIs and P values were also obtained using a bootstrap-based approach (1000 replications).
Sensitivity Analyses
As a sensitivity analysis, propensity score matching was performed. The propensity score (the likelihood of having hyperkalemia conditional on the baseline characteristics) was estimated via logistic regression. The baseline characteristics considered included age, sex, region, insurance type, diabetes, hypertension, CKD stage, dialysis treatment, heart failure, RAASi use, and CCI. Cases and controls were then matched one-to-one within strata of the propensity score. Unadjusted and adjusted 30-day and 1-year total all-cause health care costs were compared between the propensity score–matched cases and controls.
Results
Patient Characteristics
A total of 39,626 patients with hyperkalemia were identified and exactly matched to an equal number of controls (Figure 1). During the baseline period, cases were significantly older (59.71 vs. 56.49 years) and a greater proportion were men (53.29% vs. 45.55%) compared with controls (both P < 0.001). The prevalence of diabetes (36.13% vs. 23.00%) and hypertension (57.05% vs. 51.10%) was also significantly higher in cases compared with controls (both P < 0.001). Additionally, the CCI was significantly greater in cases compared with controls (1.70 vs. 1.19; P < 0.001). Dialysis treatment, CKD stage, heart failure, and RAASi use were exactly balanced as a result of the matching; 1.65% of patients (cases and controls) had dialysis treatment, 24.28% of patients had CKD, 9.56% had heart failure, and 50.79% had used RAASi. The distributions by region and health insurance type were largely similar between cases and controls (Table 1).
Figure 1.
Sample selection. ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification. aPotassium laboratory tests were identified in MarketScan laboratory database based on the Logical Observation Identifiers Names and Code 2823–3. bIf a patient had multiple potential index dates that met all of the inclusion criteria, the index date was randomly selected from the eligible potential index dates. cControls were randomly selected among eligible controls to exactly match 1:1 to cases on age group (18–64 or 65+ years), renin-angiotensin-aldosterone system inhibitors use, dialysis treatment, chronic kidney disease stage (stage 3, stage 4, stage 5, and unspecified stage), and heart failure.
Table 1.
Baseline characteristics of patients with hyperkalemia and patients without hyperkalemia
| Patient characteristicsa | Patients with hyperkalemia n = 39,626 |
Patients without hyperkalemia n = 39,626 |
P |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 59.71 (13.27) | 56.49 (15.58) | <0.001∗ |
| Gender, n (%) | <0.001∗ | ||
| Female | 18,511 (46.71) | 21,576 (54.45) | |
| Male | 21,115 (53.29) | 18,050 (45.55) | |
| Region, n (%) | <0.001∗ | ||
| Northeast | 8951 (22.59) | 7928 (20.01) | |
| North central | 8766 (22.12) | 8629 (21.78) | |
| South | 16,265 (41.05) | 16,305 (41.15) | |
| West | 5642 (14.24) | 6762 (17.06) | |
| Unknown | 2 (0.01) | 2 (0.01) | |
| Health insurance type, n (%) | <0.001∗ | ||
| Basic/Major medical | 0 (0.00) | 0 (0.00) | |
| Comprehensive | 5836 (14.73) | 4804 (12.12) | |
| Exclusive provider organization | 665 (1.68) | 625 (1.58) | |
| Health maintenance organization | 13,472 (34.00) | 11,428 (28.84) | |
| Non-capitated point-of-service | 3191 (8.05) | 2687 (6.78) | |
| Preferred provider organization | 13,417 (33.86) | 17,702 (44.67) | |
| Capitated or partially capitated point-of-service | 295 (0.74) | 121 (0.31) | |
| Consumer-driven health plan | 671 (1.69) | 660 (1.67) | |
| Health deductible health plan | 489 (1.23) | 426 (1.08) | |
| Unknown | 1590 (4.01) | 1173 (2.96) | |
| Place of service on the index date, n (%) | |||
| Inpatient | 3785 (9.55) | 383 (0.97) | <0.001∗ |
| Outpatient | 30,380 (76.67) | 20,804 (52.50) | <0.001∗ |
| Emergency department | 3132 (7.90) | 649 (1.64) | <0.001∗ |
| Pharmacy | 6523 (16.46) | 22,077 (55.71) | <0.001∗ |
| Laboratory | 26,320 (66.42) | 1988 (5.02) | <0.001∗ |
| Disease characteristics | |||
| Hyperkalemia-related comorbidities, n (%) | |||
| Chronic kidney disease (CKD) | 9620 (24.28) | 9620 (24.28) | -- |
| CKD stage 3 | 5795 (14.62) | 5795 (14.62) | |
| CKD stage 4 | 1903 (4.80) | 1903 (4.80) | |
| CKD stage 5 (including end stage) | 1191 (3.01) | 1191 (3.01) | |
| CKD stage unknown/unspecified | 731 (1.84) | 731 (1.84) | |
| Diabetes | 14,318 (36.13) | 9114 (23.00) | <0.001∗ |
| Dialysis treatment | 654 (1.65) | 654 (1.65) | -- |
| Heart failure | 3789 (9.56) | 3789 (9.56) | -- |
| Hypertension | 22,605 (57.05) | 20,249 (51.10) | <0.001∗ |
| Charlson comorbidity index, mean (SD) | 1.70 (2.04) | 1.19 (1.78) | <0.001∗ |
| Medication use | |||
| Renin-angiotensin-aldosterone system inhibitors use, n (%) | 20,128 (50.79) | 20,128 (50.79) | -- |
| Aldosterone receptor antagonists | 1939 (4.89) | 1077 (2.72) | <0.001∗ |
| Angiotensin-converting enzyme inhibitors | 13,649 (34.44) | 12,247 (30.91) | <0.001∗ |
| Angiotensin receptor blockers | 6637 (16.75) | 7951 (20.07) | <0.001∗ |
| Direct renin inhibitors | 344 (0.87) | 311 (0.78) | 0.194 |
| Sodium polystyrene sulfonate, n (%) | 833 (2.10) | 0 (0.00) | -- |
ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Demographics were measured on the index date. Disease characteristics and medication use were measured during the 6-month period before index date. Cases and controls were matched exactly 1:1 on age group (18–64 or 65+ years), renin-angiotensin-aldosterone system inhibitors use, dialysis treatment, CKD stage (stage 3, stage 4, stage 5, and unspecified stage), and heart failure.
P < 0.05. P values were estimated using generalized estimating equation models. Dash indicates that no P values were available because the patient characteristics were matched or there was a zero count in at least 1 of the cohorts.
Health Care Resource Utilization
Within the first 30 days of the study period, cases had significantly higher numbers of inpatient admissions (0.14 vs. 0.03), outpatient visits (3.33 vs. 2.28), emergency department visits (0.16 vs. 0.06), and inpatient days (1.03 vs. 0.14 days) compared with controls (all P < 0.001). More cases had at least 1 inpatient admission (12.69% vs. 2.67%), outpatient visit (94.60% vs. 76.77%), and emergency department visit (12.71% vs. 4.92%) compared with controls (all P < 0.001) (Table 2).
Table 2.
Comparison of all-cause health care resource utilization of between patients with hyperkalemia and patients without hyperkalemia within 30 days and one year of the index date
| Resource utilization | Resource utilization within 30 days |
Resource utilization within 1 year |
||||
|---|---|---|---|---|---|---|
| Patients with hyperkalemia n = 39,626 |
Patients without hyperkalemia n = 39,626 |
Difference (95% CI) | Patients with hyperkalemia n = 39,626 |
Patients without hyperkalemia n = 39,626 |
Difference (95% CI) | |
| Number of all-cause health care visits, mean (SD) | ||||||
| Inpatient admissions | 0.14 (0.37) | 0.03 (0.17) | 0.11 (0.10–0.11)∗ | 0.44 (0.99) | 0.19 (0.59) | 0.25 (0.24–0.26)∗ |
| Outpatient visits | 3.33 (3.33) | 2.28 (3.01) | 1.06 (1.01–1.10)∗ | 26.58 (31.30) | 18.53 (25.65) | 8.05 (7.65–8.44)∗ |
| Emergency department visits | 0.16 (0.49) | 0.06 (0.32) | 0.10 (0.09–0.11)∗ | 0.86 (2.29) | 0.50 (1.61) | 0.36 (0.33–0.38)∗ |
| Inpatient days per patient, mean (SD) | 1.03 (3.71) | 0.14 (1.20) | 0.89 (0.85–0.93)∗ | 3.63 (12.88) | 1.28 (6.63) | 2.35 (2.21–2.50)∗ |
| Presence of at least 1 all-cause health care visit, %, mean (SD) | ||||||
| Inpatient admissions | 12.69 (33.28) | 2.67 (16.11) | 10.02 (9.66–10.39)∗ | 26.10 (43.92) | 13.53 (34.20) | 12.57 (12.02–13.12)∗ |
| Outpatient visits | 94.60 (22.61) | 76.77 (42.23) | 17.83 (17.36–18.30)∗ | 99.58 (6.46) | 97.09 (16.82) | 2.50 (2.32–2.67)∗ |
| Emergency department visits | 12.71 (33.31) | 4.92 (21.64) | 7.79 (7.39–8.18)∗ | 35.22 (47.77) | 25.55 (43.61) | 9.67 (9.03–10.30)∗ |
CI, confidence interval.
Health care resource utilization was measured within 30 days and within 1 year of the index date.
P < 0.05. P values were computed using paired t-tests for continuous variables and McNemar tests for categorical variables.
Similarly, within the full year of the study period, cases had significantly higher numbers of inpatient admissions (0.44 vs. 0.19), outpatient visits (26.58 vs. 18.53), emergency department visits (0.86 vs. 0.50), and inpatient days (3.63 vs. 1.28 days) compared with controls (all P < 0.001). More cases had at least 1 inpatient admission (26.10% vs. 13.53%), outpatient visit (99.58% vs. 97.09%), and emergency department visit (35.22% vs. 25.55%) compared with controls (all P < 0.001) (Table 2).
A total of 10,341 cases and 5360 controls had at least 1 inpatient admission during the 1-year study period. The total numbers of inpatient admissions were 17,392 and 7490 episodes for cases and controls, respectively. The average length of stay per inpatient admission was 1.51 (95% CI 1.22–1.80) days longer for cases than controls (8.28 vs. 6.77 days) and the mean number of inpatient days per patient who was hospitalized in the 1-year study period was 4.46 (95% CI 3.86–5.06) days longer for cases compared with controls (13.93 vs. 9.46 days) (both P < 0.001) (Table 3).
Table 3.
Comparison of length of stay between patients with hyperkalemia and patients without hyperkalemia within 1 year of the index date
| Inpatient admission outcomes | Patients with hyperkalemia | Patients without hyperkalemia | Mean difference |
|---|---|---|---|
| Mean (95% CI) | |||
| Number of total inpatient admissions | 17,392 | 7490 | |
| Length of stay per inpatient admission, mean (SD) | 8.28 (12.85) | 6.77 (9.60) | 1.51 (1.22–1.80)∗ |
| Number of patients with at least 1 inpatient admission | 10,341 | 5360 | |
| Inpatient days per patient with at least 1 inpatient admission, mean (SD) | 13.93 (22.19) | 9.46 (15.73) | 4.46 (3.86–5.06)∗ |
CI, confidence interval.
Patients with at least 1 inpatient admission were included in the analysis. Inpatient admissions within 1 year of the index date were included in the analysis.
P < 0.05. P values were computed using t-tests.
There were a total of 13,895 and 5556 primary hospitalizations within 275 days of the index date for cases and controls, respectively. Relative readmission rates were at least 40.12% higher for patients with hyperkalemia compared with patients without hyperkalemia, with absolute differences of 4.34% (95% CI 3.37%–5.32%), 5.96% (95% CI 4.81%–7.11%), and 7.69% (95% CI 6.42%–8.96%) within 30, 60, and 90 days, respectively (30-day: 14.21% vs. 9.86%; 60-day: 20.71% vs. 14.74%; 90-day: 26.86% vs. 19.17%) (all P < 0.001) (Table 4).
Table 4.
Comparison of inpatient readmissionsa between patients with hyperkalemia and patients without hyperkalemia
| Inpatient readmission outcomes | Patients with hyperkalemiab n = 13,895 |
Patients without hyperkalemiab n = 5556 |
Difference (95% CI) |
|---|---|---|---|
| Presence of at least 1 inpatient readmission per hospitalization,a %, mean (SD) | |||
| Within 30 days of the discharge date of each hospitalization | 14.21 (34.91) | 9.86 (29.82) | 4.34 (3.37–5.32)∗ |
| Within 60 days of the discharge date of each hospitalization | 20.71 (40.52) | 14.74 (35.45) | 5.96 (4.81–7.11)∗ |
| Within 90 days of the discharge date of each hospitalization | 26.86 (44.32) | 19.17 (39.36) | 7.69 (6.42–8.96)∗ |
| Number of inpatient readmissions per hospitalization,a mean (SD) | |||
| Within 30 days of the discharge date of each hospitalization | 0.17 (0.44) | 0.12 (0.37) | 0.05 (0.04–0.06)∗ |
| Within 60 days of the discharge date of each hospitalization | 0.29 (0.66) | 0.20 (0.56) | 0.09 (0.07–0.11)∗ |
| Within 90 days of the discharge date of each hospitalization | 0.42 (0.86) | 0.29 (0.74) | 0.13 (0.11–0.16)∗ |
CI, confidence interval.
Hospitalizations within 275 days (365 – 90 = 275) of the index date were included in the analysis as the primary hospitalizations to ensure that readmissions within 90 days can be fully observed. All hospitalizations within the 1-year study period were considered as potential re-admissions.
The “n” represented number of primary hospitalizations among patients with hyperkalemia and patients without hyperkalemia.
P < 0.05. P values were computed using t-tests for continuous variables and χ2 tests for categorical variables.
Health Care Costs
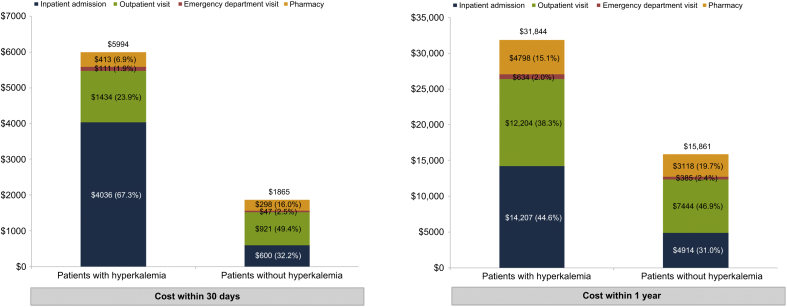
For the 30-day study period, cases incurred $4128 (95% CI $3893–$4363) higher total health care costs compared with controls ($5994 vs. $1865, P < 0.001). Costs were higher for each cost category including inpatient, emergency department, outpatient, and pharmacy (all P < 0.001). Within the full year of the study period, cases incurred $15,983 (95% CI $15,026–$16,940) higher total health care costs compared with controls ($31,844 vs. $15,861, P < 0.001). Again, costs were higher across all 4 categories (all P < 0.001) (see more details in Figure 2).
Figure 2.
Comparison of all-cause health care costs of patients with hyperkalemia versus matched patients without hyperkalemia.
The secondary multivariable regression analyses yielded largely similar results. Cases incurred $4289 (95% CI $4027–$4549) higher total health care costs compared with controls ($6347 vs. $2057) during the 30-day period and incurred $15,606 (95% CI $14,648–$16,576) higher total costs compared with controls ($33,715 vs. $18,109) during the 1-year period. Both comparisons were statistically significant (both P < 0.001).
In the sensitivity analyses using propensity score matching instead of exact matching, cases incurred $4070 (95% CI $3824–$4315) higher total health care costs compared with controls ($6014 vs. $1945, P < 0.001) during the 30-day period and $14,924 (95% CI $13,943–$15,904) higher total health care costs compared with controls ($31,719 vs. $16,795, P < 0.001) during the 1-year period. Multivariable regression analyses results remained largely similar: cases incurred $4591 (95% CI $4312–$4903) higher total health care costs compared with controls ($6695 vs. $2104, P < 0.001) during the 30-day and $16,326 (95% CI $15,273–$17,433) higher total health care costs compared with controls ($35,078 vs. $18,753, P < 0.001) during the 1-year period.
Health Care Costs in Patient Subgroups
Among all subgroups, patients with hyperkalemia incurred higher costs over both 30 days and 1 year (all P < 0.001) (Table 5). Patients with CKD stage 5 (not on dialysis) had the largest cost difference of $9685 (95% CI $7042–$12,328) within 30 days and $52,795 (95% CI $40,232–$65,357) within 1 year between cases and the matched controls (both P < 0.001) (Table 5).
Table 5.
Comparison of all-cause total health care costs of matched patients with hyperkalemia and patients without hyperkalemia within 30 days and within 1 year of the index date by subgroup
| Patient subgroup | Costs within 30 days |
Costs within 1 year |
||||||
|---|---|---|---|---|---|---|---|---|
| All patients |
All patients |
|||||||
| Number of pairs | Patients with hyperkalemia Mean |
Patients without hyperkalemia Mean |
Difference (95% CI) | Number of pairs | Patients with hyperkalemia Mean |
Patients without hyperkalemia Mean |
Difference (95% CI) | |
| Patients with CKDa and/or heart failure | 11,221 | $8165 | $2612 | $5553 ($5059–$6047)∗ | 11,221 | $48,994 | $24,861 | $24,133 ($21,748–$26,518)∗ |
| Patients with CKDa | 9620 | $7324 | $2541 | $4783 ($4227–$5339)∗ | 9620 | $46,483 | $24,625 | $21,857 ($20,079–$23,635)∗ |
| Patients with CKD stage 5a | 1191 | $13,683 | $3998 | $9685 ($7042–$12,328)∗ | 1191 | $99,616 | $46,821 | $52,795 ($40,232–$65,357)∗ |
| Patients with CKD stage 4a | 1903 | $6748 | $3019 | $3730 ($2825–$4634)∗ | 1903 | $45,911 | $29,175 | $16,736 ($11,543–$21,929)∗ |
| Patients with CKD stage 3a | 5795 | $5996 | $2116 | $3880 ($3339–$4420)∗ | 5795 | $35,592 | $19,118 | $16,474 ($14,026–$18,922)∗ |
| Patients with unspecified CKD stagea | 731 | $8994 | $2289 | $6706 ($4990–$8421)∗ | 731 | $47,736 | $20,277 | $27,459 ($20,997–$33,922)∗ |
| Patients with dialysis treatment | 654 | $22,942 | $15,959 | $6983 ($2845–$11,120)∗ | 654 | $155,346 | $130,249 | $25,097 ($8,209–$41,986)∗ |
| Patients with heart failure | 3789 | $12,718 | $4391 | $8327 ($7172–$9482)∗ | 3789 | $69,211 | $39,637 | $29,574 ($24,183–$34,965)∗ |
| Patients with diabetes | 14,318 | $6951 | $2181 | $4770 ($4392–$5148)∗ | 14,318 | $40,995 | $20,338 | $20,657 ($18,881–$22,433)∗ |
| Patients with hypertension | 22,605 | $6961 | $2255 | $4706 ($4375–$5038)∗ | 22,605 | $38,567 | $19,911 | $18,655 ($17,239–$20,071)∗ |
| Other patients | 12,384 | $4079 | $1158 | $2921 ($2576–$3266)∗ | 12,384 | $18,082 | $8656 | $9426 ($8294–$10,558)∗ |
CI, confidence interval; CKD, chronic kidney disease.
CKD and CKD stage were identified using International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes, and estimated glomerular filtration rate (eGFR). CKD was identified via diagnosis code or eGFR results indicating stage 3–5 CKD categorized as follows: stage 3 CKD was defined as having a CKD stage 3 diagnosis code (585.3) or an eGFR between 30 and 59 ml/min; stage 4 CKD was defined as having a CKD stage 4 diagnosis code (585.4) or an eGFR between 15 and 29 ml/min; stage 5 CKD was defined as having a CKD stage 5 or end-stage diagnosis code (403 except for 403.x0, 404 except for 404.x1, 585.5, 585.6), or an eGFR below 15 ml/min. The CKD subgroup excluded patients who had dialysis. Patients who had dialysis were in a subgroup that was mutually exclusive with the CKD subgroup.
P < 0.05. P values were computed using paired t-tests.
A total of 11,221 pairs of patients had CKD and/or heart failure. Compared with controls, cases incurred $5553 (95% CI $5059–$6047) higher all-cause health care costs within 30 days ($8165 vs. $2612) and $24,133 (95% CI $21,748–$26,518) higher all-cause health care costs within 1 year of the index date ($48,994 vs. $24,861) (Table 5).
After adjusting for baseline characteristics via multivariable regression analysis, there remained a significant economic burden associated with hyperkalemia among patient subgroups. For example, in patients with CKD and/or heart failure, cases incurred $6062 (95% CI $5680–$6484) higher total health care costs compared with controls ($8969 vs. $2907) during the 30-day period (P < 0.001) and incurred $25,156 (95% CI $23,529–$26,757) higher total costs compared with controls ($54,347 vs. $29,191) during the 1-year period (P < 0.001).
In conclusion, hyperkalemia is a common complication of patients with impaired renal function. Patients with heart failure or diabetes are also at increased risk of hyperkalemia.18 By some estimates, heart failure, diabetes, and kidney disease altogether impose an approximate $250 billion burden on society in the United States.25, 26, 27 Given that hyperkalemia is closely associated with these chronic diseases and that it can result in increased risk of mortality, it is important to understand to what extent hyperkalemia contributes to this significant burden. This study provides an important piece of real-word evidence to this knowledge gap.
In this study, a large US claims database (augmented with laboratory data) was used to assess the cost and health care resource utilization of hyperkalemia in the general population as well as in patient subgroups defined by hyperkalemia-related comorbidities, such as heart failure and CKD. Results of these analyses indicated that patients with hyperkalemia had substantially higher health care costs and utilization compared with matched controls both in the overall patient population and in patient subgroups. In addition, among those who had at least 1 inpatient admission, patients with hyperkalemia had longer lengths of stay and higher hospital readmission rates compared with controls.
The current study found that patients with hyperkalemia visited hospitals about twice as frequently as patients without hyperkalemia (in both the 30-day and 1-year periods), and incurred 2 to 3 times all-cause health care costs of the patients without hyperkalemia. According to our analyses, in every comorbidity-defined subgroup, patients with hyperkalemia incurred higher costs compared with matched patients without hyperkalemia. All of these results were robust, as cost differences remained largely unchanged in multivariable regression analyses. In addition, sensitivity analyses showed that results remained unchanged after matching on propensity score.
Increased inpatient admissions was the primary driver of the cost differences associated with hyperkalemia and comprised 67.34% of the cost in the short-term and 44.62% in the long-term. In particular, we identified 0.44 episodes of all-cause inpatient admissions and an inpatient cost of $14,207 during the 1-year post hyperkalemia event, which resulted in a mean all-cause inpatient cost of $35,518 per inpatient stay. This finding is consistent with the existing literature that hyperkalemia is associated with substantial inpatient cost. Dunn et al.18 reported that the average inpatient cost was $24,178 in 2011 USD (equal to $26,986 2015 USD) for inpatient admissions due to hyperkalemia. In addition, our study found that the mean length of stay per hospitalization was approximately 1.51 days longer for patients with hyperkalemia compared with patients without hyperkalemia. Furthermore, inpatient readmission rates were consistently higher among patients with hyperkalemia compared with patients without hyperkalemia for 30-, 60-, and 90-day inpatient readmission.
Although the findings were robust, the study had some limitations: (i) Comorbidities were identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes, which are used for administrative purposes; as a result, certain comorbidities may be underestimated. (ii) Race was not available from the MarketScan database and therefore could not be matched or adjusted in the analysis. (iii) The identification of hyperkalemia depended in part on the availability of serum potassium laboratory tests, which were performed by physicians’ request (in many cases done a few times a year or less). These available laboratory tests may not have accurately and fully reflected a given patient’s serum potassium level throughout the year. (iv) It is possible that some laboratory tests performed were not present in the claims data. For example, tests done in emergency departments or hospitals would not be included with the laboratories here (although such patients may very well be included in our sample via use of the diagnosis code from hospitalizations or emergency department visits for hyperkalemia). (v) The study population was limited to patients with available serum potassium laboratory tests and may not be generalizable to a population without such laboratory tests. Specifically, potassium laboratory tests may be driven by patient symptoms; therefore, the prevalence of hyperkalemia may be overestimated. However, the potassium test is part of the regular laboratory panel, so the bias is likely to be small.
This study also has a number of strengths: (i) The study benefited from the large sample size (approximately 40,000 hyperkalemia patients) of the administrative database representative of the US commercially insured population. (ii) To identify hyperkalemia via laboratory testing, this study required at least 2 laboratory tests with a serum potassium level >5.0 mEq/l, reducing the risk of false positives due to, for example, hemolysis. (iii) On the other hand, use of diagnosis codes and prescription codes of sodium polystyrene sulfonate allowed for a wider net to be cast to identify cases that identification exclusively through laboratory samples would have missed (e.g., patients who may have gone straight to an emergency department or hospital where laboratory sample data are unavailable). (iv) Patients with hyperkalemia were matched to the controls on the key hyperkalemia comorbidities (dialysis treatment, CKD stage, heart failure and RAASi use), as well as age group, to address potential confounding due to these key risk factors of both hyperkalemia and health care utilization/cost. (v) The study used multivariable regression analyses to further adjust for potential confounders, including diabetes, hypertension, CKD stage (stage 3, stage 4, stage 5, and unspecified stage), dialysis treatment, heart failure, RAASi use, age groups (<45, 45–54, 55–64, 65–74, and ≥75), gender, and CCI. Although residual confounding due to unmeasured or unobserved variable is possible, the consistent findings that hyperkalemia is associated with significant economic burden from the multivariable regression results supported the robustness of the results.
In this retrospective database study, patients with hyperkalemia had significantly higher health care costs and utilization compared with matched controls. These differences were larger in patients with CKD and/or heart failure. Patients with hyperkalemia were more likely to be hospitalized, go to the emergency department, and see a physician on an outpatient basis. Furthermore, when hospitalized, patients with hyperkalemia had longer lengths of stay and were more likely to have a readmission within 30, 60, or 90 days of discharge. In summary, hyperkalemia is associated with a significant economic burden on afflicted patients.
Disclosure
JMW is an employee of ZS Pharma, a member of the AstraZeneca Group. KAB, FM, CX, WT, and EQW are employees of Analysis Group, Inc., which has received consultancy fees from ZS Pharma, a member of the AstraZeneca Group.
Data were analyzed by Analysis Group and interpreted in collaboration with all other authors. The study sponsor was involved in all stages of the study research and manuscript preparation, but all authors participated in the design of the study and contributed to the manuscript development. All the authors vouch for the accuracy and completeness of the data reported and the adherence of the study to the protocol, and all the authors made the decision to submit the manuscript for publication.
Acknowledgments
Funding for this research was provided by ZS Pharma, a member of the AstraZeneca Group.
References
- 1.Mushiyakh Y., Dangaria H., Qavi S. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2011;1(4) doi: 10.3402/jchimp.v1i4.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehnhardt A., Kemper M.J. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26:377–384. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J., Brunelli S.M., Jensen D.E., Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford A.H. Hyperkalemia: recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37:167–175. doi: 10.1097/NAN.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 5.Jain N., Kotla S., Little B.B. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 6.McMahon G.M., Mendu M.L., Gibbons F.K., Christopher K.B. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–1842. doi: 10.1007/s00134-012-2636-7. [DOI] [PubMed] [Google Scholar]
- 7.Allon M. Hyperkalemia in end-stage renal disease: mechanisms and management. J Am Soc Nephrol. 1995;6:1134–1142. doi: 10.1681/ASN.V641134. [DOI] [PubMed] [Google Scholar]
- 8.Acker C.G., Johnson J.P., Palevsky P.M., Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158:917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]
- 9.Khanagavi J., Gupta T., Aronow W.S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir M.R., Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 11.Noize P., Bagheri H., Durrieu G. Life-threatening drug-associated hyperkalemia: a retrospective study from laboratory signals. Pharmacoepidemiol Drug Saf. 2011;20:747–753. doi: 10.1002/pds.2128. [DOI] [PubMed] [Google Scholar]
- 12.Nyirenda M.J., Tang J.I., Padfield P.L., Seckl J.R. Hyperkalaemia. BMJ. 2009;339:b4114. doi: 10.1136/bmj.b4114. [DOI] [PubMed] [Google Scholar]
- 13.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes J., Kalantar-Zadeh K., Lu J.L. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120:c8–c16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovesdy C.P., Regidor D.L., Mehrotra R. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B., Collins A.J., Reaven N. Effect of cardiovascular comorbidities on the mortality risk associated with serum potassium. Circulation. 2014;130(Suppl 2) A13320–A13320. [Google Scholar]
- 17.Torlen K., Kalantar-Zadeh K., Molnar M.Z. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272–1284. doi: 10.2215/CJN.00960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn J.D., Benton W.W., Orozco-Torrentera E., Adamson R.T. The burden of hyperkalemia in patients with cardiovascular and renal disease. Am J Manag Care. 2015;21(15 Suppl):s307–s315. [PubMed] [Google Scholar]
- 19.Chazard E., Dumesnil C., Beuscart R. How much does hyperkalemia lengthen inpatient stays? About methodological issues in analyzing time-dependant events. Stud Health Technol Inform. 2015;210:835. [PubMed] [Google Scholar]
- 20.Bolton K., Culleton B., Harvey K. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S246. [PubMed] [Google Scholar]
- 21.Romano P.S., Roos L.L., Jollis J.G. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 22.Austin P.C., Ghali W.A., Tu J.V. A comparison of several regression models for analysing cost of CABG surgery. Stat Med. 2003;22:2799–2815. doi: 10.1002/sim.1442. [DOI] [PubMed] [Google Scholar]
- 23.Barber J., Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9:197–204. doi: 10.1258/1355819042250249. [DOI] [PubMed] [Google Scholar]
- 24.Dodd S., Bassi A., Bodger K., Williamson P. A comparison of multivariable regression models to analyse cost data. J Eval Clin Pract. 2006;12:76–86. doi: 10.1111/j.1365-2753.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services. Kidney Disease Statistics for the United States. Bethesda, MD: National Kidney and Urologic Diseases Information Clearinghouse, National Institutes of Health; 2011.
- 26.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011.
- 27.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]