Abstract
Introduction
Patients with end-stage kidney disease have a high risk of 30-day readmission to hospital. These readmissions are financially costly to health care systems and are associated with poor health-related quality of life. The objective of this study was to describe and analyze the frequency, causes, and predictors of 30-day potentially avoidable readmission to hospital in patients on hemodialysis.
Methods
We conducted a retrospective cohort study using the US Renal Data System data from January 1, 2008, to December 31, 2008. A total of 107,940 prevalent United States hemodialysis patients with 248,680 index hospital discharges were assessed for the main outcome of 30-day potentially avoidable readmission, as identified by a computerized algorithm.
Results
Of 83,209 30-day readmissions, 59,045 (70.1%) resulted in a 30-day potentially avoidable readmission. The geographic distribution of 30-day potentially avoidable readmission in the United States varied by state. Characteristics associated with 30-day potentially avoidable readmission included the following: younger age, shorter time on hemodialysis, at least 3 or more hospitalizations in preceding 12 months, black race, unemployed status, treatment at a for-profit facility, longer length of index hospital stay, and index hospitalizations that involved a surgical procedure. The 5-, 15-, and 30-day potentially avoidable readmission cumulative incidences were 6.0%, 15.1%, and 25.8%, respectively.
Conclusion
Patients with end-stage kidney disease on maintenance hemodialysis are at high risk for 30-day readmission to hospital, with nearly three-quarters (70.1%) of all 30-day readmissions being potentially avoidable. Research is warranted to develop cost-effective and transferrable interventions that improve care transitions from hospital to outpatient hemodialysis facility and reduce readmission risk for this vulnerable population.
Keywords: avoidable, epidemiology, ESKD, hemodialysis, hospitalization, readmission
Hospital readmission is associated with poor quality of life and health outcomes,1, 2 as well as high costs. In the United States, an estimated $17 billion spent on return trips to the hospital can be saved annually with appropriate management.3 Given the high societal, emotional, and financial costs, research has focused on identifying patient populations at high risk for readmission, as well as developing and testing interventions to reduce this risk. For example, more than 40 randomized controlled trials have tested interventions to reduce readmission risk in patients with congestive heart failure.4
Patients with end-stage kidney disease (ESKD) on maintenance hemodialysis (HD) face particularly high rates of readmission to hospital. In 2013, 34.9% of HD patients were readmitted within 30 days of an index hospitalization.5 In comparison, 19% of the general Medicare population6 and approximately 25% of patients with congestive heart failure7 are readmitted to hospital within 30 days of an index hospital discharge. Hospitalizations in ESKD are exceptionally costly, and 38% of the nearly $30 billion in annual Medicare expenditures for ESKD is spent on acute inpatient care.5
After an index hospital discharge, a patient with ESKD will experience 1 of 3 outcomes: remain out of hospital, be readmitted to hospital, or die. Perhaps related to definitions levied by pay-for-performance programs,8 readmissions are often defined as within 30 days of an index hospitalization, and are categorized as planned or unplanned. Planned readmissions are scheduled during or shortly after the index hospitalization, and are often for a procedure, chemotherapy, transplant, or rehabilitation. Approximately 10% of all readmissions are planned in the United States.6, 9 An example scenario would be a patient admitted for a urologic procedure, with a planned readmission within 10 days for stent removal.
Of the remaining unplanned readmissions, some are unavoidable, whereas others might be avoidable with appropriate transitional and/or ambulatory care after index hospital discharge. For example, if a patient is discharged from hospital after an episode of atrial fibrillation, then readmitted within 30 days with acute cholecystitis, this readmission would be considered unplanned and also unavoidable. Conversely, if the same patient discharged from hospital after an episode of atrial fibrillation is then readmitted within 30 days with an episode of congestive heart failure, this would be considered an unplanned, but potentially avoidable readmission to hospital. The literature shows much variation regarding the proportion of potentially avoidable readmissions to hospital. In a recent systematic review, 27.1% of readmissions were deemed potentially avoidable in general medicine patients, ranging from 5% to 79%.10 Similarly, conditions in HD patients who are ambulatory-sensitive (e.g., volume overload, electrolyte imbalance) can result in readmission but may have been avoided with the appropriate transitional care on discharge.
Vest et al.11 recently published a systematic review of readmissions, and defined a potentially avoidable readmission as: “an unintended and undesired subsequent post-discharge hospitalization, where the probability is subject to the influence of multiple factors.” However, the methodology to identify an avoidable readmission varies widely in the literature, often based on subjective criteria,12, 13, 14, 15 or predefined lists of discharge categories or diagnoses.16, 17 These methodologies lack generalizability and are inadequate for use with large datasets or more sophisticated analyses. 3M Health Information Systems has developed a proprietary potentially preventable readmissions classification system,18 but its use for research purposes is limited. Halfon et al.19 derived an algorithm using administrative data (International Classification of Diseases, Ninth Revision codes and diagnosis-related group [DRG] codes) in a general medical inpatient population in Switzerland. The algorithm had 96% sensitivity and 95.7% specificity against the gold standard of chart review and has been used for research purposes to aid in the identification of predictive factors for potentially avoidable readmission in the United States.20
Despite the high risks, negative impact on patient outcomes, and financial consequences, there is a paucity of literature on the frequency and predictors of potentially avoidable readmission in ESKD,21 and lack of a standardized metric to define potentially avoidable readmission. Although some studies and reports have described all-cause,22, 23, 24, 25 or cause-specific5, 26, 27, 28, 29 readmissions in ESKD, potentially avoidable readmissions have not been previously studied. Given these gaps in the literature, we conducted an observational study using the US Renal Data System (USRDS) database to describe and analyze the frequency, causes, and predictors associated with potentially avoidable readmission.
Methods
This study was conducted and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.30
Data Source, Setting, and Participants
We conducted an observational cohort study of the USRDS database using data from the core and hospitalized datasets. Patients with Medicare as their primary insurance type, 18 to 95 years of age at day ≥91 after first ESKD service, with acute hospital discharges from January 1, 2008, to November 30, 2008, were included in the study population. All 30-day readmissions were assessed until December 31, 2008. Data were obtained on patient characteristics and comorbidities at baseline from the 2728 medical evidence form. Data on comorbid conditions were also collected from claims over a 3- to 6-month entry period (dependent on date of first ESKD service). Hospitalization claims with discharge status of “left against medical advice,” or DRG of 998, 999, or 000 (invalid or ungroupable) were excluded. Hospital discharges with primary reason for admission being rehabilitation (DRG 945, 946), psychiatric diagnosis (DRG 876 – 887), cancer (International Classification of Diseases, Ninth Revision principal discharge code of 140.xx-172.xx, 174.xx-208.xx, 230.xx-231.xx, 233.xx-234.xx), or renal transplant (DRG 652) were similarly excluded. An index hospital discharge was eligible only if it occurred during an HD treatment period, thus excluding patients on peritoneal dialysis. Patients listed as “recovered function,” with an unknown ESKD start date, who died during the index hospitalization, or with conflicting information on 1995 and 2005 medical evidence forms were also excluded. The study protocol was approved for Exempt Status by the Institutional Review Board of Northwell Health and issued a waiver of authorization as per 45 CFR 164.512 for the use and disclosure of information for research purposes.
Outcome Definitions
Thirty-Day Potentially Avoidable Readmission
The main study outcome was the proportion of index hospitalizations resulting in a potentially avoidable 30-day readmission (30-day PAR). Thirty-day PAR was identified by a computerized algorithm that uses routinely available International Classification of Diseases, Ninth Revision and DRG codes from administrative data. The algorithm was initially developed using a random sample of 3474 hospitalized patients from across Switzerland. Prediction was based on a Poisson regression model, with intra-sample sensitivity and specificity of the computerized screening algorithm reaching 96%, compared with a gold standard of systematic medical record review to identify potentially avoidable readmissions.19 A subsequent validation study of the predictive value of the computerized algorithm was then conducted, including 131,809 hospitalizations, and a random sample of 570 discharge/readmission pairs for chart review by 2 trained independent physicians.31 In this study, the predictive value was very good at 78%, for the computerized algorithm identifying potentially avoidable readmission. For a readmission to be categorized as potentially avoidable, the algorithm requires the readmission (i) is related to at least 1 diagnosis already known during the previous hospital stay; (ii) was unforeseen at the time of the previous hospital discharge; and (iii) occurs within the 30 days after the previous hospital discharge.
Thirty-Day Planned Readmission
As per the Planned Readmissions Algorithm version 2.1 published by the Centers for Medicare and Medicaid Services,32 3 principles guide the identification of a planned readmission: (i) a few specific, limited types of care are always considered planned (obstetrical delivery, transplant surgery, maintenance chemotherapy/radiotherapy/immunotherapy, rehabilitation); (ii) a planned readmission is defined as a nonacute readmission for a scheduled procedure; and (iii) admissions for acute illness or for complications of care are never planned. For this study, we defined a planned readmission using criteria for “planned readmission” from the National Quality Forum technical report on all-cause readmissions,9 or for solely vascular access reasons, as defined by USRDS analytic methods.33
Thirty-Day Unplanned and Unavoidable Readmissions
These were defined as all other hospitalizations occurring within 30 days of an index hospitalization that did not meet the definition of potentially avoidable or planned readmission.
Statistical Analysis
Patient demographic and comorbid characteristics were obtained from patient profile, hospitalization inpatient data, facility standard analysis file, and the 2728 Centers for Medicare and Medicaid Services Medical Evidence form and expressed as categorical variables. Descriptive statistics were used to describe the sample of index hospitalizations. Demographic characteristics included were gender, race, ethnicity, employment status, HD facility, HD vintage in years, and cause of ESKD. A comorbidity index, encompassing 11 comorbidities and validated for use in the ESKD population to model outcomes of mortality and hospitalization, was used to report the comorbidity burden and adjust for potential confounding of the primary outcome of 30-day PAR.34 Comorbid characteristics included in the index are atherosclerotic heart disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, other cardiac disease, chronic obstructive pulmonary disease, gastrointestinal bleed, liver disease, dysrhythmia, cancer, and diabetes. This index was used to increase stability of effect estimates and simplify comparisons. The comorbidity index outperforms the Charlson Comorbidity Index in both predictive ability and inference. Geographic-level median income was described by linking USRDS data to 2010 US census data by zip code, and unadjusted risk of 30-day potentially avoidable readmission for each state was mapped using Pitney Bowes MapInfo (Troy, NY). There were no missing zip codes in our final sample and only 8700 patients (8%) did not have income-level data and were excluded from the analysis. Because missing data were uncommon, multiple imputation was not performed.
Reason for index hospital discharge was reported using Clinical Classification Software (CCS) category. The CCS is a diagnosis and procedure categorization scheme based on International Classification of Diseases, Ninth Revision codes developed with sponsorship from the Agency for HealthCare Research and Quality for research purposes. The 5 most common single-level CCS categories of index hospital discharge that resulted in the largest proportions of 30-day PAR were identified. We then tabulated the rate, proportion, and 5 most frequent causes of 30-day PAR that resulted from each of these index hospitalization categories (defined by single-level CCS category35).
Logistic regression using generalized estimating equation methods was used to model 30-day PAR as a function of each potential risk factor of interest, with hospitalization as the unit of analysis. Generalized estimating equation estimation with robust SEs was used to account for the clustering of multiple index hospitalizations within a patient. Predictors that were individually significantly associated with 30-day PAR (P < 0.05) were included in the final multivariable model. Predictors of interest included all demographic and clinical characteristics, as well as dialysis facility type and median income. All predictors, excluding geographic-level median income and index admission primary diagnosis, were significant and included in the final model. To identify risk factors unique to PAR, we repeated these multivariable models using all readmission, all readmission + death, and PAR+ death as outcome of interest.
Time to potentially avoidable readmission was analyzed using competing risks methodology,36 because deaths, and unplanned and planned readmissions represent competing risks to the primary outcome of 30-day PAR (because the occurrence of any of these events prevents PAR from being observed). The cumulative incidence function for 30-day PAR was used to estimate the probability of a potentially avoidable readmission at 5, 10, 15, 20, 25, and 30 days after an index hospital discharge. Index hospitalizations that experienced neither death nor any type of readmission by 30 days postdischarge was considered to be censored at 30 days. In sensitivity analyses, the Kaplan-Meier method was also used, treating all events other than a potentially avoidable readmission as censored. All analyses assumed a 2-sided significance level of 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Participant and Index Hospitalization Characteristics
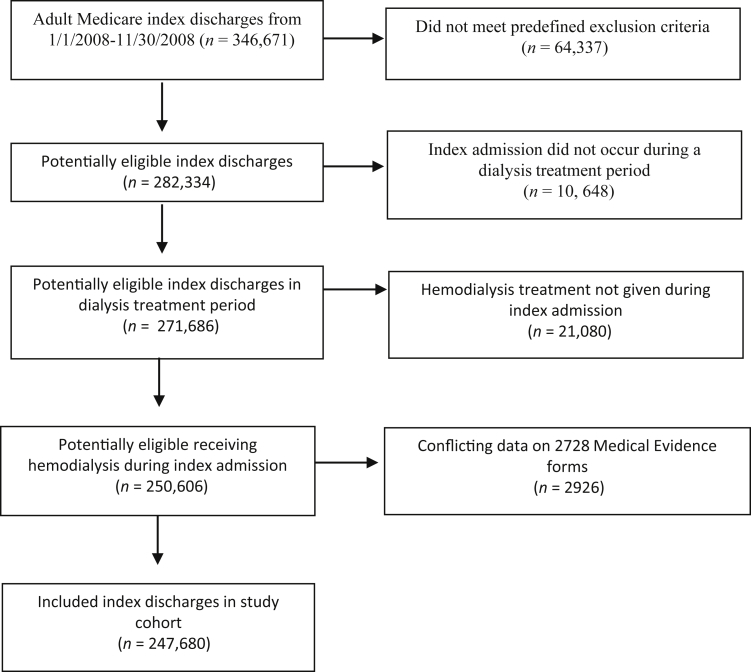
A total of 107,940 patients with 247,680 index hospitalizations met our predefined inclusion criteria, and were included in the study cohort (Figure 1). Of 346,671 possible adults, Medicare index admissions with discharges that occurred between January 1, 2008, and November 30, 2008, 64,337 admissions were excluded due to either death during the index admission, discharged after November 30, 2008, transferred in another hospital or rehabilitation center, or some other predefined exclusion criteria (see Data Source, Setting, and Participant section). Of those remaining, 10,648 admissions were removed because the index admission did not occur during a treatment period. Of those remaining, 21,080 were removed because the treatment given during the index admission was not HD. Finally of those remaining, 2,926 were removed because of conflicting data on the medical evidence forms. Patient and index hospitalization characteristics stratified by the outcome of potentially avoidable readmission are shown in Tables 1 and 2. At baseline (i.e., time of index hospitalization discharge), the median patient age was 66 years with an interquartile range of 53 to 76, 50% of patients were female, 38.9% were of black race, 13.5% were of Hispanic ethnicity, 49% had diabetes as the primary cause for ESKD, and 74.6% dialyzed at for-profit HD facilities. The median time on HD (vintage) was 2.61 years (interquartile range 1.20 to 4.87). When admitted to hospital, most patients (43%) had between 1 and 3 prior hospitalizations in the preceding 12 months, with 30% having had more than 3 prior hospitalizations in the preceding 12 months. Seventy-five percent of index hospitalizations were for a primary medical reason, as identified by DRG. The median length of stay for index hospitalizations was 4 days (interquartile range 2 to 7); however, 20% of hospitalizations (n = 48,803) had a length of stay of more than 8 days.
Figure 1.
Study flow diagram.
Table 1.
Baseline patient characteristics at index hospitalization in 107,940 patients and 247,680 hospitalizations
| Characteristics, n (col %) or median (interquartile range) | Total n = 247,680 | Potentially avoidable readmissiona (n = 59,045) |
No potentially avoidable readmissiona (n = 188,635) |
Standardized difference |
|---|---|---|---|---|
| Age, yr | 66 (53–76) | 65 (42–88) | 66 (44–88) | −0.08 |
| Gender | −0.02 | |||
| Male | 123,709 (49.9) | 29,039 (49.2) | 94,670 (50.2) | |
| Female | 123,971 (50.1) | 30,006 (50.8) | 93,965 (49.8) | |
| Race | 0.07 | |||
| White | 138,904 (56.1) | 31,925 (54.1) | 106,979 (56.7) | |
| Black | 96,361 (38.9) | 24,415 (41.4) | 71,946 (38.1) | |
| Asian | 6955 (2.8) | 1544 (2.6) | 5411 (2.9) | |
| Native American | 4015 (1.6) | 811 (1.4) | 3204 (1.7) | |
| Other | 1372 (0.6) | 330 (0.6) | 1042 (0.6) | |
| Unknown | 73 (0.0) | 20 (0.0) | 53 (0.0) | |
| Ethnicity | 0.02 | |||
| Hispanic | 33,336 (13.5) | 7,616 (12.9) | 25,720 (13.6) | |
| Non-Hispanic | 208,835 (84.3) | 50,178 (85.0) | 158,657 (84.1) | |
| Unknown | 5509 (2.2) | 1251 (2.1) | 4258 (2.3) | |
| Employment status | 0.07 | |||
| Employed/Student | 11,593 (4.7) | 2,566 (4.4) | 9,027 (4.8) | |
| Homemaker | 10,714 (4.3) | 2,484 (4.2) | 8,230 (4.4) | |
| Retired – Disability/Medical LOA | 64,332 (26.0) | 15,443 (26.2) | 48,889 (25.9) | |
| Retired – Age | 87,021 (35.1) | 19,609 (33.2) | 67,412 (35.7) | |
| Unemployed | 64,014 (25.9) | 16,570 (28.1) | 47,444 (25.2) | |
| Missing/other | 10,006 (4.0) | 2,373 (4.0) | 7633 (4.1) | |
| Geographic-level income, $ | 44,037 (35,089–57,047) | 43,661 (34,729–56,818) | 44,146 (35,239–57,154) | −0.03 |
| Dialysis vintage years | 2.61 (1.20–4.87) | 2.56 (1.16–4.78) | 2.63 (1.21–4.89) | −0.03 |
| <1 year | 51,340 (20.7) | 12,734 (21.6) | 38,606 (20.5) | 0.03 |
| 1–3 years | 85,669 (34.6) | 20,328 (34.4) | 65,341 (34.6) | |
| >3 years | 110,671 (44.7) | 25,983 (44.0) | 84,688 (44.9) | |
| ESKD primary cause | 0.03 | |||
| Glomerulonephritis/cystic kidney | 21,662 (8.8) | 4,859 (8.2) | 16,803 (8.9) | |
| Diabetes | 121,811 (49.2) | 28,886 (48.9) | 92,925 (49.3) | |
| Hypertension | 70,995 (28.7) | 17,147 (29.0) | 53,848 (28.6) | |
| Other | 33,212 (13.4) | 8,153 (13.8) | 25,059 (13.3) | |
| Dialysis facility | 0.02 | |||
| Nonprofit | 49,851 (20.1) | 11,475 (19.4) | 38,376 (20.3) | |
| Profit | 184,840 (74.6) | 44,547 (75.5) | 140,293 (74.4) | |
| Unknown | 12,989 (5.2) | 3023 (5.1) | 9966 (5.3) | |
| Comorbidity Index | 11.00 (8.00–13.00) | 11.00 (9.00–13.00) | 10.00 (7.00–13.00) | 0.31 |
| Number of prior admissions | 2.00 (0.00–4.00) | 2.00 (1.00–5.00) | 2.00 (0.00–4.00) | 0.27 |
| None | 67,400 (27.2) | 13,273 (22.5) | 54,127 (28.7) | 0.26 |
| 1–3 | 106,559 (43.0) | 22,759 (38.6) | 83,800 (44.4) | |
| >3 | 73,721 (29.8) | 23,013 (39.0) | 50,708 (26.9) |
ESKD, end-stage kidney disease; LOA, leave of absence; Q, quartile.
Percentages represent row percentage.
Table 2.
Baseline characteristics of index hospitalizations
| Characteristics n (col %) or median (interquartile range) | Total n = 247,680 |
Potentially avoidable readmissiona (n = 59,045) |
No potentially avoidable readmissiona (n = 188,635) |
Standardized difference |
|---|---|---|---|---|
| LOS Index, d | 4.00 (2.00–7.00) | 4.00 (2.00–8.00) | 4.00 (2.00–7.00) | 0.13 |
| 0–4 | 134,360 (54.3) | 29,649 (50.2) | 104,711 (55.5) | 0.12 |
| >4–8 | 64,517 (26.1) | 15,837 (26.8) | 48,680 (25.8) | |
| >8 | 48,803 (19.7) | 13,559 (23.0) | 35,244 (18.7) | |
| Type of index hospitalization | 0.05 | |||
| Primary surgical | 61,477 (24.8) | 14,261 (24.2) | 47,216 (25.0) | |
| Primary medical | 186,203 (75.2) | 44,784 (75.8) | 141,419 (75.0) | |
| Without surgical procedure | 17,469 (9.4) | 3,680 (8.2) | 13,789 (9.8) | |
| With surgical procedure | 168,734 (90.6) | 41,104 (91.8) | 127,630 (90.3) |
LOS, length of stay; Q, quartile.
Percentages represent row percentage
Characteristics of 30-Day Readmissions
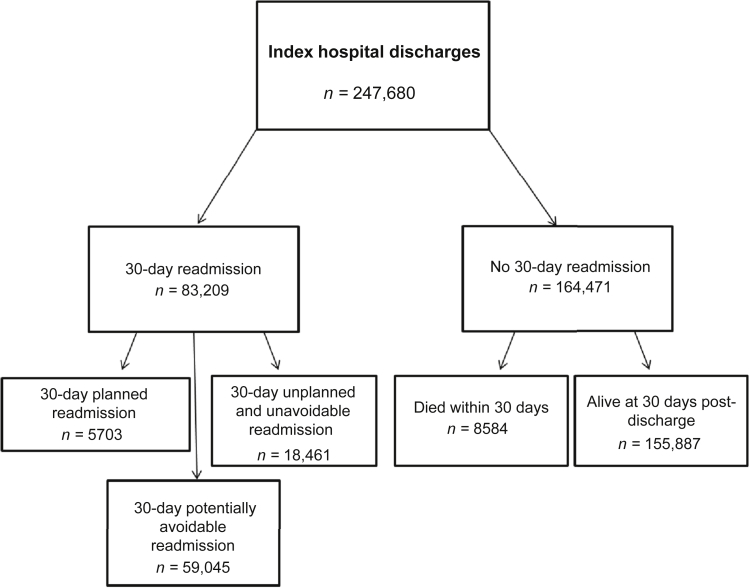
Of 247,680 index hospital discharges, 8584 patients (3.5%) died within 30 days of index hospital discharge (Figure 2). There were 83,209 index hospital discharges that resulted in a 30-day readmission (33.6%). Of these, 59,045 were classified as potentially avoidable readmissions (70.1%), 18,461 were unplanned and unavoidable readmissions (22.2%), and 5703 readmissions (6.9%) were planned.
Figure 2.
Index hospital discharge outcomes and frequencies.
Table 3 shows the 5 most frequent causes for index hospitalization that resulted in a 30-day PAR, with corresponding 30-day PAR rates, and 5 most frequent causes of 30-day PAR (by single-level CCS category). Complication of a device, implant, or graft was the most frequent index hospital discharge that resulted in a 30-day PAR, followed by congestive failure, and hypertension. The index hospital discharge cause with the highest 30-day PAR rate was diabetes mellitus with complications (28.0%), followed by congestive heart failure (27.7%). Causes of 30-day PAR are most commonly the same as reason for index hospital discharge; the most frequent 30-day PAR cause was identical to the corresponding index hospital discharge cause in all of our reported categories.
Table 3.
Most frequent causes of index hospitalizations resulting in potentially avoidable readmissiona
| Index hospitalizations (n) | Five most frequent causes of index hospitalization resulting in a 30-d potentially avoidable readmissionb | 30-d potentially avoidable readmission rate, n (row %) | Five most frequent causes for potentially avoidable readmissionb |
|---|---|---|---|
| 36,032 | Complication of device; implant or graft | 8264 (22.9) | Complication of device; implant or graft |
| Septicemia | |||
| Hypertension with complications and secondary hypertension | |||
| Congestive heart failure; nonhypertensive | |||
| Complications of surgical procedures or medical care | |||
| 19,072 | Congestive heart failure; nonhypertensive | 5290 (27.7) | Congestive heart failure; nonhypertensive |
| Hypertension with complications and secondary hypertension | |||
| Complication of device; implant or graft | |||
| Fluid and electrolyte disorder | |||
| Respiratory failure; insufficiency; arrest | |||
| 15,824 | Hypertension with complications and secondary hypertension | 4018 (25.4) | Hypertension with complications and secondary hypertension |
| Complication of device; implant or graft | |||
| Congestive heart failure; nonhypertensive | |||
| Fluid and electrolyte disorder | |||
| Diabetes mellitus with complications | |||
| 12,136 | Diabetes mellitus with complications | 3395 (28.0) | Diabetes mellitus with complications |
| Complication of device; implant or graft | |||
| Complications of surgical procedures or medical care | |||
| Hypertension with complications and secondary hypertension | |||
| Septicemia | |||
| 12,472 | Septicemia | 3131 (25.1) | Septicemia |
| Complication of device; implant or graft | |||
| Congestive heart failure; nonhypertensive | |||
| Hypertension with complications and secondary hypertension | |||
| Diabetes mellitus with complications |
Index hospitalization causes listed are in order of decreasing total number of 30-day potentially avoidable readmission.
As a percentage of index hospitalization, and identified by single-level Clinical Classification Software category as follows: Congestive Heart Failure: 108; Hypertension with Complications: 99; Fluid and Electrolyte Disorders: 55; Respiratory Failure, insufficiency or arrest: 131; Device Complications (Implant or Graft): 237; Septicemia: 2; Diabetes Mellitus with Complications: 50.
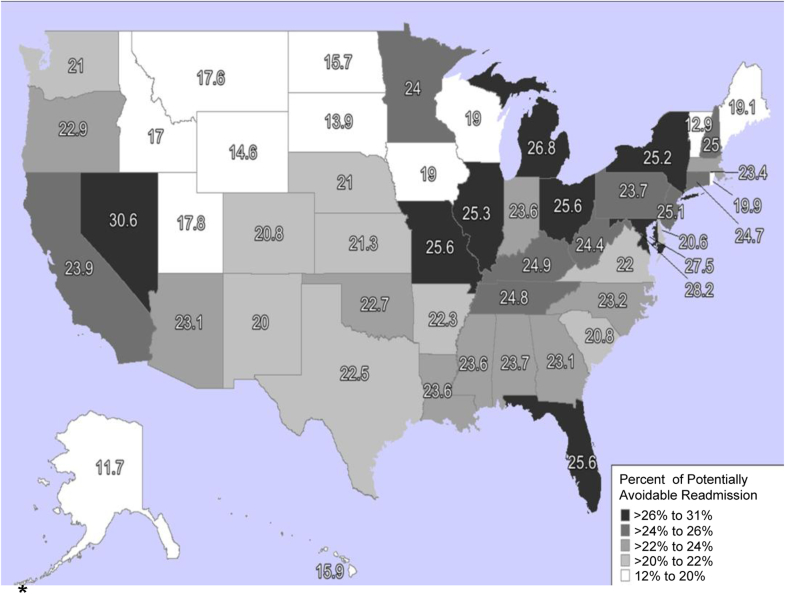
The geographic distribution of 30-day PAR varied by state, with percentages ranging from 12% to 31%, as a proportion of index hospital discharges (Figure 3). The highest 30-day PARs were in Nevada (30.6%), Washington, DC (28.3%), and Maryland (27.5%).
Figure 3.
Geographic variation in 30-day potentially avoidable readmission. ∗Percentages of 30-day potentially avoidable readmission presented as proportion of index hospitalization.
Reproduced with permission from Jonathan Amalfitano, Strategic Planning, Northwell Health, New Hyde Park, NY.
Multivariable 30-Day Potentially Avoidable Readmission
All baseline patient demographic characteristics were examined in unadjusted analysis, and the following were significantly associated with 30-day PAR (P < 0.05) and were included in the final multivariable model: age, length of stay, number of prior admissions, HD vintage years, gender, race, ethnicity, ESKD primary cause, employment status, type of admission (surgical, medical with surgery, medical without surgery), dialysis facility, and comorbidity index. Geographic-level median income and index admission primary diagnosis were not significantly associated with 30-day PAR and therefore not included in the final multivariable model.
In adjusted analyses that controlled for patient comorbidities using a validated comorbidity index,34 patient characteristics associated with increased odds of 30-day PAR included greater number of prior hospitalizations (odds ratio [OR] 1.40; 95% confidence interval [CI] 1.36–1.45 for >3 compared with none), black race (OR 1.06; 95% CI 1.03–1.08 compared with white race), unemployed status (OR 1.07; 95% CI 1.01–1.13 compared with employed/student), HD at a for-profit HD facility (OR 1.05; 95% CI 1.02–1.07 compared with nonprofit facility), and higher comorbidity index (OR 1.19; 95% CI 1.18–1.19 per 2-unit increase). Older age (OR 0.93; 95% CI 0.92–0.94 per 10-year age increase) and longer HD vintage (OR 0.77; 95% CI 0.75–0.80 for >3 years compared with <1 year) was associated with decreased risk of 30-day PAR (Table 4). Index hospitalization characteristics associated with higher risk 30-day PAR included longer length of index hospital stay (OR 1.30; 95% CI 1.26–1.34 for >8 days compared with 0–4 days), and index hospitalizations that involved a surgical procedure. Compared with surgical index hospitalizations, medical index hospitalizations that did not involve surgery had a lower risk of 30-day PAR (OR 0.86; 95% CI 0.82–0.90) (Table 4). Additionally, medical admissions with a surgical procedure had greater odds of PAR as compared with medical admissions without a surgical procedure (OR 1.08, 95% CI 1.03–1.13).
Table 4.
Multivariable logistic regression model of association of potentially avoidable readmission association with patient and index hospitalization characteristics among prevalent patients with ESKD on hemodialysis in the United States
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Age, yr (unit = 10 yr) | 0.93 (0.92–0.94) | <0.001 |
| Length of stay of index hospitalization, d | ||
| 0–4 | Ref | – |
| >4–8 | 1.12 (1.10–1.16) | <0.001 |
| >8 | 1.30 (1.26 1.34) | <0.001 |
| Number of prior admissions (12 months prior) | ||
| None | Ref | – |
| 1–3 | 1.03 (1.00–1.06) | 0.02 |
| >3 | 1.40 (1.36–1.45) | <0.001 |
| Dialysis vintage, yr | ||
| <1 | Ref | – |
| 1–3 | 0.84 (0.81–0.87) | <0.001 |
| >3 | 0.77 (0.75–0.80) | <0.001 |
| Gender | ||
| Male | Ref | – |
| Female | 1.03 (1.01–1.06) | 0.003 |
| Race | ||
| White | Ref | – |
| Black | 1.06 (1.03–1.08) | <0.001 |
| Asian | 1.07 (0.99–1.14) | 0.05 |
| Native American | 0.88 (0.81–0.97) | 0.01 |
| Other | 1.13 (0.98–1.31) | 0.09 |
| Unknown | 1.12 (0.63–1.98) | 0.70 |
| Ethnicity | ||
| Non-Hispanic | Ref | – |
| Hispanic | 1.01 (0.97–1.04) | 0.75 |
| Unknown | 0.84 (0.76–0.94) | 0.0014 |
| ESKD primary cause | ||
| Glomerulonephritis/cystic kidney | Ref | – |
| Diabetes | 0.99 (0.95–1.04) | 0.81 |
| Hypertension | 1.05 (1.01–1.10) | 0.01 |
| Other | 1.12 (1.07–1.18) | <0.001 |
| Employment status | ||
| Employed/Student | Ref | – |
| Homemaker | 1.02 (0.95–1.10) | 0.61 |
| Retired – Disability/Medical LOA | 0.98 (0.94–1.04) | 0.67 |
| Retired – Age | 1.02 (0.96–1.08) | 0.49 |
| Unemployed | 1.07 (1.01–1.13) | 0.02 |
| Unknown/Other | 1.07 (0.98–1.17) | 0.15 |
| Type of index admission | ||
| Surgical | Ref | – |
| Medical without surgery | 0.85 (0.81–0.89) | <0.001 |
| Medical with surgery | 0.91 (0.89–0.94) | <0.001 |
| Dialysis facility | ||
| Nonprofit | Ref | – |
| Profit | 1.05 (1.02–1.07) | 0.0013 |
| Unknown | 0.99 (0.95–1.05) | 0.85 |
| Comorbidity Index (unit = 2) | 1.19 (1.18–1.19) | <0.001 |
CI, confidence interval; ESKD, end-stage kidney disease; LOA, leave of absence; OR, odds ratio.
To assess for unique risk factors for PAR, models were repeated using all readmission, all readmission + death and PAR + death as the main outcomes of interest (Supplementary Tables S1–S3). When comparing the original model with the “all readmission” model, findings were similar with the addition that geographic-level median income was now significant (OR 1.01; 95% CI 1.00–1.01; P = 0.0110) for every $10,000 increase in income. When comparing the original model to “all readmission + death,” findings were similar with the exception that age was no longer significant. When comparing the original model to PAR + death, findings are similar with the exception of increasing age associated with the increased risk of the composite outcome of PAR + death (OR 1.01; 95% CI 1.00–1.02; P = 0.0110). Finally, in our original analysis, surgical index admissions were associated with the highest risk of 30-day PAR. However, medical index admissions have the highest risk when death is included as a composite outcome with either the “all readmission” or “PAR” outcome.
Time to Potentially Avoidable Readmission
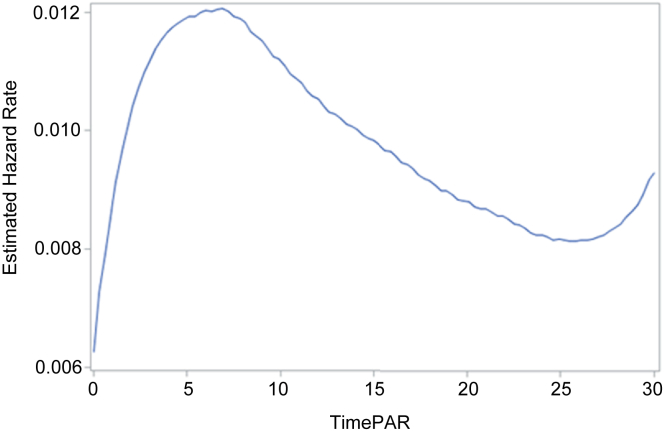
The cumulative incidence function was estimated with competing risks data. The 5-, 15-, and 30-day potentially avoidable readmission cumulative incidences were 6.0%, 15.1%, and 23.8%, respectively. Using standard survival analysis procedures (Kaplan-Meier product-limit method) the 5-, 15-, and 30-day failure estimates of potentially avoidable readmission were 6.1%, 15.9%, and 25.8%, respectively. It is possible that the different methods yield similar results due to the relatively small number of deaths (3.5%), unplanned readmissions (7.5%), and planned readmissions (2.3%), representing only 13.2% of the total index hospitalizations. The instantaneous hazard rate for potentially avoidable readmission varied with length of time from index hospital discharge. The estimated hazard rate increased for approximately the first week postdischarge then decreased until day 25 with a subsequent recurrent increase (Figure 4).
Figure 4.
Hazard function for potentially avoidable readmission.
Discussion
Using data from a national longitudinal database of US HD patients, this study explored the frequency, causes, predictors, and time to potentially avoidable readmission to hospital within 30 days of an index hospital discharge in patients with ESKD receiving HD treatment. Nearly three-quarters (70.1%) of all 30-day readmissions may have been potentially avoidable. Geographic frequency of 30-day PAR varied by state. The most common medical index hospital discharge condition that resulted in a 30-day PAR was heart failure. The most common surgical index hospital discharge condition that resulted in a 30-day PAR was a vascular procedure. In adjusted analysis, characteristics associated with higher 30-day PAR included younger age, shorter HD vintage, greater number of prior hospitalizations, black race, unemployed status, HD treatment at a for-profit center, and longer length of index hospital stay. Increasing age was associated with PAR + death, suggesting that older patients are more likely to die and younger patients are more likely to be readmitted for PAR. The estimated hazard rate for potentially avoidable readmission varied from day 0 to day 30 postdischarge.
Observational studies have described risk factors for readmission to hospital in patients with ESKD, including anemia,37, 38 low serum albumin,37, 39 and comorbid conditions, such as cerebrovascular accident, peripheral vascular disease, and depression.40 Given the observational study designs and variable definitions of readmission, comparison across studies is limited. A recent study by Harel et al.41 using administrative data from Ontario, Canada, reported 17% of in-center HD patients were readmitted, 27% visited the emergency department, and 7.5% died with 30 days of an index hospital discharge. Although this study extends the understanding of postdischarge resource utilization and patient outcomes to include emergency room visits, comparison with the current US in-center HD population is limited. The proportion of patients with 30-day readmission is substantially lower than observed in the United States since the USRDS reports a 35.2% all-cause 30-day readmission risk.5 Harel et al.41 proposed several reasons for this observed difference in 30-day readmission risk between Canada and the United States, including variations in HD care provision, reimbursement policies, and HD unit location in hospitals versus free-standing units. Prospective studies are required to test these hypotheses in controlled trials. Another recent study by Flythe et al.42 assessed risk factors for 30-day readmission to hospital, and excluded scheduled readmissions for vascular access or other planned procedures (as determined by admission history and physical review). Unlike our study, Flythe et al.42 did not distinguish “unplanned but unavoidable” readmissions from potentially avoidable readmissions. Of 349 included patients, 32.1% had a 30-day readmission. Similar to our study, comorbid illness and greater number of hospitalizations in the prior 12 months were associated with 30-day readmission. Prescription medication changes (from higher to lower number at index hospital discharge), and weekend discharge day were identified by Flythe et al.42 as associated with 30-day readmissions, as possibly actionable risk factors for 30-day readmission. Unlike Flythe et al.,42 we identified several patient-related risk factors, including younger age, female gender, black race, and shorter dialysis vintage, which were not identified by those authors. These characteristics may be associated with patients who are particularly ill and/or disadvantaged and thus prone to potentially avoidable 30-day readmission. Although the computerized algorithm we used cannot perfectly distinguish avoidable from unavoidable readmissions, we created a cohort enriched with avoidable readmissions to better identify risk factors for potentially avoidable readmission in HD patients than would otherwise be possible. A future full validation study in HD patients should be performed, given that our results identify a potentially important performance gap.
Previous studies have described associations between interventions and lower risk of hospital readmission in HD patients. Chan et al.24 reported that hemoglobin monitoring, erythropoietin dose adjustments, and vitamin D administration after hospital discharge were associated with a 15% lower risk of readmission than others. Erickson et al.25 reported a 3.5% reduction in the probability of readmission with one additional nephrologist provider visit in the month following a hospital discharge. These observational study results suggest that higher intensity of outpatient care in the period following a hospital discharge could reduce the risk of a subsequent readmission. However, the components of such a targeted intervention require characterization and study through prospective controlled trials.
The lack of evidence-based strategies to reduce readmission risk in HD patients is disquieting, in light of the Standardized Readmission Ratio component of the End-Stage Renal Disease Quality Incentive Program.43 This program is a “pay-for-performance” program administered by the Centers for Medicare and Medicaid Services. Beginning in payment year 2017, a high Standardized Readmission Ratio rate could contribute to a total performance score that causes payment reductions to HD facilities. In other high-risk populations, such as skilled nursing facility residents, pay-for-performance readmission quality measures attempt to include only potentially avoidable readmissions16 to reduce the misaligned incentives to defer hospitalization of a skilled nursing facility resident for elective or beneficial care.
Our results provide the groundwork for several avenues of future research aimed at reducing avoidable readmissions in HD patients. First, we demonstrate a substantial difference in risk between readmissions considered potentially avoidable and those that were unplanned and also unavoidable. We show that the estimated hazard rate of 30-day PAR varies with a late rise at 25 days post hospital discharge, suggesting that the postdischarge 30-day window conventionally used to identify readmissions may be inappropriately short. Thus, research is required to develop a standardized measure of avoidable readmission tailored to HD patients with the appropriate time span after hospital discharge. This will facilitate comparison of results across research studies and provide a meaningful target for improvement through prospective interventional studies. Second, our study identifies several factors associated with increased risk of 30-day PAR in HD patients that can be used in subsequent patient-level studies for the derivation and validation of a clinical prediction model. This would benefit clinicians and policy makers by focusing intensive postdischarge care on patients at highest risk of readmission, and facilitate researchers in enrolling HD patients at highest risk into clinical trials testing interventions to reduce readmission risk. Third, our reported baseline risk estimates of potentially avoidable readmission can be used in sample size calculations for future randomized controlled trials. Finally, the wide geographic variation we report across the United States implies that improvement in potentially avoidable readmission risk may be possible; however, more detailed study is required to elucidate the processes and practices which effectively reduce potentially avoidable readmissions that are nationally or regionally transferrable.
In this study, the outcome of 30-day PAR was determined using a computer algorithm for administrative data validated in a general medicine population. There are several reasons that rationalize the use of this algorithm in the HD population, including the following: (i) the proportion of potentially avoidable readmission we report is similar to studies of other patient populations. A MedPac report using 3M classification software identified 76% of all 30-day readmissions as potentially preventable from 2005 Medicare discharge claims data.44 A single-center study of acute general medical hospitalizations deemed 71% of readmissions potentially avoidable.15 Finally, a recent systematic review of potentially avoidable readmissions found 8 studies with more than 50% of readmissions classified as potentially avoidable.10 (ii) Our analysis identifies several predictors of potentially avoidable readmission which are consistent with the algorithm developed by Halfon et al.,19 including previous hospitalizations, high comorbidity index, and long length of stay. (iii) Although this algorithm was originally derived and validated in Switzerland, it has been used with good to excellent discriminatory power and calibration in US general medical populations,20 and in US populations with comorbid disease, including cancer45 and chronic kidney disease.46
Our study should be qualified by several potential limitations. (i) We underestimated the proportion of planned 30-day readmissions, as hospital discharges for rehabilitation, psychiatric reasons, cancer treatments, and renal transplants were excluded. Our study observed that 6.8% of all 30-day readmissions were planned, compared with approximately 10% in the general medicine population.6 (ii) Exclusion of these planned hospital stays slightly reduced the number of denominator index hospital discharges and thus modestly increased the reported proportion of 30-day PAR. Exclusion of these hospital discharges was an intentional component of our study design to limit the index cohort to acute hospitalizations of patients who were in a more homogeneous “at-risk” set for potentially avoidable readmission upon discharge. (iii) Data from 2008 were used for this analysis, and some trends in ESKD care may not reflect current practice. However, all-cause 30-day readmission risk has remained relatively fixed since 2008, ranging from 35.2% to 36.3% as indicated in annual USRDS data reports,5, 47, 48 lending validity to our results in the present day. (iv) We are limited by the use of a national database, where medical evidence forms and claims-based data may have misclassified variables for medical conditions and encounters. In particular, the 2728 Medical Evidence report has very good to excellent specificity, but comorbid conditions are underreported.49 Given our use of administrative data, we did not have access to laboratory values and medication utilization, which would augment the risk predictors identified in this study.
ESKD patients on maintenance HD are at high risk for 30-day readmission, and a large proportion of these readmissions may have been prevented with optimized transitional care. The ESKD patient’s requisite follow-up in the outpatient HD unit provides a unique opportunity to streamline and facilitate hospital discharge processes and coordinate outpatient care. Given the high financial costs to health systems and emotional and societal costs to patients and their caregivers, future research should focus on developing and testing cost-effective interventions to reduce this high risk of readmission and improve patient outcomes.
Disclosure
KK-Z was sponsored by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases mentoring award K24-DK091419 to support early-career investigators. KK-Z has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, ASN, AstraZeneca, Aveo, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, NIH, NKF, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma. All the other authors declared no competing interests.
Acknowledgments
The authors thank Dr. Yves Eggli, Institute of Health Economics and Management, University of Lausanne, for applying the SqLape computerized algorithm to identify potentially avoidable readmissions, and Jonathan Amalfitano, Director of Strategic Analysis, Northwell Health, for mapping geographic variation of potentially avoidable readmissions.
This study was presented in abstract form as a poster presentation at the American Society of Nephrology 2016 Annual General Meeting, and the Academy Health 2017 Annual General Meeting.
The authors thank the USRDS for supplying the data used for this study. The interpretation and reporting of these USRDS data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
This work was conducted with support from the Empire Clinical Research Investigator Program New York State Department of Health (support for AM).
Footnotes
Table S1. Multivariable logistic regression model of association of all readmission association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Table S2. Multivariable logistic regression model of association of all readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Table S3. Multivariable logistic regression model of association of potentially avoidable readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Multivariable logistic regression model of association of all readmission association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Multivariable logistic regression model of association of all readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Multivariable logistic regression model of association of potentially avoidable readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
References
- 1.Osman I.M., Godden D.J., Friend J.A. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52:67–71. doi: 10.1136/thx.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond T.A.S., Lander S., Gorodeski E. Patient-reported health related quality of life (EQ-5D) predicts readmission risk. J Am Coll Cardiol. 2012;59 E1890–E1890. [Google Scholar]
- 3.Robert Wood Johnson Foundation. The revolving door: a report on US hospital readmissions. 2013. Available at: http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Accessed February 19, 2015.
- 4.Phillips C.O., Wright S.M., Kern D.E. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure:a meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 5.USRDS 2014 Hospitalizations. 2015. Available at: http://www.usrds.org/2014/view/v2_04.aspx. Accessed June 12, 2015.
- 6.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 7.Joynt K.E., Jha A.K. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes. 2011;4:53–59. doi: 10.1161/CIRCOUTCOMES.110.950964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medicaid Readmissions Reduction Program. Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed October 21, 2015.
- 9.National Quality Forum. Patient outcomes: all-cause readmissions. expedited review 2011. 2012. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=71674. Accessed October 19, 2015.
- 10.van Walraven C., Bennett C., Jennings A. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391–E402. doi: 10.1503/cmaj.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vest J.R., Gamm L.D., Oxford B.A. Determinants of preventable readmissions in the United States: A systematic review. Implement Sci. 2010;5:88. doi: 10.1186/1748-5908-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddone E.Z., Weinberger M., Horner M. Classifying general medicine readmissions. Are they preventable? Veterans Affairs Cooperative Studies in Health Services Group on Primary Care and Hospital Readmissions. J Gen Intern Med. 1996;11:597–607. doi: 10.1007/BF02599027. [DOI] [PubMed] [Google Scholar]
- 13.Madigan E.A., Schott D., Matthews C.R. Rehospitalization among home healthcare patients: results of a prospective study. Home Healthc Nurse. 2001;19:298–305. doi: 10.1097/00004045-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Phelan D., Smyth L., Ryder M. Can we reduce preventable heart failure readmissions in patients enrolled in a Disease Management Programme? Ir J Med Sci. 2009;178:167–171. doi: 10.1007/s11845-009-0332-6. [DOI] [PubMed] [Google Scholar]
- 15.Shalchi Z., Saso S., Li H.K. Factors influencing hospital readmission rates after acute medical treatment. Clin Med (Lond) 2009;9:426–430. doi: 10.7861/clinmedicine.9-5-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Providigm LLC. Development of potentially avoidable readmission and functional outcome SNF quality measures. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.568.5308&rep=rep1&type=pdf. Accessed October 30, 2015.
- 17.Graham H., Livesley B. Can readmissions to a geriatric medical unit be prevented? Lancet. 1983;1:404–406. doi: 10.1016/s0140-6736(83)91513-1. [DOI] [PubMed] [Google Scholar]
- 18.3M Health Information Systems. Potentially preventable readmissions classification system. Available at: http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed October 12, 2015.
- 19.Halfon P., Eggli Y., van Melle G. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587. doi: 10.1016/s0895-4356(01)00521-2. [DOI] [PubMed] [Google Scholar]
- 20.Donzé J., Aujesky D., Williams D. Potentially avoidable 30-day hospital readmissions in medical patients:derivation and validation of a prediction model. JAMA Intern Med. 2013;173:632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 21.Mathew A.T., Strippoli G.F., Ruospo M. Reducing hospital readmissions in patients with end-stage kidney disease. Kidney Int. 2015;88:1250–1260. doi: 10.1038/ki.2015.307. [DOI] [PubMed] [Google Scholar]
- 22.Sood M.M., Miller L., Komenda P. Long-term outcomes of end-stage renal disease patients admitted to the ICU. Nephrol Dial Transplant. 2011;26:2965–2970. doi: 10.1093/ndt/gfq835. [DOI] [PubMed] [Google Scholar]
- 23.Weinhandl E.D., Nieman K.M., Gilbertson D.T. Hospitalization in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. Am J Kidney Dis. 2015;65:98–108. doi: 10.1053/j.ajkd.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Chan K.E., Lazarus J.M., Wingard R.L. Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int. 2009;76:331–341. doi: 10.1038/ki.2009.199. [DOI] [PubMed] [Google Scholar]
- 25.Erickson K.F., Winkelmayer W.C., Chertow G.M. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25:2079–2087. doi: 10.1681/ASN.2013080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engemann J.J., Friedman J.Y., Reed S.D. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005;26:534–539. doi: 10.1086/502580. [DOI] [PubMed] [Google Scholar]
- 27.Nissenson A.R., Dylan M.L., Griffiths R.I. Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am J Kidney Dis. 2005;46:301–308. doi: 10.1053/j.ajkd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Beaubrun A.C., Kilpatrick R.D., Freburger J.K. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24:1461–1469. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troidle L., Eisen T., Pacelli L. Complications associated with the development of bacteremia with. Staphylococcus aureus. Hemodial Int. 2007;11:72–75. doi: 10.1111/j.1542-4758.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 30.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 31.Halfon P., Eggli Y., Prêtre-Rohrbach I. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44:972–981. doi: 10.1097/01.mlr.0000228002.43688.c2. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare and Medicaid Services. Measure methodology. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html. Accessed September 12, 2015.
- 33.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- 34.Liu J., Huang Z., Gilbertson D.T. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 35.Elixhauser A, Steiner CA, Whittington C, et al. Clinical classifications for health policy research: hospital inpatient statistics, 1995. Healthcare Cost and Utilization Project, HCUP 3 Research Note. Rockville, MD: Agency for Health Care Policy and Research; 1998. AHCPR Pub. No. 98–0049.
- 36.Lin G, So Y, Johnston G, eds. Analyzing Survival Data with Competing Risks Using SAS® Software. Available at: https://support.sas.com/resources/papers/proceedings12/344-2012.pdf. Accessed November 27, 2017.
- 37.Di Napoli A., Pezzotti P., Di Lallo D. Determinants of hospitalization in a cohort of chronic dialysis patients in central Italy. J Nephrol. 2005;18:21–29. [PubMed] [Google Scholar]
- 38.Xia H., Ebben J., Ma J.Z. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10:1309–1316. doi: 10.1681/ASN.V1061309. [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe W., Khan I.H., Prescott G.J. Hospitalization in the first year of renal replacement therapy for end-stage renal disease. QJM. 2003;96:899–909. doi: 10.1093/qjmed/hcg155. [DOI] [PubMed] [Google Scholar]
- 40.Powe N.R., Griffiths R.I., Watson A.J. Effect of recombinant erythropoietin on hospital admissions, readmissions, length of stay, and costs of dialysis patients. J Am Soc Nephrol. 1994;4:1455–1465. doi: 10.1681/ASN.V471455. [DOI] [PubMed] [Google Scholar]
- 41.Harel Z., Wald R., McArthur E. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. doi: 10.1681/ASN.2014060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flythe J.E., Katsanos S.L., Hu Y. Predictors of 30-day hospital readmission among maintenance hemodialysis patients: a hospital's perspective. Clin J Am Soc Nephrol. 2016;11:1005–1014. doi: 10.2215/CJN.11611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Medicare and Medicaid Services (CMS). End-stage renal disease quality incentive program (ESRD QIP). 2015. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/05_LawsandRegs.html. Accessed October 9, 2015.
- 44.MedPAC. Report to the Congress: Promoting Greater Efficiency in Medicare. Medicare Payment Advisory Committee, 2007. Available at: http://medpac.gov/docs/default-source/reports/Jun07_EntireReport.pdf. Accessed December 4, 2015.
- 45.Donzé J.D., Lipsitz S., Schnipper J.L. Risk factors and patterns of potentially avoidable readmission in patients with cancer. J Oncol Pract. 2017;13:e68–e76. doi: 10.1200/JOP.2016.011445. [DOI] [PubMed] [Google Scholar]
- 46.Donzé J., Lipsitz S., Bates D.W. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. doi: 10.1136/bmj.f7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Renal Data System. 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Ann Arbor, MI: National Institute of Diabetes and Digestive and Kidney Diseases, 2012.
- 48.USRDS. 2011 Hospitalization. 2013. Available at: http://www.usrds.org/2013/pdf/v2_ch3_13.pdf. Accessed November 2, 2014.
- 49.Longenecker J.C., Coresh J., Klag M.J. Validation of comorbid conditions on the end stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable logistic regression model of association of all readmission association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Multivariable logistic regression model of association of all readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.
Multivariable logistic regression model of association of potentially avoidable readmission + death association with patient and index hospitalization characteristics among prevalent patients with end-stage renal disease on hemodialysis in the United States.