Abstract
BACKGROUND
Social stressors such as social relationship deficits have been increasingly linked to chronic disease outcomes, including cancer. However, critical gaps exist in our understanding of the nature and strength of such links, as well as the underlying biological mechanisms relating social relationships to cancer progression and survival.
METHODS
Utilizing novel questionnaire and biomarker data from the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC), the present study examines the associations between diverse measures of social support and mortality risk among individuals with cancer (N=1,004). We further assess the role of multiple serum markers of inflammation—including high sensitivity C-reactive protein (CRP), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF)—as potential mediators in the social relationship-cancer link.
FINDINGS
Findings revealed that one’s appraisal of their social support was associated with cancer mortality, such that individuals reporting higher levels of social support satisfaction had lower mortality risk than individuals reporting lower levels of satisfaction. The amount of support received, on the other hand, was not predictive of cancer survival. We further found evidence that inflammatory processes may undergird the link between social support satisfaction and mortality among individuals with cancer, with individuals reporting higher levels of social support satisfaction having lower levels of CRP, IL-6, and TNF-α.
CONCLUSIONS
These results provide new knowledge of the biosocial processes producing population disparities in cancer outcomes.
IMPACT
Our study offers new insights for intervention efforts aimed at promoting social connectedness as a means for improving cancer survival.
INTRODUCTION
Research across disciplines documents the critical role of social relationships in affecting health and disease risk. Social relationship deficits have been linked to a wide range of outcomes, including higher rates of cardiovascular disease (1, 2), depression (3, 4), and infection (5). The stress associated with social isolation, social relationship strain, and low levels of social support have been further linked to a host of biological markers of disease risk, including biomarkers of inflammation (6, 7) and metabolic function (8). The magnitude of the documented associations between social relationship deficits and mortality is substantial, with the health risks associated with isolation being comparable to that of smoking and exceeding those of other documented risk factors of mortality, such as obesity and physical inactivity (9–11). In addition to the detrimental health consequences of social isolation and social relationship stress, research also provides overwhelming evidence of the critical nature of positive social relationship experiences—namely, high levels of social connectedness and perceived social support—in maintaining and improving health across the life course (12–14). In this way, the experiences individuals have within the context of social relationships can serve to promote or impede health and well-being.
Despite a growing body of research in this area, critical gaps in our understanding of the links between social relationships and health and disease risk remain. First, few studies examine how social relationships are related to cancer survival, and—as a result—there is limited understanding of the mechanisms producing such links. The lack of research linking social relationships to cancer outcomes is of critical population health importance both because cancer is the second leading cause of death in the U.S. and because there is increasing evidence that social relationships are consequential for cancer biology and survival (15). Animal studies have linked social isolation to tumor growth and metastases (16), and studies of humans have further linked lower levels of social embeddedness to poorer survival from breast (17), colorectal (18), and lung cancer (19). On the other hand, research suggests that having larger social networks and higher levels of social support is associated with declines in cancer mortality (20). Still, whether or how social relationship characteristics relate to cancer related outcomes remains largely unexplored.
Furthermore, the biological mechanisms undergirding the potential links between social relationship characteristics and cancer survival also remain to be better understood. Increasingly, researchers have highlighted the potential role of inflammation in explaining the associations between different dimensions of social relationships and cancer etiology and prognosis (21). Chronic, systemic inflammation is a critical determinant of all stages of carcinogenesis—including initiation, promotion, and progression (22)—and cancer prognosis (23, 24). Further, exposure to chronic stress, such as the stress associated with long-term social isolation or relationship strain, has been linked to increased inflammation (7, 10, 25). On the other hand, high levels of social integration and support have been linked to decreased inflammation, which suggests that social relationships can improve or sustain health by buffering against the up-regulation of physiological responses to stress exposure (26–28). Given that cancer is a pro-inflammatory disease and that systemic inflammation has been increasingly linked to carcinogenesis and prognosis, assessing whether and how social relationships affect cancer mortality through inflammatory processes may shed new light on better prevention and intervention strategies.
In response to social stressors, activity in the autonomic nervous system, hypothalamic-pituitary-adrenal (HPA) axis, and sympathetic nervous system (SNS) increases, resulting in the release of a number of biochemical mediators of stress, such as cortisol, catecholamines, and neuropeptides. Prolonged exposure to these mediators resulting from chronic activation of physiological stress response systems can alter inflammatory gene expression and promote inflammation in the tumor microenvironment (29–31). Molecular studies suggest that the pathways linking stress exposure to tumor growth and progression are primarily activated by adrenergic receptors (ADRs), which are found on many different types of cancer cells and in the cells surrounding the tumor microenvironment. In particular, macrophages, which are a class of immune cells that both participate in tumor progression and cause chronic inflammation, may play an essential role in linking stress, inflammation, and cancer (30). Further, the chronic inflammation that results from prolonged activation of physiological stress response systems can impair immune functions (32), which may be especially harmful for individuals with cancer, as compromised immune states may create an environment conducive to cancer progression (19, 31, 33, 34). Still, while social relationship characteristics such as social integration and social support have been linked to overall health, immune function, and inflammatory response generally, whether inflammatory processes mediate the link between social relationships and cancer outcomes has not been sufficiently explored.
Second, previous literature on the links between social relationships and health, broadly, and cancer, specifically, is generally limited in its measures of social relationships. Research on the associations between social relationships and health generally considers two primary dimensions of social relations: social integration and social support. Whereas social integration generally refers to the structural dimension of social relationships by reflecting the size, scope, and connectedness of social networks, social support typically indicates the functional dimension of relationships by reflecting the level of support an individual receives or perceives from their network (12, 35). Most studies of the impact of social relations on health and disease risk focus on the structural dimension of social relationships by utilizing indicators such as living arrangements (36), marital status (37), and social network size (38). As a result, less is known about the role of the functional dimension of social relationships, including levels of social support and satisfaction with support, affect health and mortality risk. Individuals can still perceive isolation or loneliness despite having many social ties; conversely, having one or two close social connections may lead to greater levels of perceived support (35). Further, research suggests that perceiving or receiving high levels of support from social network members—regardless of the size or scope of network ties—may dampen the physiological arousal in response to social stress and thus improve health and disease outcomes (8). Still, because most studies of social relationships and health focus on the structural dimension of social relationships, less is known about how the support that one derives from their relationships relate to health, generally, and cancer mortality, specifically. Further, studies on social support and health rely predominately on measures indicating the level of support one receives without consideration of the perceived quality of that support. As such, consideration of multiple aspects of social relationships is critical to advancing understanding of how individuals’ social ties—as well as their assessments of those relationships—produce population disparities in cancer outcomes.
In sum, previous research documents social relationship gradients in health and disease, and studies also offer evidence of inflammation-cancer links. However, these two bodies of research have not been fully integrated to examine how social conditions—such as different aspects of social support—”get under the skin” through inflammatory pathways to affect cancer survival. Using cohort study data from the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC) study (2010–2016), the present study breaks new ground by examining how diverse measures of social support are associated with cancer mortality. In particular, we assess how two indicators of social support—namely, satisfaction with social support (e.g., support quality) and level of social support (e.g., support quantity)—relate to cancer mortality. Additionally, utilizing recently assayed biomarker data, we further assess the links between social relationship support and inflammatory markers to gauge whether inflammatory processes may help to explain the associations between social support and cancer survival. By integrating social and biological data on a cohort of cancer survivors, this study provides new evidence of how supportive social relationships impact cancer outcomes and offers critical insights into intervention efforts aimed at improving cancer survival.
DATA AND MEASURES
Data from this study comes from the University of North Carolina at Chapel Hill Health Registry/Cancer Survivorship Cohort (HR/CSC), which integrates clinical, epidemiological, and interview data with repositories of biologic specimens. Consistent with the American Cancer Society, this study recognizes cancer survivorship as including individuals who have received a cancer diagnosis, regardless of treatment status (39). The HR/CSC is a hospital-based registry of incident and prevalent adult cancer survivors age 18 years of age and older, with appointments in outpatient oncology clinics between 2010 and 2016, that speak English or Spanish. Patients who could not provide informed consent or who could not complete the interview questionnaires were excluded. The analytic sample for the current study was further restricted to HR/CSC participants who had a pathologically confirmed cancer diagnosis, completed baseline questionnaires, had abstracted medical records, available serum, blood draw was outside the treatment window, and had complete data on the variables used in the analysis as of October 2015. Participants with blood drawn “outside the treatment” window included those whose blood was drawn prior to treatment, or had no treatment, or whose blood was drawn more than 56 days after surgery and/or greater than 240 days after chemotherapy or radiation. We also excluded participants who were diagnosed more than 90 days after consent. Together, these restrictions resulted in an analytic sample group of 1,104 participants. A cohort discovery flow chart outlining sample inclusion/exclusion criteria is provided in Supplementary Figure S1. All study protocols for the HR/CSC were IRB approved (UNC IRB#09-0605) and all participants provided informed consent.
Outcomes for this study include all-cause mortality (1=died), which was ascertained from North Carolina Vital Records. Of the respondents who died during the period of observation, a majority (110 respondents, representing approximately 68 percent of all deaths) had cancer as the cause of death. Supplementary analysis using cancer-specific mortality as an outcome yielded substantively similar results to models using all-cause mortality as the outcome. Given that models utilizing all-cause mortality had greater statistical power, we used all-cause mortality as the key outcome of interest in the study. Other outcomes include continuous measures of four key biomarkers of inflammation: high sensitivity C-reactive protein (CRP) (from 0.1mL serum), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF) (from 0.1mL serum). The biomarkers of inflammation were measured in HR/CSC participant blood serum samples using the Bio-Plex 200, a multiplex immunoassay system based on the Luminex technology. For each individual marker, samples with levels that were below the detection level or out of range exceeding the maximum level were excluded from the analyses (CRP: 113 cases excluded; IL-6: ; TNF- α: 12 cases excluded; VEGF: 89 cases excluded). In the final analyses, all measures of inflammation are log transformed to account for skewness.
Our measures of social relationship quality were derived from questions asked as part of the HR/CSC Baseline Questionnaires, a computer assisted telephone interview administered by trained study staff following enrollment and reflect the functional dimension of social relationships. The measure of satisfaction with social support comes from the Patient-Reported Outcomes Measurement Information System – Global (PROMIS-Global). Study participants were administered PROMIS questionnaire v1.0 Global, which is a 10-item scale that measures the domains of physical function, fatigue, pain, emotional distress, and social health (40). Our measure of satisfaction with social support was ascertained by asking study participants “In general, how would you rate your satisfaction with your social activities and relationships?” The measure is coded so that “1” indicates poor satisfaction and “5” indicates excellent satisfaction. Level of social support was measured by the social/family well-being subscale of the Functional Assessment of Cancer Therapy – General Population (FACT-GP), version 4, which is a 21-item scale that measures health-related quality of life (41, 42). Our measure of level of social support is a composite measure indicating participants’ perceived level of support from family, friends, and partners. For each individual indicator of perceived support, we created a dummy variable where “1” indicated membership in the highest quartile of support. We then summed the dummy indicators to derive a continuous score of quantity of overall support as well as a dichotomized variable with a value of “1” representing high support as indicated by being in the top quartile.
Other covariates include age, sex, race, marital status, education, smoking status, physical activity, alcohol use, and cancer characteristics such as cancer stage and an incident/prevalent case status (incident status indicates a participant who consented to participate in the study less than 30 days after diagnosis or less than 90 days before diagnosis; prevalent status indicates a participant who consented 30 or more days after diagnosis). Details regarding the coding of the covariates are presented in Table 1.
Table 1.
Sample Descriptive Statistics
| Mean/% | Number of deaths |
N | Death rate (deaths/1,000 person-years) |
|
|---|---|---|---|---|
| Variable | ||||
| Death (1=died) | 15.96 | 161 | 1,009 | 39.74 |
| Satisf. social support | ||||
| Poor | 3.67 | 10 | 37 | 59.39 |
| Fair | 8.92 | 18 | 90 | 55.19 |
| Good | 29.83 | 56 | 301 | 45.73 |
| Very good | 35.68 | 47 | 360 | 35.18 |
| Excellent | 21.90 | 25 | 221 | 28.61 |
| Level of social support | ||||
| Low | 60.57 | 90 | 590 | 32.11 |
| High | 39.43 | 61 | 384 | 62.11 |
| Age (baseline) | 60.21 | 1,009 | - | |
| Sex | ||||
| Male | 27.65 | 64 | 279 | 54.51 |
| Female | 72.35 | 92 | 730 | 33.44 |
| Race/ethnicity | ||||
| White | 79.88 | 133 | 806 | 42.25 |
| Non-white | 20.12 | 23 | 203 | 29.60 |
| Marital status | ||||
| Not married | 34.69 | 64 | 350 | 53.27 |
| Married | 65.31 | 92 | 659 | 33.78 |
| Smoking | ||||
| Non-smoker | 91.97 | 141 | 928 | 38.49 |
| Smoker | 8.03 | 15 | 81 | 57.40 |
| Physical activity level | ||||
| No physical activity | 23.89 | 53 | 241 | 65.33 |
| Low | 31.12 | 36 | 314 | 29.09 |
| Moderate | 24.78 | 40 | 250 | 39.59 |
| High | 20.22 | 27 | 204 | 31.18 |
| Alcohol use | ||||
| Non-drinker | 31.81 | 55 | 321 | 51.12 |
| Drinker | 68.19 | 101 | 688 | 35.45 |
| Education | ||||
| <HS | 6.44 | 13 | 65 | 61.02 |
| HS | 21.9 | 35 | 221 | 43.09 |
| Some college | 27.45 | 44 | 277 | 41.83 |
| BA+ | 44.2 | 64 | 446 | 34.63 |
| Stage | ||||
| 0, I, II | 68.48 | 68 | 691 | 24.58 |
| III | 22.79 | 59 | 230 | 68.96 |
| IV | 8.72 | 29 | 88 | 95.76 |
| Incident/Prevalent | ||||
| Prevalent | 51.44 | 92 | 519 | 31.60 |
| Incident | 48.56 | 64 | 490 | 63.11 |
| Cancer site | ||||
| Breast | 22.5 | 26 | 227 | 20.73 |
| Colon | 6.64 | 15 | 67 | 44.61 |
| Uterine | 21.41 | 17 | 216 | 37.27 |
| Prostate | 4.06 | 5 | 41 | 19.64 |
| Skin | 7.73 | 12 | 78 | 43.61 |
| Other | 37.66 | 81 | 380 | 60.04 |
ANALYTIC STRATEGY
We employ a multi-staged analysis to examine the associations between social relationship quality, cancer mortality, and biomarkers of inflammation. First, we conduct univariate and bivariate descriptive analyses, which both describe our sample characteristics and offer preliminary evidence of the associations between the covariates, inflammation, and mortality. We then assess the associations between the two measures of social relationship quality and mortality using Cox proportional hazard models for time-to-event analyses. We examine the associations between the two measures of social relationship quality and mortality separately and jointly, fitting the models in a step-wise fashion by adjusting first for age, sex, and race and then the full set of covariates. Next, to assess of the associations between social relationship quality variables and each of the four inflammatory markers, we estimate step-wise multivariate regression models first adjusting for age, sex, race and then the full set of covariates. To probe whether inflammatory processes may mediate the association between social relationship quality and cancer mortality, we first utilize Cox proportional hazard models to assess the associations of the four inflammatory markers and mortality individually and then jointly. Finally, we use Cox proportional hazard models to estimate the associations of social relationship quality and mortality, adjusting for each marker of inflammation individually and then jointly.
RESULTS
Descriptive statistics for the sample are presented in Table 1. Figure 1 presents the distribution of the biomarkers of inflammation by sex. In Figure 2, we present the bivariate associations between our measures of social support and mortality using Kaplan-Meier survival curves. In both Table 1 and Figure 2, we document a gradient in the association between satisfaction with social support and mortality risk, with individuals reporting poor satisfaction with their social support having higher death rates than individuals reporting higher levels of satisfaction with their social support (p<0.001). As reported in Figure 2, we do not find statistical evidence of a social gradient in mortality risk by level of social support either measured continuously or dichotomously. The subsequent analyses used the dichotomized level of support only because it yielded a better model fit.
Figure 1. Biomarker Descriptive Statistics by Sex.
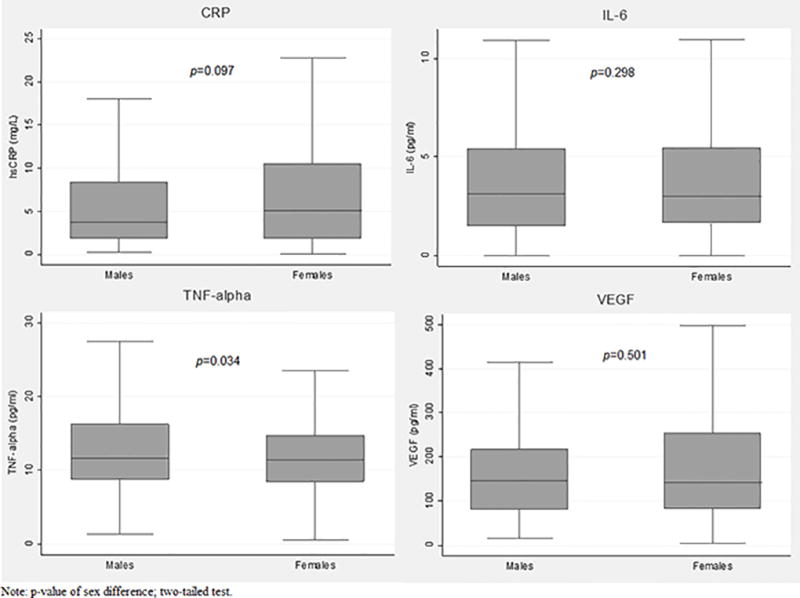
Figure 1 contains an image of four box plots displaying the distributions of the biomarkers of inflammation, stratified by sex. The upper left quadrant of the figure presents box plots for the distribution of C-reactive protein (CRP) for men and women. The upper right quadrant of the figure presents box plots for the distribution of interleukin-6 (IL-6) for men and women. The bottom left quadrant of the figure presents box plots for the distribution of tumor necrosis factor alpha (TNF-alpha) for men and women. The bottom right quadrant of the figure presents box plots for the distribution of vascular endothelial growth factor (VEGF) for men and women.
Figure 2. Kaplan-Meier Survival Estimates of Associations of Social Support with Survival.
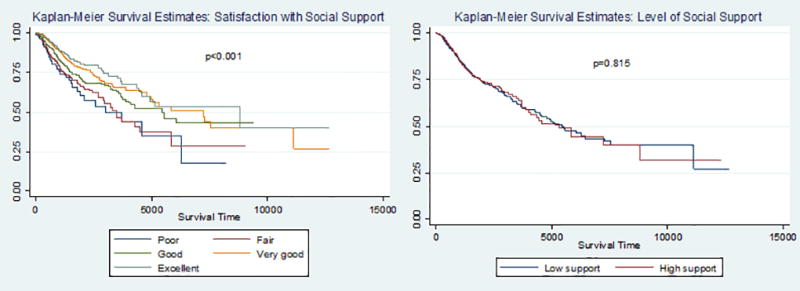
Figure 2 contains an image of two Kaplan-Meier survival estimates of the associations of the two measures of social support with survival. The left side of the figure presents the Kaplan-Meier survival estimates by level of satisfaction with support. The right side of the figure presents the Kaplan-Meier survival estimates by level of social support.
Results of the Cox proportional hazards models examining the relationships between the measures of social support and mortality are presented in Table 2. Model 1 is the basic adjusted model that controls for age, sex, and race, and Model 2 is the fully adjusted model that includes the full set of covariates. Adjusting for time since diagnosis did not show that it has impact beyond the incidence/prevalence status. While we again find no associations between level of social support and mortality risk, we find that satisfaction with social support is significantly associated with mortality risk, such that increases in satisfaction with social support are associated with decreased mortality risk in the basic adjusted model (HR=0.808, 95% CI: 0.700–0.931, p=0.003). In the fully adjusted model, this association was attenuated with the inclusion of the measure of physical activity, in particular, suggesting that physical activity may serve as a mechanism linking satisfaction with social support and mortality. Consistent with these results, supplementary models that jointly adjusted for satisfaction with support and level of support and also adjusted for age, sex, and race indicated that only satisfaction with support had significant associations with mortality risk.
Table 2.
Associations of Social Support and Mortality Risk
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
|
|
|
|||
| Social support measure | ||||
| Satisfaction with social support | 0.808 | 0.003 | 0.892 | 0.157 |
| (0.700 – 0.931) | (0.762 – 1.045) | |||
| N=1,004
|
||||
| Level of social support (1=high) | 0.928 | 0.668 | 0.966 | 0.844 |
| (0.661 – 1.304) | (0.683 – 1.366) | |||
| N=970 | ||||
Notes: Results of Cox proportional hazards models. Hazard ratios with 95% confidence intervals and corresponding p-values are presented. Model 1 adjusts for age, sex, and race. Model 2 adjusts for age, sex, race, marital status, education, smoking, physical activity, alcohol use, cancer stage, and incident/prevalent case status.
Table 3 includes results of the multivariate regression models of the associations between the measures of social support and the biomarkers of inflammation. For each biomarker, models with the subscript “a” are the basic adjusted models that include controls for age, sex, and race, and models with the subscript “b” are the fully adjusted models that include the full set of covariates. Consistent with the mortality analyses presented in Table 2, we find that satisfaction with social support is more strongly associated with the biomarkers of inflammation than level of social support. In Models 1a and 2a, we find that increases in satisfaction with support are associated with lower levels of log CRP (−0.044, p=0.090) and IL-6 (−0.090, p=0.011), respectively. We also find a relationship between satisfaction with support and TNF-α that persists across both the basic (−0.043, p=0.004) and fully adjusted (−0.036, p=0.025) model, where those reporting poor satisfaction with support also have higher levels of inflammation than individuals reporting higher levels of satisfaction with social support.
Table 3.
Associations of Social Support and Biomarkers of Inflammation
| Log CRP | Log IL-6 | Log TNF-α | Log VEGF | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 1b | Model 2a | Model 2b | Model 3a | Model 3b | Model 4a | Model 4b | |||||||||
|
|
||||||||||||||||
| Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
Coeff. (SE) |
p- value |
|
|
|
||||||||||||||||
| Social support | ||||||||||||||||
| Satisfaction with social support | −0.044 | 0.090 | −0.002 | 0.950 | −0.090 | 0.011 | −0.032 | 0.391 | −0.043 | 0.004 | −0.036 | 0.025 | −0.037 | 0.158 | −0.038 | 0.174 |
| (0.026) | (0.027) | (0.035) | (0.038) | (0.015) | (0.016) | (0.026) | (0.028) | |||||||||
| N=906 | N=847 | N=999 | N=926 | |||||||||||||
|
|
||||||||||||||||
| Level of social support (1=high) | −0.039 | 0.481 | −0.016 | 0.766 | −0.099 | 0.199 | −0.068 | 0.368 | −0.083 | 0.012 | −0.076 | 0.022 | 0.046 | 0.417 | 0.029 | 0.612 |
| (0.055) | (0.055) | (0.077) | (0.076) | (0.033) | (0.033) | (0.057) | (0.057) | |||||||||
| N=872 | N=823 | N=965 | N=894 | |||||||||||||
Notes: Results of OLS regression models. Coefficient estimates, standard errors, and corresponding p-values are presented. Models with the subscript “a” adjust for age, sex, and race. Models with the subscript “b” adjust for age, sex, race, marital status, education, smoking, physical activity, alcohol use, cancer stage, and incident/prevalent case status.
We find no statistically significant associations between level of social support and CRP, VEGF, or IL-6. However, as reported in Table 3, we find that individuals reporting high levels of support have lower levels of TNF-α in both the basic (−0.083, p=0.012) and fully adjusted (−0.076, p=0.022) models.
Results of the Cox proportional hazard models estimating the associations between the inflammatory markers and mortality indicated that all four individual inflammatory markers were positively associated with mortality, with individuals having higher levels of inflammation being at increased risk of mortality (see Supplementary Table S2 for model results). In particular, the markers of CRP and IL-6 had particularly strong associations with mortality in both the independent and joint models. Given that CRP and IL-6 were strongly associated with both satisfaction with social support and mortality risk, these results suggest that these two inflammatory makers could play a role in linking satisfaction with support and cancer mortality risk.
Finally, Table 4 includes results from the Cox proportional hazards models that adjust for the markers of inflammation both individually and jointly, with basic adjustments for age, sex, and race. These models assess whether inflammatory processes may mediate the associations between social support and mortality. Given that results from the previous stages of analysis indicated a particularly strong association between satisfaction with social support and mortality risk, the findings in Table 4 focus solely on the associations between satisfaction with social support, inflammation, and mortality risk. Consistent with the results in Table 2, results from Models 1–6 of Table 4 indicate a protective benefit of increasing satisfaction with social support on mortality risk. Across Models 2, 3, and 4, we also document positive associations between log CRP (HR=1.878, 95% CI: 1.475 – 2.391, p<0.001), log IL-6 (HR=1.342, 95% CI: 1.163 – 1.548, p<0.001), log TNF-α (HR=1.532, 95% CI: 1.016 – 2.310, p=0.042), and VEGF (HR=1.286, 95% CI: 1.013–1.633, p=0.039) and mortality, respectively, with higher levels of inflammation putting individuals at an increased mortality risk. Further, including the measures of log CRP, log IL-6, and TNF- α in Models 2–4 reduced the statistical significance of the association between satisfaction with social support and mortality risk in the respective models. When all four measures of inflammation are jointly included in Model 6, the measure of satisfaction with social support is only marginally associated with mortality risk, while log CRP and log IL-6 maintain associations with mortality. Together, the results presented in Supplementary Table S2 and Table 4 offer preliminary evidence that inflammatory processes may mediate the association between satisfaction with social support and mortality among individuals with cancer.
Table 4.
Associations of Satisfaction with Social Support and Biomarkers of Inflammation and Mortality Risk
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
|
|
|
||||||||||||
| Satisfaction w/social support | 0.792 | 0.005 | 0.849 | 0.053 | 0.836 | 0.035 | 0.806 | 0.010 | 0.788 | 0.004 | 0.859 | 0.076 |
| (0.673 – 0.931) | (0.720 – 1.002) | (0.708 – 0.987) | (0.684 – 0.949) | (0.669 – 0.928) | (0.727 – 1.106) | |||||||
| Log CRP | 1.878 | <0.001 | 1.642 | <0.001 | ||||||||
| (1.475 – 2.391) | (1.257 – 1.143) | |||||||||||
| Log IL-6 | 1.342 | <0.001 | 1.182 | 0.078 | ||||||||
| (1.163 – 1.548) | (0.981 – 1.424) | |||||||||||
| Log TNF-α | 1.532 | 0.042 | 1.075 | 0.740 | ||||||||
| (1.016 – 2.310) | (0.701 – 1.650) | |||||||||||
| Log VEGF | 1.286 | 0.039 | 1.139 | 0.296 | ||||||||
| (1.013 – 1.633) | (0.892 – 1.456) | |||||||||||
Notes: Hazard ratios with 95% Cis and corresponding p-values. Models adjust for age, sex, and race.
DISCUSSION
Social stressors such as social relationship deficits have been increasingly linked to cancer outcomes (17, 18, 34). However, critical gaps exist in our understanding of the nature and strength of such links, as well as the underlying biological mechanisms relating social relationships to cancer progression and survival. This study provides new insights into the biosocial linkages undergirding the associations between social relationships and one key cancer outcome in a large sample of cancer patients.
Findings from this study offer two key contributions to the literature. First, we find that satisfaction with support, more than overall level of social support, is particularly relevant to cancer survival. As indicated in Tables 1–4, we document that cancer patients reporting greater levels of satisfaction with their social support report have lower risk of mortality compared to cancer patients with lower levels of satisfaction with their support. By contrast, we found no associations between overall level of social support and mortality risk. As discussed, supplementary analyses utilizing alternative operationalizations of the measure of level of social support, including a continuous measure and dichotomous measures employing different cutpoints, provided similar findings, such that cancer patients’ perception of their overall level of social support did not affect their mortality risk. In this way, findings from this study suggest that one’s subjective appraisal of their social relationship support is more important to cancer prognosis and survival than the actual level of social support received. Population-based studies have documented a strong and consistent association between levels of social support and a host of outcomes (8, 10, 43, 44), yet our study did not find evidence of this association. These findings may have theoretical and methodological underpinnings. Our analytic sample is comprised of individuals who have received a cancer diagnosis, who may require a particular type of support, different from the general population. Receiving a cancer diagnosis and undergoing cancer treatment may invoke a particular set of life stressors for individuals, placing new and different demands and responsibilities on spouses, partners, friends, and family that are different from the everyday stressors and strains of the overall population (45). We conducted supplementary analyses that tested whether those with incident and prevalent status received differential health protections from their satisfaction with their social support. Though we did not find statistical evidence of an interaction, stratified models suggested that subjective appraisal of social support may be more protective for individuals with recent diagnoses—i.e., the incident cases. In this way then, the quality of social relationships and the cancer patients’ subjective appraisal of these sources of support may become particularly important for their psychosocial functioning and survival as they handle the stress associated with diagnosis. While more research is needed, findings from this study highlight the essential nature of subjective appraisal of social ties for promoting positive outcomes for individuals with cancer.
Second, this study also offers preliminary evidence that the protective effect of satisfaction with social support on mortality risk may be related to its ability to reduce inflammatory responses in cancer patients. While most prior population research on inflammation is restricted to markers of systemic inflammation such as CRP, this study used multiple markers involved in angiogenesis and tumor growth, including IL-6, TNF-α, VEGF, as well as CRP. We documented consistent associations between satisfaction with social support and biomarkers of inflammation, as presented in Table 3, such that individuals reporting higher levels of satisfaction with their social relationships also had lower levels of CRP, TNF-α, and IL-6. Our results were also suggestive that inflammatory processes may mediate the association between social relationships and mortality. We found that the markers of inflammation were positively associated with mortality risk. As we further reported in Table 4, including the markers of inflammation in models assessing the association between satisfaction with social support and mortality risk reduced the statistical significance of the associations between satisfaction with support and mortality. Formal mediation tests using a bias-corrected bootstrapping method (6, 46, 47) were also suggestive that CRP and IL-6, in particular, accounted for a considerable portion of the association between satisfaction with support and mortality risk, though our formal mediation analyses were limited by relatively low statistical power. Research documents that inflammation is a critical determinant of all stages of carcinogenesis (22) and cancer prognosis (23, 24). Studies further indicate that exposure to social stressors upregulate inflammatory response (10, 21, 25) and that social relationships can help to buffer against the health-harming physiological consequences of stress exposure, including inflammation (48). Our study breaks new ground by combining these disparate bodies of research to offer preliminary evidence that inflammatory processes may help to explain the documented links between social relationships and cancer survival. By altering inflammatory gene expression, promoting inflammation in the tumor microenvironment, and impairing immune functions (29–31, 49), exposure to social relationship stress and social isolation may have particularly deleterious consequences for individuals with and at greatest risk for cancer. Conversely, receiving or perceiving high quality social support might improve outcomes for cancer survivors by decreasing inflammation.
This study is not without limitations. Data for this study came from a hospital-based registry data of cancer patients in North Carolina, which may limit our ability to make generalizations to cancer patients in other geographic locations. Further, our sample consists of a slightly higher proportion of Whites as compared to the total U.S. population and also has higher levels of educational attainment (50). To the extent that our sample consisted of relatively high proportions of Whites and highly educated individuals, our results may be conservative given positive associations between high SES and social support, in particular (47). Continued research with diverse samples will improve understanding of population disparities in social relationships, inflammation, and cancer outcomes. Additionally, while we offer preliminary evidence that inflammatory processes may undergird the association between social relationship satisfaction and mortality, more research with larger samples is needed to provide more robust tests for mediation. Further, the lack of measures on the structural dimension of social relationships, such as the number of social ties, restricts our ability to compare and contrast the relative importance of quantity and quality of relationships in affecting cancer outcomes. The lack of patient-reported prospective, longitudinal data on respondents also limits our ability to make causal inferences. Finally, because we cannot fully account for comorbidities at the time of blood draw, confounding of inflammation levels and comorbidity is a possibility. While our study breaks new ground in linking social relationships, inflammation, and cancer mortality, we urge future research to build on our findings to further probe these associations.
Given that cancer is increasingly a leading cause of death in the United States, understanding the social and biological mechanisms that shape cancer outcomes is of pressing scientific and population health concern. Findings from this study offer new evidence of the essential nature of social relationships in promoting health and survival among individuals with cancer, which has both clinical and therapeutic implications. Our findings suggest that the development and maintenance of social relationships is of critical health importance for individuals with cancer, particularly as they confront new and intense demands associated with diagnosis and treatment. Additionally, by improving knowledge of the inflammatory mechanisms relating specific social relationships to survival, this study offers new insights of how and why the presence of positive, supportive social ties may be particularly essential for individuals with cancer, especially those with cancer types affected by immune disorders. Together, findings from this study suggest that the nurturing of social relationships should be a vital element of cancer prevention, treatment, and control efforts.
Supplementary Material
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Berkman LF, Vaccarino V, Seeman T. Gender differences in cardiovascular morbidity and mortality: The contribution of social networks and support. Annals of Behavioral Medicine. 1993 [Google Scholar]
- 2.Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002 Apr 15;155(8):700–9. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- 3.George LK, Blazer DG, Hughes DC, Fowler N. Social support and the outcome of major depression. Br J Psychiatry. 1989 Apr;154:478–85. doi: 10.1192/bjp.154.4.478. [DOI] [PubMed] [Google Scholar]
- 4.Heikkinen R, Kauppinen M. Depressive symptoms in late life: a 10-year follow-up. Arch Gerontol Geriatr. 2004;38(3):239–50. doi: 10.1016/j.archger.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24(3):297. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- 6.Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav. 2013;54(2):182–202. doi: 10.1177/0022146513485244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YC, Schorpp K, Harris KM. Social support, social strain and inflammation: Evidence from a national longitudinal study of US adults. Soc Sci Med. 2014;107:124–35. doi: 10.1016/j.socscimed.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YC, Boen C, Mullan Harris K. Social relationships and hypertension in late life: evidence from a nationally representative longitudinal study of older adults. J Aging Health. 2015 Apr;27(3):403–31. doi: 10.1177/0898264314551172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):578–83. doi: 10.1073/pnas.1511085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 12.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51(6):843–57. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–4. [PubMed] [Google Scholar]
- 14.Yang Y, Li T, Ji Y. Impact of social integration on metabolic functions: evidence from a nationally representative longitudinal study of US older adults. BMC Public Health. 2013;13(1):1210. doi: 10.1186/1471-2458-13-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penwell LM, Larkin KT. Social support and risk for cardiovascular disease and cancer: A qualitative review examining the role of inflammatory processes. Health Psychology Review. 2010;4(1):42–55. [Google Scholar]
- 16.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression throughout the life span. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(Special Issue 1):32–41. doi: 10.1093/geronb/60.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat. 2013;137(1):261–71. doi: 10.1007/s10549-012-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda A, Kawachi I, Iso H, Iwasaki M, Inoue M, Tsugane S. Social support and cancer incidence and mortality: the JPHC study cohort II. Cancer Causes & Control. 2013;24(5):847–60. doi: 10.1007/s10552-013-0147-7. [DOI] [PubMed] [Google Scholar]
- 19.Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009–18. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol. 2010;75(2):122–37. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YC, Li T, Frenk SM. Social network ties and inflammation in US adults with cancer. Biodemography and social biology. 2014;60(1):21–37. doi: 10.1080/19485565.2014.899452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028(1):1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 24.Marx J. Inflammation and cancer: the link grows stronger: research into a long-suspected association between chronic inflammation and cancer reveals how the immune system may be abetting tumors. Science. 2004;306(5698):966–9. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among US adults. Ann Epidemiol. 2006;16(2):78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005 Dec 20;102(51):18757–62. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loucks EB, Berkman LF, Gruenewald TL, Seeman TE. Relation of social integration to inflammatory marker concentrations in men and women 70 to 79 years. Am J Cardiol. 2006;97(7):1010–6. doi: 10.1016/j.amjcard.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 29.De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature reviews. Cancer. 2006;6(1):24. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 30.Jin Shin K, Jin Lee Y, Ryoul Yang Y, Park S, Suh P, Yung Follo M, et al. Molecular mechanisms underlying psychological stress and cancer. Curr Pharm Des. 2016;22(16):2389–402. doi: 10.2174/1381612822666160226144025. [DOI] [PubMed] [Google Scholar]
- 31.Tilan J, Kitlinska J. Sympathetic Neurotransmitters and Tumor Angiogenesis-Link between Stress and Cancer Progression. J Oncol. 2010;2010:539706. doi: 10.1155/2010/539706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finch CE. The Biology of Human Longevity:: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic Press. 2010 [Google Scholar]
- 33.Kiecolt-Glaser JK, Robles T, Heffner K, Loving T, Glaser R. Psycho-oncology and cancer: psychoneuroimmunology and cancer. Annals of Oncology. 2002;13(suppl_4):165–9. doi: 10.1093/annonc/mdf655. [DOI] [PubMed] [Google Scholar]
- 34.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. The lancet oncology. 2004;5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 35.Kiecolt-Glaser JK, Gouin J, Hantsoo L. Close relationships, inflammation, and health. Neuroscience & Biobehavioral Reviews. 2010;35(1):33–8. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarwari A, Fredman L, Langenberg P, Magaziner J. Prospective study on the relation between living arrangement and change in functional health status of elderly women. Am J Epidemiol. 1998;147(4):370–8. doi: 10.1093/oxfordjournals.aje.a009459. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Goldman N. Mortality differentials by marital status: an international comparison. Demography. 1990;27(2):233–50. [PubMed] [Google Scholar]
- 38.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 39.Glossary: Definitions and Phonetic Pronunciations [Internet] 2017 Available from: https://www.cancer.org/cancer/glossary.html#alpha-s.
- 40.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research. 2009;18(7):873–80. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cella D. FACT-GP (Version 4) 1997 Copyright 1987. [Google Scholar]
- 42.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993 Mar;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310. [PubMed] [Google Scholar]
- 44.Uchino BN, Cacioppo JT, Kiecolt-Glaser J. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119(3):488. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 45.Visser A, Huizinga GA, van der Graaf, Winette TA, Hoekstra HJ, Hoekstra-Weebers JE. The impact of parental cancer on children and the family: a review of the literature. Cancer Treat Rev. 2004;30(8):683–94. doi: 10.1016/j.ctrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Judd CM, Kenny DA. Process analysis: Estimating mediation in treatment evaluations. Eval Rev. 1981;5(5):602–19. [Google Scholar]
- 47.Stringhini S, Berkman L, Dugravot A, Ferrie JE, Marmot M, Kivimaki M. Socioeconomic status, structural and functional measures of social support, and mortality the British whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–83. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16(7):729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finch CE. The Biology of Human Longevity:: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic Press. 2010 [Google Scholar]
- 50.QuickFacts: Population estimates, July 1, 2016 [Internet] 2016 Available from: https://www.census.gov/quickfacts/fact/table/US/PST045216.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


