Abstract
Objectives
The aim of this study was to evaluate the effects of herbal extracts on bone regeneration. Two known samples were screened.
Materials and Methods
We previously established a rat calvaria defect model using a combination of collagen scaffold and herbal extracts. An 8 mm diameter trephine bur with a low-speed dental hand piece was used to create a circular calvaria defect. The experimental group was divided into 4 classifications: control, collagen matrix, Danshen with collagen, and Ge Gan with collagen. Animals in each group were sacrificed at 4, 6, 8, and 10 weeks after surgery, and bone regeneration ability was evaluated by histological examination.
Results
Results revealed that both Danshen and Ge Gan extracts increased bone formation activity when used with collagen matrix. All groups showed almost the same histological findings until 6 weeks. However, after 6 weeks, bone formation activity proceeded differently in each group. In the experimental groups, new bone formation activity was found continuously up to 10 weeks. In the Danshen and Ge Gan groups, grafted materials were still present until 10 weeks after treatment, as evidenced by foreign body reactions showing multinucleated giant cells in chronic inflammatory vascular connective tissue.
Conclusion
Histological analyses showed that Danshen and Ge Gan extractions increased bone formation activity when used in conjunction with collagen matrix.
Keywords: Bone regeneration, Herb extract, Danshen, Ge Gan, Rat calvaria defect
I. Introduction
Bone defects are caused by tumors, cysts, infections, trauma, and aging. They play a significant role in mastication, as well as in esthetics. Slow bone regeneration in such defects can pose therapeutic problems. Various methods are currently used to obtain adequate defect closure including autogenous bone grafting, allogenic bone grafting, and application of growth factors, polymeric membranes, and enamel matrix derivatives1,2,3,4,5,6.
Herbal medicines have been used for centuries to treat a variety of diseases and conditions including promotion of fractured bone healing and bone diseases such as osteoporosis7. Puerariae radix, also known as Ge Gen, comes from the root of Pueraria lobata; this commonly used traditional medicine has been demonstrated to decrease loss in bone density8 as well as treat fever9, liver diseases10, and cardiovascular disease11. Danshen or Radix salvia miltiorrhiza is another common traditional medicine that has been used to treat cardiovascular disease, improve perfusion of ischemic myocardium, and enhance blood circulation12,13,14. Several studies have examined the effectiveness of Danshen in promoting healing in bone fracture15,16.
Like aspirin (from leaves of the willow tree, nonsteroidal antiinflammatory drug), Taxol (from the cortex of Taxus brevifolia, chemotherapy drug), and Stillen (from leaves of Artemisia argyi, antiulcerant), several studies using herbal medicine have been and are still being performed. The aim of this study was to evaluate the osteogenic capacity of Ge Gen and Danshen extracts and to establish a screening system of herbal medicines.
II. Materials and Methods
1. Experimental materials and animals
Forty-eight Sprague-Dawley male rats, 8 weeks old, weighing 250 g were selected for the study. Rat size increases rapidly during this stage, providing a favorable model for studying normal bone healing. Before the study, the animals were housed in an environmentally controlled animal facility for 1 week for acclimatization.
An 8 mm diameter trephine bur with a low-speed dental hand piece was used to create circular calvaria defects. Collagen membranes (Rapi-Plug; Dalim Tissen, Seoul, Korea) were used to fill the defects in the experimental group.
Animal selection and management, surgical protocol, and preparation followed routines approved by the Institutional Animal Care and Use Committee of Inha University (INHA 160802-426).
2. Preparation of herb extraction
Crude Danshen and Ge Gan were extracted with 70% methanol reflux for 3 hours at 70°C. The liquid/solid ratio was 1:10. The extracts were lyophilized and sealed in sterilized tubes. Before starting the experiment, 4 mL distilled water was added to 4 g of each lyophilized powder. The collagen matrix was soaked for 15 minutes before grafting in the Danshen and Ge Gan groups.
3. Surgical procedure
Surgery was conducted on all rats under sterile conditions. General anesthesia was induced by intramuscular injection of 10 mg/kg xylazine HCl and 40 mg/kg ketamine HCl and was maintained by injection of the same mixture in half doses. Rat skulls were shaved and sterilized using 70% ethanol. An injection of 2% lidocaine HCl 1.8% containing 1:100,000 epinephrine was administrated locally at the operation site to reduce bleeding.
The bone surface of the calvaria was exposed by a 3 cm longitudinal midline incision along the sagittal suture. In each parietal bone, a standardized round defect was created using an 8 mm diameter trephine bur in a low-speed hand piece. To prevent overheating of the bone, the operation site was irrigated continuously with sterile saline solution.
The defects were grafted with different materials. In the Danshen and Ge Gan groups, the defects were filled with a collagen matrix containing Danshen or Ge Gan extract. In the collagen group, the defects were filled with collagen matrix containing saline; in the control group, the defects were left untreated. The graft materials were prepared 15 minutes before grafting.
All experimental areas were closed with 4.0 black surgical silks. Postoperatively, all rats received an intramuscular injection of 25,000 IU/kg penicillin G once daily for 3 days.
4. Histological preparation
Animals were sacrificed by CO2 inhalation at 4, 6, 8, and 10 weeks after surgery. Defects and surrounding tissues were removed for histological preparation and immediately fixed by immersion in 10% neutral buffered formalin.
The specimens was dehydrated in 70% ethanol and then embedded in methacrylate-based resin. Polymerization of the embedding solution occurred in a photopolymerization unit (EXAKT Apparatebau, Norderstedt, Germany) with exposure to daylight for 2 hours and to ultraviolet light for 10 hours. Each polymerized block was affixed to the vacuum head of the EXAKT macrocutter, and sections were sliced to a thickness of approximately 100 µm. Sections were ground and polished using the EXAKT micro grinder to a thickness of 15 µm, mounted on microscope slides, stained with hematoxylin and eosin, and coverslipped. Bone healing patterns were observed under a light microscope (BX50; Olympus, Tokyo, Japan).
III. Results
In total, 4 study groups underwent microscopic evaluation based on the injected biomaterial. Each group was tested at four time intervals, and none of them showed complete filling of the defect with new bone.
In all groups, the 4 and 6 week time points showed almost the same histological findings. Activated osteoblasts rimming newly formed woven bone (Fig. 1; double arrows) were observed in the bone repair areas. Osteocytes were clearly distinguishable at the inner parts of newly formed bone, indicating direct bone repair. The presence of granulation tissue was characterized by numerous newly formed small blood vessels, an intense inflammatory cell infiltrate, and immature connective tissue with large amounts of fibroblasts and immature collagen fiber bundles in the center of the bone defect area.
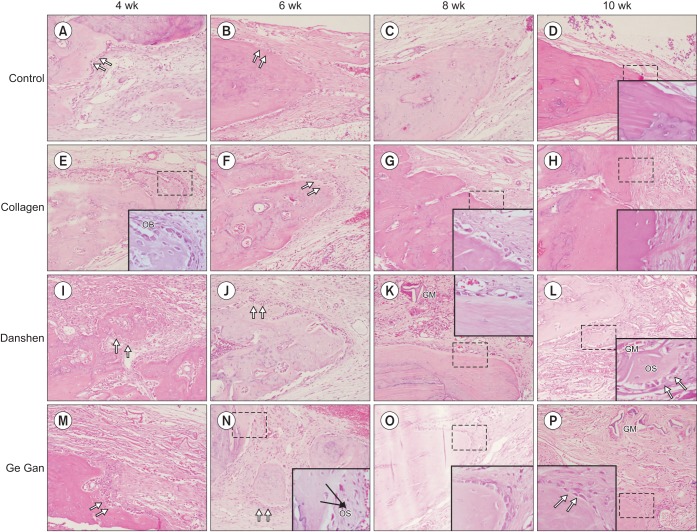
Fig. 1. Representative H&E sections depicting the new bone formation at 4, 6, 8, and 10 weeks for each groups (H&E staining, A–P: ×200, inset: ×1,000). (OB: osteoblasts [double arrows], GM: grafted material, OS: osteoid).
Interestingly, bone formation activity was attenuated at different time points in each group. In the control group, bone formation was reduced at 6 weeks. Fibroblast and collagen fiber volume density significantly increased in the first 6 weeks, followed by a reduction in the subsequent periods (8 and 10 weeks). In the collagen group, activated osteoblasts rimming newly formed woven bone were seen at 6 weeks, but from 8 weeks on, peripheral osteoid and osteoblastic rimming were markedly reduced. By 10 weeks, a mineralized bone margin was found without osteoblastic rimming. In contrast, in the Danshen and Ge Gan groups, osteoid formation with osteoblasts rimming was found continuously in the trabecular bone surface at the edges and the center of the defect at 8 and 10 weeks, respectively.(Fig. 1)
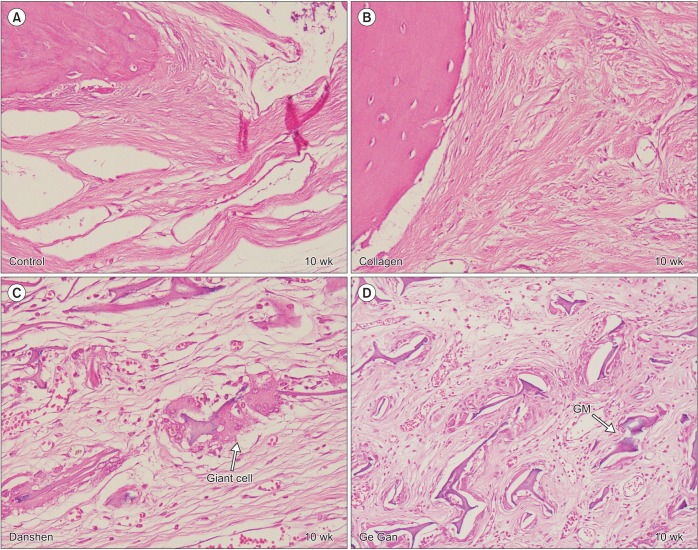
Grafted biomaterials were resorbed at different time points for each group. For example, in the collagen group, only one section showed graft material at 10 weeks; most of them totally resorbed in 6 weeks. At 10 weeks, the collagen group showed a defect area filled with fibroblastic dense collagen tissue without inflammation. However, in the Danshen and Ge Gan groups, grafted materials were present until 10 weeks, as evidenced by a foreign body reaction showing multinucleated giant cells in chronic inflammatory vascular connective tissue.(Fig. 2)
Fig. 2. Representative H&E section depicting healing process in defect area at 10-week trial (H&E staining, A–D: ×400). A, B. Fibroblastic dense collagen tissue without inflammation in control and collagen group. C, D. Grafted materials (GMs) are surrounded by foreign body reaction showing multinucleated giant cells in chronic inflammatory vascular connective tissue in Danshen and Ge Gan groups, respectively.
These results were divided into seven classifications and are summarized in Table 1.
Table 1. Histological findings at 4, 6, 8, and 10 weeks for each groups.
| Group | Trial time (wk) | Inflammation | Granulation tissue | Fibrosis | Osteoid | Osteoblastic rimming | Mineralized bone | Foreign body reaction |
|---|---|---|---|---|---|---|---|---|
| Control | 4 | + | + | + | + | + | + | − |
| 6 | ± | ± | + | + | + | + | − | |
| 8 | ± | ± | + | − | − | + | − | |
| 10 | ± | ± | + | − | − | + | − | |
| Collagen | 4 | + | + | + | + | + | + | − |
| 6 | + | + | + | + | + | + | − | |
| 8 | − | − | + | − | ± | + | − | |
| 10 | − | − | + | − | − | + | − | |
| Danshen | 4 | + | + | + | + | + | + | − |
| 6 | + | + | + | + | + | + | − | |
| 8 | + | + | + | + | + | + | + | |
| 10 | + | + | + | ± | ± | + | + | |
| Ge Gan | 4 | + | + | + | + | + | + | − |
| 6 | + | + | + | + | + | + | − | |
| 8 | ± | ± | + | ± | ± | + | + | |
| 10 | ± | ± | + | ± | ± | + | + |
(+: positive, −: negative)
IV. Discussion
The demand for use of alloplastic materials for bone repair is increasing since there are limitations in grafting autogenous bone. Autogenous bone grafts require additional surgery and are associated with donor site morbidity, including infection, pain, and hematoma17. To develop more effective bone graft materials with enhanced osteogenic properties, alloplastic materials are combined with osteoinductive substances. Many in vitro and in vivo studies have shown the positive effects of herbs on bone formation by the promotion of osteoblastic proliferation and inhibition of osteoclastic formation18. However, questions regarding, mechanism of action, main compounds, and experimental systems remain. Thus, it is worth evaluating herbal extracts to analyze their osteogenic enhancement properties.
Bone is a highly vascularized tissue. To maintain homeostasis and regeneration, the development of microvasculature and microcirculation is crucial19; this process is known as angiogenesis. The formation of new vasculature transports oxygen and nutrients to bone tissues that can differentiate into osteoblasts, chondroclasts, and osteoclasts20. Many experiments have shown that endothelial cells provide a microvasculature during bone remodeling, and this angiogenesis is necessary for bone formation21,22. Hence, the processes of osteogenesis and angiogenesis are closely correlated. Danshen and Ge Gan have both been reported to have angiogenesis and osteogenesis activity23.
Danshen, or Radix salvia miltiorrhiza, is a common traditional medicine that has been used to treat cardiovascular disease, improve perfusion of ischemic myocardium, and enhance blood circulation. Several studies have examined the effectiveness of Danshen in promoting osteogenesis. Chin et al.24 reported an in vivo study showing that Danshen enhanced bone remodeling by regulating the gene expression of alkaline phosphatase (ALP), osteocalcin (OCN), osteoprotegerin (OPG), and receptor activator of nuclear factor kappa-B ligand (RANKL). An in vivo study by Wong and Rabie16 found that Danshen significantly promoted new bone formation when an extract in collagen matrix was placed in bone defects. Lay et al.25 showed that Danshen extract and salvianolic acid B (a component of Danshen) enhanced angiogenesis in a murine SVEN 1 ras (SVR) endothelial cell line by up-regulating vascular endothelial growth factor (VEGF) and VEGF-R2 gene expression.
Ge Gan or Puerariae radix comes from the root of Pueraria lobata and has been shown to have effects on decreasing loss in bone density. Wang et al.8 examined the role of Ge Gan in bone metabolism by feeding OVX mice a diet containing different doses of the herb. They suggest that Ge Gan may prevent osteoporosis in post-menopausal women. Huh et al.26 found that Ge Gan increased the mRNA expression of VEGF and dose-dependently increased ALP activity related to osteoblastic activity. Zhang et al.27 found that Ge Gan was able to induce angiogenesis in an ischemic myocardial infarction model.
In this study, we observed several interesting results. Bone formation activity was attenuated at different time points in each group. In the control group, bone formation was reduced at 6 weeks. In the collagen group, osteoid and osteoblastic rimming were found until 8 weeks. In the Danshen and Ge Gan groups, bone formation was found at 10 weeks. This comparison showed that Danshen and Ge Gan extractions increased bone formation activity when used with a collagen matrix. The mechanism of this effect is likely angiogenesis. However, in the Danshen and Ge Gan groups, grafted materials were still present at 10 weeks, as evidenced by foreign body reaction showing multinucleated giant cells in chronic inflammatory vascular connective tissue. Thus, angiogenesis mechanisms cannot rule out the inflammation effect, and further study is needed to understand the mechanism of inflammatory vascular connective tissue.
Pryor et al.28 evaluated the validity of radiographic evaluations of bone formation in a critical-size rat calvaria osteotomy defect model. Bilateral, calvaria osteotomy defects in rats treated with a platelet-rich plasma preparation or control treatments were evaluated by radiographic and histometric measures following a 4- or 8-week healing interval. They reported low accuracy of radiographic evaluations employed in identifying and characterizing bone filling in the rat calvaria osteotomy defects. Nyan et al.29 treated rat calvaria defects using calcium sulfate and simvastatin. They observed the smallest amount of bone formation at 2 and 4 weeks but remarkable bone formation at 8 weeks. According to their findings, bone formation in the experimental group seemed to be delayed as a result of soft tissue inflammation, but graft materials appeared to promote bone formation in spite of the inflammatory response. Yoon et al.30 implanted adipose-derived stem cells (ADSCs) with polymer constructs in rat calvaria defect. They reported that, at 12 weeks post-implantation, all of the cell-loaded defects were partly filled with newly formed bone. Partial solid osseous bridging of the defect was also noted. In this study, all groups showed the same level of activated osteoblasts and rimmed newly formed woven bone until 6 weeks. After 6 weeks, bone formation activity was different in each group. In the experimental groups, bone formation activity was found continuously up to 10 weeks. Our results suggest that it is appropriate to evaluate the time of new bone formation by histologic observation 8 to 10 weeks after surgery.
V. Conclusion
In this in vivo experiment on rat calvarial bone defect, histological analyses showed that Danshen and Ge Gan extractions increased bone formation activity when used with collagen matrix. The mechanism of this effect is likely angiogenesis. However, it is possible that this behavior is simply an inflammation effect.
Footnotes
Authors' Contributions: Conception and design: D.H.L. and I.K.K. Performed the experiments: D.H.L., H.Y.C., J.H.S., and J.M.J. Analysis and interpretation of data: D.H.L., I.K.K., and J.K. Writing, review, and/or revision of the manuscript: D.H.L. and I.K.K. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate: Animal selection and management, surgical protocol, and preparation followed routines approved by the Institutional Animal Care and Use Committee of Inha University (INHA 160802-426).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Park JC, Kim YH, Choi HS, Oh JS, Shin SH, Kim YD. The rate and stability of mandibular block bone graft in recent 5 years. Maxillofac Plast Reconstr Surg. 2017;39:21. doi: 10.1186/s40902-017-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burg KJ, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 3.Gomes KU, Carlini JL, Biron C, Rapoport A, Dedivitis RA. Use of allogeneic bone graft in maxillary reconstruction for installation of dental implants. J Oral Maxillofac Surg. 2008;66:2335–2338. doi: 10.1016/j.joms.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Peng W, Kim IK, Cho HY, Seo JH, Lee DH, Jang JM, et al. The healing effect of platelet-rich plasma on xenograft in peri-implant bone defects in rabbits. Maxillofac Plast Reconstr Surg. 2016;38:16. doi: 10.1186/s40902-016-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lekovic V, Camargo PM, Weinlaender M, Nedic M, Aleksic Z, Kenney EB. A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol. 2000;71:1110–1116. doi: 10.1902/jop.2000.71.7.1110. [DOI] [PubMed] [Google Scholar]
- 6.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmöller M, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZQ, Li JL, Sun YL, Yao M, Gao J, Yang Z, et al. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evid Based Complement Alternat Med. 2013;2013:356260. doi: 10.1155/2013/356260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Wu J, Chiba H, Umegaki K, Yamada K, Ishimi Y. Puerariae radix prevents bone loss in ovariectomized mice. J Bone Miner Metab. 2003;21:268–275. doi: 10.1007/s00774-003-0420-z. [DOI] [PubMed] [Google Scholar]
- 9.Chueh FS, Chang CP, Chio CC, Lin MT. Puerarin acts through brain serotonergic mechanisms to induce thermal effects. J Pharmacol Sci. 2004;96:420–427. doi: 10.1254/jphs.fp0040424. [DOI] [PubMed] [Google Scholar]
- 10.Overstreet DH, Lee YW, Rezvani AH, Pei YH, Criswell HE, Janowsky DS. Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang LY, Zhao AP, Chai XS. Effects of puerarin on cat vascular smooth muscle in vitro. Zhongguo Yao Li Xue Bao. 1994;15:180–182. [PubMed] [Google Scholar]
- 12.Chang PN, Mao JC, Huang SH, Ning L, Wang ZJ, On T, et al. Analysis of cardioprotective effects using purified Salvia miltiorrhiza extract on isolated rat hearts. J Pharmacol Sci. 2006;101:245–249. doi: 10.1254/jphs.fpj05034x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 15.Liu YR, Qu SX, Maitz MF, Tan R, Weng J. The effect of the major components of Salvia Miltiorrhiza Bunge on bone marrow cells. J Ethnopharmacol. 2007;111:573–583. doi: 10.1016/j.jep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Wong RW, Rabie AB. Effect of Salvia miltiorrhiza extract on bone formation. J Biomed Mater Res A. 2008;85:506–512. doi: 10.1002/jbm.a.31577. [DOI] [PubMed] [Google Scholar]
- 17.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Wong RW, Rabie AB. Traditional Chinese medicines and bone formation--a review. J Oral Maxillofac Surg. 2006;64:828–837. doi: 10.1016/j.joms.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Schmid J, Wallkamm B, Hämmerle CH, Gogolewski S, Lang NP. The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin Oral Implants Res. 1997;8:244–248. doi: 10.1034/j.1600-0501.1997.080311.x. [DOI] [PubMed] [Google Scholar]
- 20.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 21.Rabie AB, Shum L, Chayanupatkul A. VEGF and bone formation in the glenoid fossa during forward mandibular positioning. Am J Orthod Dentofacial Orthop. 2002;122:202–209. doi: 10.1067/mod.2002.125991. [DOI] [PubMed] [Google Scholar]
- 22.Augustin G, Antabak A, Davila S. The periosteum. Part 1: anatomy, histology and molecular biology. Injury. 2007;38:1115–1130. doi: 10.1016/j.injury.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Chin A, Zhang L, Lu J, Wong RW. The role of traditional Chinese medicines in osteogenesis and angiogenesis. Phytother Res. 2014;28:1–8. doi: 10.1002/ptr.4959. [DOI] [PubMed] [Google Scholar]
- 24.Chin A, Yang Y, Chai L, Wong RW, Rabie AB. Effects of medicinal herb Salvia miltiorrhiza on osteoblastic cells in vitro. J Orthop Res. 2011;29:1059–1063. doi: 10.1002/jor.21376. [DOI] [PubMed] [Google Scholar]
- 25.Lay IS, Chiu JH, Shiao MS, Lui WY, Wu CW. Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Med. 2003;69:26–32. doi: 10.1055/s-2003-37034. [DOI] [PubMed] [Google Scholar]
- 26.Huh JE, Yang HR, Park DS, Choi DY, Baek YH, Cho EM, et al. Puerariae radix promotes differentiation and mineralization in human osteoblast-like SaOS-2 cells. J Ethnopharmacol. 2006;104:345–350. doi: 10.1016/j.jep.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Chen S, Shen Y, Yang D, Liu X, Sun-Chi AC, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29:945–950. doi: 10.1248/bpb.29.945. [DOI] [PubMed] [Google Scholar]
- 28.Pryor ME, Susin C, Wikesjö UM. Validity of radiographic evaluations of bone formation in a rat calvaria osteotomy defect model. J Clin Periodontol. 2006;33:455–460. doi: 10.1111/j.1600-051X.2006.00921.x. [DOI] [PubMed] [Google Scholar]
- 29.Nyan M, Sato D, Oda M, Machida T, Kobayashi H, Nakamura T, et al. Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. J Pharmacol Sci. 2007;104:384–386. doi: 10.1254/jphs.sc0070184. [DOI] [PubMed] [Google Scholar]
- 30.Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]