Recently two overviews have appeared on cancer risks and surveillance in Beckwith-Wiedemann syndrome (BWS) [Maas et al., 2016; Mussa et al., 2016]. This has prompted us as an international group of researchers to initiate a similar study of “isolated hemihyperplasia” (IHH; OMIM 23500), as IHH shares with BWS the body asymmetry and risk to develop cancer [Clericuzio and Martin, 2009].
However, a major problem in designing this study is the definition and various terms that are used. The most commonly used terms are “isolated hemihyperplasia” and “isolated hemihypertrophy”, usually defined as proposed by Clericuzio and Martin [2009]: “asymmetric regional body overgrowth because of an underlying abnormality of cell proliferation in individuals without any other underlying diagnosis” to which the authors added as comments “there are no widely accepted criteria for defining IHH as distinct from normal growth variation in children, and therefore the pragmatic case definition is that IHH should be apparent ‘from the end of the bed’.”
We propose here to designate a novel term for this finding, formatted in accordance with the standards set by the Elements of Morphology [Allanson et al., 2009].
Lateralized Overgrowth
Definition: Significant increase in the length and/or girth of most or all of one side of the body compared to its contralateral side.
Synonyms: Segmental overgrowth
Replaces: Hemihyperplasia; Hemihypertrophy
Comments:
We replaced the term ‘hemi” as it is seemingly indicating that the overgrowth should be present at the same half of the body. However, the overgrowth can also be present in body parts that differ in body laterality.
The use of the terms “hyperplasia” or “hypertrophy” are problematic as this is a histologic description while histological proof is only rarely available. The term “overgrowth” indicates the same phenomenon without suggesting the histologic specificity. Furthermore, it is usually not known whether the difference in size between the left and right body half is caused by an overdevelopment of one body half or instead underdevelopment of the other body half, or a combination of these. For example, patients with asymmetry may be proven to carry H19 hypomethylation, which is mostly associated with undergrowth [Russo et al., 2016]. We acknowledge that typically the sign that is clinically apparent (‘from the end of the bed’) is the overdevelopment, and that underdevelopment may or may not be present. The terms “unilateral overgrowth” or “lateralized overgrowth” describe better what can be visualized during physical exam. We prefer the term ‘lateralized’ as the term ‘unilateral’ seemingly indicates the overgrowth is present on one side while overgrowth can be crossed or restricted to different sides of the body. We also considered the term “asymmetrical overgrowth” but then overgrowth in the upper versus the lower body segment would also fulfill this description, and entities such as various forms of lipodystrophy would fulfill the description as well while these constitute a different type of disorders.
The definition does not specify the component(s) of the increased size (bone, connective tissue, blood vessels, muscles, etc.) as any combination can occur. It should however exclude edema as this is not overgrowth.
Overgrowth of an organ (including asymmetry of kidneys) may or may not be present and is not a prerequisite for the finding.
We refrain deliberately from defining the term ‘significant’ which we use in the definition. Our clinical experience suggests that determining a degree of asymmetry between left and right side of the body by inspection, palpation or measurements is not sufficiently reliable. Additionally, the previous definition ‘from the end of the bed’ may not account for clinically apparent but more subtle cases. We estimate that in clinical practice differences in length or girth of 10% can usually be determined, and experienced physicians may even determine smaller differences. However, we prefer not to use subjective criteria in the definition that are dependent on the experience of the physician. Objective techniques such as measurements using 3D imaging technology and DEXA scanning may be more useful and need to be studied in more detail [Waelchli et al., 2015; Bazzocchi et al., 2016; Ng et al., 2016]. Techniques that allow the combination of soft and skeletal tissue measurements may prove to be especially useful [Ibrahim et al., 2016]. As physiological variations in symmetry exist between populations and body parts if determined by direct measurements of bones [Auerbach and Ruff, 2006], normal values using such techniques need to be developed as well. Until results of such studies are available, we propose to leave it to the discretion of the examining clinician to decide if a difference in size between left and right side of (or part of) the body is significant.
Using this definition of lateral overgrowth, we subsequently redefine the diagnosis of “isolated (or non-syndromic) hemihyperplasia” and “isolated (or non-syndromic) hemihypertrophy” as “isolated lateralized overgrowth.” Isolated lateralized overgrowth is lateralized overgrowth in the absence of a recognizable pattern of major or minor malformations, dysplasias, or morphologic variants [Hennekam et al., 2013]. We recognize that isolated lateralized overgrowth is likely genetically heterogeneous and may be part of currently unrecognized patterns of malformation. Subdivision into separate entities may become possible if sufficiently large numbers of affected individuals are carefully studied clinically and molecularly, as was accomplished with Proteus syndrome (PMID 16883308), CLOVES syndrome (PMID 17963221), and ‘Hemihyperplasia’ with multiple lipomatosis (HHML, PMID 9781913). These entities were split based on careful phenotyping before their etiologic basis was discovered. This may allow separation of groups of affected individuals based on their molecular background or based on a pattern of concomitant subtle features that becomes evident. Such subdivisions may also indicate differences in nature, age of onset, reaction to various management schemes, prognosis, and risks for cancer in the affected individuals and their families, similar to what the recent studies in BWS have shown [Maas et al., 2016; Mussa et al., 2016].
In summary, this definition of lateralized overgrowth is a necessary first step towards characterization and determination of diagnostic subdivisions within the category of isolated lateralized overgrowth to thereby determine additional related clinical features and molecular etiology.
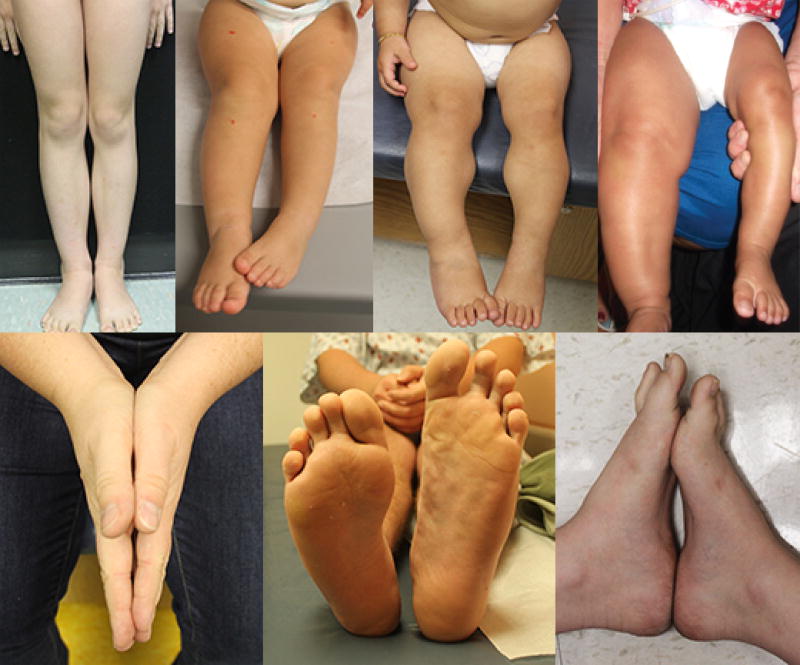
Figure 1.
A series of examples of individuals with isolated lateralized overgrowth.
References
- Allanson JE, Biesecker LG, Carey JC, Hennekam RC. Elements of morphology: introduction. Am J Med Genet A. 2009;149A:2–5. doi: 10.1002/ajmg.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BM, Ruff CB. Limb bone bilateral asymmetry: variability and commonality among modern humans. J Hum Evol. 2006;50:203–218. doi: 10.1016/j.jhevol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Bazzocchi A, Ponti F, Albisinni U, Battista G, Guglielmi G. DXA: Technical aspects and application. Eur J Radiol. 2016;85:1481–1492. doi: 10.1016/j.ejrad.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Clericuzio CL, Martin RA. Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med. 2009;11:220–222. doi: 10.1097/GIM.0b013e31819436cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC, Biesecker LG, Allanson JE, Hall JG, Opitz JM, Temple IK, Carey JC, Elements of Morphology C. Elements of morphology: general terms for congenital anomalies. Am J Med Genet A. 2013;161A:2726–2733. doi: 10.1002/ajmg.a.36249. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Suttie M, Bulstrode NW, Britto JA, Dunaway D, Hammond P, Ferretti P. Combined soft and skeletal tissue modelling of normal and dysmorphic midface postnatal development. J Craniomaxillofac Surg. 2016;44:1777–1785. doi: 10.1016/j.jcms.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas SM, Vansenne F, Kadouch DJ, Ibrahim A, Bliek J, Hopman S, Mannens MM, Merks JH, Maher ER, Hennekam RC. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A. 2016;170:2248–2260. doi: 10.1002/ajmg.a.37801. [DOI] [PubMed] [Google Scholar]
- Mussa A, Molinatto C, Baldassarre G, Riberi E, Russo S, Larizza L, Riccio A, Ferrero GB. Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J Pediatr. 2016;176:142–149. e141. doi: 10.1016/j.jpeds.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Ng BK, Hinton BJ, Fan B, Kanaya AM, Shepherd JA. Clinical anthropometrics and body composition from 3D whole-body surface scans. Eur J Clin Nutr. 2016;70:1265–1270. doi: 10.1038/ejcn.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Calzari L, Mussa A, Mainini E, Cassina M, Di Candia S, Clementi M, Guzzetti S, Tabano S, Miozzo M, Sirchia S, Finelli P, Prontera P, Maitz S, Sorge G, Calcagno A, Maghnie M, Divizia MT, Melis D, Manfredini E, Ferrero GB, Pecile V, Larizza L. A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver-Russell and Beckwith-Wiedemann syndromes. Clin Epigenetics. 2016;8:23. doi: 10.1186/s13148-016-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelchli R, Williams J, Cole T, Dattani M, Hindmarsh P, Kennedy H, Martinez A, Khan S, Semple RK, White A, Sebire N, Healy E, Moore G, Kinsler VA. Growth and hormone profiling in children with congenital melanocytic naevi. Br J Dermatol. 2015;173:1471–1478. doi: 10.1111/bjd.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]