Abstract
Hepatocellular carcinoma (HCC) has limited treatment options. Molecular analysis of its mutational landscape may enable the identification of novel therapies. However, biopsy is not routinely performed in HCC. The utility of analyzing cell-free circulating tumor DNA (ctDNA) by next-generation sequencing (NGS) is not established.
We performed 32 ctDNA NGS analyses on 26 patients; 10 of these patients had tissue NGS (236 to 626 genes). ctDNA was evaluated using an assay that detects single nucleotide variants, amplifications, fusions, and specific insertion/deletion alterations in 54 to 70 genes.
The ctDNA demonstrated that 23 of 26 patients (88.5%) had ≥ 1 characterized alteration, and all these individuals had ≥ 1 potentially actionable alteration. The most frequently mutated gene was TP53 (16 of 26 patients, 61.5%). There were 47 unique characterized molecular alterations amongst 18 total gene alterations (variants of unknown significance (VUSs) excluded). ctDNA and tissue NGS frequently showed different profiles, perhaps due to length of time between tissue and blood samples (median = 370 days (range, 29 to 876 days)). Serial ctDNA evaluation in an illustrative patient treated with capecitabine demonstrated emergence of a new TP53 alteration after progression.
In conclusion, ctDNA profiling is feasible in advanced HCC, and serial assessment using ctDNA NGS can reveal genomic changes with time. NGS of ctDNA provides a minimally invasive alternative for identifying potentially actionable gene alterations and potential molecular targeted therapies. Dynamic changes in molecular portfolio associated with therapeutic pressure in difficult-to-biopsy patients can be observed.
Keywords: hepatocellular carcinoma, liquid biopsy, circulating tumor DNA, next-generation sequencing, molecular targeted therapy
Introduction
Hepatocellular carcinoma (HCC), the most common form of liver cancer, is caused mainly by hepatitis B or C (HVB or HVC), but may also stem from metabolic toxins such as alcohol or conditions like metabolic syndrome (1,2). Common forms of treatment for HCC include resection, transplantation, and chemotherapy (3,4). Although small, localized tumors of early stage HCC patients allow for successful treatment, individuals with advanced stage HCC are much harder to treat (3). The tumors of advanced stage HCC patients are larger and more numerous, making surgery a high-risk treatment option with increased potential for operative and post-operative complications (3). In addition, even aggressive therapies such as transplantation are associated with a high incidence of recurrence (5).
Circulating tumor DNA (ctDNA) may yield information pertinent to identifying genomic alterations in shed DNA from tumors. When necrotic or apoptotic tumor cells decompose and release DNA, ctDNA in the bloodstream can be analyzed, reflecting a so-called “liquid biopsy” (6). The ctDNA can be isolated via centrifugation, amplified and interrogated (7). In comparison to healthy individuals, patients with cancer show an increased concentration of ctDNA (8).
Liquid biopsies may be especially useful in patients with HCC because tissue biopsies may carry morbidities in these fragile patients. Herein we describe the results and clinical implications of liquid biopsies in 26 patients with advanced HCC.
Methods
Patients
ctDNA was isolated from blood samples of 26 patients with advanced HCC utilizing Guardant360, a commercially available ctDNA sequencing panel (Guardant Health in Redwood, CA (http://www.guardanthealth.com). This study was conducted and consents obtained in accordance with the University of California, San Diego (UCSD) Moores Cancer Center Internal Review Board requirements (NCT02478931). The UCSD Moores Cancer Center Molecular Tumor Board and UCSD Liver Cancer Group Tumor Board were used as resources for sharing and reviewing patient cases when deemed necessary.
Next-Generation Sequencing
Liquid biopsies
The digital sequencing required for this study was conducted in a College of American Pathologists (CAP)-accredited and Clinical Laboratory Improvement Amendment (CLIA)-certified clinical laboratory. Liquid biopsies require two 10mL samples of blood, which collectively provide 5ng of DNA for ctDNA analysis. Liquid biopsy panel had each base sequenced at average raw coverage depth of 8,000× with a minimum average base coverage of 3,000× and a minimum Qscore of 20; Tissue biopsy coverage depth was 500×. The lowest detection mutation fraction is 0.1% for ctDNA and 5% for tissue biopsy. Detailed methods for both liquid biopsy ctDNA and tissue NGS have been previously published (9,10). There were 32 blood specimens in the study (N = 26 patients). A 54-gene panel was used for one sample; a 68-gene panel for 28 samples; and a 70-gene panel for 3 samples (Supplemental Tables 1, 2, and 3). One patient had 3 blood samples collected, four patients had 2 blood samples collected, and the remaining twenty-one patients had a single blood sample.
Tissue molecular profiling
In this study, tissue NGS was performed in 10 patients. In nine patients, a 236-gene panel was utilized (FoundationOne™, Cambridge, Massachusetts, http://www.foundationone.com); one patient, a 626-gene panel (Molecular Health, http://www.molecularhealth.com).
Definition of Potentially Actionable Alterations
A potentially actionable alteration is defined as a genomic alteration which produces a protein product that may directly serve as the primary target of an antibody, or the target, at low 50% inhibitory concentration (IC50), of a small molecule inhibitor. If an immediate downstream effector of a gene product alteration can be modulated, that gene was also considered potentially actionable. Finally, gene products that can be targeted because of their differential expression in tumor versus normal cells were considered actionable, regardless of impact on function.
Results
Patient Demographics
Twenty-six patients with HCC were analyzed (20 (76.9%) men) (Table 1). Their median age was 65 years (range, 44 to 74 years). Risk factors for HCC included HBV, HCV, alcohol, and metabolic syndrome, with HCV being the most common (17 patients (65.4%)). Regarding Barcelona clinic liver cancer (BCLC) staging (11), stage C was the most frequent (46.2%). Child-Pugh classification (12) was most often Stage B (50.0%), and patients most commonly had an ECOG (13) performance status of 2 (42.3%).
Table 1.
Demographic and baseline characteristics of 26 patients with HCC
| Variable | N=26 |
|---|---|
|
| |
| Median age - year | 65 (range 44–74) |
|
| |
| Sex – no. (%) | |
| Men | 20 (76.9%) |
|
| |
| * BCLC stage – no. (%) | |
| B | 3 (11.5%) |
| C | 12 (46.2%) |
| D | 3 (11.5%) |
|
| |
| * Child-Pugh class – no. (%) | |
| A | 4 (15.4%) |
| B | 13 (50.0%) |
| C | 7 (26.9%) |
| N/A | 2 (7.7%) |
|
| |
| ECOG performance status – no. (%) | |
| 0 | 2 (7.7%) |
| 1 | 8 (30.8%) |
| 2 | 11 (42.3%) |
| 3 | 5 (19.2%) |
|
| |
| Risk Factors: All patients – no. (%) | |
| Hepatitis B | 1 (3.8%) |
| Hepatitis C | 17 (65.4%) |
| Alcohol | 3 (11.5%) |
| Metabolic Syndrome | 5 (19.2%) |
|
| |
| Number of patients with tissue next generation sequencing | 10 (38.4%) |
|
| |
| Risk Factors: Ten patients with both liquid and tissue biopsy – no. (%) | N=10 |
| Hepatitis B | 0 (0.0%) |
| Hepatitis C | 8 (80.0%) |
| Alcohol | 1 (10.0%) |
| Metabolic Syndrome | 1 (10.0%) |
|
| |
| Number of patients with ≥ 1 characterized alteration (%) | 23 (88.5%) |
Abbreviations: BCLC = Barcelona Clinic Liver Cancer Staging; ECOG = Eastern Cooperative Oncology Group; HCC = hepatocellular carcinoma
Data not available for all patients
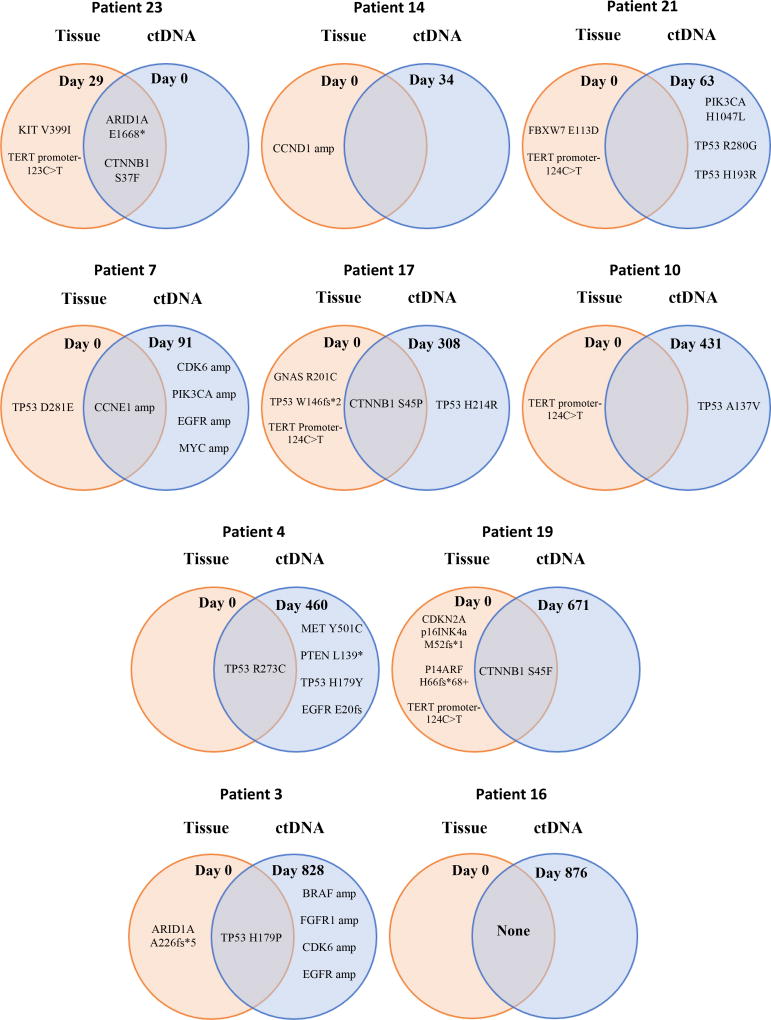
Genomic data was collected on the 26 patients via liquid biopsy for every patient and tissue biopsy for 10 (38.5%) of the patients (Figure 1A and B, Table 2). In 9 of the 10 patients with both tissue and liquid biopsy, the tissue predated the liquid biopsy (Figure 2).
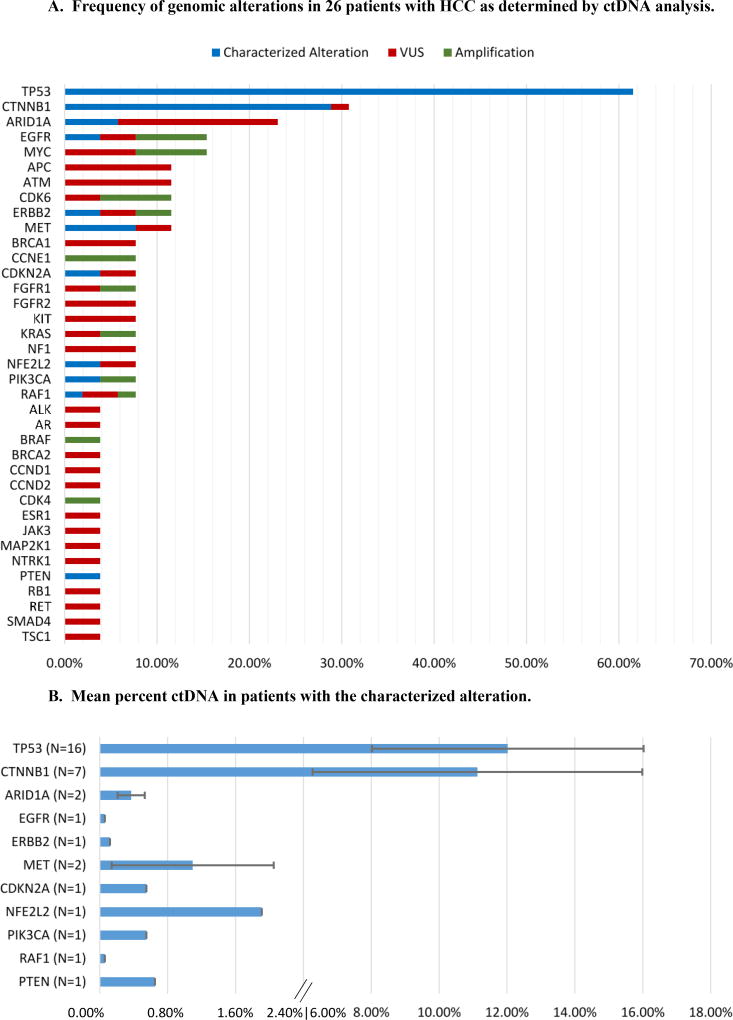
Figure 1.
A: Frequency of genomic alterations in 26 patients with HCC as determined by ctDNA analysis. Chart shows the percent of patients with the alteration.
Abbreviations: ctDNA = circulating tumor DNA; HCC = hepatocellular carcinoma; VUS = variant of unknown significance
B: Mean percent ctDNA in patients with the characterized alteration. N represents the number of patients with the alteration. The mean ± SE of %ctDNA is depicted. Only patients with non-zero results for each particular characterized alteration were included in the calculations for that given alteration. Percent ctDNA is calculated as a fraction of cell free DNA.
Table 2.
Risk factors, genomic alteration pattern, and percent ctDNA in 26 patients with HCC
| Patient | Risk Factor |
Tissue NGS/Molecular** |
Relative Date of Tissue NGS |
Relative Date of Serial ctDNA |
ctDNA Characterized Alterations (%ctDNA) |
ctDNA VUS (%ctDNA) |
AFP Levels (ng/mL) (Normal range, <15ng/ml)*** |
Child-Pugh Score (Class) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | HBV | N/A | N/A | Day 0* | TP53 R249S 33.31% | >60500.0 | 11 (C) | |
|
| ||||||||
| 2 | HCV | N/A | N/A | Day 0 | CTNNB1 D32N 0.22% | CTNNB1 S29T 0.23% | 870 | 11 (C) |
| EGFR D837Y 0.20% | ||||||||
|
| ||||||||
| 3 | HCV | TP53 H179P | Day 0 | Day 828 | TP53 H179P 37.77% | ARID1A A226P 1.11% | N/A | N/A |
| ARID1A A226fs*5 | BRAF amplification | |||||||
| FGFR1 amplification | ||||||||
| CDK6 amplification | ||||||||
| EGFR amplification | ||||||||
|
| ||||||||
| 4 | HCV | TP53 R273C | Day 0 | Day 460 | MET Y501C 2.05% | 13480 | 11 (C) | |
| PTEN L139* 0.21% | ||||||||
| TP53 R273C 1.18% | ||||||||
|
|
|
|||||||
| Day 592 | PTEN L139* 0.65% | |||||||
| TP53 R273C 1.01% | ||||||||
| TP53 H179Y 0.18% | ||||||||
| EGFR E20fs 0.06% | ||||||||
|
|
|
|||||||
| Day 624 | PTEN L139* 0.15% | |||||||
| TP53 R273C 1.63% | ||||||||
| TP53 H179Y 0.13% | ||||||||
|
| ||||||||
| 5 | HCV | N/A | N/A | Day 0 | TP53 R175H 29.51% | ARID1A Q2207R 0.55% | 374000 | 11 (C) |
| CTNNB1 S33C 13.02% | ||||||||
|
| ||||||||
| 6 | HCV | N/A | N/A | Day 0 | CDKN2A R80* 0.55% | 10 | 9 (B) | |
| CTNNB1 G34V 1.35% | ||||||||
|
| ||||||||
| 7 | Metabolic Syndrome | AKT2 amplification | Day 0 | Day 91 | CCNE1 amplification | 448100 | 6 (A) | |
| CCNE1 amplification | CDK6 amplification | |||||||
| PIK3CA amplification | ||||||||
| TP53 D281E | EGFR amplification | |||||||
| MYC amplification | ||||||||
|
| ||||||||
| 8 | Metabolic Syndrome | N/A | N/A | Day 0 | CTNNB1 H36P 12.09% | MAP2K1 V60M 0.67% | 3 | 8 (B) |
| RAF1 I634F 0.12% | ATM R337C 0.27% | |||||||
| TP53 H179R 0.13% | ALK R1347Q 0.12% | |||||||
| RAF1 amplification | ||||||||
|
|
|
|
||||||
| Day 156 | CTNNB1 H36P 31.54% | MAP2K1 V60M 0.35% | ||||||
| TP53 R273H 0.18% | ||||||||
| TP53 H179R 0.12% | ||||||||
| RAF1 amplification | ||||||||
|
| ||||||||
| 9 | HCV | N/A | N/A | Day 0 | TP53 H193R 0.92% | APC N1919K 0.26% | 13 | 6 (A) |
| ERBB2 V1128I 8.71% | ||||||||
|
| ||||||||
| 10 | HCV | RB1 loss exons 7–17 | Day 0 | Day 431 | ARID1A Q171H 0.31% | 7360 | 7 (B) | |
| BRCA2 F1219L 0.42% | ||||||||
|
|
|
|
||||||
| TERT promoter −124C>T | Day 634 | TP53 A138V 0.12% | BRCA1 R866C 0.19% | |||||
| BRCA2 F1219L 0.12% | ||||||||
|
| ||||||||
| 11 | Alcohol | N/A | N/A | Day 0 | TP53 R273C 12.08% | APC T683I 2.74% | 8990 | 10 (C) |
| ERBB2 amplification | ||||||||
| MYC amplification | ||||||||
|
|
|
|
||||||
| Day 133 | TP53 R273C 40.57% | APC T683I 10.09% | ||||||
| ERBB2 amplification | ||||||||
| MYC amplification | ||||||||
|
| ||||||||
| 12 | Metabolic Syndrome | N/A | N/A | Day 0 | TP53 S241F 0.13% | ESR1 P592R 0.10% | 4 | 9 (B) |
|
| ||||||||
| 13 | Alcohol | N/A | N/A | Day 0 | ARID1A Q268* 0.21% | APC S2533F 0.10% | 72 | 7 (B) |
| ARID1A E1531K 0.27% | ||||||||
| RAF1 E278K 0.23% | ||||||||
| SMAD4 V354E 1.77% | ||||||||
|
| ||||||||
| 14 | Alcohol | CCND1 amplification | Day 0 | Day 34 | NFE2L2 F37S 0.48% | 75 | 8 (B) | |
| FGFR2 G570S 0.12% | ||||||||
| ERRFI1 S302fs*10 | CCND1 E135K 0.10% | |||||||
| VEGFA amplification | ||||||||
| FGF19 amplification | ||||||||
|
| ||||||||
| 15 | HCV | N/A | N/A | Day 0 | CDK6 H297Y 0.27% | N/A | N/A | |
| MYC R98W 0.30% | ||||||||
|
| ||||||||
| 16 | HCV | No alterations | Day 0 | Day 876 | ARID1A E2078K 0.12% | 1.9 | 7 (B) | |
|
| ||||||||
| 17 | HCV | CTNNB1 S45P | Day 0 | Day 308 | CTNNB1 S45P 15.35% | CCND2 D264G 1.26% | 5 | 10 (C) |
| GNAS R201C | TP53 H214R 0.29% | NF1 G2499S 0.19% | ||||||
| TP53 W146fs*2 | FGFR2 K539E 0.13% | |||||||
|
|
|
|
||||||
| IRF2 W46* | Day 350 | CTNNB1 S45P 26.04% | CCND2 D264G 1.58% | |||||
| TERT promoter −124C>T | TP53 H214R 0.39% | RET K710N 0.17% | ||||||
|
| ||||||||
| 18 | HCV | N/A | N/A | Day 0 | ERBB2 R143Q 0.12% | CDKN2A S12L 0.38% | 1256 | 8 (B) |
| TP53 H178D 2.27% | KIT D975N 0.11% | |||||||
| NF1 R2179C 0.10% | ||||||||
|
| ||||||||
| 19 | HCV | CDKN2A p16INK4a M52fs*1 | Day 0 | Day 671 | CTNNB1 S45F 2.85% | 1899 | 8 (B) | |
| p14ARF H66fs*68+ | ||||||||
| CTNNB1 S45F | ||||||||
| KEAP1 R260* | ||||||||
| RBM10 Q518* | ||||||||
| TERT promoter −124C>T | ||||||||
|
| ||||||||
| 20 | Metabolic Syndrome | N/A | N/A | Day 0 | TP53 K139N 0.69% | ATM R337C 0.43% | 2134 | 7 (B) |
| JAK3 M576K 0.44% | ||||||||
|
| ||||||||
| 21 | HCV | FBXW7 E113D – subclonal | Day 0 | Day 63 | PIK3CA H1047L 0.55% | KRAS I24L 2.29% | 55 | 7 (B) |
| TP53 R280G 2.90% | ||||||||
| CDKN2A/B loss | TP53 H193R 2.57% | |||||||
| AXIN1 R395P – subclonal | ||||||||
| TERT promoter −124C>T | ||||||||
|
| ||||||||
| 22 | Metabolic Syndrome | N/A | N/A | Day 0 | TP53 C176F 7.95% | MET P4S 6.48% | 2 | 6 (A) |
|
| ||||||||
| 23 | HCV | KIT V399I | Day 29 | Day 0 | ARID1A E1668* 0.53% | ATM N3003S 0.35% | 17.2 | 6 (A) |
| ARID1A E1668* | CTNNB1 S37F 0.75% | BRCA1 N941D 1.35% | ||||||
| CTNNB1 S37F | ||||||||
| TERT promoter −124C>T | ||||||||
|
| ||||||||
| 24 | HCV | N/A | N/A | Day 0 | TP53 R249S 31.96% | FGFR1 E464K 0.20% | 571000 | 11 (C) |
| TP53 R196Q 0.19% | ||||||||
| KRAS amplification | ||||||||
| CCNE1 amplification | ||||||||
| CDK4 amplification | ||||||||
|
| ||||||||
| 25 | HCV | N/A | N/A | Day 0 | MET I491T 0.14% | 2 | 8 (B) | |
|
| ||||||||
| 26 | HCV | N/A | N/A | Day 0 | AR R608Q 0.68% | KIT D975N 0.27% | 3 | 7 (B) |
| CTNNB1 T41A 2.35% | MYC S154L 0.18% | |||||||
| NFE2L2 E82G 1.91% | NTRK1 E413K 0.15% | |||||||
| TP53 R248G 2.03% | RB1 M704R 1.69% | |||||||
| TSC1 E1101K 0.11% | ||||||||
Abbreviations: ctDNA = circulating tumor DNA; HBV = hepatitis B; HCC = hepatocellular carcinoma; HCV = hepatitis C; N/A = not available; VUS = variant of unknown significance
Day 0 represents the original test date.
All patients had genomic testing by Foundation One (http://foundationone.com/), except for patient #16, whose testing was performed by Molecular Health (626 genes)
AFP level closest in date to ctDNA blood draw was chosen
Figure 2. Venn diagrams showing the overlap between tissue NGS and ctDNA NGS for characterized genomic alterations.
The relative dates between the collection of the tissue NGS sample and ctDNA sample are also presented with Day 0 representing the date of the first test. The samples are ordered according to relative number of days between tests. Only genes that were assayed in both ctDNA and tissue NGS tests are included on the Venn diagrams. For patients with serial ctDNA tests, all unique alterations over the serial testing dates were included once in the diagram.
Genomic testing and alterations found in ctDNA
In total, alterations in 37 different genes were observed, including characterized alterations and VUSs. Only 18 different genes were involved at least once with a characterized alteration. The median number of genes with characterized alterations per patient was 1.5 (range, 0 to 5). Twenty-three of 26 patients (88.5%) had at least one characterized altered gene; the three patients without characterized alterations had VUSs. In total, there were 47 distinct characterized molecular alterations amongst the 23 patients with characterized anomalies.
The most common genetic alterations (including variants of unknown significance (VUSs)) occurred in the TP53 gene, a master regulator of apoptosis and the cell cycle, affecting 16 (61.5%) of the patients. The second most common alteration affected the CTNNB1 gene (8 (30.8%) of the patients), a key regulator of the Wnt pathway (Figure 1A). ARID1A is a subunit of the SWN/SNF complex, an epigenetic regulator, and was altered in 23.1% of patients (N = 6). The rest of the genomic alterations occurred at low frequency and affected oncogenes and tumor suppressor genes such as EGFR, MYC, APC, ATM, CDK6, ERBB2, RAF1, BRCA1, FGFR1, KRAS, PIK3CA, ALK, and BRAF. A visual breakdown of all observed genetic alterations and the patients with whom they corresponded has been provided (Supplemental Figure 1).
Thirteen patients (50.0%) had a genomic portfolio that was identical to at least one other patient: 6 patients with only TP53 gene alterations; 2 patients with only CTNNB1 gene alterations; 2 patients with only TP53 and CTNNB1 gene alterations; and 3 patients that had no characterized genomic alterations. However, no patients were identical at the molecular level, since patients with, for instance, TP53 anomalies, had distinct loci mutated.
Percent ctDNA found in liquid biopsies and correlations with AFP and Child Pugh class
The highest mean mutant allele frequency of ctDNA was seen in TP53 mutation (N = 16 patients; mean ± standard error (SE) = 12.0 ± 4.0%) (Figure 1B). This was followed by CTNNB1 with 11.1± 4.9% (N = 7 patients). Other genes that had mutant allele frequency of greater than 1.0% were MET and NFE2L2; all other characterized genomic alterations had less than 1.0% mutant allele frequency of ctDNA.
Percent ctDNA correlated well with AFP (P <0.001). We did not find a significant correlation between Child Pugh class and %ctDNA, perhaps because of the small number of patients in each subgroup (Table 2).
Potential actionability: Examples of possible targeted therapies
Among altered genes that included characterized alterations (N = 18 genes), 16 (88.9%) were potentially actionable (Table 3). All 23 patients (88.5% of 26 patients studied) had at least one potentially actionable alteration in their ctDNA. The median (range) number of potentially actionable ctDNA alterations was 1.5 (0 to 5). For example, CTNNB1 encodes beta catenin, a key regulator of the Wnt pathway. Sulindac and celecoxib demonstrate inhibitory activity against this pathway; furthermore, experimental therapies are in development (NCT02675946; CGX1321). Multiple agents, such as erlotinib and cetuximab, are currently approved by the Food and Drug Administration (FDA) for targeting the EGFR receptor tyrosine kinase. CDK6 is a regulator of cell cycle that may be impacted by palbociclib, a CDK 4/6 inhibitor.
Table 3.
Potential actionability: Examples of possible targeted therapies for specific characterized genomic alterations
| Genomic Aberration |
Examples of possible treatments |
|---|---|
|
| |
| ARID1A | ARID1A is possibly targetable with EZH2 inhibitor (EPZ-6438*, NCT01897571) through a synthetic lethal mechanism (23). |
|
| |
| BRAF | BRAF is targetable by BRAF inhibitors such as vemurafenib and dabrafenib (24). |
|
| |
| CCNE1 | Synthetic lethal screen showed CCNE1 amplified cells required ubiquitin pathway and was sensitive to proteasome inhibitor bortezomib. |
| CCNE1 mutations may be actionable with bortezomib, a proteasome inhibitor (25). | |
|
| |
| CDK4 | CDK4 may be targetable by CDK4/6 inhibitors such as palbociclib (26). |
|
| |
| CDK6 | Palbociclib is a CDK4/6 inhibitor (26). |
|
| |
| CDKN2A | CDKN2A genomic alterations result in increased CDK4/6 and are theoretically actionable with palbociclib, a CDK4/6 inhibitor (27). |
|
| |
| CTNNB1 | In preclinical study using hepatocellular carcinoma cell lines, sorafenib was capable of inhibiting Wnt/β- catenin signaling (28). |
| Sulindac (NSAIDs) also targets this pathway (29). | |
| CTNNB1 may also be targeted by gamma secretase inhibitors; these inhibitors target Notch(30). | |
|
| |
| EGFR | EGFR amplification is targetable with cetuximab, an anti-EGFR therapy (31). EGFR alterations are actionable with erlotinib (31). |
|
| |
| ERBB2 | ERBB2 alterations are actionable with lapatinib and trastuzumab (32). |
|
| |
| FGFR1 | FGFR1 aberrations are potentially targetable with lenvatinib (33). |
|
| |
| KRAS | KRAS mutations may be actionable with trametinib and other MEK inhibitors (34). |
|
| |
| MET | MET is targetable by cabozantinib (35). |
|
| |
| MYC | MYC can increase CDK4 levels and thus MYC is potentially targetable with the CDK4/6 inhibitor palbociclib (36). |
| BET inhibitors downregulate MYC transcription (NCT01943851)(37). | |
|
| |
| PIK3CA | PIK3CA mutations are actionable with everolimus and other mTOR inhibitor (38). |
|
| |
| PTEN | PTEN mutations are actionable with everolimus, a mTOR inhibitor (39). |
|
| |
| TP53 | TP53 genomic alterations correlate with increased VEGF-A expression (40). A retrospective study suggests that patients with TP53 mutations had longer progression-free survival with bevacizumab-containing therapies when compared to non-bevacizumab containing regimen (median 11.0 versus 4.0 months [p < 0.0001]) (41). |
| Another report indicates that TP53 mutations are associated with improvement in all outcome parameters when using VEGF/VEGFR inhibitors but that improvement is not seen in TP53 wild-type patients (42). | |
| Finally, TP53 mutations have been associated with better outcomes in sarcoma patients treated with the VEGFR inhibitor pazopanib (43). | |
| TP53 may also be targetable by WEE1 inhibitors (44). | |
Abbreviations: NSAID = nonsteroidal anti-inflammatory drug
Comparison of ctDNA and Tissue NGS Results
Ten patients had both tissue and ctDNA NGS testing and are shown on Figure 2. Nine patients (Table 2, cases #3, 4, 7, 10, 14, 17, 19, 21, 23) had characterized alterations in either their tissue NGS, ctDNA NGS, or both. One patient had no characterized alterations in either tissue or ctDNA NGS (Table 2, case #16). Examining alterations that were assayed in both the tissue and ctDNA NGS, six genomic alterations were found in both tissue and ctDNA; 18 alterations were found only in ctDNA; and 14 alterations were found only in tissue. The median time interval between tissue and liquid biopsy for ctDNA for the ten patients was 370 days (range, 29 to 876 days). The concordance for the three most commonly altered genes was as follows: TP53, 50.0%; CTNNNB1, 100.0%; and ARID1A, 90.0%.
Serial analysis of ctDNA and monitoring of treatment effect
A total of five patients (19.2%) had multiple liquid biopsies for ctDNA performed, with a median of 133 days between tests. The percent ctDNA for alterations varied between test dates. Further, only one of the five patients with multiple tests had an identical portfolio of genomic ctDNA alterations between tests. These patients were on therapy between tests.
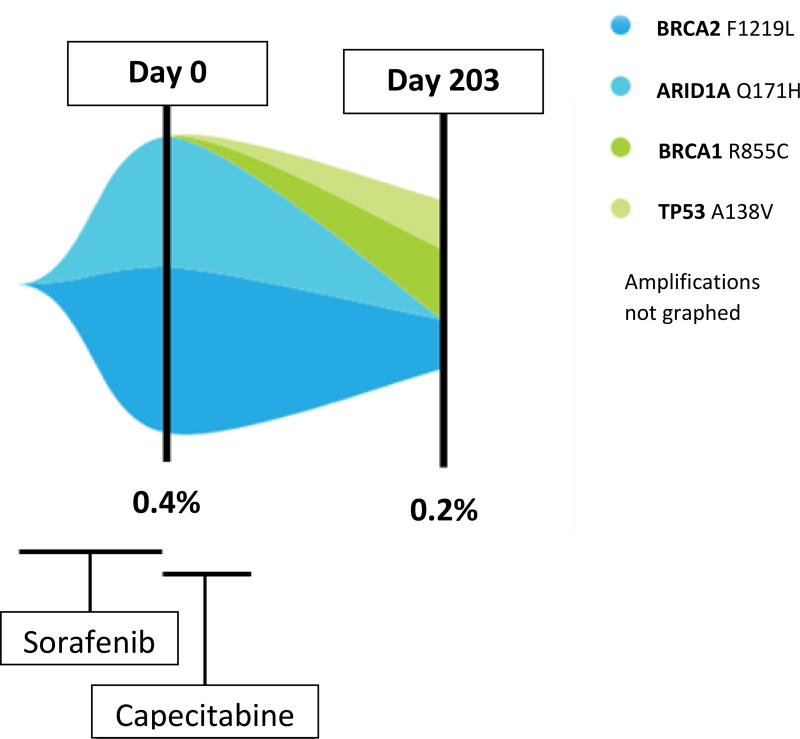
ctDNA can be measured at multiple time points during the course of treatment. An example in Figure 3 demonstrated that the alteration profile changes over time. This patient was diagnosed with BCLC Stage B HCC and received multiple locoregional therapies. The patient also started sorafenib; however, his disease metastasized to regional lymph nodes. Initial analysis of ctDNA revealed two genomic alterations: BRCA2 F1219L and ARID1A Q171H. The patient started metronomic dose capecitabine (14). The following ctDNA analysis, which occurred after capecitabine treatment was stopped due to toxicity and progression, demonstrated reduction of mutant allele fraction in two VUSs: BRCA2 F1219L (0.42% to 0.12%) and disappearance of ARID1AQ171H alteration (0.31% to 0%). However, there were two new aberrations emerged: BRCA1 R866C (VUS) and TP53 A138V (characterized alteration). Because of the patient’s deteriorating general condition and increased bilirubin, the patient chose hospice care.
Figure 3. Serial analysis of ctDNA (Table 2, patient #10).
This figure shows the serial analysis of one patient over 203 days. Two serial ctDNA biopsies were conducted. The patient had been treated with sorafenib, but disease had shown progression. The patient was then treated with capecitabine starting on Day 0, which represents the date of the first liquid biopsy. The patient came off therapy because of side effects and progression. Liquid biopsy later shows a decrease in the original ctDNA alterations but emergence of new alterations. The X-axis represents time. The Y-axis represents the fraction of mutant allele frequency of each genomic alterations. The ARID1A and BRCA2 ctDNA alterations (both VUSs) decreased but BRCA1 (VUS) and TP53 alterations emerged.
Discussion
This study investigated ctDNA analysis in 26 patients with advanced HCC. Twenty-three patients (88.5%) had at least one characterized alteration, and, in each of these individuals, there was at least one potentially druggable alteration. This observation suggests the potential clinical utility for ctDNA assessment. This utility may be especially relevant in HCC because biopsies are often not performed for diagnosis, at least in part because of their potential for complications.
In total, there were 47 distinct characterized molecular alterations involving 18 genes amongst the 23 patients with characterized anomalies. ctDNA was, however, detected in all 26 patients, albeit only VUSs were seen in three of the patients. This rate of detection is comparable to that in a study of patients with lung cancer that showed that ctDNA alterations were discernible in 83% of participants (15).
The most common alterations in our study were in TP53, CTNNB1 and ARID1A. The literature suggests that these genes are also commonly found to be abnormal by tissue NGS of HCC (16). Four of five patients with metabolic syndrome, 10 of 17 patients with HCV, and the one patient with HBV had a TP53 mutation (Table 2). Previously, HBV-related HCC has been associated with TP53 mutations (17). Other genes that were occasionally aberrant (characterized alterations) and of possible interest from a treatment point of view include EGFR, ERBB2, PIK3CA/PTEN, CDK4/CDKN2A, and BRAF as well as KRAS (Figure 1A, Table 3)(16,18). Indeed, 16 of the 18 genes with characterized alterations were potentially pharmacologically tractable. Clinical utility of these treatment options in genomically-matched patient populations is currently being investigated in basket trials (e.g., NCI-MATCH (NCT02465060), ASCO TAPUR (NCT02693535)). Of interest, however, no two patients were identical at the molecular level. This observation may complicate the use of genomics for choosing therapy, at least in traditionally designed trials. Ultimately, treatment choices may need to be individualized.
The highest percent ctDNA was seen with TP53 mutations (mean ± standard error (SE) = 12.0 ± 4.0%) (Figure 1B). This observation may be significant because TP53 overexpression is an strong indicator of poor prognosis for HCC patients (19). The second highest percent ctDNA was seen with CTNNB1 at 11.1± 4.9%. All other genes had a percent ctDNA of less than 2.0%. Previous studies have suggested that higher percent ctDNA correlated with a worse prognosis (20).
We attempted to determine if higher AFP levels correlated with higher percentage ctDNA. Percent ctDNA correlated well with AFP (P <0.001). This may not be surprising since both percent ctDNA and AFP correlate inversely with prognosis (21). We did not find a significant correlation between Child Pugh class and %ctDNA, perhaps because of the small number of patients in each subgroup.
A comparison of liquid and tissue NGS was also performed in this study. Data from ctDNA NGS was collected in all 26 patients, while data from tissue NGS was collected in only 10 of the patients (38.5%). The concordance levels for the three most commonly altered genes, TP53, CTNNB1, and ARID1A, are 50.0%, 100.0%, and 90.0%, respectively. These concordance rates are similar to those in previous studies that compare tissue and ctDNA (22). On the other hand, of alterations evaluated in both tissue and ctDNA assays, 18 alterations were found only in ctDNA NGS and 14 alterations were found only in tissue NGS. The median time between tissue and ctDNA sequencing was 370 days (range, 29 to 876 days), during which many new genomic alterations could have occurred. Previous reports indicate that the concordance of tissue and ctDNA sequencing depends on the time interval between two tests (the longer the interval, the less the concordance) consistent with the notion of genomic evolution (20). Other reasons for discrepancies between ctDNA and tissue genomics include technical factors, suppression of ctDNA clones as a result of therapy, or due to the fact that ctDNA includes shed tumor DNA from multiple sites, while tissue includes genomic alterations in only the site biopsied.
ctDNA may also change with time under therapeutic pressure. For instance, prior results have shown that either urine or blood ctDNA may be suppressed by therapy (18). In our patient, dynamic change of ctDNA mutant allele fraction can be seen as demonstrated in Figure 3. Serial ctDNA analysis captured dynamic molecular events wherein ARID1A and BRCA2 VUS mutant allele fraction decreased on capecitabine while BRCA1 VUS and TP53 characterized alterations emerged (with the follow up ctDNA assessed after patient was taken off drug due to progressive disease), perhaps reflecting the effects of the chemotherapy on various cancer clones.
There are some limitations to this study. First the ctDNA panel grew bigger with time (Supplemental Tables 1, 2, and 3). However, the most commonly altered genes were seen in the original 54 gene panel (ARID1A being an exception). On the other hand, of the 32 ctDNA tests performed, only one sample was tested with the initial 54 gene panel; 28 samples were tested with the 68 gene panel; and three samples, 70 genes. Second, the number of patients in our study is relatively small. Future studies of larger numbers of patients are warranted. Finally, serial ctDNA sampling is required in more patients. Although one of our patients showed emergence of new ctDNA alterations under therapeutic pressure (Figure 3), in other patients, treatment was performed at outside institutions and correlations with serial sampling were not possible. Additional studies should focus on large serial sampling studies are of interest.
In summary, our observations suggest that ctDNA analysis via liquid biopsy is feasible in HCC and frequently reveals diverse, potentially actionable genomic alterations. Dynamic changes in ctDNA after treatment can also be observed with serial assessment. Further investigation of ctDNA clinical utility in larger cohorts of patients and correlation with therapeutic outcome is warranted in this difficult-to-biopsy cancer.
Supplementary Material
Acknowledgments
Dr. Kurzrock has research funding from Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant, as well as consultant fees from XBiotech, and Actuate Therapeutics and an ownership interest in CureMatch Inc.
Funding and Acknowledgement: Funded in part by the National Cancer Institute grant P30 CA016672 (Razelle Kurzrock), and the Joan and Irwin Jacobs Fund (philanthropic fund).
Footnotes
Conflict of Interest: The other authors have nothing to disclose.
References
- 1.Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11(5):383–93. doi: 10.1111/j.1365-2893.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 2.Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108(1):222–8. doi: 10.1038/bjc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7(1):42–9. doi: 10.1080/13651820410024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PJ. Non-surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7(1):50–5. doi: 10.1080/13651820410024076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10(4):534–40. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 6.Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clinica chimica acta; international journal of clinical chemistry. 2001;313(1–2):139–42. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 7.Ono A, Fujimoto A, Yamamoto Y, Akamatsu S, Hiraga N, Imamura M, et al. Circulating Tumor DNA Analysis for Liver Cancers and Its Usefulness as a Liquid Biopsy. CMGH Cellular and Molecular Gastroenterology and Hepatology. 2015;1(5):516–34. doi: 10.1016/j.jcmgh.2015.06.009. http://dx.doi.org/10.1016/j.jcmgh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anker P, Mulcahy H, Chen XQ, Stroun M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999;18(1):65–73. doi: 10.1023/a:1006260319913. [DOI] [PubMed] [Google Scholar]
- 9.Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PloS one. 2015;10(10):e0140712. doi: 10.1371/journal.pone.0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 12.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 14.Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18(12):1256–7. doi: 10.1634/theoncologist.2013-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villaflor V, Won B, Nagy R, Banks K, Lanman RB, Talasaz A, et al. Biopsy-free circulating tumor DNA assay identifies actionable mutations in lung cancer. Oncotarget. 2016;7(41):66880–91. doi: 10.18632/oncotarget.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–11. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1):149. doi: 10.1186/s12943-017-0712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husain H, Melnikova VO, Kosco K, Woodward B, More S, Pingle SC, et al. Monitoring Daily Dynamics of Early Tumor Response to Targeted Therapy by Detecting Circulating Tumor DNA in Urine. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan P, Ji YN. Prognostic significance of TP53 expression for patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg Nutr. 2014;3(1):11–7. doi: 10.3978/j.issn.2304-3881.2014.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwaederle M, Husain H, Fanta PT, Piccioni DE, Kesari S, Schwab RB, et al. Use of Liquid Biopsies in Clinical Oncology: Pilot Experience in 168 Patients. Clin Cancer Res. 2016;22(22):5497–505. doi: 10.1158/1078-0432.CCR-16-0318. [DOI] [PubMed] [Google Scholar]
- 21.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–32. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamurthy N, Spencer E, Torkamani A, Nicholson L. Liquid Biopsies for Cancer: Coming to a Patient near You. J Clin Med. 2017;6(1) doi: 10.3390/jcm6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21(3):231–8. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci U S A. 2013;110(48):19489–94. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadoo KA, Gucalp A, Traina TA. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer (Dove Med Press) 2014;6:123–33. doi: 10.2147/BCTT.S46725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Adams RP, Swain SM. Does CDKN2A loss predict palbociclib benefit? Curr Oncol. 2015;22(6):e498–501. doi: 10.3747/co.22.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18(18):4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasper B, Strobel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682–93. doi: 10.1634/theoncologist.2010-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes DP, Kummar S, Lazar AJ. New, tolerable gamma-secretase inhibitor takes desmoid down a notch. Clin Cancer Res. 2015;21(1):7–9. doi: 10.1158/1078-0432.CCR-14-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheler JJ, Tsimberidou AM, Falchook GS, Zinner RG, Hong DS, Fok JY, et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations. Mol Cancer Ther. 2013;12(10):2167–75. doi: 10.1158/1535-7163.MCT-12-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman B, Stein S, Casey MA, Newstat BO. Lapatinib in combination with capecitabine in the management of ErbB2-positive (HER2-positive) advanced breast cancer. Biologics. 2008;2(1):61–5. doi: 10.2147/btt.s1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manchado E, Weissmueller S, Morris JP, Chen C-C, Wullenkord R, Lujambio A, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016 doi: 10.1038/nature18600. advance online publication http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature18600.html#supplementary-information. [DOI] [PMC free article] [PubMed]
- 35.Hage C, Rausch V, Giese N, Giese T, Schonsiegel F, Labsch S, et al. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. doi: 10.1038/cddis.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uras IZ, Walter GJ, Scheicher R, Bellutti F, Prchal-Murphy M, Tigan AS, et al. Palbociclib treatment of FLT3-ITD+ AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6. Blood. 2016;127(23):2890–902. doi: 10.1182/blood-2015-11-683581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–17. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohseni M, Park BH. PIK3CA and KRAS mutations predict for response to everolimus therapy: now that's RAD001. J Clin Invest. 2010;120(8):2655–8. doi: 10.1172/JCI44026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets. 2014;15(1):65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwaederle M, Lazar V, Validire P, Hansson J, Lacroix L, Soria JC, et al. VEGF-A Expression Correlates with TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Antiangiogenesis Therapy. Cancer Res. 2015;75(7):1187–90. doi: 10.1158/0008-5472.CAN-14-2305. [DOI] [PubMed] [Google Scholar]
- 41.Said R, Hong DS, Warneke CL, Lee JJ, Wheler JJ, Janku F, et al. P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget. 2013;4(5):705–14. doi: 10.18632/oncotarget.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheler JJ, Janku F, Naing A, Li Y, Stephen B, Zinner R, et al. TP53 Alterations Correlate with Response to VEGF/VEGFR Inhibitors:Implications for Targeted Therapeutics. Mol Cancer Ther. 2016 doi: 10.1158/1535-7163.MCT-16-0196. [DOI] [PubMed] [Google Scholar]
- 43.Koehler K, Liebner D, Chen JL. TP53 mutational status is predictive of pazopanib response in advanced sarcomas. Ann Oncol. 2016;27(3):539–43. doi: 10.1093/annonc/mdv598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghiasi N, Habibagahi M, Rosli R, Ghaderi A, Yusoff K, Hosseini A, et al. Tumour suppressive effects of WEE1 gene silencing in breast cancer cells. Asian Pac J Cancer Prev. 2014;14(11):6605–11. doi: 10.7314/apjcp.2013.14.11.6605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.