Abstract
Objectives
WHO recommends HIV viral-load (VL) testing six months after antiretroviral therapy (ART) initiation and every 12 months thereafter, but cost prohibits routine, universal VL testing in many developing countries. We sought to devise a targeted approach to routine VL monitoring that could reduce cost and identify those at low risk for virologic failure (VF).
Methods
We analyzed screening data from a clinical trial enrolling adults on ART in Malawi. We identified risk factors associated with VF, and employed the Knill-Jones method to assign summary score identifying persons at lower risk for VF.
Results
Among 957 adults, prevalence of VF was 9.4%. Factors independently associated with VF included; age < 38 years (OR 3.44, 95% CI 2.01 – 5.89), ART duration > 2.5 years (OR 2.98, 95% CI 1.79 – 4.96), ART adherence < 95% (OR 1.76, 95% CI 1.06 – 2.94), CD4 count < 200 cells/µL (OR 5.94, 95% CI 3.27 – 10.78), hemoglobin < 13 g/dl (OR 2.76, 95% CI 1.70 – 4.50) and CD8 count > 885 cells/µL (OR 2.10, 95% CI 1.28 – 3.44). Our VF prediction summary score included all factors above except CD8 count and was fairly accurate with validated area-under-receiver-operating-characteristic-curve of 0.76. Implementation could reduce VL testing by 65%.
Conclusion
A simple score incorporating age, ART duration and adherence, and CD4 count can accurately identify adults at low-risk for VF in a sub-Saharan-African setting. In areas with high ART utilization and limited VL testing capacity, a targeted approach could optimize routine VL monitoring while identifying adults in need of alternate ART regimens.
Keywords: HIV, virologic failure, viral load monitoring, Malawi, antiretroviral therapy
Introduction
Sub-Saharan Africa is home to 25.8 million people living with HIV (PLHIV) infection with over 12 million accessing life-saving anti-retroviral therapy (ART) administered in the context of national HIV programs.1 WHO recommends viral load (VL) monitoring six months after ART initiation and every 12 months thereafter for adults on ART.2 In settings where VL testing is not widely available, virologic failure (VF) is monitored using clinical and immunological criteria.3 Clinical criteria include development of a new or recurrent WHO stage 4 clinical event after 6 months of ART with good adherence. Immunological criteria include a decrease in CD4 count to baseline or below, or persistent CD4 levels <100 cells/µL.2 Used alone, clinical and immunological criteria poorly predict VF.4, 5, 6, 7 Delayed identification of VF leads to accumulation of resistance mutations, transmission of resistant strains,8, 9, 10, 11, 12 and increased morbidity and mortality in individuals on failing regimens.6, 13, 14, 15, 16 Inaccurate clinical and immunological monitoring leads to unnecessary switching to second line ART regimens that are more expensive, toxic, and complex to procure.6, 13, 14, 16, 17, 18, 19
In an effort to maximize the impact of strained public health resources, routine VL monitoring in Malawi is scheduled at 6 and 24 months after ART initiation, then once every 2 years.20 Logistics, high technical demands and cost of routine VL monitoring are challenging for low income countries like Malawi where more than 600,000 people on ART strain a limited healthcare system.20, 21, 22 In 2015, only one-quarter of Malawian ART patients had a VL test.23
The availability of a screening tool that incorporates readily available data to identify individuals at low risk for VF could reduce the costs and inefficiencies associated with routine VL monitoring in all patients on ART.18, 24 Therefore, we set out to develop a practical VF prediction tool that could be used by health care workers in Malawi and similar low-income country settings for routine testing of adults on first line ART who otherwise do not meet criteria for viral load testing. The tool is meant for use in a relatively healthy population of adults on ART, as persons with a new WHO Stage 3 or 4 defining condition would routinely undergo viral load testing in this setting.
Materials and Methods
Study design and population
This cross sectional study analyzed screening data from a randomized clinical trial among adults on antiretroviral therapy in a semi-urban region of Blantyre, Malawi (ClinicalTrials.gov Identifier NCT01650558).25 We screened adult PLHIV at Ndirande Health Center ART Clinic in Blantyre, Malawi. We obtained informed consent from non-pregnant adults on first line ART for at least 6 months and cotrimoxazole prophylaxis for at least 2 months and without evidence of active WHO Stage 3 or 4 illness.25 Screening laboratory specimens for HIV viral load, CD4, full blood count (FBC), alanine transaminase (ALT) and creatinine were drawn from participants without severe acute illness, active tuberculosis, ongoing secondary antibiotic prophylaxis, or contraindications to chloroquine or anti-folate drugs.25 Standard first-line ART for this population included a non-nucleoside reverse transcriptase inhibitor (nevirapine or efavirenz) and two nucleoside/nucleotide reverse transcriptase inhibitors (stavudine, tenofovir, or zidovudine plus lamivudine). We defined VF according to WHO guidelines2 at >1,000 copies/mL.
Study Procedures
We assessed HIV VL using the Abbott real time HIV-1 assay (Des Plaines, IL, USA), CD4 and CD8 cell counts using the Becton-Dickinson FacsCount (San Jose, CA, USA), and full blood count using a Beckman Coulter counter (Miami, FL, USA) to determine hemoglobin and absolute neutrophil count (ANC). We analyzed ALT and creatinine using a Beckman Coulter AU480 biochemistry analyzer (Miami, FL, USA). Data were captured on paper case report forms, reviewed via routine quality control checks and then transcribed into an electronic database. The number of missed ART doses since the last clinic visit approximately 3 months prior was self-reported by participants and considered adequate if ≥95% of doses were taken.
Statistical Analysis
We analyzed data using STATA version 13.0 (Stata Corporation, College Station, TX, USA) and R version 2.15.3 (The R Foundation for Statistical Computing, Vienna, Austria; rms package). Bivariate logistic regression was used to identify factors associated with VF. Potential risk factors included in the analysis were; gender, age in years, body mass index (BMI), duration on ART, ART adherence, hemoglobin, ALT, ANC, CD4 cell count, CD8 cell count, and creatinine. Using a forward inclusion stepwise approach, we developed a multivariable logistic model including variables associated with VF in bivariate analysis at a predetermined P-value of ≤ 0.1, namely: age, sex, ART duration (months), ART adherence (defined as adequate if ≥95% doses taken, or inadequate if <95% doses taken in the last 3 months), hemoglobin, ANC, ALT, creatinine, CD8 and CD4 cell count.8, 14, 15, 16, 17, 18, 19, 21 All continuous variables except CD4 count were assigned a data-derived threshold to maximize sensitivity and specificity for VF. For CD4 count, we used a threshold of 200 cells/µL because of its clinical relevance.
To establish a model to predict VF risk, we used the Knill-Jones method to assign numerical values to risk factors.26 We first developed the model using a tool derivation dataset from the first group of screened participants by calculating the logarithm of the likelihood ratio of each variable and converted to the nearest integer to give a value to each risk factor. To assess validity, performance of this model was then internally validated using data from the next set of participants screened for the clinical trial but not included in tool’s derivation dataset. We summarized the numeric values assigned to each of the identified risk factors, establishing a VF prediction tool with four threshold scores.
Ethics
The study was approved by the University of Malawi, College of Medicine Research Ethics Committee (COMREC) and the University of Maryland Institutional Review Board. Written informed consent was obtained from each participant.
Results
Baseline characteristics
We enrolled 957 participants from November 2012 to February 2014 for the derivation cohort and 186 participants from March 2014 to October 2014 for the validation cohort. The derivation and the validation cohorts differed with regard to gender, age, time on ART, poor adherence to ART, hemoglobin and CD4 count (Table 1), while the prevalence of VF in the two groups was not significantly different.
Table 1.
Comparison of baseline characteristics in derivation and validation groups
| Derivation (n = 957) | Validation (n = 186) | P-value | ||
|---|---|---|---|---|
| Clinical | Male gender (%) | 249 (26%) | 63 (34%) | 0.02 |
| Mean age – years (SD) | 39.6 (9.16) | 38.0 (9.95) | 0.03 | |
| Mean Body Mass Index (SD) | 22.4 (3.71) | 22.2 (3.34) | 0.45 | |
| Median months on ART (IQR) | 34 (18 – 60) | 27 (13 – 51) | <0.01 | |
| ART adherence <95% (%) | 526 (55%) | 128 (69%) | <0.01 | |
| Laboratory | Mean hemoglobin – g/dL (SD) | 13.2 (1.78) | 12.9 (1.92) | 0.02 |
| Median ALT – IU/L (IQR) | 23 (19 – 32) | 23 (16 – 32) | 0.08 | |
| Median ANC – ×103/µL (IQR) | 1.9 (1.43 – 2.60) | 1.8 (1.36 – 2.53) | 0.94 | |
| Mean CD4 – cells/µL (SD) | 499.9 (265.92) | 448.5 (263.76) | 0.02 | |
| Mean CD8 – cells/µL (SD) | 884.5 (384) | 908.5 (336.81) | 0.47 | |
| VL >1,000 copies/ml (%) | 90 (9.40%) | 21 (11.3%) | 0.43 | |
| Mean Creatinine – mg/dL (SD) | 0.69 (0.19) | 0.72 (0.17) | 0.02 | |
SD – standard deviation; IQR – interquartile range; ART – antiretroviral therapy; ALT – alanine transaminase; ANC – absolute neutrophil count; VL – viral load.
Risk Factors for VF
Factors associated with VF in bivariate analysis include age <38 years (OR 2.83, 95% CI 1.74 – 4.61), >2.5 years on ART (OR 1.91, 95% CI 1.21 – 3.04), inadequate ART adherence (OR 2.05, 95% CI 1.28 – 3.27), hemoglobin <13 g/dl (OR 3.00, 95% CI 1.89 – 4.77), ALT >33 U/L (OR 0.54, 95% CI 0.29 – 1.01), ANC <1,300 cells/µL (OR 2.10, 95% CI 1.30 – 3.38), CD4 count <200 cells/µL (OR 4.80, 95% CI 2.88 – 8.02) and CD8 >855 cells/µL (OR 1.72, 95% CI 1.11 – 2.66). Gender (OR 0.64, 95% CI 0.37 – 1.12), body mass index (OR 0.73, 95% CI 0.34 – 1.56), and creatinine (OR 0.56, 95% CI 0.19 – 1.64) were not associated with VF in the bivariate analysis (Table 2).
Table 2.
Risk factors for virologic failure and contribution to prediction tool
| Univariate analysis | Multivariate analysis | Score if factor present |
||||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |||
| Clinical | Male gender | 0.64 (0.37, 1.12) | 0.12 | |||
| Age < 38 years | 2.83 (1.74, 4.61) | <0.01 | 3.44 (2.01, 5.89) | <0.01 | 1 | |
| Body Mass Index <18.5 | 0.73 (0.34, 1.56) | 0.42 | ||||
| ART duration >2.5 years | 1.91 (1.21, 3.04) | 0.01 | 2.98 (1.79, 4.96) | <0.01 | 1 | |
| ART adherence <95% | 2.05 (1.28, 3.27) | <0.01 | 1.76 (1.06, 2.94) | 0.03 | 1 | |
| Laboratory | Hemoglobin <13 g/dL | 3.00 (1.89, 4.77) | <0.01 | 2.76 (1.70, 4.50) | <0.01 | 1 |
| ALT >33 U/L | 0.54 (0.29, 1.01) | 0.05 | 0.64 (0.32, 1.25) | 0.19 | ||
| ANC <1.3 ×103/µL | 2.10 (1.30, 3.38) | <0.01 | 2.50 (1.44, 4.23) | 0.08 | ||
| CD4 <200 cells/µL | 4.80 (2.88, 8.02) | <0.01 | 5.94 (3.27, 10.78) | <0.01 | 2 | |
| CD8 >885cells/µL | 1.72 (1.11, 2.66) | 0.02 | 2.10 (1.28, 3.44) | 0.01 | 1* | |
| Creatinine > 0.69 mg/dL | 0.56 (0.19, 1.64) | 0.30 | ||||
ART – antiretroviral therapy; ALT – alanine transaminase; ANC – absolute neutrophil count
CD8 not included in score based prediction tool as CD4 served as proxy
In multivariable analysis, age <38 years (OR 3.44, 95% CI 2.01 – 5.89), >2.5 years on ART (OR 2.98, 95% CI 1.79 – 4.96), adherence <95% (OR 1.76, 95% CI 1.06 – 2.94), hemoglobin <13 g/dl (OR 2.76, 95% CI 1.70 – 4.50), CD4 count <200 cells/µL (OR 5.94, 95% CI 3.27 – 10.78) and CD8 >855 cells/µL (OR 2.10, 95% CI 1.28 – 3.44) remained independently associated with VF (Table 2).
Construction and validation of the virologic failure prediction model and tool
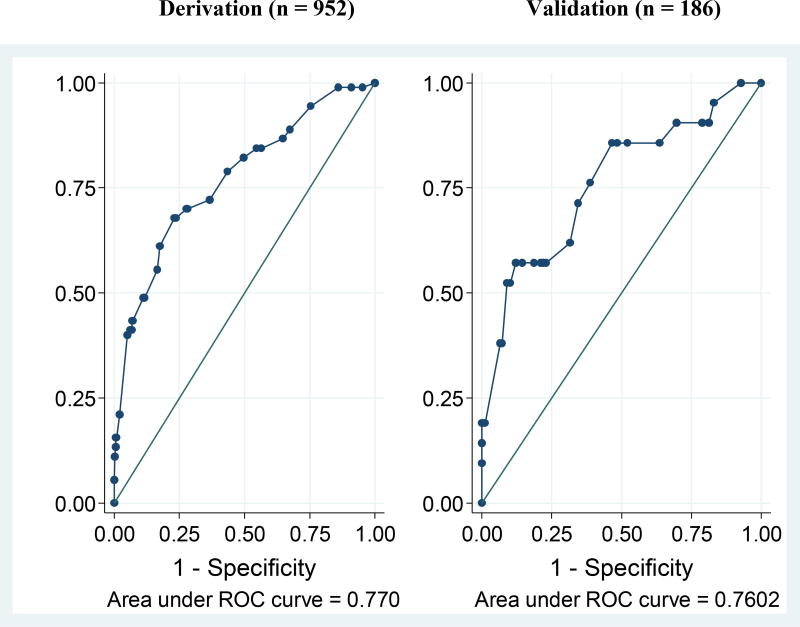
Using the Knill-Jones method of constructing prediction models to estimate adjusted likelihood ratios, and the derivation dataset, we assigned numerical values to each of the variables that demonstrated significance in multivariable analysis (Table 2). All variables that were independently predictive of VF contributed a value of one except CD4 count, which earned two. We then applied the model to predict VF in the derivation and validation datasets. The sensitivity and specificity of the model in predicting VF when the total point score was ≥ 1 was similar in both datasets (Figure 1) as the area under receiver operating characteristic curve (AUROC) was 0.77 in the derivation set and 0.76 in the validation set (p-value = 1.0). A model that did not include CD8 values showed similar results (AUROC 0.77 for derivation dataset).
Figure 1.
Derivation (n = 952) Validation (n = 186)
The five variables included in the final model (age < 38 years, ART duration > 2.5 years, ART adherence < 95%, hemoglobin < 13 g/dL, and CD4 < 200 cells/µL) were incorporated in our final VF prediction tool. Using this tool, an individual’s score is calculated by the sum of the numerical values of each of the five variables; totals can range from 0 to 6 (Table 2).
Determining the threshold of the prediction tool score for virologic failure
We then explored the most useful threshold score that could identify patients with very low VF risk while minimizing the risk of missing patients with VF. At a threshold score of ≥ 1, the tool shows 100% sensitivity for VF, but would also require that 96% of individuals undergo VL testing. At a threshold score of ≥ 2, the tool demonstrated 90% sensitivity for VF. The AUROC of 0.77 represented averting 266/957 (27.8%) VL tests, which would have missed only 9/90 (10%) of patients with VF. Raising the cut-off to ≥ 3 decreased the sensitivity to 75.6%, with an AUROC of 0.72. Under this scenario, 626/957 (65%) VL tests could be averted but missing 22/90 (24%) of patients with VF. Among patients predicted not to have VF at this higher threshold, the probability of having a VL of <1000 copies/mL was 604/626 (96.5%) (Table 3).
Table 3.
Performance of different thresholds of the score of the prediction tool for virologic failure
| Score* | Sensitivity (%) | Specificity (%) | n | PPV (%) | NPV (%) | AUROC | % need VL test |
VF missed (%) |
|---|---|---|---|---|---|---|---|---|
| ≥ 1 | 100.0 | 3.7 | 920 | 9.7 | 100 | 0.7694 | 96.1 | 0/90 |
| ≥ 2 | 90.0 | 29.7 | 687 | 11.8 | 96.6 | 0.7651 | 71.8 | 9/90 (10%) |
| ≥ 3 | 75.6 | 69.7 | 329 | 20.7 | 96.5 | 0.7164 | 34.8 | 22/90 (24%) |
| ≥ 4 | 38.9 | 93.0 | 95 | 36.8 | 93.6 | 0.6681 | 9.9 | 55/90 (61%) |
Variables included in tool: age <38 years, ART duration >2.5 years, ART adherence <95%, hemoglobin <13 g/dL, and CD4 <200 cells/µL
PPV – positive predictive value; NPV – negative predictive value; AUROC – area under receiver operator curve; VL – viral load
Discussion
Our study findings demonstrate a 9.4% prevalence of VF in clinically stable adults who had been on ART for at least 6 months and who had no symptoms suggestive of WHO stage 3 or stage 4 illness. Targeting VL testing to the minority of adults on ART at highest risk of VF where the recommended VL monitoring is not widely accessible, reasonably justifies developing a strategy that will identify individuals at low risk of VF, and then excluding them from VL testing.
The independent risk factors for VF in our population were younger age, longer ART duration, sub-optimal self-reported ART adherence, lower hemoglobin, lower CD4 count and higher CD8 count. These findings are consistent with those in other studies from resource-limited settings.24, 27, 28, 29, 30
We developed a VF prediction tool from these clinical and laboratory risk factors that can select clinically stable adults on first line ART who may be exempted from VL testing at routine VL monitoring milestones. Of the score thresholds that we considered, two had potential merit for use in routine VL monitoring. Using a threshold of ≥ 2 (sensitivity for VF = 90%), the tool would have precluded 266/957 (28%) of VL tests in our cohort but failed to predict the need for VL testing in 9/266 patients (3.4%). At a lower sensitivity threshold of ≥3 (sensitivity = 75.6%), the tool would have reduced nearly 2/3 of VL tests but missing nearly one quarter (24%) of patients with VF.
Previously published tools used in Cambodia and Lesotho24, 27, 30 were developed in cohorts with relatively high prevalence of VF, and were optimized to identify patients with high risk of VF for VL testing. In addition they were determined on the basis of prospectively collected laboratory data, including trends of 6-monthly CD4 count and hemoglobin; these were considered not to be feasible in Malawi and similar settings18, 24, 30. In contrast, our prediction tool is designed to identify individuals with very low VF risk, and to be used at a routine VL testing milestone for ART patients who are clinically stable. Our VF prediction tool is appropriate for our setting as the included parameters are readily available. The identified risk factors for VF in our model showed reasonable but suboptimal predictive performance (AUROC 0.77), leaving room for further refinement to achieve higher predictive accuracy through studies in similar settings.
Development and application of tools that predict reduced risk of VF face several challenges. Identifying the optimal balance between sensitivity and specificity in clinically stable patients is difficult. Both of the thresholds we evaluated led to substantial saving on VL tests -- but would fail to identify patients with VF. Such missed opportunities to diagnose VF before clinical or immunological criteria manifest are extremely detrimental and may lead to emergence and spread of ART-resistant virus, clinical deterioration and mortality.3, 9, 12, 31 If patients who were exempted from VL testing would automatically qualify at the next planned routine VL testing date, those who were not provided VL testing due to the incorrect assignment of low risk would have a maximum delay of VF diagnosis of two years under WHO guidelines - but this could be as long as four years under Malawi guidelines. A second challenge is that calculating the correct score of the VF prediction tool is relatively complicated and thus subject to human error, especially in settings where ART services are delegated to health workers with limited education and training. This could be overcome by incorporation of the calculation in an electronic medical record system or through a simple mobile phone-based calculator.
Routine VL monitoring benefits both patients and ART programs by reducing the number of persons being wrongly switched to second-line therapy and the associated increased expense and toxicity.32 VL-informed care of PLHIV motivates good ART adherence, prevents VF and can lead to less frequent follow-up required for those who are stable and suppressed, resulting in more capacity to follow-up those who are unsuppressed: promoting ART adherence and timely switching to second-line ART.33 However, in the fourth quarter of 2015, only 27% of the estimated 75,000 patients on ART in Malawi who met criteria for routine VL monitoring had a VL test done.23 Viral load testing is logistically and technically demanding, and expensive. A lack of clinician awareness of the long-term individual patient benefit and the role of VL monitoring in prolonging longevity of ART treatment programs may also contribute to limited implementation of VL testing.21
Several strategies have been proposed to make routine VL monitoring more cost-effective, including use of dried blood spot (DBS) samples, simplifying sampling, storage and transport, and the use of pooled plasma samples.3 Use of a VF prediction tool to distinguish clinically stable, very low-risk adults who might not benefit from VL testing is another potential cost saving strategy.3, 34 Our VF prediction tool has the potential to save a substantial percentage of VL tests among a very large and increasing number of patients on ART and therefore holds important cost-saving promise, but formal cost-effectiveness studies are required to support this.
Our targeted approach to routine VL monitoring employs a combination of readily available clinical and laboratory variables to identify those at very low risk of VF among clinically stable PLHIV on first line ART. The tool may be used to exclude patients from VL testing in a routine VL monitoring program, and has the potential to reduce the number of VL tests by more than half with a small risk of missing VF. Future studies should further refine and validate the approach and determine its practicality, optimal timing, acceptability and cost-effectiveness.
Acknowledgments
We are grateful to clients of the Ndirande ART clinic who volunteered to participate in the study and to the Blantyre Malaria Project Ndirande Research Clinic staff for their contribution to this study. We are also grateful to the National Institute of Health, Division of AIDS for sponsoring the ongoing clinical trial. We analyzed screening data from a clinical trial sponsored by a grant from the U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS, to Miriam K. Laufer, MD, MPH (U01AI089342).
References
- 1.Global AIDS Update Geneva, Switzerland. Vol. 16 Geneva Switzerland: 2016. Joint United Nations Programme on HIV/AIDS. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. [Google Scholar]
- 2.Organization. WH. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 [PubMed] [Google Scholar]
- 3.Roberts T, Bygrave H, Fajardo E, Ford N. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc. 2012;15:17324. doi: 10.7448/IAS.15.2.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherford GW, Anglemyer A, Easterbrook PJ, Horvath T, Vitoria M, Penazzato M, Doherty MC. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. Aids. 2014;28(Suppl 2):S161–9. doi: 10.1097/QAD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 5.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankale JL, Dieng-Sarr A, Agbaji O, Onwujekwe DI, Gashau W, Nkado R, Ekong E, Okonkwo P, Murphy RL, Kanki PJ. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53:1283–90. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oosterhout JJv, Brown L, Weigel R, Kumwenda JJ, Mzinganjira D, Saukila N, Mhango B, Hartung T, Phiri S, Hosseinipour MC. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health volume. 2009;14:856–861. doi: 10.1111/j.1365-3156.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 7.Waruru A, Muttai H, Ng’ang’a L, Ackers M, Kim A, Miruka F, Erick O, Okonji J, Ayuaya T, Schwarcz S. Positive Predictive Value of the WHO Clinical and Immunologic Criteria to Predict Viral Load Failure among Adults on First, or Second-Line Antiretroviral Therapy in Kenya. PLoS ONE. 2016;11:e0158881. doi: 10.1371/journal.pone.0158881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseinipour MC, Oosterhout JJv, Weigel R, Phiri S, Kamwendo D, Parkin N, Fiscus SA, Nelson JAE, Eron JJ, Kumwenda J. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vekemans M, John L, Colebunders R. When to switch for antiretroviral treatment failure in resource-limited settings? AIDS. 2007;21:1205–6. doi: 10.1097/QAD.0b013e3281c617e8. [DOI] [PubMed] [Google Scholar]
- 10.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22:2097–106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersen PS, Brox IK, Naman E, Bruun JN, Dyrhol-Riise AM, Trøseid M, Johannessen A. Antiretroviral treatment failure predicts mortality in rural Tanzania. Int J STD AIDS. 2015;26:633–639. doi: 10.1177/0956462414548460. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV Drug Resistance During First- and Second-Line Antiretroviral Therapy in Resource-Limited Settings. The J Infect Dis. 2013;207:S49–S56. doi: 10.1093/infdis/jit107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castelnuovo B, Sempa J, Agnes KN, Kamya MR, Manabe YC. Evaluation of WHO criteria for viral failure in patients on antiretroviral treatment in resource-limited settings. AIDS Res Treat. 2011 doi: 10.1155/2011/736938. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore DM, Awor A, Downing R, Kaplan J, Montaner JS, Hancock J, Were W, Mermin J. CD4+ T-cell count monitoring does not accurately identify HIV-infected adults with virologic failure receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;49:477–84. doi: 10.1097/QAI.0b013e318186eb18. [DOI] [PubMed] [Google Scholar]
- 15.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008;22:1971–7. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SJ, Nakigozi G, Newell K, Ndyanabo A, Galiwongo R, Boaz I, Quinn TC, Gray R, Wawer M, Serwadda D. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23:697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanapathipillai R, McGuire M, Mogha R, Szumilin E, Heinzelmann A, Pujades-Rodrı´guez M. Benefit of viral load testing for confirmation of immunological failure in HIV patients treated in rural Malawi. Trop Med Int Health. 2011;16:1495–1500. doi: 10.1111/j.1365-3156.2011.02874.x. [DOI] [PubMed] [Google Scholar]
- 18.Lutgarde Lynen M, Sokkab An M, Olivier Koole M, Sopheak Thai M, Seilavath Ros M, Paul De Munter M, Delphine Sculier M, Line Arnould M, Katrien Fransen M, Joris Menten M, Marleen Boelaert M, PhD, Jef Van den Ende M, PhD, Robert Colebunders M., PhD An Algorithm to Optimize Viral Load Testing in HIV-Positive Patients With Suspected First-Line Antiretroviral Therapy Failure in Cambodia. J Acquir Immune Defic Syndr. 2009;52:40–48. doi: 10.1097/QAI.0b013e3181af6705. [DOI] [PubMed] [Google Scholar]
- 19.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, Frank I, Maartens G, Nachega JB. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malawi MoH. Clinical Management of HIV in Children and Adults. Lilongwe: Ministry of Health, Malawi; 2016. [Google Scholar]
- 21.Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis : an official publication of the Infectious Diseases Society of America. 2016;62:1043–1048. doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecher S, Ellenberger D, Kim AA, Fonjungo PN, Agolory S, Borget MY, Broyles L, Carmona S, Chipungu G, De Cock KM, Deyde V, Downer M, Gupta S, Kaplan JE, Kiyaga C, Knight N, MacLeod W, Makumbi B, Muttai H, Mwangi C, Mwangi JW, Mwasekaga M, Ng'Ang AL, Pillay Y, Sarr A, Sawadogo S, Singer D, Stevens W, Toure CA, Nkengasong J. Scale-up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries. MMWR Morb Mortal Wkly Rep. 2015;64:1287–90. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health GoM. Malawi Intergrated HIV Program Report October – December 2015. Lilongwe: Ministry of Health, Malawi; 2015. pp. 26–27. [Google Scholar]
- 24.Labhardt ND, Lejone T, Poka M, Ehmer J, Pfeiffer K, Kiuvu PZ, Lynen L. A clinical prediction score in addition to WHO criteria for anti-retroviral treatment failure in resource-limited settings-experience from Lesotho. PLoS One. 2012;7:e47937. doi: 10.1371/journal.pone.0047937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurens M, Mungwira R, Nyirenda O, Divala T, Kanjala M, Muwalo F, Mkandawire F, Tsirizani L, Nyangulu W, Mwinjiwa E, Taylor T, Mallewa J, Blackwelder W, Plowe C, Laufer M, van Oosterhout J. TSCQ study: a randomized, controlled, open-label trial of daily trimethoprim-sulfamethoxazole or weekly chloroquine among adults on antiretroviral therapy in Malawi: study protocol for a randomized controlled trial. Trials. 2016;17:322. doi: 10.1186/s13063-016-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knill-Jones R. Diagnostic systems as an aid to clinical decision making. Br Med J (Clin Res Ed) 1987;295:1392–1396. doi: 10.1136/bmj.295.6610.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griensven Jv, Phan V, Thai S, Koole O, Lynen L. Simplified Clinical Prediction Scores to Target Viral Load Testing in Adults with Suspected First Line Treatment Failure in Phnom Penh, Cambodia. PLOS One. 2014;1:e87879. doi: 10.1371/journal.pone.0087879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouyannis M, Menten J, Kiragga A, Lynen L, Robertson G, Castelnuovo B, Manabe YC, Reynolds SJ, Robertsa L. Development and validation of systems for rational use of viral load testing in adults receiving first-line ART in sub-Saharan Africa. AIDS. 2011;25:1627–1635. doi: 10.1097/QAD.0b013e328349a414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meriki HD, Tufon KA, Afegenwi MH, Nyindem BA, Atanga PN, Anong DN, Cho-Ngwa F, Nkuo-Akenji T. Immuno-haematologic and virologic responses and predictors of virologic failure in HIV-1 infected adults on first-line antiretroviral therapy in Cameroon. Infect Dis Poverty. 2014;3 doi: 10.1186/2049-9957-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan V, Thai S, Koole O, Menten J, Meheus F, van Griensven J, Lynen L. Validation of a clinical prediction score to target viral load testing in adults with suspected first-line treatment failure in resource-constrained settings. J Acquir Immune Defic Syndr. 2013;62:509–16. doi: 10.1097/QAI.0b013e318285d28c. [DOI] [PubMed] [Google Scholar]
- 31.Estill J, Egger M, Johnson LF, Gsponer T, Wandeler G, Davies MA, Boulle A, Wood R, Garone D, Stringer JS, Hallett TB, Keiser O. Monitoring of antiretroviral therapy and mortality in HIV programmes in Malawi, South Africa and Zambia: mathematical modelling study. PLoS One. 2013;8:e57611. doi: 10.1371/journal.pone.0057611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandormael AM, Boulware DR, Tanser FC, Barnighausen TW, Stott KE, de Oliveira T. Brief Report: Virologic Monitoring Can Be a Cost-Effective Strategy to Diagnose Treatment Failure on First-Line ART. J Acquir Immune Defic Syndr. 2016;71:462–6. doi: 10.1097/QAI.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, Bonner K, Rousseau C, Garnett G, Cambiano V, Nakagawa F, Ford D, Bansi-Matharu L, Miners A, Lundgren JD, Eaton JW, Parkes-Ratanshi R, Katz Z, Maman D, Ford N, Vitoria M, Doherty M, Dowdy D, Nichols B, Murtagh M, Wareham M, Palamountain KM, Chakanyuka Musanhu C, Stevens W, Katzenstein D, Ciaranello A, Barnabas R, Braithwaite RS, Bendavid E, Nathoo KJ, van de Vijver D, Wilson DP, Holmes C, Bershteyn A, Walker S, Raizes E, Jani I, Nelson LJ, Peeling R, Terris-Prestholt F, Murungu J, Mutasa-Apollo T, Hallett TB, Revill P. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528:S68–S76. doi: 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutstein SE, Hosseinipour MC, Kamwendo D, Soko A, Mkandawire M, Biddle AK, Miller WC, Weinberger M, Wheeler SB, Sarr A, Gupta S, Chimbwandira F, Mwenda R, Kamiza S, Hoffman I, Mataya R. Dried blood spots for viral load monitoring in Malawi: feasible and effective. PLoS One. 2015;10:e0124748. doi: 10.1371/journal.pone.0124748. [DOI] [PMC free article] [PubMed] [Google Scholar]