Abstract
Background
Estrogen metabolism in premenopausal women may be related to early life body fatness.
Methods
Premenopausal women participating in the Nurses’ Health Study II recalled their body fatness at ages 5, 10, and 20 years using a validated 9-level pictogram. Fifteen estrogens and estrogen metabolites (EM) were measured using liquid chromatography-tandem mass spectrometry in luteal phase urines from 603 women aged 32-54 years. Geometric means of individual EM, metabolic pathway groups, and pathway ratios were examined by body fatness categories using linear mixed models.
Results
Body fatness at each age was inversely associated with adult concentrations of all EM combined, parent estrogens (estrone, estradiol), and the 2-hydroxylation pathway. Women in the top (vs. bottom) category of body fatness at age 10 had 21% lower levels of all EM (P-trend=0.003), 24% lower parent estrogens (P-trend=0.002), and 36% lower 2-pathway (P-trend=0.0003). Body fatness at age 10 was inversely associated with 2-catechols (35% lower, P-trend=0.0004) and 2-methylated catechols (30% lower, P-trend=0.002). After adjusting for premenopausal body mass index (BMI), these associations remained inverse but were attenuated; only parent estrogens remained statistically significant (21% lower, P-trend=0.01). Body fatness at ages 5 and 20 were similarly, but more weakly, associated with estrogen pathways.
Conclusions
Estimates of body fatness during early life were inversely associated with premenopausal levels of all EM combined, parent estrogens, and 2-pathway estrogen metabolites. These relationships were not fully explained by adult BMI.
Impact
These findings inform investigations of diseases linked to early life body fatness and estrogen metabolism.
Keywords: estrogens, body size, early life, premenopause
Introduction
Both body fatness and endogenous estrogen have been implicated in the etiology of breast cancer 1-4. However, the relationship between body fatness and endogenous estrogen exposure is complex in that it differs over the life course. Heavier postmenopausal women have higher concentrations of circulating estrogens, specifically estrone sulfate, estrone, and estradiol, than lighter women5-7; heavier premenopausal women have lower circulating estrogen concentrations than lighter women 8,9; and heavier premenarcheal girls have circulating estrogen concentrations similar to those of lighter girls 10. The dynamic relationship between body fatness and estrogen levels across the life course may be due to differences in the sources of estrogen production. Throughout life, estrogens are produced peripherally by the conversion of androgens in adipose tissue 11. However, during a woman’s reproductive years, estrogen is primarily produced centrally along the hypothalamic-pituitary-ovarian (HPO) axis, with 90% of estrogen coming from the ovaries 11. Because childhood and adolescence include the years when the HPO axis is developing, body fatness during this critical window may have a persistent impact on estrogen production and influence adult estrogen levels. On the other hand, premenopausal mid-luteal estrogens are associated inversely with breast cancer risk, suggesting that estrogen metabolism is also important in breast cancer etiology.1
The parent estrogens, estradiol and estrone, are irreversibly metabolized by hydroxylation at the 2-, 4- or 16-positions of the steroid ring 12. 2- and 4-pathway catechol estrogens (2-hydroxyestrone, 2-hydroxyestradiol, and 4-hydroxyestrone) can be further metabolized into methylated catechol estrogens (2-methoxyestradiol, 2-methoxyestrone, 2-hydroxyestrone-3-methyl ether, 4-methoxy estrone, and 4-methoxyestradiol). In the 16-pathway, 16α-hydroxyestrone is metabolized primarily to estriol, as well as 17-epiestriol, 16-epiestriol, and 16-ketoestradiol. In a case-control study of premenopausal breast cancer nested within the Nurses’ Health Study II cohort, we measured these two estrogens and 13 estrogen metabolites (all 15 referred to as EM) in premenopausal luteal phase urines and found that estrogen metabolism differs among individuals at different risk for breast cancer 1. Urinary levels of estrone and estradiol were strongly associated with reduced premenopausal breast cancer risk; urinary levels of 2-pathway and 4-pathway metabolites were moderately associated with reduced risk; urinary levels of 16-pathway metabolites were not related to risk. Subsequently, in a series of cross-sectional studies including only the controls from the case-control study, we explored the relationships in premenopausal women of individual EM and EM profiles, measured in luteal phase urines, to established and proposed breast cancer risk factors, including reproductive and menstrual factors 13, smoking 14, physical activity 15, caffeine 16, alcohol 17, dietary fat and fiber 18, analgesic use 19, mammographic density20 and adult BMI and height 21. Adult premenopausal BMI, assessed close to the time of urine collection, was strongly, statistically significantly and inversely associated with all EM combined and the 2-hydroxylation pathway 21. To explore how early life body fatness is related to adult estrogen production and metabolism, we now investigate whether body fatness during childhood and adolescence is associated with premenopausal urinary EM levels, and if these associations are independent of adult BMI.
Materials and Methods
Study Population
The Nurses’ Health Study II (NHSII) is a prospective cohort study that tracks health outcomes in 116,430 registered nurses who completed comprehensive health and lifestyle questionnaires biannually beginning in 1989. Between 1996 and 1999, 29,611 participants between the ages of 32 and 54 years who were cancer-free provided blood and urine samples, and 18,521 of those were premenopausal women who had not used oral contraceptives, been pregnant or breast-fed during the six months before sample collection. The current analysis includes 603 women who were either selected as controls in a nested breast cancer case-control study (n=493) 1 or included in a biomarker reproducibility study (n=110) 22. Informed consent was implied by receipt of completed questionnaires and urine samples. The study was approved by the Committee on the Use of Human Subjects in Research at Harvard School of Public Health and Brigham and Women’s Hospital and was conducted in accordance with the Belmont Report.
Early Life Somatotype and Covariate Assessment
In 1989, our study participants, aged 25-43 years, characterized their body fatness at ages 5, 10, and 20 using a 9-level pictogram 23, which had been validated among older women as a reasonably accurate assessment of body fatness at young ages 24. We created ordinal variables for increasing body fatness and grouped the five larger categories into one group because of small numbers in each category; these scores are presented as 1, 2, 3, 4, and 5+.
Data on potential covariates were collected from the biennial study questionnaires as well as a supplemental questionnaire administered at the time of urine collection. Age at menarche, weight at age 18, and attained height were reported on the 1989 questionnaire. Reproductive history and oral contraceptive use were also collected on the 1989 questionnaire and updated on subsequent biennial questionnaires. Information on menstrual cycle regularity and length at present and during adolescence was obtained in 1993. Physical activity was collected on both the 1997 and 2001 questionnaires, and we calculated the average of these two time periods. We also calculated the average of the alcohol and caffeine intakes reported in the 1995 and 1999 questionnaires. Participants recorded their current weight, smoking status, and details of the urine collection, such as date and time and whether it was a first morning void, using the supplemental questionnaire. BMI at the time of urine collection was calculated as weight in kilograms divided by attained height (from 1989 questionnaire) in meters squared.
Participants confirmed the start date of the menstrual period immediately following specimen collection by mailing in postcards. Luteal day was determined using backward dating from confirmed date of next menstrual cycle. We considered women with plasma luteal progesterone levels ≥400 ng/dL to have donated samples in an ovulatory cycle, while participants with levels below this cutoff point were defined as donating in an anovulatory cycle.
Measurement of Urinary Estrogens and Estrogen Metabolites
Participants collected first morning urine specimens without preservative during the luteal phase of their menstrual cycle. Urine samples were shipped on ice via overnight courier to the biorepository and stored in aliquots in liquid nitrogen freezers until time of assay. The Laboratory of Proteomics and Analytical Technologies, Frederick National Laboratory for Cancer Research (Frederick, MD) received 0.5 ml of frozen urine for the measurement of 15 EM.
The details of the LC-MS/MS method used to measure the 15 EM have been previously described 25. Glucuronide and sulfate conjugates of each EM were hydrolyzed enzymatically and measured together with any unconjugated EM. The precision of this method was determined by the inclusion of masked replicate quality control samples in each batch. The overall laboratory coefficients of variation (CVs), which included both within- and between-batch variability, were less than 7% for each EM, except for 4-methoxyestrone (CV=17%) and 4-methoxyestradiol (CV=15%), which were present in urine at the lowest concentrations of all EM1. The lower limit of quantitation for each EM was approximately 150 fmol/mL urine.
Urinary creatinine was measured in three batches: the first at the Endocrine Core Laboratory at Emory University (Atlanta, GA), the second at the laboratory of Dr. Vincent Ricchiuti at Brigham and Women’s Hospital (Boston, MA), and the third at the laboratory of Dr. Nader Rifai at Boston Children’s Hospital (Boston, MA). Overall CVs in each lab were less than 9.2%.
Plasma progesterone was assayed by chemiluminescence immunoassay using the Immulite Auto-Analyzer (Diagnostic Products Corp.). Overall CVs were ≤17%, while within-batch CVs were ≤4%.
Statistical Analyses
EM were analyzed individually, in groups representing metabolic pathways, and as ratios of metabolic pathways (all listed in Table 2). We standardized urinary concentrations for individual EM and metabolic pathway groups (pmol/mL) by urinary creatinine concentrations (mg/mL) to account for urine volume and dilution. We log-transformed individual EM, metabolic pathway groups, and pathway ratios. Statistical outliers for each measure were identified using the generalized extreme Studentized deviate many-outlier approach26 and removed from analyses. The number of outliers ranged from 0 to 9 for all individual EM except 2-methoxyestradiol (16 outliers).
In primary analyses, we used linear mixed models to calculate adjusted geometric means of individual EM, metabolic pathway groups, and pathway ratios by category of body fatness at ages 5, 10, and 20. Test for trend analyses were conducted by modeling the five body fatness scores as a continuous exposure variable and calculating the Wald statistic. Multivariable models were adjusted for age at urine collection (continuous), urine collection during ovulatory cycle (yes/no), luteal phase at urine collection (≤5, 6-7, 8-9, ≥10 days to next period), first morning urine (yes/no), combined age at first birth/parity (nulliparous, age at first birth<25 years/1–2 children, age at first birth 25-29 years/1–2 children, age at first birth ≥30 years/1–2 children, age at first birth <25 years/≥3 children, age at first birth≥25 years/≥3 children), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, 27–41.9, ≥42 MET-hr/week), alcohol intake (nondrinker, <1.49, 1.50-4.85, >4.85 g/day), quartiles of caffeine intake (<70, 70-186, 187-359, >359 mg/day), current smoker (yes/no), current menstrual cycle length (<26, 26-31, ≥32 days), and current menstrual cycle regularity (extremely regular, very regular, regular, usually/always irregular). The main analyses were also modeled including BMI at the time of urine collection as a continuous variable and the covariates listed above. We also considered other potential confounders, including age at menarche, menstrual cycle length and regularity during high school, menstrual cycle regularity at ages 18-22, time until cycles became regular, and previous use of oral contraceptives. However, adjustment for these variables did not appreciably change our results and they were not retained in final models. For example, the absolute % change in the geometric means for each individual EMs when we adjusted body size at age 10 by age at menarche was consistently <1%. Finally, models based on total pmoles of individual EM and metabolic pathway groups, without standardization for urinary creatinine, gave results similar to creatinine-adjusted models.
Secondary analyses were restricted to women with urine collected in an ovulatory cycle, women who provided the urine sample during luteal days 4–10, and women who had not smoked in the 30 days prior to urine collection.
All P values were two-sided and considered to be statistically significant if ≤0.05. Analyses were conducted with SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Body fatness at ages 5 and 10 were highly correlated with each other [Spearman correlation coefficient (r) = 0.84] but only moderately correlated with body fatness at age 20 (Spearman r = 0.51 and 0.59, respectively). Twelve percent of the women reported that they were light (pictogram ≤2) at all three ages while 25% reported they were heavy (pictogram ≥4) at all three ages. Body fatness at ages 5, 10, and 20 were less correlated with adult premenopausal BMI (at the time of urine collection) than with each other (Spearman r for premenopausal BMI with ages 5, 10, and 20 = 0.28, 0.34, and 0.34, respectively). The 603 urine samples included in this analysis came from a population of mostly Caucasian women (97%) with a median age of 43 y. The majority (86%) of the urine samples were collected in mid-luteal phase, 4 to 10 days before the next menstrual cycle. Women who were heavier during childhood, based on the body fatness they recalled for age 10, were more likely to report an earlier age at menarche, regular periods within one year of menarche, and regular menstrual cycles during high school and ages 18-22 (Table 1). As adults, these women were also heavier and more likely to consume caffeinated beverages and smoke.
Table 1.
Characteristics of the premenopausal study population (n=603) by body fatness at age 101
| Recalled body fatness at 10 years old | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5+ | |
| N | 101 | 191 | 151 | 91 | 63 |
| Caucasian, % | 94 | 97 | 97 | 97 | 98 |
| Age at urine collection,y | 42.7(4.0) | 42.7(4.0) | 42.8(3.8) | 42.7(3.5) | 44.0(3.4) |
| BMI at urine collection, kg/m2 | 23.2 (3.8) | 23.6(3.7) | 25.1(4.9) | 26.9(5.8) | 30.3(6.9) |
| Height, m | 1.66(0.06) | 1.65(0.07) | 1.65(0.07) | 1.65(0.07) | 1.67(0.07) |
| Parous, % | 81 | 84 | 84 | 83 | 73 |
| Age at first birth, y | 26.8(4.2) | 26.6(4.1) | 26.2(4.8) | 26.8(5.0) | 28.1(5.7) |
| Physical activity, MET-hr/wk | 22.0(23.3) | 22.2(21.2) | 20.4(16.8) | 23.0(23.7) | 19.9(17.1) |
| Alcohol intake, g/day | 4.0(6.9) | 4.0(6.5) | 3.2(4.5) | 4.3(5.8) | 4.8(7.7) |
| Caffeine intake, mg/day | 208(180) | 217(194) | 257(226) | 241(174) | 267(205) |
| Current smoker, % | 4 | 4 | 9 | 5 | 8 |
| Current menstrual cycle length of 26-31 days, % | 59 | 65 | 75 | 66 | 65 |
| Current menstrual cycles regular, % | 96 | 96 | 95 | 96 | 95 |
| Age at menarche | 13.2(1.4) | 12.8(1.3) | 12.5(1.5) | 11.9(1.3) | 12.1(1.4) |
| Regular menstrual cycles within 1 year of menarche, % | 36 | 46 | 46 | 51 | 60 |
| Regular menstrual cycles during high school, % | 80 | 90 | 91 | 88 | 95 |
| Regular menstrual cycles during ages 18-22, % | 83 | 91 | 92 | 93 | 94 |
| Sample collected during ovulatory cycle, % | 95 | 91 | 86 | 88 | 85 |
| Sample collected 4-10 days before next period, % | 88 | 84 | 84 | 90 | 83 |
| First morning urine sample, % | 78 | 80 | 85 | 75 | 79 |
| Creatinine, mg/L | 1021(504) | 1217(608) | 1158(564) | 1076(586) | 1093(691) |
Participants recalled their body fatness at age 10 using a 9-level pictogram; from these reports we created ordinal variables for increasing body fatness as 1, 2, 3, 4, and 5+, with 5+ combining the five highest levels.
Values are means (SD) or percentages.
Numbers do not sum to 603 due to missing values for body fatness.
Body fatness at ages 5, 10 and 20 were each inversely associated with all EM combined, grouped parent estrogens, the 2-hydroxylation pathway, 2-catechols, and 2-methylated catechols after adjusting for covariates (Tables 2-4). In general, trends for these metabolic groups were most pronounced at age 10 and weakest at age 5. Geometric means for all EM combined for the heaviest women (pictograms 5+), compared to the leanest women (pictogram 1), were 12% lower (P-trend=0.03) for age 5 years, 21% lower (P-trend=0.003) for age 10 years, and 10% lower (P-trend=0.03) for age 20 years. Geometric means for parent estrogens for the heaviest, compared to leanest, women were 11% lower for age 5 (P-trend=0.02), 24% lower for age 10 (P-trend=0.002), and 24% lower for age 20 (P-trend=0.01). Geometric means for the 2-hydroxylation pathway for the heaviest, compared to leanest, women were 21% lower for age 5 (P-trend=0.01), 36% lower for age 10 (P-trend=0.0003), and 36% lower for age 20 (P-trend=0.004).
Table 3.
Multivariable-adjusted1 geometric means of premenopausal estrogen metabolism measures by body fatness at age 10
| Geometric mean by body fatness pictogram | P-trend1,2 | P-trend2,3 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5+ | |||
| N4 | 101 | 191 | 151 | 91 | 63 | ||
| Individual and grouped estrogens/estrogen metabolites (EM) (pmol/mg creatinine) | |||||||
| All EM combined | 221.2 | 197.9 | 199.8 | 186.7 | 173.7 | 0.003 | 0.10 |
| Parent estrogens | 47.0 | 42.0 | 40.2 | 40.1 | 35.9 | 0.002 | 0.01 |
| Estrone | 31.0 | 27.4 | 26.4 | 27.9 | 22.9 | 0.01 | 0.03 |
| Estradiol | 14.8 | 13.5 | 13.5 | 12.2 | 12.5 | 0.02 | 0.01 |
| Catechols | 76.8 | 65.8 | 63.1 | 61.4 | 50.2 | 0.001 | 0.23 |
| 2-catechols | 66.4 | 57.2 | 53.7 | 52.1 | 41.9 | 0.0004 | 0.18 |
| 2-Hydroxyestrone | 59.1 | 50.4 | 47.3 | 45.7 | 36.6 | 0.0003 | 0.15 |
| 2-Hydroxyestradiol | 6.3 | 6.0 | 5.4 | 5.4 | 4.5 | 0.01 | 0.45 |
| 4-catechols | |||||||
| 4-Hydroxyestrone | 7.1 | 6.3 | 6.0 | 6.1 | 5.5 | 0.10 | 0.28 |
| Methylated catechols | 12.2 | 11.2 | 9.6 | 10.4 | 8.5 | 0.001 | 0.17 |
| Methylated 2-catechols | 11.7 | 10.9 | 9.2 | 9.9 | 8.2 | 0.002 | 0.17 |
| 2-Methoxyestrone | 9.4 | 8.3 | 7.2 | 8.0 | 6.5 | 0.004 | 0.26 |
| 2-Methoxyestradiol | 0.78 | 0.72 | 0.66 | 0.75 | 0.59 | 0.07 | 0.63 |
| 2-Hydroxyestrone-3-methyl ether | 1.4 | 1.3 | 1.2 | 1.2 | 1.1 | 0.045 | 0.55 |
| Methylated 4-catechols | 0.28 | 0.21 | 0.23 | 0.24 | 0.17 | 0.04 | 0.62 |
| 4-Methoxyestrone | 0.17 | 0.14 | 0.15 | 0.15 | 0.11 | 0.052 | 0.99 |
| 4-Methoxyestradiol | 0.06 | 0.05 | 0.05 | 0.06 | 0.04 | 0.22 | 0.90 |
| 2-Hydroxylation Pathway | 79.3 | 69.2 | 63.7 | 62.7 | 51.0 | 0.0003 | 0.13 |
| 4-Hydroxylation Pathway | 8.2 | 7.1 | 7.0 | 6.7 | 6.4 | 0.06 | 0.21 |
| 16-Hydroxylation Pathway | 66.3 | 63.8 | 62.9 | 62.9 | 62.3 | 0.52 | 0.97 |
| 16α-Hydroxyestrone | 11.9 | 11.0 | 11.3 | 9.8 | 9.4 | 0.06 | 0.40 |
| Estriol | 27.7 | 28.4 | 26.9 | 30.2 | 29.9 | 0.49 | 0.65 |
| 17-Epiestriol | 1.4 | 1.7 | 1.5 | 2.0 | 1.4 | 0.62 | 0.39 |
| 16-Ketoestradiol | 14.0 | 13.3 | 13.3 | 13.3 | 11.4 | 0.10 | 0.85 |
| 16-Epiestriol | 5.8 | 5.7 | 5.8 | 5.7 | 5.6 | 0.83 | 0.70 |
| Ratios (pmol/pmol) | |||||||
| 2-Hydroxyestrone/16α-Hydroxyestrone | 4.9 | 4.3 | 4.0 | 4.4 | 4.3 | 0.36 | 0.89 |
| 4-Pathway/2-Pathway | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 | 0.14 | 0.90 |
| 2-Pathway/16-Pathway | 1.19 | 1.04 | 0.94 | 0.96 | 0.86 | 0.01 | 0.25 |
| 2,4-Pathway/16-Pathway | 1.3 | 1.2 | 1.1 | 1.1 | 1.0 | 0.03 | 0.32 |
| 2-Catechols/Methylated 2-catechols | 5.6 | 5.1 | 5.5 | 5.0 | 4.9 | 0.18 | 0.51 |
| 4-Catechols/Methylated 4-catechols | 26.0 | 29.3 | 26.2 | 24.8 | 34.4 | 0.63 | 0.69 |
| Catechols/Methylated catechols | 6.3 | 5.7 | 6.2 | 5.7 | 5.7 | 0.40 | 0.67 |
| 2-Pathway/Parent estrogens | 1.7 | 1.6 | 1.5 | 1.5 | 1.4 | 0.01 | 0.96 |
| 4-Pathway/Parent estrogens | 0.16 | 0.16 | 0.17 | 0.15 | 0.17 | 0.99 | 0.56 |
| 16-Pathway/Parent estrogens | 1.4 | 1.5 | 1.6 | 1.6 | 1.5 | 0.34 | 0.17 |
| Parent estrogens/2-, 4-, 16-Pathways | 0.28 | 0.28 | 0.27 | 0.28 | 0.29 | 0.75 | 0.14 |
Adjusted for age at urine collection, ovulatory cycle, luteal phase of sample, first morning urine sample, age at first birth/parity, physical activity, alcohol intake, caffeine intake, current smoker, current menstrual cycle length, and current menstrual cycle regularity.
Tests for trend were conducted by modeling the five body fatness scores as a continuous exposure and calculating the Wald statistic. Statistically significant P for trend are shown in bold.
Additionally adjusted for BMI (continuous) at urine collection
Six participants did not report body fatness at age 10.
Table 2.
Multivariable-adjusted1 geometric means of premenopausal estrogen metabolism measures by body fatness at age 5
| Geometric mean by body fatness pictogram | P-trend1,2 | P-trend2,3 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5+ | |||
| N4 | 117 | 196 | 164 | 73 | 43 | ||
| Individual and grouped estrogens/estrogen metabolites (EM) (pmol/mg creatinine) | |||||||
| All EM combined | 223.7 | 189.2 | 194.6 | 180.0 | 197.7 | 0.03 | 0.30 |
| Parent estrogens | 49.8 | 39.1 | 39.8 | 37.3 | 44.1 | 0.02 | 0.06 |
| Estrone | 33.0 | 25.2 | 26.7 | 24.9 | 29.2 | 0.06 | 0.15 |
| Estradiol | 15.4 | 12.7 | 13.4 | 11.7 | 13.7 | 0.03 | 0.01 |
| Catechols | 78.8 | 60.7 | 63.2 | 54.4 | 62.9 | 0.01 | 0.50 |
| 2-catechols | 67.9 | 52.3 | 53.5 | 46.3 | 52.5 | 0.01 | 0.44 |
| 2-Hydroxyestrone | 60.5 | 46.1 | 47.0 | 40.6 | 46.2 | 0.01 | 0.39 |
| 2-Hydroxyestradiol | 6.4 | 5.5 | 5.3 | 5.1 | 5.6 | 0.11 | 0.89 |
| 4-catechols | |||||||
| 4-Hydroxyestrone | 7.6 | 5.9 | 6.2 | 5.4 | 6.8 | 0.18 | 0.34 |
| Methylated catechols | 12.6 | 10.5 | 9.9 | 9.1 | 11.1 | 0.02 | 0.45 |
| Methylated 2-catechols | 12.2 | 10.1 | 9.5 | 8.7 | 10.9 | 0.03 | 0.45 |
| 2-Methoxyestrone | 9.7 | 7.7 | 7.4 | 7.0 | 8.7 | 0.05 | 0.61 |
| 2-Methoxyestradiol | 0.82 | 0.66 | 0.70 | 0.61 | 0.77 | 0.20 | 0.83 |
| 2-Hydroxyestrone-3-methyl ether | 1.5 | 1.3 | 1.3 | 1.1 | 1.5 | 0.13 | 0.58 |
| Methylated 4-catechols | 0.24 | 0.22 | 0.22 | 0.20 | 0.16 | 0.03 | 0.46 |
| 4-Methoxyestrone | 0.15 | 0.15 | 0.14 | 0.12 | 0.10 | 0.03 | 0.65 |
| 4-Methoxyestradiol | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.26 | 0.89 |
| 2-Hydroxylation Pathway | 81.1 | 63.5 | 63.9 | 55.5 | 64.1 | 0.01 | 0.37 |
| 4-Hydroxylation Pathway | 8.6 | 6.7 | 6.9 | 6.3 | 7.9 | 0.17 | 0.36 |
| 16-Hydroxylation Pathway | 69.2 | 63.4 | 60.0 | 64.1 | 66.4 | 0.41 | 0.67 |
| 16α-Hydroxyestrone | 12.4 | 10.7 | 10.7 | 9.6 | 10.5 | 0.09 | 0.34 |
| Estriol | 28.7 | 28.8 | 26.4 | 29.3 | 30.4 | 0.96 | 0.81 |
| 17-Epiestriol | 1.5 | 1.7 | 1.5 | 1.8 | 1.6 | 0.76 | 0.59 |
| 16-Ketoestradiol | 15.3 | 12.8 | 12.8 | 12.6 | 13.4 | 0.09 | 0.57 |
| 16-Epiestriol | 6.0 | 5.7 | 5.7 | 5.5 | 6.7 | 0.87 | 0.93 |
| Ratios (pmol/pmol) | |||||||
| 2-Hydroxyestrone/16α-Hydroxyestrone | 4.8 | 4.2 | 4.1 | 4.2 | 4.7 | 0.63 | 0.78 |
| 4-Pathway/2-Pathway | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 | 0.29 | 0.73 |
| 2-Pathway/16-Pathway | 1.2 | 1.0 | 1.0 | 0.9 | 1.0 | 0.16 | 0.83 |
| 2,4-Pathway/16-Pathway | 1.3 | 1.1 | 1.1 | 1.0 | 1.2 | 0.23 | 0.94 |
| 2-Catechols/Methylated 2-catechols | 5.5 | 5.1 | 5.2 | 5.3 | 4.8 | 0.28 | 0.67 |
| 4-Catechols/Methylated 4-catechols | 32.4 | 26.3 | 27.1 | 29.4 | 45.1 | 0.37 | 0.98 |
| Catechols/Methylated catechols | 6.3 | 5.7 | 5.9 | 6.0 | 5.6 | 0.46 | 0.73 |
| 2-Pathway/Parent estrogens | 1.6 | 1.6 | 1.5 | 1.5 | 1.5 | 0.11 | 0.50 |
| 4-Pathway/Parent estrogens | 0.16 | 0.16 | 0.17 | 0.16 | 0.17 | 0.86 | 0.51 |
| 16-Pathway/Parent estrogens | 1.4 | 1.6 | 1.5 | 1.6 | 1.4 | 0.69 | 0.47 |
| Parent estrogens/2-, 4-, 16-Pathways | 0.29 | 0.27 | 0.28 | 0.28 | 0.31 | 0.55 | 0.37 |
Adjusted for age at urine collection, ovulatory cycle, luteal phase of sample, first morning urine sample, age at first birth/parity, physical activity, alcohol intake, caffeine intake, current smoker, current menstrual cycle length, and current menstrual cycle regularity.
Tests for trend were conducted by modeling the five body fatness scores as a continuous exposure and calculating the Wald statistic. Statistically significant P for trend are shown in bold.
Additionally adjusted for BMI (continuous) at urine collection.
Ten participants did not report body fatness at age 5.
Table 4.
Multivariable-adjusted1 geometric means of premenopausal estrogen metabolism measures by body fatness at age 20
| Geometric mean by body fatness pictogram | P-trend1,2 | P-trend1,3 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5+ | |||
| N4 | 20 | 160 | 231 | 126 | 65 | ||
| Individual and grouped estrogens/estrogen metabolites (EM) (pmol/mg creatinine) | |||||||
| All EM combined | 208.1 | 213.9 | 194.5 | 186.7 | 187.8 | 0.03 | 0.35 |
| Parent estrogens | 49.1 | 43.6 | 40.7 | 39.0 | 37.4 | 0.01 | 0.04 |
| Estrone | 32.6 | 28.3 | 26.4 | 25.5 | 25.8 | 0.07 | 0.19 |
| Estradiol | 14.9 | 14.2 | 13.4 | 12.6 | 12.9 | 0.07 | 0.02 |
| Catechols | 92.2 | 70.6 | 61.3 | 60.0 | 58.1 | 0.01 | 0.63 |
| 2-catechols | 79.1 | 61.0 | 52.9 | 51.5 | 46.9 | 0.003 | 0.45 |
| 2-Hydroxyestrone | 71.2 | 53.7 | 46.6 | 45.4 | 40.7 | 0.003 | 0.39 |
| 2-Hydroxyestradiol | 7.0 | 6.3 | 5.6 | 5.2 | 5.2 | 0.02 | 0.81 |
| 4-catechols | |||||||
| 4-Hydroxyestrone | 8.1 | 6.8 | 5.8 | 5.6 | 7.1 | 0.40 | 0.75 |
| Methylated catechols | 12.5 | 11.2 | 10.3 | 9.7 | 9.3 | 0.02 | 0.69 |
| Methylated 2-catechols | 12.1 | 10.8 | 9.9 | 9.2 | 8.9 | 0.02 | 0.64 |
| 2-Methoxyestrone | 9.9 | 8.4 | 7.8 | 7.3 | 7.1 | 0.03 | 0.70 |
| 2-Methoxyestradiol | 0.70 | 0.73 | 0.67 | 0.68 | 0.65 | 0.35 | 0.68 |
| 2-Hydroxyestrone-3-methyl ether | 1.5 | 1.3 | 1.3 | 1.2 | 1.1 | 0.15 | 0.85 |
| Methylated 4-catechols | 0.31 | 0.24 | 0.22 | 0.24 | 0.20 | 0.19 | 0.80 |
| 4-Methoxyestrone | 0.20 | 0.15 | 0.14 | 0.14 | 0.12 | 0.07 | 0.91 |
| 4-Methoxyestradiol | 0.08 | 0.05 | 0.05 | 0.06 | 0.05 | 0.53 | 0.61 |
| 2-Hydroxylation Pathway | 90.0 | 72.7 | 64.2 | 61.4 | 57.4 | 0.004 | 0.48 |
| 4-Hydroxylation Pathway | 9.2 | 7.6 | 6.6 | 6.4 | 8.3 | 0.52 | 0.98 |
| 16-Hydroxylation Pathway | 60.1 | 67.6 | 63.9 | 61.7 | 59.0 | 0.20 | 0.39 |
| 16α-Hydroxyestrone | 9.7 | 11.1 | 10.2 | 11.0 | 10.4 | 0.91 | 0.32 |
| Estriol | 24.5 | 30.8 | 30.5 | 25.6 | 26.9 | 0.16 | 0.055 |
| 17-Epiestriol | 1.6 | 1.5 | 1.4 | 1.8 | 1.7 | 0.09 | 0.03 |
| 16-Ketoestradiol | 13.7 | 13.6 | 13.0 | 13.1 | 12.0 | 0.23 | 0.81 |
| 16-Epiestriol | 5.6 | 5.8 | 5.6 | 5.5 | 5.8 | 0.82 | 0.58 |
| Ratios (pmol/pmol) | |||||||
| 2-Hydroxyestrone/16α-Hydroxyestrone | 6.9 | 4.6 | 4.4 | 4.0 | 4.0 | 0.06 | 0.27 |
| 4-Pathway/2-Pathway | 0.10 | 0.10 | 0.10 | 0.11 | 0.12 | 0.03 | 0.54 |
| 2-Pathway/16-Pathway | 1.5 | 1.0 | 1.0 | 0.9 | 1.0 | 0.23 | 0.79 |
| 2,4-Pathway/16-Pathway | 1.7 | 1.2 | 1.1 | 1.1 | 1.2 | 0.35 | 0.62 |
| 2-Catechols/Methylated 2-catechols | 5.9 | 5.5 | 5.2 | 5.3 | 5.1 | 0.28 | 0.79 |
| 4-Catechols/Methylated 4-catechols | 26.2 | 27.1 | 25.8 | 24.7 | 37.8 | 0.34 | 0.93 |
| Catechols/Methylated catechols | 6.7 | 6.2 | 5.8 | 5.9 | 6.2 | 0.60 | 0.99 |
| 2-Pathway/Parent estrogens | 1.8 | 1.7 | 1.6 | 1.5 | 1.5 | 0.08 | 0.33 |
| 4-Pathway/Parent estrogens | 0.18 | 0.17 | 0.15 | 0.17 | 0.19 | 0.49 | 0.18 |
| 16-Pathway/Parent estrogens | 1.2 | 1.6 | 1.6 | 1.6 | 1.5 | 0.85 | 0.60 |
| Parent estrogens/2-, 4-, 16-Pathways | 0.31 | 0.27 | 0.28 | 0.28 | 0.28 | 0.86 | 0.09 |
Adjusted for age at urine collection, ovulatory cycle,luteal phase of sample, first morning urine sample, age at first birth/parity, physical activity, alcohol intake, caffeine intake,, current smoker, current menstrual cycle length, and current menstrual cycle regularity.
Tests for trend were conducted by modeling the five body fatness scores as a continuous exposure and calculating the Wald statistic. Statistically significant P for trend are shown in bold.
Additionally adjusted for BMI (continuous) at urine collection
One participant did not report body fatness at age 20.
To further explore the associations of early life body fatness with patterns of metabolism across pathways, we systematically examined pathway ratios. Metabolic patterns were most pronounced for age 10. Body fatness at age 10 was associated with reduced 2-hydroxylation as demonstrated by inverse associations for the following ratios: 2-pathway/16-pathway (P-trend=0.01), 2,4-pathway/16-pathway (P-trend=0.03), and 2-pathway/parent estrogens (P-trend=0.01) (Table 3). Body fatness at ages 5 and 20 were also inversely associated with the 2-pathway/16-pathway and 2-pathway/parent estrogens ratios, though the trends were not statistically significant (Tables 2 and 4).
The inverse association of age 10 body fatness with parent estrogens remained statistically significant after the addition of adult premenopausal BMI to the model (P-trend=0.01) (Table 3; Supplemental Table 1); in addition, adjustment for adult BMI only slightly attenuated the decrease in parent estrogens in the heaviest compared to leanest women (from 24% to 21% lower). Both estrone and estradiol remained statistically significantly inversely associated with age 10 body fatness after addition of adult BMI. The inverse associations of parent estrogens with body fatness at ages 5 and 20 also remained relatively strong after adjustment for adult BMI (9% lower comparing heaviest to leanest women; P-trend=.06 and 22% lower; P-trend=0.04, respectively) (Tables 2 and 4; Supplemental Tables 2 and 3). However, the other estrogen metabolism pathway associations that we consistently noted across early life were substantially attenuated and trends were no longer statistically significant with adjustment for adult premenopausal BMI. For example, without adjusting for adult BMI, the 2-hydroxylation pathway was 36% lower in the heaviest, compared with the leanest, women, at age 10 (P-trend=0.0003); this association remained inverse but was weakened after adjusting for adult BMI (19% lower; P-trend=0.13) (Table 3; Supplemental Table 1). Similarly, the associations of all EM combined, 2-catechols, and 2-methylated catechols with body fatness at age 10 remained inverse, but were noticeably weakened after adjustment (22%, 37%, and 30% lower, respectively, in the heaviest, compared with leanest, women before adjustment for adult BMI and 14%, 19%, and 15% lower, respectively, after adjustment) (Table 3; Supplemental Table 1).
The joint and independent relationships of selected estrogen metabolic pathways, as well as estrone and estradiol, with body fatness at age 10 and premenopausal adult BMI are shown in Supplemental Table 4. For both parent estrogens and estradiol, the influence of age 10 body fatness, controlled for adult BMI, was more pronounced than the influence of adult BMI, controlled for age 10 body fatness.
We summarize in Figure 1 how body fatness at ages 5, 10, and 20 and adult premenopausal BMI are associated with estrogen metabolism pathways during the premenopausal years. For most of the metabolic pathways, the most pronounced differences in mean urinary premenopausal concentrations, comparing the heaviest to leanest girls, were noted for body fatness at age 10 (second bar). For parent estrogens and 2-hydroxylation pathway metabolites, the % differences increased between body fatness at ages 5 and 10, but then remained relatively unchanged between ages 10 and 20. Only two of the trends in premenopausal estrogen pathway concentrations associated with early life body fatness remained statistically significant when premenopausal BMI was added to the models: the inverse associations of parent estrogens with body fatness at ages 10 and 20.
Figure 1.
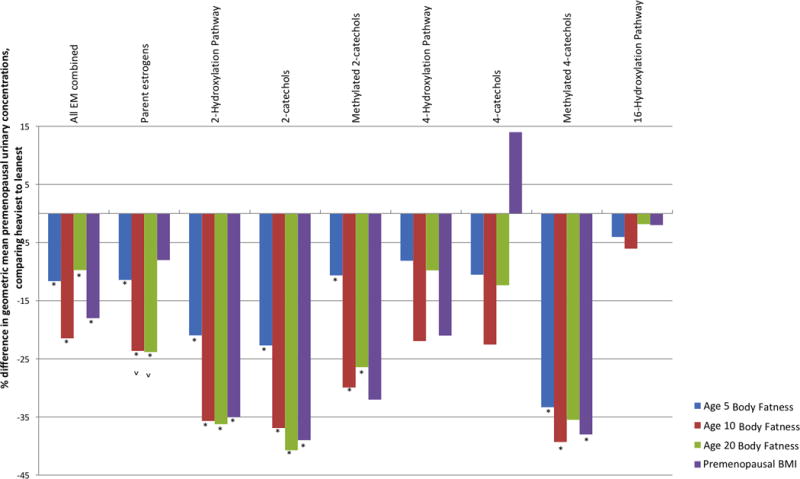
Relative difference in premenopausal estrogen metabolism pathways, comparing the heaviest to leanest based on recalled body fatness at ages 5, 10 and 20 and BMI in the premenopausal years. Geometric means for urinary concentrations of each metabolic pathway for the heaviest (pictograms 5+ or 30+ kg/m2) and leanest women (pictogram 1 or <22 kg/m2) were calculated using linear mixed models, adjusted for age at urine collection, ovulatory cycle, luteal phase of sample, first morning urine sample, age at first birth/parity, physical activity, alcohol intake, caffeine intake, current smoker, current menstrual cycle length, and current menstrual cycle regularity. Tests for trend were conducted by modeling the body fatness categories as a continuous exposure and calculating the Wald statistic. The estrogen metabolism pathways with statistically significant trends are marked with an asterisk (*). Trends that remained significant after addition of adult premenopausal BMI to the model are marked by a caret (ˆ)
We also tested if body fatness at ages 5, 10, or 20 attenuated the previously reported relationships between adult premenopausal BMI and estrogen metabolism pathways and ratios 21, but all associations remained relatively unchanged.
In sensitivity analyses, our results for body fatness at ages 5, 10 and 20 did not change when only including women with urine samples collected during ovulatory cycles (n=537), women with mid-luteal urine samples collected 4–10 days prior to the next menstrual cycle (n=516), or women who were not smoking at the time of urine collection (n=560). We also compared different ways of correcting for urine dilution in the statistical models. In the analyses shown, individual EM and grouped EM concentrations (pmol/mL urine) were divided by creatinine concentrations (mg/mL urine). Individual EM and grouped EM concentrations were also entered directly into the models as the outcome variables with creatinine as a covariate. These methods produced similar results.
Discussion
Estimates of body fatness during early life were statistically significantly associated with lower urinary concentrations of all EM combined, parent estrogens, and 2-hydroxylation pathway metabolites during the premenopausal years. These associations were most apparent for body fatness at age 10. The relationships were not fully explained by adult premenopausal BMI at the time of urine collection. In particular, the inverse association of early life body fatness with premenopausal parent estrogen levels persisted and remained statistically significant or marginally significant after adjustment for premenopausal BMI, suggesting early life growth patterns can influence estrogen exposure later in life.
Our finding that early life body fatness was associated with lower urinary concentrations of 2-pathway estrogen metabolites is consistent with the associations between early adult BMI, based on recalled weight and height for age 18, and estrogen metabolism previously observed in this population 21. The relative difference comparing the heaviest to leanest women was higher using recalled body fatness at age 20 (36% difference for pictograms 5+ compared to pictogram 1; p-trend = 0.0003) than using recalled BMI at age 18 (10% difference for BMI ≥25 kg/m2 compared to BMI <20 kg/m2; p-trend = 0.10). Recalled BMI at age 18 was moderately correlated with recalled body fatness at age 20 (Spearman r=0.68). The stronger contrast observed for 2-pathway estrogen metabolites when relying on pictograms to characterize body fatness can be attributed to several reasons. First, the pictograms may be more valid than recalled BMI at discriminating overweight/obese from slender individuals 27. Second, the pictograms are sensitive to body shape, which may measure an important aspect of body fatness, specifically body fat distribution, that BMI does not capture. Finally, the percent of the study population falling into the extreme categories being compared differs for the two measures. More women were in the lowest BMI at age 18 group than in the age 20 pictograms (36% of study population compared to 3%) 21 suggesting that BMI at age 18 may include several of the lighter pictograms.
The inverse associations of body fatness at ages 5, 10, and 20 with premenopausal urinary concentrations of parent estrogens were attenuated, but not eliminated, when we additionally controlled for adult premenopausal BMI (p-trend, after adjustment for adult BMI = 0.06, 0.01, and 0.04, respectively). Indeed, the association of premenopausal urinary parent estrogens with adult premenopausal BMI (p-trend = 0.29)22 was weaker than the associations with the three measures of early life body fatness (all p-trend ≤ 0.02). Surprisingly, BMI at age 18 was not as strongly associated with premenopausal urinary parent estrogens (p-trend, without adjustment for adult BMI = 0.17) 21 as body fatness at age 18 (p-trend, without adjustment for adult BMI = 0.01). It is conceivable that early life body shape and fat distribution, which pictograms capture better than BMI, contribute to determining estrogen production and metabolism in the premenopausal years. In a previously published analysis in this study population, age 10 body fatness, based on the same pictograms used in the current analysis, was statistically significantly inversely associated with premenopausal plasma concentrations of estrone sulfate, a measure of total estrogen production, in both the follicular and luteal phase (p-trend = 0.05 and 0.02, respectively)8. Furthermore, these associations with early life fatness persisted even after adjusting for adult premenopausal BMI, which, like the urinary results, supports the independent contribution of early life body fatness to estrogen production in the premenopausal years.
In a recent study of serum concentrations of 12 estrogens and estrogen metabolites measured by LC-MS/MS in 12 lean and 23 obese prepubertal girls, 8-10 years of age, estrone sulfate, the most abundant of the analytes, was nonsignificant lower in the obese girls, but estradiol was statistically significantly higher28. While we assessed the influence of early life fatness on estrogen metabolism in the premenopausal years, this previous study demonstrated its association with estrogen metabolism measured much earlier, prior to the onset of puberty28.
The strongest associations of early life body fatness with premenopausal levels of parent estrogens occurs at age 10. This age corresponds with the onset of puberty, usually marked by breast development, which typically occurs two to four years before menarche.29 There is debate as to whether breast development reflects the activation of the hypothalamic-pituitary-ovarian (HPO) axis or increased peripheral conversion of androgens to estrogens in adipose tissue. Most likely, however, both central and peripheral production of estrogens contribute to the physical manifestation of breast development, whereas menarche and the completion of puberty occur once the ovary is the prime source of estrogen. Since the effects of body fatness on estrogen profiles were not explained by age at menarche, estrogen production may be influenced by body fatness specifically during the entire pubertal transition, not just at menarche. Heavier girls are more likely to develop breasts earlier than lighter girls30, and increased body fat mass is also related to shorter time intervals between the onset of breast development and menarche 31. Therefore, early life body fatness may modulate adult estrogen exposure production and metabolism through its influence on the timing and tempo of the pubertal transition when the HPO axis is maturing. However, the associations of adult estrogen metabolism profiles with early life body fatness were not confounded, and thus not explained, by the exact age at menarche.
Our finding that childhood body fatness may independently lead to reduced parent estrogen levels in the premenopausal years may help explain the recognized association of childhood body fatness with reduced premenopausal breast cancer incidence 32-34. In a récent pooled analysis of individual participant data from seven prospective studies, prediagnostic circulating levels of estrone and estradiol were modestly associated with premenopausal breast cancer risk (statistically significant 20-30% increases in risk with doubling in concentration) 2. Thus, childhood body fatness, if it contributes to reduced estrogen exposure in the premenopausal years, may also contribute to reduced breast cancer risk. However, in our prior study, urinary estrone and estradiol were each strongly inversely associated with risk of premenopausal breast cancer1. The interrelationships of circulating and urinary estrogen concentrations in individual women, as well as the relationships to levels in breast tissue, are not well understood1.
The strengths of our study include a large study population of more than 600 women; well-timed mid-luteal urine samples; and the precise, accurate quantification of 15 individual EM using high-throughput LC-MS/MS. A notable strength of our study is that we have measures of perceived body fatness at three ages in early life, as well as recalled BMI at age 18 and reported BMI at the time of urine collection. The use of pictograms as a measure of body fatness during childhood and adolescence has been validated. In a longitudinal study which measured weight and height in participants during childhood and adolescence and later asked the same participants as adults to choose pictograms reflecting early life fatness, the correlations in women for ages 5, 10, and 20 were 0.6, 0.7, and 0.8, respectively.24 However, how accurately pictograms also capture early life body shape and body fat distribution, which may also modulate estrogen levels35, has not been assessed.
The primary limitation of our study is its cross-sectional design, which precludes the ability to test for causal relationships. We conducted multiple statistical tests; and although we emphasized associations with estrogen metabolism pathways and not individual estrogens and estrogen metabolites, it is possible that some of the statistically significant associations we detected were due to chance. Another limitation is that we only measured EM in urine samples during mid-luteal phase, and the relationship between early body fatness and follicular levels may differ from what we observed here. Moreover, the relationships between urinary estrogen metabolism patterns and circulating estrogen metabolism patterns has yet to be established. Lastly, the majority of participants in NHSII are White and of a limited range of socioeconomic status, and so our findings may not be generalizable to other populations, however the underlying biological associations are not likely to vary in different populations.
In conclusion, early life body fatness was inversely associated with adult premenopausal urinary levels of all estrogens/estrogen metabolites combined, parent estrogens, and 2-pathway estrogen metabolites. These relationships were not fully explained by adult BMI. The inverse association with premenopausal parent estrogen concentrations remained particularly strong. The persistent association of early life body fatness with reduced adult levels of urinary parent estrogens may be due to the programming of estrogen production and metabolism during puberty, which is a time when primary estrogen production is shifting from peripheral to central production.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by UM1 CA176726 (Walter C. Willett) and R01 CA67262 (Susan E. Hankinson) from the National Cancer Institute (NCI). It was also supported by the Intramural Research Program of the NCI Division of Cancer Epidemiology and Genetics and with the federal funds of the NCI awarded under Contract HHSN261200800001E to Leidos Biomedical Research, Inc. (Formerly SAIC-Frederick, Inc.). Julia Sisti was supported by training grants R25 CA098566 and T32 CA900137.”
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Eliassen AH, Spiegelman D, Xu X, et al. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer research. 2012;72(3):696–706. doi: 10.1158/0008-5472.CAN-11-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Key TJ, Appleby PN, Reeves GK, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. The Lancet Oncology. 2013;14(10):1009–1019. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. American journal of epidemiology. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 4.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. Journal of the National Cancer Institute. 2011;103(3):250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. European journal of endocrinology / European Federation of Endocrine Societies. 2004;150(2):161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 6.Bezemer ID, Rinaldi S, Dossus L, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer causes & control : CCC. 2005;16(5):561–572. doi: 10.1007/s10552-004-7472-9. [DOI] [PubMed] [Google Scholar]
- 7.Key TJ, Appleby PN, Reeves GK, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. British journal of cancer. 2011;105(5):709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tworoger SS, Eliassen AH, Missmer SA, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(12):2494–2501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- 9.Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. Journal of the National Cancer Institute. 1996;88(11):756–758. doi: 10.1093/jnci/88.11.756. [DOI] [PubMed] [Google Scholar]
- 10.Baer HJ, Colditz GA, Willett WC, Dorgan JF. Adiposity and sex hormones in girls. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(9):1880–1888. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- 11.Melmed S, Williams RH. Williams Textbook of Endocrinology. Elsevier/Saunders; 2011. [Google Scholar]
- 12.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Fortner RT, Hankinson SE, Schairer C, Xu X, Ziegler RG, Eliassen AH. Association between reproductive factors and urinary estrogens and estrogen metabolites in premenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(6):959–968. doi: 10.1158/1055-9965.EPI-12-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu F, Caporaso NE, Schairer C, et al. Urinary concentrations of estrogens and estrogen metabolites and smoking in caucasian women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(1):58–68. doi: 10.1158/1055-9965.EPI-12-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews CE, Fortner RT, Xu X, Hankinson SE, Eliassen AH, Ziegler RG. Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. The Journal of clinical endocrinology and metabolism. 2012;97(10):3724–3733. doi: 10.1210/jc.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisti JS, Hankinson SE, Caporaso NE, et al. Caffeine, Coffee, and Tea Intake and Urinary Estrogens and Estrogen Metabolites in Premenopausal Women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(8):1174–1183. doi: 10.1158/1055-9965.EPI-15-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman TJ, Sisti JS, Hankinson SE, Xu X, Eliassen AH, Ziegler R. Alcohol Consumption and Urinary Estrogens and Estrogen Metabolites in Premenopausal Women. Hormones & cancer. 2016;7(1):65–74. doi: 10.1007/s12672-015-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H, Smith-Warner SA, Tamimi RM. Dietary Fat and Fiber Intakes Are Not Associated with Patterns of Urinary Estrogen Metabolites in Premenopausal Women. 2015;145(9):2109–2116. doi: 10.3945/jn.115.212779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortner RT, Oh H, Daugherty SE, et al. Analgesic use and patterns of estrogen metabolism in premenopausal women. Hormones & cancer. 2014;5(2):104–112. doi: 10.1007/s12672-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand KA, Eliassen AH, Hankinson SE, et al. Urinary estrogens and estrogen metabolites and mammographic density in premenopausal women. Breast cancer research and treatment. 2012;136(1):277–287. doi: 10.1007/s10549-012-2240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Eliassen AH, Xu X, et al. Body size in relation to urinary estrogens and estrogen metabolites (EM) among premenopausal women during the luteal phase. Hormones & cancer. 2012;3(5–6):249–260. doi: 10.1007/s12672-012-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliassen AH, Ziegler RG, Rosner B, et al. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(11):2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stunkard A, Sorensen T, Schulsinger F. Use of the Danish adoption register for the study of obesity and thinness. In: Kety S, Rowland L, Sidman S, Mathysee S, editors. The genetics of neurological and psychiatric disorders. New York City: Raven Press; 1983. pp. 115–120. [PubMed] [Google Scholar]
- 24.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. American journal of epidemiology. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Veenstra TD, Fox SD, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Analytical Chemistry. 2005;77(20):6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- 27.Keshtkar AA, Semnani S, Pourshams A, et al. Pictogram use was validated for estimating individual’s body mass index. Journal of clinical epidemiology. 2010;63(6):655–659. doi: 10.1016/j.jclinepi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Mauras N, Santen RJ, Colon-Otero G, et al. Estrogens and Their Genotoxic Metabolites Are Increased in Obese Prepubertal Girls. The Journal of clinical endocrinology and metabolism. 2015;100(6):2322–2328. doi: 10.1210/jc.2015-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biro FM, Lucky AW, Simbartl LA, et al. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J Pediatr. 2003;142(6):643–646. doi: 10.1067/mpd.2003.244. [DOI] [PubMed] [Google Scholar]
- 30.Biro FM, Greenspan LC, Galvez MP, et al. Onset of Breast Development in a Longitudinal Cohort. Pediatrics. 2013 doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. The Journal of clinical endocrinology and metabolism. 1992;75(2):442–446. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- 32.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. The New England journal of medicine. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 33.Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sorensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast cancer research : BCR. 2014;16(1):R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. American journal of epidemiology. 2010;171(11):1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Ridder CM, Bruning PF, Zonderland ML, et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. The Journal of clinical endocrinology and metabolism. 1990;70(4):888–893. doi: 10.1210/jcem-70-4-888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


