Abstract
Previously, we have shown that the Arabidopsis det2 (deetiolated2) mutant is defective in the biosynthesis of brassinosteroids (BR) and that DET2 (a steroid 5α-reductase) acts early in the proposed BR biosynthetic pathway. In this paper we present further biochemical characterization of det2. We have undertaken metabolic experiments with 2H-labeled substrates of intermediates involved in the formation of campestanol from campesterol, and quantitative analysis of intermediates in Arabidopsis wild type and det2. The results of these studies indicate the early operating steps of BR biosynthesis as: campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol in Arabidopsis, with det2 deficient in the conversion of 4-en-3-one to 3-one. We have also detected these intermediates in the formation of campestanol from campesterol and their metabolic conversions using cultured cells of Catharanthus roseus. These studies confirmed the biosynthetic sequence of events from campesterol to campestanol as was found in Arabidopsis. As such, the originally proposed biosynthetic pathway should be modified.
Since the isolation of BL from pollen extracts of Brassica napus (Grove et al., 1979), over 40 analogs (collectively called BRs) have been isolated from various plant species (Fujioka and Sakurai, 1997a; Clouse and Sasse, 1998; Fujioka, 1999). Their biosynthesis, however, remained unknown until recently. Studies of the biosynthetic pathway of BRs using cultured cells of Catharanthus roseus have revealed that BL is biosynthesized from campesterol by two parallel branched pathways (Fujioka and Sakurai, 1997b; Sakurai and Fujioka, 1997). Moreover, a number of BR-deficient dwarf mutants of Arabidopsis, pea, and tomato have been identified (det2 [Li et al., 1996, 1997; Fujioka et al., 1997]; dwf1/dim [Takahashi et al., 1995; Klahre et al., 1998; Choe et al., 1999a]; cpd [Szekeres et al., 1996]; dwf4 [Azpiroz et al., 1998; Choe et al., 1998]; dwf7 [Choe et al., 1999b]; lkb [Nomura et al., 1997]; tomato dwarf [Bishop et al., 1996, 1999]). These mutants exhibit intriguing phenotypes such as dwarfism and de-etiolation, and the findings of BR mutants led to wide acceptance of essential roles for BRs in plant growth and development (Yokota, 1997; Altmann, 1998; Clouse and Feldmann, 1999).
One such BR-deficient mutant, det2 (deetiolated2), was originally identified as being involved in light-regulated development (Chory et al., 1991). When grown in the dark, det2 exhibits inhibition of hypocotyl growth, expansion of cotyledons, development of primary leaf buds, and accumulation of anthocyanins (Chory et al., 1991). When grown in the light, det2 has a short stature, dark-green leaves, reduced male fertility and apical dominance, and delayed senescence and flowering (Chory et al., 1991, 1994). The DET2 gene has been cloned and shown to encode a protein with similar properties to the mammalian steroid 5α-reductases (Li et al., 1996, 1997). det2 mutant phenotypes can be rescued by application of BL, suggesting that det2 is deficient in BR biosynthesis (Li et al., 1996). In fact, the levels of endogenous BRs in det2 are below 10% of the wild type, indicating that endogenous BR levels are closely linked to the loss of activity of DET2 (Fujioka et al., 1997). Since it was hypothesized that det2 is blocked in the conversion of campesterol to campestanol in the proposed biosynthetic pathway, we examined this step in detail. As expected, in feeding experiments using [2H6]campesterol, no conversion of [2H6]campesterol to [2H6]campestanol was observed in det2, whereas the wild type converted [2H6]campesterol to [2H6]campestanol (Fujioka et al., 1997). In det2 the endogenous level of campestanol was greatly reduced, however, campesterol did not accumulate (Fujioka et al., 1997). Furthermore, only 3-oxo-Δ4,5-steroids were shown to be the substrates of recombinant DET2 (Li et al., 1997). From these observations we speculated that campestanol was not formed directly from campesterol, but was formed via a 3-oxo-Δ4,5-steroid. More detailed analysis revealed that 4-en-3-one was present in Arabidopsis, and this steroid accumulated in det2 (Fujioka et al., 1997). Thus, our previous studies suggest that campestanol is biosynthesized via 4-en-3-one and 3-one and that the defective step in det2 is the conversion of 4-en-3-one to 3-one. However, the other proposed intermediates in the conversion of campesterol to campestanol and their metabolic conversions remain unknown.
In this study we have undertaken more detailed analyses to provide conclusive evidence for our proposal that the DET2 reductase uses a 3-oxo-Δ4,5-steroid as a substrate. To that end, we have conducted metabolic studies and quantitative analysis of the sterols involved in the conversion of campesterol to campestanol. We demonstrate that conversion of campesterol to campestanol proceeds via 4-en-3β-ol, 4-en-3-one, and 3-one, and that det2 is defective in the conversion of 4-en-3-one to 3-one. Furthermore, we have also conducted metabolic experiments and quantitative analyses of these intermediates in cultured cells of C. roseus. The C. roseus study confirms the operation of the biosynthetic sequence, campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol. Together, these results refine the original proposed pathway for BL and provide firm evidence for the precise block in det2 mutants.
MATERIALS AND METHODS
Arabidopsis Seedling Cultures
Wild-type Arabidopsis ecotype Columbia (Col-0) and the det2-1 (here called det2) mutant (Chory et al., 1991) were used in this study. Wild-type and det2 seedlings were germinated and grown on one-half-concentrated Murashige and Skoog medium containing 1% agar and 1% Suc in the light at 22°C. Seven days after they were sown, the seedlings (wild type, 5 seedlings; det2, 40 seedlings) were transferred to a 200-mL flask containing 30 mL of Murashige and Skoog medium supplemented with 3% Suc. The seedlings were incubated at 22°C in the light on a shaker (110 rpm). After 7 d in culture, 2H-labeled substrates were added aseptically to each 200-mL flask, and seedlings were allowed to grow under the same conditions.
V208 Cell Cultures
The cultured cells of Catharanthus roseus (V208) have been previously described (Sakurai and Fujioka, 1996). Cells were grown in a 200-mL flask containing 60 mL of Murashige and Skoog medium supplemented with 3% Suc at 27°C by shaking at 100 rpm in the dark.
GC-MS and GC-SIM Analyses
The GC-MS and GC-SIM analyses were carried out under the following conditions: an Automass mass spectrometer (model JMS-AM150, JEOL) connected with a gas chromatograph (model 5890A-II, Hewlett-Packard), EI (70 eV), source temperature 210°C, DB-5 column (15 m × 0.25 mm, 0.25-μm film thickness, J&W Scientific, Folsom, CA), injection temperature 250°C, column temperature program: 80°C for 1 min, then raised to 320°C at a rate of 30°C min−1, and held on this temperature for 5 min; interface temperature 250°C, carrier gas He, flow rate 1 mL min−1, splitless injection. Samples were trimethylsilylated with N-methyl-N-trimethylsilyltrifluoroacetamide at 80°C for 30 min.
Metabolism of [2H6]Sterols
A MeOH solution of 10 μg/10 μL [2H6]campesterol was added to Arabidopsis seedlings grown in liquid medium. The seedlings were incubated for 2 d at 22°C in the light on a shaker (110 rpm). After incubation the cultures were extracted with MeOH, and the extract was partitioned three times between CHCl3 (25 mL) and water (50 mL). The CHCl3-soluble fraction was purified by a silica gel cartridge (Sep-Pak Vac Silica, 1 g, Waters) with 10 mL of CHCl3 and an ODS cartridge (Sep-Pak Plus C18, Waters) with 20 mL of MeOH. The eluent was subjected to ODS-HPLC (Senshu Pak ODS-4150-N; 10 × 150 mm, Senshu Scientific Co., Ltd., Tokyo) at a flow rate of 2 mL min−1 with 100% MeOH. Fractions were collected every 0.5 min (Rt, 14–21 min). Main fractions of each sterol were as follows: 4-en-3-one (Rt, 16.5–17 min), 4-en-3β-ol (Rt, 17–17.5 min), campesterol (Rt, 18–18.5 min), campestanol (Rt, 19.5–20 min), and 3-one (Rt, 20–20.5 min). 4-en-3-one and 3-one fractions were analyzed by GC-MS without derivatization. 4-en-3β-ol, campesterol, and campestanol fractions were subjected to GC-MS analysis after trimethylsilylation. The experiments with other substrates, [2H6]4-en-3β-ol, [2H6]4-en-3-one, and [2H6]3-one (each 10 μg), were carried out in the same manner, but incubation times were for 4 d. The fresh weight of seedlings after incubation was approximately 10 g.
For V208 cultured cells, each substrate (250 μg of [2H6]campesterol, 100 μg of [2H6]4-en-3β-ol, 100 μg of [2H6]4-en-3-one, and 100 μg of [2H6]3-one) was aseptically added to the cultures at log phase (7 or 8 d old). After incubation for 5 d at 27°C by shaking at 100 rpm in the dark, extraction, purification, and analysis were performed under the same conditions as described above. The fresh weight of cultured cells after incubation was about 15 g. The conversion rate was calculated as a percentage of the amount of each metabolite versus the amount of substrate added to the culture.
Quantitative Analysis of Endogenous Sterols
Arabidopsis seedlings (wild type, det2) were germinated and grown for 12 d on one-half-concentrated Murashige and Skoog medium containing 0.8% agar and 1% Suc under a 16-h light/8-h dark regime at 22°C. Seedlings were collected and extracted with MeOH-CHCl3 (4:1). Ten micrograms of [2H6]campesterol, 100 ng of [2H6]4-en-3β-ol, 1 μg of [2H6]4-en-3-one, 10 ng of [2H6]3-one, and 200 ng of [2H6]campestanol were added to the extract (500 mg fresh weight equivalent) as internal standards. Purification was performed under the same conditions as described in the metabolic studies. Each fraction was subjected to GC-MS analysis. The endogenous levels of each sterol were determined as the ratio of the peak areas of molecular ions for the endogenous sterol and for the internal standard.
For V208 cultured cells, log-phase cells (8 d old, 7.7 g fresh weight) were collected and extracted with MeOH. Fifty micrograms of [2H6]campesterol, 500 ng of [2H6]4-en-3β-ol, 50 μg of [2H6]4-en-3-one, 500 ng of [2H6]3-one, and 30 μg of [2H6]campestanol were added to the extract as internal standards. The MeOH extract was purified and analyzed as described above.
Synthesis of Sterols
4-en-3-one and [26,28-2H6]4-en-3-one were synthesized from campesterol and [26,28-2H6]campesterol, respectively. A mixture of 200 mg, 0.499 mmol of campesterol (kindly supplied by Tama Biochemical Co.), 3 mL of 1-methyl-4-piperidone, 200 mg, 0.979 mmol of aluminum isopropoxide, and 15 mL of toluene was heated at 120°C under an argon atmosphere for 6 h. The reaction mixture was cooled and extracted with EtOAc. The extract was successively washed with 2 m HCl, a saturated NaHCO3 solution and brine, and dried over anhydrous MgSO4. Filtration and removal of the solvent gave a crude product, which was applied to a column of silica gel (2 cm i.d. × 20 cm, 70–230 mesh, Merck, Darmstadt, Germany). Elution with toluene-EtOAc (100:1 to 50:1, v/v) gave 4-en-3-one (126 mg, 63%), melting point 97°C to 98°C (MeOH), 1H-NMR (CDCl3) δ: 0.711 (3H, s), 0.773 (3H, d, J = 6.7 Hz), 0.803 (3H, d, J = 7.0 Hz), 0.852 (3H, d, J = 6.7 Hz), 0.908 (3H, d, J = 6.7 Hz), 1.181 (3H, s), 5.72 (1H, s). EI-MS m/z: 398 (M+, 68), 383 (9), 356 (22), 341 (6), 313 (6), 300 (5), 275 (23), 229 (45), 124 (100). HR-EI-MS; calculated for C28H46O (M+): 398.3551, found: 398.3540. In the same manner, [26,28-2H6]campesterol (44 mg, kindly supplied by Tama Biochemical Co.) was converted to [26,28-2H6]4-en-3-one (26.3 mg, 60%), melting point 97°C to 98°C (MeOH), 1H-NMR (CDCl3) δ: 0.710 (3H, s), 0.907 (3H, d, J = 6.7 Hz), 1.181 (3H, s), 5.72 (1H, s). EI-MS m/z: 404 (M+, 100), 389 (10), 362 (25), 346 (7), 319 (5), 306 (7), 281 (23), 229 (47), 124 (87). HR-EI-MS; calculated for C28H40OD6 (M+): 404.3927, found: 404.3922.
4-en-3β-ol and [26,28-2H6]4-en-3β-ol were synthesized from 4-en-3-one and [26,28-2H6]4-en-3-one, respectively. Sodium borohydride (15 mg, 0.397 mmol) was added to a solution of 89 mg, 0.224 mmol of 4-en-3-one and 100 mg, 0.268 mmol of cerium (III) chloride heptahydrate in 4 mL of tetrahydrofuran and 4 mL of MeOH, and the mixture was stirred at room temperature for 30 min. Two milliliters of water was added to the reaction mixture and the whole was extracted with diethyl ether. The organic phase was washed with brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. A crude product was applied to two plates of silica gel (20 × 20 cm, 0.5 mm thickness, Merck). The plates were developed with toluene-EtOAc (10:1, v/v). The silica gel band with RF 0.28 to 0.33 was scraped off and eluted with EtOAc. Filtration and removal of the solvent in vacuo gave 4-en-3β-ol (59.5 mg, 66%), melting point 127.5°C to 128°C (MeOH), 1H-NMR (CDCl3) δ: 0.678 (3H, s), 0.771 (3H, d, J = 6.7 Hz), 0.801 (3H, d, J = 6.7 Hz), 0.849 (3H, d, J = 6.7 Hz), 0.898 (3H, d, J = 6.7 Hz), 1.049 (3H, s), 4.15 (1H, m), 5.27 (1H, d, J = 1.5 Hz). EI-MS m/z: 400 (M+, 39), 382 (100), 367 (15), 342 (11), 330 (34), 315 (7), 301 (5), 274 (9), 261 (9), 255 (25). HR-EI-MS; calculated for C28H48O (M+): 400.3707, found: 400.3716. In the same manner, [26,28-2H6]4-en-3-one (12.3 mg) was converted to [26,28-2H6]4-en-3β-ol (8.0 mg, 65%), melting point 127°C to 128°C (MeOH), 1H-NMR (CDCl3) δ: 0.678 (3H, s), 0.896 (3H, d, J = 6.7 Hz), 1.048 (3H, s), 4.15 (1H, m), 5.27 (1H, d, J = 1.5 Hz). EI-MS m/z: 406 (M+, 34), 388 (100), 372 (13), 348 (10), 336 (23), 280 (5), 255 (15). HR-EI-MS; calculated for C28H42OD6 (M+): 406.4084, found: 406.4080.
(24R)-24-Methyl-5α-cholestan-3β-ol and [26,28-2H6](24R)-24-methyl-5α-cholestan-3β-ol were synthesized from campesterol and [26,28-2H6]campesterol, respectively. Campesterol (308 mg, 0.769 mmol) was dissolved in 20 mL of EtOH, and 40 mg of 10% Pd-C was added. The mixture was stirred at 40°C to 50°C under a hydrogen atmosphere for 2 h. The catalyst was filtered off and the filtrate was concentrated in vacuo to give a crude product. Recrystallization from EtOH gave 230 mg, 74% (24R)-24-methyl-5α-cholestan-3β-ol, melting point 145°C to 146°C (EtOH), 1H-NMR (CDCl3) δ: 0.650 (3H, s), 0.770 (3H, d, J = 6.7 Hz), 0.800 (3H, d, J = 6.7 Hz), 0.802 (3H, s), 0.848 (3H, d, J = 7.0 Hz), 0.894 (3H, d, J = 6.4 Hz), 3.59 (1H, m). EI-MS m/z: 402 (M+, 100), 387 (19), 369 (9), 345 (4), 276 (6), 248 (13), 233 (57), 215 (48). HR-EI-MS; calculated for C28H50O (M+): 402.3864, found: 402.3865. In the same manner, 37 mg of [26,28-2H6]campesterol was converted quantitatively to 37 mg of [26,28-2H6](24R)-24-methyl-5α-cholestan-3β-ol, melting point 145°C to 146°C (EtOH), 1H-NMR (CDCl3) δ: 0.648 (3H, s), 0.802 (3H, s), 0.893 (3H, d, J = 6.3 Hz), 3.59 (1H, m). EI-MS m/z: 408 (M+, 28), 390 (100), 375 (38), 337 (43), 257 (29), 233 (21), 215 (57). HR-EI-MS; calculated for C28H44OD6 (M+): 408.4240, found: 408.4248.
3-one and [26,28-2H6]3-one were synthesized from (24R)-24-methyl-5α-cholestan-3β-ol and [26,28-2H6](24R)-24-methyl-5α-cholestan-3β-ol, respectively. Five drops of Jones reagent was added to a solution of 26 mg, 64.7 μmol of (24R)-24-methyl-5α-cholestan-3β-ol in 5 mL of acetone and 2 mL of n-hexane, and the mixture was stirred at room temperature for 15 min. Five milliliters of water was added to the reaction mixture, and the whole was extracted with diethyl ether. The organic phase was washed with water, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. A crude product was applied to a plate of silica gel (20 × 20 cm, 0.5 mm thickness). The plate was developed with toluene-EtOAc (10: 1, v/v). The silica gel band with RF 0.55 to 0.62 was scraped off and eluted with EtOAc. Filtration and removal of the solvent in vacuo gave 22 mg, 85% 3-one, melting point 152°C to 153°C (MeOH), 1H-NMR (CDCl3) δ: 0.679 (3H, s), 0.772 (3H, d, J = 6.7 Hz), 0.802 (3H, d, J = 7.0 Hz), 0.850 (3H, d, J = 6.7 Hz), 0.901 (3H, d, J = 6.4 Hz), 1.006 (3H, s). EI-MS m/z: 400 (M+, 81), 385 (13), 246 (13), 231 (100), 217 (25). HR-EI-MS; calculated for C28H48O (M+): 400.3707, found: 400.3704. In the same manner, 10 mg of [26,28-2H6](24R)-24-methyl-5α-cholestan-3β-ol was converted to 6.0 mg of [26,28-2H6]3-one, melting point 151°C to 152°C (MeOH), 1H-NMR (CDCl3) δ: 0.678 (3H, s), 0.900 (3H, d, J = 6.7 Hz), 1.006 (3H, s). EI-MS m/z: 406 (M+, 100), 391 (17), 246 (13), 231 (78), 217 (24). HR-EI-MS; calculated for C28H42OD6 (M+): 406.4084, found: 406.4082.
RESULTS
Metabolism of 2H-Labeled Intermediates Involved in the Formation of Campestanol from Campesterol in Arabidopsis Seedlings
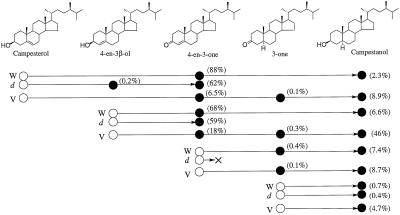
To examine the blocked step of BR biosynthesis in det2 and biosynthetic sequence between campesterol and campestanol in detail, feeding experiments with 2H6-labeled intermediates were undertaken. After incubation, purified sterol fractions were analyzed by GC-MS. The results are summarized in Figure 1.
Figure 1.
Metabolism of 2H-labeled sterols in Arabidopsis (wild type and det2) and C. roseus (V208 cultured cells). ○, Substrate; •, metabolite; W, Arabidopsis wild type; d, Arabidopsis det2; and V, C. roseus V208 cultured cells. Values in parentheses indicate conversion rates.
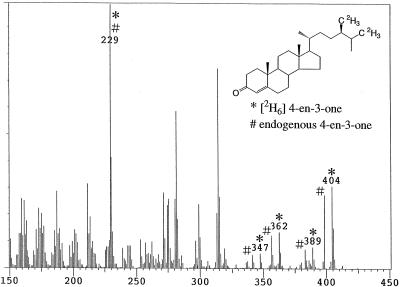
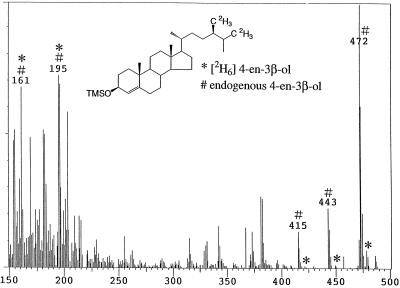
When [2H6]campesterol was fed to wild-type seedlings, [2H6]4-en-3-one was detected as the major metabolite (conversion rate: 88%). A mass spectrum derived from a mixture of endogenous 4-en-3-one and [2H6]4-en-3-one (a metabolite of [2H6]campesterol) was obtained. Prominent ion peaks were as follows (*: metabolite, #: endogenous, Fig. 2): m/z 404* [31%], 398# [28%], 389* [8%], 383# [8%], 362* [14%], 356# [12%], 347* [6%], 341# [5%], and 229*# [100%]. [2H6]Campestanol was detected as a minor metabolite (conversion rate: 2.3%, m/z 480* [3%], 474# [21%], 465* [3%], 459# [24%], 423* [1%], 417# [10%], 390* [2%], 384# [19%], 375* [4%], 369# [28%], and 215*# [100%]). In contrast, in det2, although [2H6]4-en-3-one was detected as a major metabolite (conversion rate: 62%), [2H6]campestanol was not detected. However, [2H6]4-en-3β-ol, which was not detected in the wild type, was identified in det2 (conversion rate: 0.2%, m/z 478* [7%], 472# [100%]. 449* [1%], 443# [22%], 421* [1%], 415# [14%], 388* [2%], 382# [26%], and 255*# [12%], Fig. 3).
Figure 2.
GC-MS analysis of 4-en-3-one fraction obtained from feeding [2H6]campesterol to the Arabidopsis wild type. *, Metabolite; #, endogenous.
Figure 3.
GC-MS analysis of 4-en-3β-ol fraction obtained from feeding [2H6]campesterol to the Arabidopsis det2 mutant. *, Metabolite; #, endogenous.
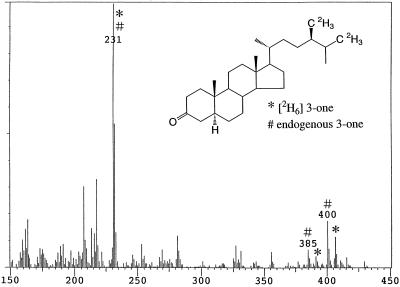
When [2H6]4-en-3β-ol was fed to seedlings, both [2H6]4-en-3-one (conversion rate: 68%, Fig. 4) and [2H6]campestanol (conversion rate: 6.6%) were detected in wild type; however, only [2H6]4-en-3-one (conversion rate: 59%) was detected in det2. One-fourth of the substrate added to the culture remained unmetabolized in both the wild type and det2. Likewise, when [2H6]4-en-3-one was fed to seedlings, [2H6]3-one (conversion rate: 0.4%) and [2H6]campestanol (conversion rate: 7.4%) were detected in the wild type, whereas no conversion was observed in det2. However, feeding [2H6]3-one to seedlings resulted in the detection of [2H6]campestanol in both the wild type and det2 (conversion rate: 0.7% and 0.4%, respectively). In both feedings of [2H6]4-en-3-one and [2H6]3-one, most of each substrate remained unmetabolized (more than 80%). [2H6]4-en-3-one apparently was more rapidly converted to [2H6]campestanol than [2H6]3-one. Exogenously applied [2H6]3-one might be less accessible to the metabolic site than [2H6]3-one converted from [2H6]4-en-3-one in the seedlings.
Figure 4.
GC-MS analysis of 3-one fraction obtained from feeding [2H6]4-en-3-one to the Arabidopsis wild type. *, Metabolite; #, endogenous.
These results show that the conversion from campesterol to campestanol is not a single step, but proceeds via 4-en-3-one and 3-one. Moreover, the precise defective step in det2 can be defined as the conversion of 4-en-3-one to 3-one. 4-en-3β-ol was also identified and this compound was metabolized to 4-en-3-one and campestanol, suggesting that the biosynthetic sequence of campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol operates in Arabidopsis (Fig. 5).
Figure 5.
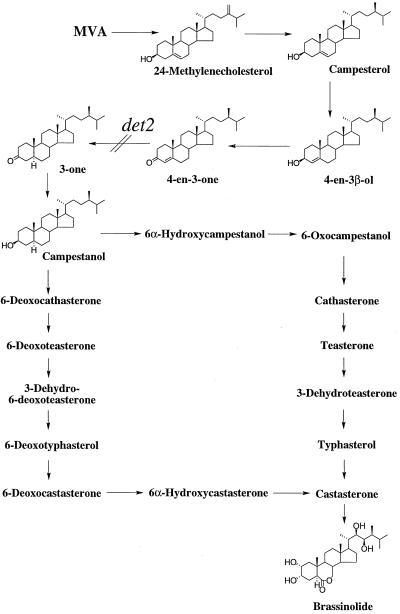
Proposed biosynthetic pathway for BL indicating the blocked step in the det2 mutant. Mevalonic acid.
Metabolism of 2H-Labeled Intermediates Involved in the Formation of Campestanol from Campesterol in C. roseus V208 Cells
Previously, Suzuki et al. (1995) examined metabolism of 13C- and/or 14C-labeled campesterol in cultured cells of C. roseus. In their study a major metabolite of campesterol was identified to be campestanol. We have re-examined the conversion of campesterol to campestanol in V208 cultured cells. The results were similar to those observed in Arabidopsis wild type (Fig. 1).
When feeding [2H6]campesterol to cultured cells, [2H6]4-en-3-one, [2H6]3-one, and [2H6]campestanol were detected as metabolites (conversion rate: 6.5%, 0.1% and 8.9%, respectively). When [2H6]4-en-3β-ol was fed to cells, [2H6]4-en-3-one, [2H6]3-one, and [2H6]campestanol were detected as metabolites (conversion rate: 18%, 0.3% and 46%, respectively). One-third of the substrate added to the culture remained unmetabolized. Upon addition of [2H6]4-en-3-one to cells, [2H6]3-one and [2H6]campestanol were detected (conversion rate: 0.1% and 8.7%, respectively). From the feedings of [2H6]3-one, [2H6]campestanol was detected as a metabolite (conversion rate: 4.7%). In both feedings of [2H6]4-en-3-one and[2H6]3-one, most of each substrate remained unmetabolized (more than 80%). In all metabolic experiments in this series, corresponding endogenous sterols were identified along with 2H-labeled metabolites. These results indicate that the biosynthetic sequence of campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol also operates in C. roseus (Fig. 5).
Quantitative Analysis of Endogenous Levels of Intermediates between Campesterol and Campestanol
To confirm the blocked step of BR biosynthesis in det2, we measured quantitatively the endogenous levels of intermediates between campesterol and campestanol in Arabidopsis seedlings (wild type and det2) using GC-SIM with 2H-labeled internal standards. Endogenous levels of intermediates in V208 cultured cells were also determined.
The levels of intermediates are summarized in Table I. In Arabidopsis, although endogenous 4-en-3β-ol, 4-en-3-one, and 3-one were detected in both wild type and det2, the levels of these sterols were different. In det2 the levels of 4-en-3-one and 4-en-3β-ol were 4 and 6 times higher than in the wild type. On the contrary, the level of 3-one in det2 was less than 20% of the wild-type level. The analysis confirmed that DET2 acts in the 5α-reduction of 4-en-3-one. In V208 cultured cells all these intermediates were also detected, however, the levels of 4-en-3-one, 3-one and campestanol were much higher than those of Arabidopsis seedlings.
Table I.
Sterol content in Arabidopsis seedlings (wild type and det2) and C. roseus cultured cells (V208)
| Sterol | Wild Type | det2 | V208 |
|---|---|---|---|
| ng/g fresh wt | |||
| Campesterol | 23,000 | 15,000 | 59,000 |
| 4-en-3β-ol | 42 | 243 | 37 |
| 4-en-3-one | 394 | 1,395 | 8,000 |
| 3-one | 17 | 3 | 259 |
| Campestanol | 473 | 61 | 10,000 |
DISCUSSION
Recently, the notion that BRs are essential for plant growth and development has been widely accepted by the discovery of BR dwarf mutants of Arabidopsis, pea, and tomato (Yokota, 1997; Altmann, 1998; Clouse and Feldmann, 1999). The observation that only BRs could rescue the mutant phenotypes to the wild type provided convincing evidence that BRs are indeed essential plant hormones for normal plant growth and development. However, there is limited biochemical data for the precise blocks in these BR biosynthetic mutants. Quantitative analysis of intermediates of BR biosynthesis and metabolic experiments with labeled intermediates provide firm biochemical evidence for BR-deficient mutants, and allow determination of the defective step in BR biosynthesis. Previously, we showed that det2 is blocked in the conversion of campesterol to campestanol leading to BL biosynthesis (Fujioka et al., 1997). The study also suggested that the conversion of campesterol to campestanol is not a single step, but composed of the biosynthetic sequence of campesterol → 4-en-3-one → 3-one → campestanol. In this report we have undertaken more detailed metabolic analyses of the intermediates that occur between campesterol and campestanol.
In Arabidopsis wild-type seedlings, 4-en-3-one and campestanol were identified as metabolites of campesterol. 3-one and campestanol were detected as metabolites of 4-en-3-one, and campestanol was detected as a metabolite of 3-one. These results provide evidence for the operation of the following biosynthetic sequence in Arabidopsis: campesterol → 4-en-3-one → 3-one → campestanol in Arabidopsis. Recently, co-occurrence of campesterol, 4-en-3-one, 3-one, and campestanol in the seeds of Cannabis sativa, wheat, and foxtail millet has also been demonstrated (Takatsuto et al., 1997, 1999; Takatsuto and Kawashima, 1998), which support the operation of the above biosynthetic sequence.
In det2, 4-en-3β-ol and 4-en-3-one were identified as the metabolites of campesterol, and 4-en-3-one was identified as a metabolite of 4-en-3β-ol. 3-one and campestanol were not detected from feeding of 4-en-3-one in det2, although endogenous campestanol was detected. Quantitative analysis of these sterols revealed that the endogenous level of 4-en-3-one in det2 is 4-fold higher than the wild type, whereas the level of 3-one is less than 20% of the wild-type level. These results clearly indicate that the deficient step of BR biosynthesis in det2 is the conversion of 4-en-3-one to 3-one; in other words, DET2 catalyzes the 5α-reduction of 4-en-3-one.
Enzymes that catalyze conversion from 3β-hydroxy-Δ5,6-steroid to 3-oxo-Δ4,5-steroid in bacteria and mammals have been reported. In bacteria, 3β-hydroxysteroid dehydrogenase and Δ5,6-Δ4,5 isomerase exist as two separate proteins (Talalay and Wang, 1955). On the other hand, in mammals two enzymatic activities appear to reside within a single protein (Lachance et al., 1990). The enzymatic conversion from 3β-hydroxy-Δ5,6-steroid to 3-oxo-Δ4,5-steroid is generally believed to involve a primary oxidation of the 3β-hydroxy group by 3β-hydroxysteroid dehydrogenase, followed by a rearragement of the double bond. 4-en-3β-ol was identified as a metabolite of campesterol in det2, and this compound was detected as an endogenous sterol in Arabidopsis and C. roseus in this study. In addition, the conversion of 4-en-3β-ol to 4-en-3-one was definitely demonstrated in both Arabidopsis and C. roseus. Therefore, the present study indicates that 4-en-3β-ol is involved in the conversion of campesterol to 4-en-3-one, and isomerization of Δ5,6 to Δ4,5 may occur before dehydrogenation of 3β-hydroxy-Δ5,6-steroid at least in Arabidopsis and C. roseus.
Our findings (natural occurrence of 4-en-3β-ol and the conversions of campesterol to 4-en-3β-ol and of 4-en-3β-ol to 4-en-3-one) may represent an alternative pathway for the conversion of campesterol to 4-en-3-one. If this pathway is ubiquitous and a major one in the plant kingdom, it would mean that the conversion from 3β-hydroxy-Δ5,6-steroid to 3-oxo-Δ4,5-steroid is regulated differently in at least three different kinds of organisms, namely mammals, bacteria, and plants. However, the present study does not deny the generally accepted mechanism of the conversion from 3β-hydroxy-Δ5,6-steroid to 3-oxo-Δ4,5-steroid. Plants may also have a similar mechanism, i.e. the conversion of campesterol to 4-en-3-one could proceed via a 3-oxo-Δ5,6-steroid. Identification of these enzymes in plants will provide the requisite information to answer this important question.
In summary, we have provided further biochemical information on the BR-deficient mutant det2. Reduction of endogenous levels of BRs in det2 (Fujioka et al., 1997) is closely linked to the loss of activity of the DET2 gene (Li et al., 1997). The present study clearly demonstrates that det2 is defective in the 5α-reduction step (4-en-3-one to 3-one) leading to BL biosynthesis. det2 is now a well-characterized BR-deficent mutant by biochemical, physiological, and molecular studies, and will be an important tool for BR biosynthesis studies. In addition, our present data allow us to propose that the biosynthetic scheme campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol is ubiquitous in the plant kingdom. To test our proposal, further analysis, including identification and characterization of the enzymes controlling these proposed steps and isolation of BR-deficient mutants blocked in these steps, will be necessary. Together with our previous studies, the present study refines the original proposed pathway for BL and defines precisely the blocked step in the det2 mutant.
Abbreviations:
- 3-one
(24R)-24-methyl-5α-cholestan-3-one
- 4-en-3β-ol
(24R)-24-methylcholest-4-en-3β-ol
- 4-en-3-one
(24R)-24-methylcholest-4-en-3-one
- BL
brassinolide
- BR
brassinosteroid
- EI
electron ionization
- EtOAc
ethyl acetate
- GC-SIM
GC-selected ion monitoring
- HR
high resolution
- ODS
octadecylsilane
- Rt
retention time
Footnotes
This work was supported by the President's Special Research Grant from the Institute of Physical and Chemical Research (RIKEN) to S.F., a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports, and Culture of Japan to S.F. (no. 10460050), and a grant from the U.S. Department of Agriculture to J.C. (no. 96-35301-3153). J.C. is an investigator of the Howard Hughes Medical Institute.
LITERATURE CITED
- Altmann T. Recent advances in brassinosteroid molecular genetics. Curr Opin Plant Biol. 1998;1:378–383. doi: 10.1016/s1369-5266(98)80259-8. [DOI] [PubMed] [Google Scholar]
- Azpiroz R, Wu Y, LoCascio JC, Feldmann KA. An Arabidopsis brassinosteorid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Harrison K, Jones JDG. The tomato DWARF gene isolated by heterologous transposon tagging encodes the first member of a new cytochrome P450 family. Plant Cell. 1996;8:959–969. doi: 10.1105/tpc.8.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S and others. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999a;119:897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA. The Arabidopsis dwf7/ste1 mutant is defective in the Δ7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999b;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis. Plant Physiol. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Feldmann KA. Molecular genetics of brassinosteroid action. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids. Tokyo: Springer-Verlag; 1999. pp. 163–190. [Google Scholar]
- Clouse SD, Sasse JM. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Fujioka S. Natural occurrence of brassinosteroids in the plant kingdom. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids. Tokyo: Springer-Verlag; 1999. pp. 21–45. [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Brassinosteroids. Nat Prod Rep. 1997a;14:1–10. doi: 10.1039/np9971400001. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Biosynthesis and metabolism of brassinosteroids. Physiol Plant. 1997b;100:710–715. [Google Scholar]
- Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Jr, Steffen GL, Flippen-Anderson JL, Cook JC., Jr Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance Y, Luu-The V, Labrie C, Simard J, Dumont M, de Launoit Y, Guerin S, Leblanc G, Labrie F. Characterization of human 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase gene and its expression in mammalian cells. J Biol Chem. 1990;265:20469–20475. [PubMed] [Google Scholar]
- Li J, Biswas M, Chao A, Russel D, Chory J. Conservation of function between mammalian and plant steroid 5α-reductase. Proc Natl Acad Sci USA. 1997;94:3554–3559. doi: 10.1073/pnas.94.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Fujioka S. Catharanthus roseus (Vinca rosea): in vitro production of brassinosteroids. In: Bajaj YSP, editor. Biotechnology in Agriculture and Forestry, Vol 37: Medicinal and Aromatic Plants IX. Berlin: Springer-Verlag; 1996. pp. 87–96. [Google Scholar]
- Sakurai A, Fujioka S. Studies on biosynthesis of brassinosteroids. Biosci Biotech Biochem. 1997;61:757–762. doi: 10.1271/bbb.61.757. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Inoue T, Fujioka S, Saito T, Takatsuto S, Yokota T, Murofushi N, Yanagisawa T, Sakurai A. Conversion of 24-methylcholesterol to 6-oxo-24-methylcholestanol, a putative intermediate of the biosynthesis of brassinosteroids, in cultured cells of Catharanthus roseus. Phytochemistry. 1995;40:1391–1397. [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabodopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua NH. The DIMINUTO gene of Arabdidopsis is involved in regulating cell elongation. Genes Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Takatsuto S, Kawashima T. Identification of 24-methyl-5α-cholestan-3-one and 24-methylcholest-4-en-3-one in the seeds of Cannabis sativa L. J Jpn Oil Chem Soc. 1998;47:783–786. [Google Scholar]
- Takatsuto S, Kawashima T, Noguchi T, Fujioka S, Sakurai A. Identification of teasterone and phytosterols in the lipid fraction from seeds of Cannabis sativa L. J Jpn Oil Chem Soc. 1997;46:1499–1504. [Google Scholar]
- Takatsuto S, Kosuga N, Abe B, Noguchi T, Fujioka S, Yokota T. Occurrence of potential brassinosteroid precursor steroids in seeds of wheat and foxtail millet. J Plant Res. 1999;112:27–33. [Google Scholar]
- Talalay P, Wang VS. Enzymatic isomerization of Δ5-3-ketosteroid. Biochim Biophys Acta. 1955;18:300–301. doi: 10.1016/0006-3002(55)90079-2. [DOI] [PubMed] [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997;2:137–143. [Google Scholar]