Abstract
BACKGROUND
The P3 component of the event-related potential (ERP) has been particularly useful in alcohol research for identifying endophenotypes of alcohol use disorder (AUD) risk in sober subjects. However, practice and/or fatigue reduces P3 amplitude, limiting the ability to ascertain acute and adaptive effects of alcohol exposure. Here, we report acute alcohol effects on P3 amplitude and latency using an adaptive stop signal task (aSST).
METHODS
One hundred and forty eight nondependent moderate to heavy social drinkers, age 21 to 27, participated in 2 single-blind, alcohol or placebo, counterbalanced sessions approximately one week apart. During each session, subjects performed an adaptive stop signal task (aSST) at (1) baseline, (2) upon reaching the target 60 mg/dL breath alcohol concentration or at the equivalent time during the placebo session, and (3) approximately 135 minutes later while the breath alcohol concentration was clamped. Here, we report on differences between baseline and first subsequent measurements across the experimental sessions. During each aSST run, the stop signal delay (SSD, the time between stop and go signals) adjusted trial-by-trial based on the subject’s performance.
RESULTS
The aSST reliably generated a STOP P3 component that did not change significantly with repeated task performance. The pre-infusion SSD distribution was bimodal, with mean values several hundred msec apart (FAST: 153 msec and SLOW: 390 msec). This suggested different response strategies: FAST SSD favoring “going” over “stopping,” and SLOW SSD favoring “stopping” over “going”. Exposure to alcohol at 60 mg/dL differentially affected the amplitude and latency of the STOP P3 according to SSD group. Alcohol significantly reduced P3 amplitude in the SLOW SSD compared to FAST SSD group, but significantly increased P3 latency in the FAST SSD compared to SLOW SSD group.
CONCLUSIONS
The aSST is a robust and sensitive task for detecting alcohol induced changes in inhibition behavior as measured by the P3 component in a within subject design. Alcohol was associated with P3 component changes which varied by SSD group, suggesting a differential effect as a function of task strategy. Overall, the data support the potential utility of the aSST in the detection of alcohol response related AUD risk.
Keywords: P300, Event-related potential (ERP), response inhibition, response strategy, alcohol
INTRODUCTION
Scalp Event-Related Potentials (ERPs) reflect neurophysiological responses to discrete events. ERPs are characterized by several key components (e.g., P2, N2, P3, P3a, P3b) related to the neural network response to stimuli and cognitive events. In contrast to fMRI, which can locate sources of neural activity but provide meager temporal resolution, ERPs provide rich temporal information about neural processing activity, but less spatial resolution. The scalp topography and temporal properties of ERPs using P3 paradigms obtained in a sober state have proven useful as endophenotypes of risk for alcohol use disorder (AUD) (e.g., Euser et al., 2012, Justus et al., 2001, Kamarajan et al., 2005, Porjesz, et al., 2005) and for neural systems research in general (e.g., Di Giorgio et al., 2015, Motlagh et al., 2016, Downes et al., 2017).
Our laboratory seeks responses to alcohol that are novel endophenotypes of alcohol use disorder (AUD) risk, using intravenous alcohol administration to prescribe identical brain exposures in all subjects (Plawecki et al., 2008, Ramchandani et al., 1999b, O’Connor et al., 1998). ERPs are promising candidates among the various behavioral, subjective, and neurophysiologic responses to alcohol exposure. The P3 component is particularly appealing because it is genetically influenced, varies substantially in morphology across subjects (yet very little within subjects), reflects cognitive processes known to be impaired by exposure to alcohol, and has been shown to be associated with AUD risk in the sober state (Cohen et al., 1997, Perlman et al., 2013). Alcohol exposure is believed to reduce maximum P3 amplitude (|P3|), lengthen latency (λP3) to |P3|, and perhaps shift the scalp locus of peak P3 activity (if alcohol has a differential hemispheric influence on P3 generators) (Lukas et al., 1990, Martin and Garfield, 2006, Lewis et al., 2013).
Demonstrating a reliable alcohol effect on P3 activity is difficult. Detection of an alcohol effect on cognitive components of an ERP ideally include a comparison of intoxicated performance to baseline activity within an individual, thus requiring repetition of the paradigm. However, standard P3 paradigms are sensitive to practice and fatigue effects, as both stimulus novelty and importance influence the evoked response to target stimuli (Segalowitz et al., 2001). Specifically, reduced novelty from repetition alters the P3. Thus, most studies of alcohol’s influence on P3 activity implement an across subject design (Lewis et al., 2013, Sanchez-Roige et al., 2016, Easdon et al., 2005).
To isolate the effect of alcohol on individuals, our study design compares the acute alcohol response during a clamped breath alcohol concentration (BrAC) to baseline, accounting for changes from the same measure performed during a separate, counterbalanced placebo infusion session. Thus, our clamping protocol requires that each subject performs an ERP paradigm a minimum of 4 times, compounding the influence of fatigue and practice on the results. Our prior studies reflect this challenge, and have not shown within-subject changes in P3 after alcohol as compared to placebo, likely due to practice effects. As documenting the sensitivity of P3 ERP paradigms to alcohol remains of high interest for detecting potential endophenotypes of AUD risk, alternate approaches are needed.
Inhibitory capacity, as well as alcohol’s effect upon it, is important to identify risk factors associated with developing an AUD. A predisposition toward risky and premature responding (impulsivity) has been repeatedly demonstrated to be a risk factor for AUD (e.g., de Wit, 2009, Dick et al., 2010, Jentsch et al., 2014, Lejuez et al., 2010, Verdejo-Garcia et al., 2008). Stop Signal task (SST) performance requires inhibiting learned reflexes (i.e., inhibit the pre-potent motor response for the GO stimulus when followed by the STOP stimulus) and is thought to be a marker of impulsivity. Oral alcohol challenge affects impulsivity, but with variable effects across exposures, tasks, and populations (e.g., Reed et al., 2012, Ortner et al., 2003, Finn et al., 1999, Dougherty et al., 2008, Reynolds et al., 2006, Fillmore et al., 2008). Brain activity induced by the SST alone, as well as affected by alcohol, may also be informative in studying AUD risk (Kareken et al., 2013, Weafer et al., 2015). Under the horse-race model of the SST, the GO and STOP responses are considered to be independent processes, with successful inhibition determined by the difference in their completion. However, data suggests that, “subjects can make proactive response-strategy adjustments on a trial by trial basis” (Aron, 2011; Verbruggen and Logan, 2009) making interpretation more challenging.
The scalp ERP of the SST is also complex. Each SST trial always includes a fixation and a pure GO stimulus, with a subset of trials including a STOP stimulus following the GO signal after a stop signal delay (SSD, msec). Each stimulus elicits a brain response reflected in the EEG recorded from the scalp. Inherent to the SST paradigm, some of these evoked brain responses overlap in time, necessitating various filtering approaches to separate them (e.g., Bekker et al., 2005; de Jong et al., 1990; Dimoska et al., 2006; Dimoska et al., 2003; Pliszka et al., 2000; Woldorff, 1993). The literature reports a relationship between STOP P3 amplitude during response inhibition and experimental alcohol consumption (Jones et al., 2013), as well as electrophysiological differences in “fast” versus “slow” responders (van Boxtel et al., 2001, Band et al., 2003, Dimoska et al., 2006). However, none of these studies used a within-subject design to dissociate the influences of practice versus brain exposure to alcohol and none of the ERP alcohol challenge studies used intravenous infusion of alcohol to overcome the nearly 3-fold variation in BrAC trajectories following ingestion of standard oral doses of alcohol (Ramchandani et al., 1999b).
We examined how exposure to alcohol at 60 mg/dL affects the spatio-temporal features of the average electrophysiological response to STOP signal stimuli. This response reflects the relative import of the stimulus, and possibly the behavioral strategy used. Further, knowledge of the SSD delay (between the onsets of GO and STOP stimuli) offers the chance to derive the P3 component response to STOP prompts that are uncontaminated by the unfinished response to the GO stimulus.
Here we examine the placebo-controlled, within-subject initial response to alcohol in STOP signal P3 component using a real-time, adaptive SST task reported elsewhere in brain imaging work by our group (aSST; Kareken et al., 2013; Weafer, et al., 2015). aSST adjusts the SSD on each STOP trial, lengthening it by 50 msec after a subject successfully withholds the GO response following a STOP stimulus or shortening the SSD if the subject responded despite the STOP stimulus. Over many trials, the task ideally finds the SSD at which subjects respond to 50% of the STOP prompts with the correct behavioral inaction. Subjects performed the aSST thrice in each of two sessions: at an infusion-free baseline, following acquisition of a clamped 60 mg% target BrAC (or at a matched time-point during the placebo infusion), and again after prolonged alcohol exposure. We hypothesized that alcohol would alter the STOP P3, and that these changes would be eventually useful as an endophenotypic markers of AUD risk. Here we report the effect of acute alcohol on |P3| and λP3 as defined by changes across baseline and upon reaching the target BrAC, and accounting for similar changes across a placebo infusion session.
METHODS
GPRA Study Design
The parent study was designed to examine the effects of single nucleotide polymorphisms (SNPs) previously associated with AUD risk and other known risk factors on the effects of a controlled brain exposure to alcohol for several dependent measures. Appraisals included resting state EEG, aSST, saccadic eye movements, and the subjective response to alcohol (reported in Kosobud et al., 2015); a subsample of these same subjects subsequently went on to repeat the aSST during fMRI under alcohol and placebo infusions (Kareken et al., 2013, Weafer et al., 2015). The current analysis focuses on detection of an acute alcohol effect on the aSST STOP P3 ERP component.
Subjects
DSM-IV non-dependent, heavy-drinking, European American subjects, aged 21–27 years, were recruited using community advertisements and interviewed. Following BrAC measurement to confirm sobriety and the subject’s signing of an informed consent approved by the Indiana University Institutional Review Board, subjects completed portions of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), completed a 30-day timeline followback to assess recent drinking history (TLFB; Sobell et al., 1988), and provided blood and urine samples for assessment of liver function, drugs of abuse screening, and, in females, pregnancy screening. Inclusion criteria were consumption of at least 17 drinks in the past month, good health as determined by medical self-report and brief nursing assessment, and review of laboratory test results. Exclusion criteria included self-reported current or prior serious disease (including central nervous system, cardiovascular, respiratory, gastrointestinal, hepatic, renal, or endocrine), positive hepatitis or HIV test, alcoholism in the biological mother during pregnancy, current or prior history of severe alcohol-induced flushing reactions, current or prior history of DSM-IV Axis-I psychiatric conditions including alcohol or drug dependence but not alcohol abuse, use of medications known to interact with alcohol within 2 weeks of study initiation, and females who were or intended to become pregnant. In addition, subjects were excluded if, on the days of testing, they had a positive BrAC, presence of illicit drugs on urine drug screen, or, for females, a positive urine pregnancy test.
General Procedure
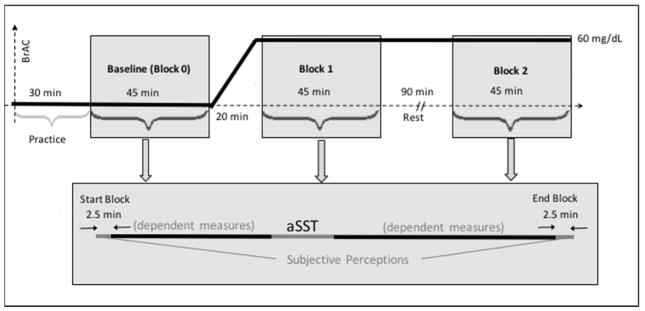
Each subject undertook 2 single-blind, intravenous-infusion study sessions at least 3 days but targeted 7 days apart receiving 6% ethanol in half-normal saline during one session and vehicle only at a comparable infusion rate profile during the other. Infusate order was counter-balanced across subjects. Subjects were advised that they would receive alcohol during one or both visits. On both study days, the subject arrived at the laboratory by 7:00 AM and underwent a brief physical exam, as well as breathalyzer and urine testing. We offered a 550 calorie breakfast, after which nurses placed a 20-gauge indwelling venous catheter in an antecubital vein of each arm, flushed with saline, and capped with a heparin lock. Lab technicians provided instruction on the various tasks to be performed, and fitted a 64-channel EASYCAP® electrode cap (EASYCAP GmbH, Germany). Infusion sessions began at approximately 10:15 AM. Testing in each session comprised three 45-minutes blocks during which multiple tasks were administered in a fixed order (Figure 1); only electrophysiological aSST results for Baseline (Block 0) and the subsequent measurement (Block 1) are reported here. Baseline was obtained just prior to the infusion; subjects were aware that they had not yet received alcohol. Block 1 began either 20 minutes later (placebo session) or after the 60 mg/dL alcohol clamp was established and stable for 5 minutes [occurring across the entire sample at 18.8 ± 0.01 minutes (mean ± SEM)]. The aSST was performed approximately 17 minutes into each experimental block.
Fig. 1.
Experimental Design. Adaptive Stop Signal Task (aSST) was performed in two consecutive runs (4.5 min/run), in a fixed order with other dependent measures, and began approximately 17 minutes within each of three 45 minute blocks during the infusion sessions. Sub-block aSST assessments were analyzed together. Block 0, or Baseline, occurred before infusion of either alcohol or placebo. Block 1 was collected beginning 20 minutes after the initiation of alcohol/placebo infusion and Block 2 initiated 90 minutes after the completion of Block 1. Sessions were scheduled nominally 7 days apart.
Experimental Alcohol Exposure
BrAC closely approximates the arterial alcohol concentration (Gomez et al., 2012), and therefore the brain’s exposure to alcohol, as the brain is a high-flow, low-volume organ. However, studies of responses attributable to changes in BrAC are complicated when oral dosing is used because individuals show significant variation in rise, peak, and fall of BrAC (Ramchandani et al., 1999b). Intravenous (IV) infusion of alcohol, based on an individual’s physiologically based pharmacokinetic parameters, assures identical BrAC trajectories among subjects by circumventing absorption kinetics and compensating for individual variation in distribution and elimination kinetics (Plawecki et al., 2008; Ramchandani et al., 1999b). Using IV infusion to “clamp” BrAC at a specific concentration is ideal for examining the acute effects of alcohol without complications from inter-subject variability in BrAC, or from Pavlovian associations that come from the oral ingestion of alcohol. As developed by our laboratory, the intravenous alcohol infusion methodology has been successfully used to prescribe alcohol exposures in many experimenter-specified and alcohol self-administration experiments (e.g., Cyders et al., 2016, Junger et al., 2016, Kareken et al., 2010, Oberlin et al., 2015, Stangl et al., 2017, Yoder et al., 2009).
The alcohol infusate was prepared by the Indiana University Hospital research pharmacy. Individualized infusion rates were computed and delivered by our Computer-assisted Alcohol Infusion System (CAIS, Zimmermann et al., 2008) using a transformation of the subject’s age, height, weight, and gender into the parameters of a physiologically based pharmacokinetic model of alcohol distribution and elimination (O’Connor et al., 1998; Plawecki et al., 2008; Ramchandani et al., 1999b). That infusion rate profile, in conjunction with BrAC measurement feedback, achieved and maintained a BrAC of 60.8 ± 0.1 mg/dL (Mean ± SEM; intended target 60.0 mg/dL) over Block 1 dependent measures assessment, assessed with an Alcotest meter, model 7410 or 6510 (Draeger, Irving, TX).
Stop Signal Task
We programmed and administered the aSST (Kareken et al. 2013, as adapted from Rubia et al., 2003) using E-Prime® 2.0 software (Psychology Software Tools Inc., Sharpsburg, PA) to provide consistency across experimental environments of the parent study. At baseline, Block 1, and Block 2, each subject completed 2 runs of the aSST (divided to allow BrAC testing but immediately subsequent), with each sub-block consisting of 60 “pure GO” trials and 30 “STOP” trials. We instructed subjects to respond as quickly and as accurately as possible to each GO trial. STOP trials included a red up-pointing arrow, after a green horizontal GO stimulus, indicating the need to inhibit the button press associated with the GO response. An adaptive staircase algorithm, with inter-trial interval of 3000 msec and SSD initiated at 250 msec, adjusted the delay in 50-msec increments to target a stop inhibition success rate of 50%. The duration of the fixation cross, GO, and STOP stimuli were 700 msec, 250 msec, and 250 msec respectively. A combination GO-STOP stimulus appeared during any time periods of overlap between the GO and STOP stimuli. Preliminary analysis demonstrated no clear difference across sub-blocks, so they were subsequently combined to increase trial numbers. Combining sub-blocks, each block comprised a total of 120 pure GO trials and 60 STOP trials.
Scalp EEG Data Collection
Dependent measures, including all electrophysiologic data, were collected with the subject seated in a comfortable, reclining chair located in a sound attenuated RF-shielded room (IAC, Industrial Acoustics, Bronx, NY). Fifty-nine leads in the expanded 10–20 system were referenced to the bridge of the nose with the ground placed at the midline forehead. Separate leads were placed for heart beat and horizontal and vertical eye movement detection. Electrode impedance was maintained below 5kΩ. EEG activity was recorded with Synamps2® EEG amplifiers connected to a 24-bit analog-to digital converter and sampled at 1000 kHz and using NeuroScan® (Neurosoft, Inc., El Paso, TX) PC-based data collection software.
Data Reduction
One hundred forty-eight subjects contributed aSST session data for ERP analysis. The continuous EEG records were visually screened for identification of bad leads, which were then removed with a nearest neighbor correction algorithm (Buchsbaum et al., 1982, Shepard, 1968). Matlab® based programs were then used to synchronize the E-prime and Neuroscan event records and identify eye blink artifacts as well as artifact-free blocks within the record for Neuroscan Spatial Filtering. GO and STOP stimuli were epoched, non-phase shifted, bandpass filtered, linearly detrended, baseline corrected, subjected to voltage thresholding then sorted and averaged based on stimulus type. We employed an approach to minimize impact of response overlap that was modeled on the Adjacent Response Filtering, ADJAR, procedure (Woldorff, 1993, Dimoska et al., 2006). Our modified ADJAR took advantage of distinct GO trials and the assumption of independence of the GO and STOP processes. We identified the sequence of artifact-free STOP trials to be used in computing the ERP and the corresponding array of SSD for each trial in the sequence. We jittered the subject’s own pure GO ERP according to the SSD sequence to create a Blurred GO response for that block’s usable STOP trials. Finally, we subtracted the Blurred GO response from the contaminated average STOP response and baseline corrected the result in the [−200, 0] msec pre-stimulus interval.
Data Analysis Strategy
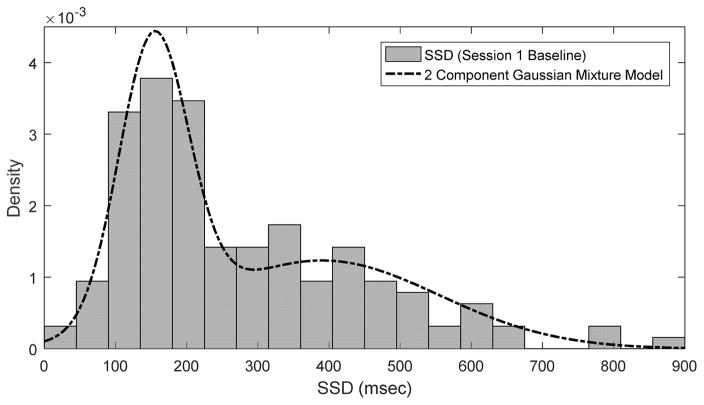
Review of the average SSD distribution, from all available data revealed an unexpected bimodal distribution, with short (FAST) and long (SLOW) SSDs (Supplemental Figure 1). The SSD data from the baseline blocks retained this bimodal character and was modeled as a two component Gaussian Mixture via the Matlab® fitgmdist function (Figure 2). Three groups were thus defined based on their first exposure to the aSST task (baseline of the first session, independent of infusate type): the ‘FAST’ group comprised subjects with a SSD less than or equal to the mean of the shorter SSD group (153 msec). The ‘SLOW’ group had an SSD greater than or equal to the mean of the longer SSD group (390 msec). The INTERMEDIATE (IM) group had SSDs that fell between the SLOW and FAST groups.
Fig. 2.
Comparison of Session 1 Baseline Stop Signal Delay Density and 2 Component Gaussian Mixture Model.
P3 Extraction Procedure
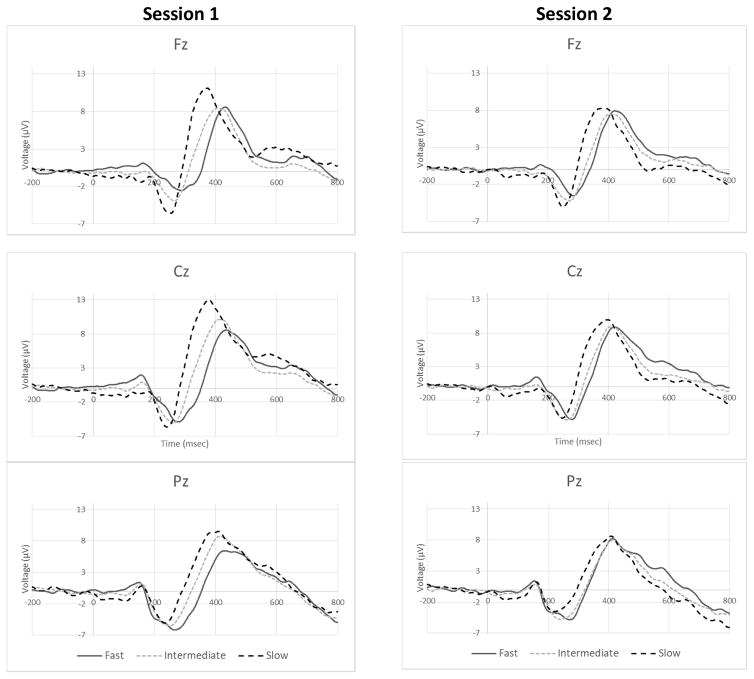
A spatial mask for computation of P3 amplitude (|P3|) at a corresponding latency (λP3) in each ERP was created based on the intermediate (IM) SSD group grand mean STOP ERPs (Figure 3 and Supplemental Figure 3). We chose a set of 16 adjacent leads, comprising the 5 midline leads {Fz – Pz}, their nearest 10 off-midline neighbors, and posterior lead POz to reduce data dimensionality. The |P3| at a specific λP3 was defined as the average voltage within that spatial distribution. A static topographic display of each ERP’s voltage over time and space was examined for the latency of characteristic maximal centro-midline signature of P3 activity. Then, a computerized search for maximum spatial mask voltage within a ± 20 msec window about that approximation, defined the analytic variables |P3| and λP3 for that ERP. The masking procedure removed sensitivity of |P3| to midline shifts in P3 activity associated with distinct occurrences of P3a and P3b sub-component activity. In addition, the conservative masking procedure reduced the spatio-temporal peak P3 amplitude to accommodate rational sensitivity to the spatial extent of P3 activity at λP3.
Fig. 3.
Baseline Grand Mean STOP ERPs. Grand Mean STOP ERPs at electrode locations Fz (Top), Cz (Middle), and Pz (Bottom) are displayed for each of the 3 SSD groups for Baseline Session 1 (Left) and Session 2 (Right). Each waveform reflects application of the modified ADJAR decontamination procedure.
Calculation of an Alcohol Effect
Consistent with our other examinations of the response to alcohol as a function of constant BrAC, we defined the initial response to alcohol (IRA) or placebo (IRP) infusion as the value of the dependent variable at Block 1 (alcohol target reached) – Block 0 (non-infusion baseline; Kosobud et al., 2015; Morzorati et al., 2002; Ramchandani et al., 1999a). The overall alcohol effect was defined as (IRA – IRP), to account for changes present in both the alcohol and placebo sessions. Subjects with an IM SSD (those who did not fall within the inclusion parameters for either the FAST or SLOW groups), having served as a model to define the P3 region of interest, were excluded from further analysis. For inclusion in the final analytical database, subjects required 4 blocks of valid aSST electrophysiological data: subjects not completing a session were excluded. Outlier data (greater than 2 standard deviations from overall mean for |P3| and λP3 alcohol effects independently) and subjects with unrecognizable ERP morphology in one or more of the blocks were removed from further analysis. One hundred and fourteen subjects had complete data. No group was preferentially impacted by the outlier removal and data review processes. Final group sizes, after outlier, incomplete data, and poor data removal, were 25 and 24 for FAST and SLOW SSD groups respectively (Table 1). Upon visual inspection, these groups demonstrated clear differences in baseline grand mean ERP morphology (Figure 3) and λP3, but not |P3| (Figure 4a–b), as well as differences in the effect of alcohol (Figure 5).
Table I.
Subject Demographics and Alcohol Exposure by FAST and SLOW SSD Subgroups.
Subject distributions did not differ for any groups (p ≥ 0.09).
| Subject numbers by group | FAST | SLOW | P |
|---|---|---|---|
| Females (n = 24) | 12 | 12 | >0.99 |
| Males (n = 25) | 13 | 12 | |
| Age | 24.2 ± 0.4 | 23.2 ± 0.4 | 0.09 |
| Height (in cm) | 174.1 ± 2.0 | 174.3 ± 1.7 | 0.92 |
| Weight (in kg) | 74.4 ± 2.8 | 78.5 ± 2.8 | 0.3 |
| Education (in yrs) | 15.8 ± 0.4 | 15.7 ± 0.3 | 0.78 |
| Total Drinks (30 days) | 54.7 ± 4.8 | 69.5 ± 10.1 | 0.16 |
| Total Drinking Days (30 days) | 11.5 ± 0.7 | 13.5 ± 1.2 | 0.13 |
| Drinks/Drinking Day (30 days) | 4.4 ± 0.3 | 5.1 ± 0.5 | 0.22 |
| Heavy Drinking Day (30 days) | 4.9 ± 0.6 | 5.7 ± 1.1 | 0.45 |
| Time to 60 mg/dL (min) | 13.7 ± 0.3 | 14.0 ± 0.5 | 0.56 |
| Average BrAC across block 1 clamp (mg/dL) | 60.9 ± 0.3 | 61.0 ± 0.3 | 0.84 |
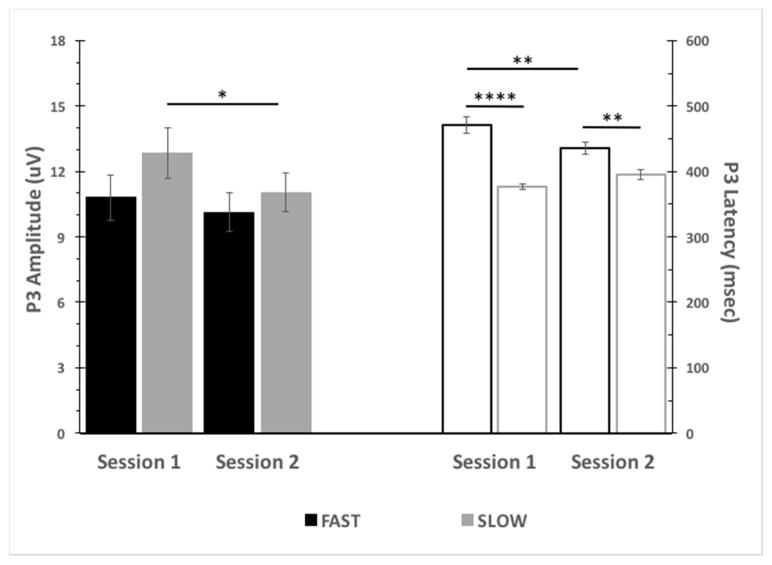
Fig. 4.
Baseline P3 Amplitude and Latency by Session and SSD group. P3 Amplitude (Left, Closed Bars): No baseline P3 amplitude differences between FAST and SLOW SSD groups was observed across sessions. SLOW SSD group baseline P3 amplitude was significantly reduced in Session 2 versus Session 1. P3 Latency (Right, Open Bars): FAST SSD group baseline P3 latency was significantly longer than that of SLOW SSD group across sessions. FAST SSD group baseline P3 latency was significantly reduced in Session 2 versus Session 1. (* p<0.05; **p<0.01; ****p<0.0001).
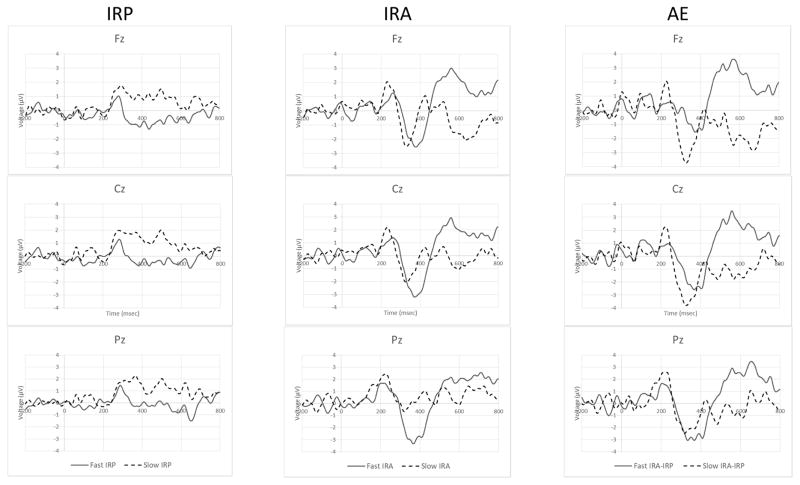
Fig. 5.
Differences of Grand Mean STOP ERPs. Tempero-spatial differences between FAST and SLOW SSD groups in the initial responses to placebo administration (IRP), initial responses to alcohol exposure at 60mg/dL (IRA), and the alcohol effect (AE) calcualted as the difference of differences (IRA-IRP)are displayed. None of these signals were actually measured and the comparison is meant only to illustrate group effects observed in association with alcohol exposure.
Statistical analyses were performed using GraphPad Prism 7 for Mac (GraphPad Software, La Jolla, CA) for demographic and baseline differences between SSD groups and IBM SPSS Statistics 24 (IBM, Armonk, New York) for multivariate analysis of variance. We assessed demographic differences between the FAST and SLOW groups using t-tests (for quantitative traits) or chi-squared tests (for qualitative traits). For each session, we assessed baseline differences in |P3| and λP3 for SSD groups using 2-tailed t-tests. Analyses across the two sessions employed 2-way repeated measures analysis of variance (ANOVAs) with SSD group and session as factors. Post-hoc comparisons were performed using Bonferroni correction. The dependent variable was the effect of alcohol on the initial response for |P3| and λP3, as measured by Alcohol Effect = IRA-IRP. No correlation between the |P3| alcohol effect and λP3 alcohol effect was observed (r = 0.02, p = 0.91). A multivariate analysis of variance (MANOVA) assessed differences in the two SSD groups (FAST vs SLOW) on the alcohol effect for |P3| and λP3.
RESULTS
Behavior and Electrophysiology
FAST and SLOW SSD groups did not differ on any demographic variables (ps > 0.09) (Table 1). Baseline STOP |P3| did not significantly differ by SSD group in either infusion session [Session 1: t(47) = 1.3, p = 0.20; Session 2: t(47) = 0.7, p = 0.47]. A significant smaller baseline |P3| occurred [F(1, 47) = 6.58, p = 0.01] for SLOW SSD for session 2 compared to session 1 (p = 0.02). A significantly longer baseline λP3 obtained for the group with FAST compared to SLOW SSD in both infusion sessions [session 1: t(47) = 6.72, p < 0.0001; session 2: t(47) = 3.23, p < 0.01]. A significant effect of session on baseline |P3| was observed [F(1, 47) = 6.58, p = 0.01] with post hoc analysis indicating a significant reduction in |P3| for SLOW SSD at session 2 baseline compared to session 1 baseline (p = 0.02). A significant session x SSD interaction for baseline λP3 was observed [F(1, 47) = 20.62, p < 0.0001] with post hoc analysis indicating a significant reduction in λP3 for FAST SSD at session 2 compared to session 1 (p < 0.001) (Figure 4).
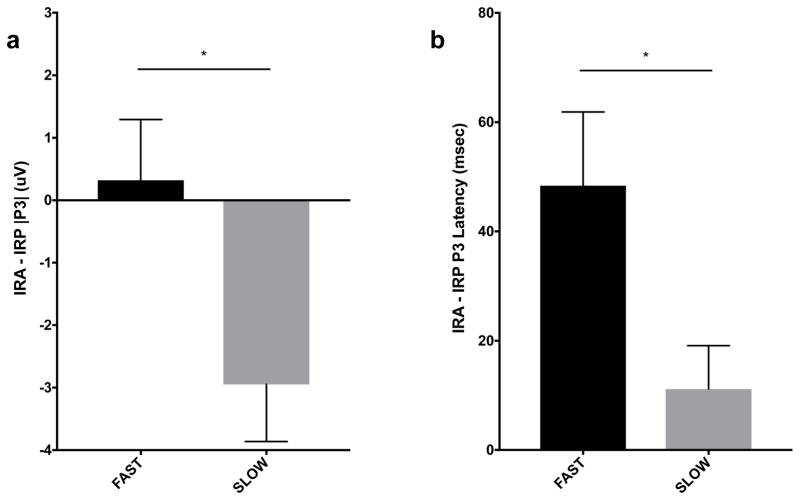
We observed homogeneity of covariance matrices of the alcohol effect (Box’s M p = 0.09) across the SSD groups. The multivariate alcohol effect was significant by SSD group, [F(2, 46) = 6.30, p < 0.01, partial η2 = 0.25], reflecting a significantly reduced |P3| for the SLOW compared to FAST SSD group [F(1, 47) = 5.98, p < 0.02, partial η2 = 0.11], and a significantly longer λP3 for the FAST compared to SLOW SSD group [F(1, 47) = 5.56, p = 0.02, partial η2 = 0.11] (Figure 6a–b).
Fig. 6.
Effect of Alcohol on STOP P3 Amplitude and Latency. Alcohol significantly decreased |P3| for SLOW SSD compared to FAST SSD group (a). Alcohol significantly lengthened P3 latency for FAST SSD compared to SLOW SSD group (b). (* p < 0.05)
DISCUSSION
We examined the effect of a precisely-controlled, acute alcohol exposure on ERP component P3 indices following a cue for response inhibition in an adaptive SST task. We employed a within-subject, placebo-controlled design to identify changes attributable to alcohol exposure. We made use of a real-time adaptive SST algorithm to reduce the well-known confound of practice and fatigue on the STOP ERP. Inspection of the STOP Signal Delays yielded a clear bimodal distribution, suggesting two distinct strategies for performing the task. Similar to prior reports of “fast” and “slow” SSRT response and SSD groups, we identified two groups with consistent response strategies (FAST and SLOW) and a larger INTERMEDIATE group which likely reflected a response strategy that reflected a mixture of the strategies (Dimoska et al., 2006, Verbruggen and McLaren, 2016, Greenhouse and Wessel, 2013). The FAST SSD group (SSD mean of 117 ± 6 msec) appeared to utilize a response strategy that favored going over stopping while the SLOW SSD group (SSD mean of 518 ± 20 msec) appeared to use a conservative strategy that emphasized stopping over going. As suggested by Dimoska et al., 2006 and Greenhouse and Wessel, 2013, these proposed response strategies for the SSD groups were supported by baseline STOP P3 differences as well as the observed difference in pure GO |P3| (unanalyzed but utilized within the modified ADJAR procedure, see Supplemental Figure 4). We subsequently hypothesized that the effect of alcohol on the two strategies would be apparent in STOP P3 component amplitude and latency.
To our knowledge, this is the first SST-based electrophysiological study to demonstrate a within-subject effect of alcohol on conservative estimates of ERP component activity. We observed significant alcohol effects on P3 amplitude and latency that differed depending on the response strategy implemented. Although all subjects were provided the same task instructions in an identical manner, we do not have specific data why subjects chose a specific strategy. FAST subjects appeared to use a response strategy that favors rapid go responses, resulting in a STOP |P3| that was not significantly influenced by either practice or acute alcohol exposure, but with a significantly longer λP3 than the SLOW subjects. In contrast, subjects in the SLOW group appeared to implement a response strategy that prioritizes stopping over going, and exposure to 60 mg/dL alcohol was associated with changes opposite to that demonstrated by the FAST group: a significantly reduced STOP |P3|, but no impact on λP3. Consequently, we hypothesize that the influence of alcohol impacts one’s approach to solving a problem, rather than the solution of the problem itself. The dichotomy may represent an important indicator of AUD risk. We are currently conducting analyses on other ERP components derived from the SST suggested by our results and intend to test for associations between risk factors (gender, recent drinking history, family history, candidate genes) and SSD related ERP parameters once complete.
The findings are subject to limitations. The traditional SST paradigm, like most other ERP paradigms, is not entirely immune to practice effects across repeated testing (Manuel et al., 2013). Thus, the effect of practice on the resulting P3 ERP can still be confounded with the effect of alcohol administration due to the need for at least one replication of the task (SST P3 with and without alcohol). Although the aSST used here is designed to minimize this effect, we did observe baseline |P3| and λP3 differences for the SLOW and FAST groups respectively between Session 1 and 2. These ERP changes, associated with repeating the task over three experimental blocks in each of 2 sessions, supports our analytical methodology that intentionally accounted for the confound. Another limitation of this work is brain exposure to alcohol at mid-morning rather than in the evening when the sample population is more likely to have had experience with drinking alcohol. On the other hand, subjects were also unlikely to have previously experienced the purely pharmacological effects of alcohol. Thus, we chose to sacrifice a more ecologically valid schedule for the precision of exposure afforded by our intravenous alcohol infusion technique and within the practical limitations of scheduling these sessions.
To examine the effect of acute alcohol exposure on the inhibitory ERP distinct from that of practice, a modified ADJAR procedure was used to remove the overlap of the preceding GO ERP signal from the STOP prior to the determination of any alcohol effect. Indeed, the resultant STOP ERP waveforms were visibly distinct and improved (see Supplemental Figures 2 and 4). The widely-held assumption of independence between the STOP and GO processes is inherent in the decontamination procedure. If these processes are not independent, our, and many other, decontamination strategies would instead introduce error into the analysis. Consequently, the assumption of STOP and GO independence should be considered a potentially significant limitation. Further, any ADJAR based decontamination procedure will differentially impact the STOP signal ERPs as a function of SSD. Indeed, the procedural effect of STOP ERP decontamination on P3 activity was greater in the order of FAST to IM to SLOW groups (See Supplemental Figure 2). Other procedures have been reported to accomplish decontamination (see review in Ihrke et al., 2009). However, the structure of our data, including number of relevant stimuli, their overlap, and sub-block design, prevented their implementation. Overall, we recommend application of the modified ADJAR procedure for understanding ERP activity from any version of the SST.
Our use of a spatial mask to define |P3|, while advantageous from a data reduction perspective, precluded a more refined spatial analysis. It is commonly accepted that the P3 is comprised of two positive potentials that occur in close temporal proximity (P3a and P3b). The early P3a component has a frontal distribution and peaks around 240 – 280 msec after the stimulus. The later P3b component has a more parietal distribution that peaks around 250 – 500 msec after the stimulus (Squires et al., 1975). Both components are thought to reflect the updating of working memory with new information (Polich & Kok, 1995). Specifically, the P3a has been hypothesized to reflect attentional processing that initiates the inhibition of ongoing activity (Polich, 2007), though further research is needed to verify that hypothesis. Grand Mean ERPs (see Figure 5) suggest the potential for detecting separate P3a and P3b subcomponents. It is conceivable that the alcohol-induced increase in P3 latency for FAST subjects and reduced |P3| for SLOW subjects reflects differential alcohol effects upon these subcomponents. That possibility might help identify which strategy that individual members of the INTERMEDIATE SSD group of subjects were using. However, our conservative method for defining |P3| precluded testing such a hypothesis. Further analysis of the STOP ERP is needed to fully ascertain the effect of alcohol on response inhibition (e.g. does alcohol have a different effect on ERPs from correctly inhibited motor responses to STOP cues when compared to uninhibited motor responses?). Finally, the cognitive-neurophysiological aspects of the stop signal task are complex and extend beyond the response inhibition processing hypothesized to be represented in P3. Inspection of the spatio-temporal signatures in our database suggests the possibility of SSD group and drug influences on P1, N2, CNV, and a late bi-frontal activity of opposite polarity.
More generally, the two session within-subject design and SSD-based analytical approach proved analytically complex and costly from a data retention perspective. However, subtracting the effect of repetition allowed for a more robust phenotype. To be included in the final analysis, subjects were stratified according to clear response types and needed to provide high quality data across four experimental blocks. Further, subjects may alter their strategy across and within experimental runs in ways that may or may not depend on experimental intervention, potentially confounding our results. Efforts to better identify the strategy subgroups, even potentially within an experimental run, is thus a clear area of need.
In summary, we documented an effect of precisely controlled alcohol exposure at 60mg/dL on what we perceive to be two distinct strategies used to produce the P3 component of scalp ERPs cued by a visual stimulus signaling the need for response inhibition. Our findings may be eventually useful in searching for the expression of risk for developing an AUD associated with impulsivity. The ability to inhibit a pre-potent response is an integral part of normal cognitive functioning. Even moderate alcohol exposure can compromise conflict monitoring depending on the nature of the response strategy or stimulus salience. Speed of inhibition might be compromised when a pre-potent response is prioritized (i.e., such as might be the case in the FAST SSD group > SLOW SSD group comparison). On the other hand, conflict processing (i.e., |P3| amplitude) could be compromised when stopping is prioritized (i.e., SLOW SSD group). The ERP data here make clear that such subject-level task approaches may need to be accounted for to more completely understand the underlying neurodynamics, with and without alcohol.
Supplementary Material
Supplemental Figure 1. Comparison of SSD Density for All Sessions and Blocks and the Session 1 Baseline (S1B0) 2 Component Gaussian Mixture Model. The model, defined by the first exposure to the task independent of infusion type, closely approximates the overall SSD distribution.
Supplemental Figure 2. Go-Stop Stimulus ERP Overlap Decontamination. Session 1 Baseline Grand Mean Stop ERPs before (dashed) after (solid) applying the modified ADJAR decontamination procedure to individual data. The impact of decontamination is displayed for electrode Cz across the Fast (Top), Intermediate (Middle), and Slow (Bottom) Stop Signal Delay groups. As applied to this data, the modified ADJAR decontamination procedure attempts to remove any overlap between the STOP stimulus response and the preceding GO stimulus response. Consequently, it is expected that as the amount of time between those stimuli decreases (alternatively, as Stop Signal Delay decreases), the effect of decontamination on the STOP signal ERP will become more pronounced. In agreement with those expectations, the Fast group (top) demonstrates the greatest change, the Intermediate group a moderate change, and the Slow group the least change in STOP signal ERP morphology.
Supplemental Figure 3. P3 Amplitude Mask Generation. Scalp distribution of Session 1 Baseline decontaminated P3 Amplitude Voltages at (300, 350, 400) and (450, 500, 550) msec of the Intermediate Stop Signal Delay Group showing a midline distribution. The set of comprising the 5 midline leads {Fz – Pz}, their nearest 10 off-midline neighbors, and posterior lead POz was chosen to reduce data dimensionality. The moment when the average voltages in the mask peaked was defined as the single P3 latency in all leads.
Supplemental Figure 4. Baseline Grand Mean of PURE GO ERPs (no STOP signal) at electrode locations Fz (Top), Cz (Middle), and Pz (Bottom) are displayed for each of the 3 SSD groups for Session 1 (Left) and Session 2 (Right). The timing of the substantial P3 components indicated the need for removing their influence on the assessment of the brain’s response to the subsequent STOP stimuli.
An Adaptive Stop Signal Task was used to examine within-subject alcohol ERP changes
Stop Signal Delay distribution was bimodal, implying different response strategies
Alcohol was associated with different P3 ERP changes by Stop Signal Delay group
Acknowledgments
Supported by: NIH grant P60 AA007611 (SOC, DAK) and the Gary R. Helman Postdoctoral Research Fellowship (KAW).
We gratefully acknowledge the efforts of Victor Vitvitskiy, Liying Chen, and Promita Hazra for assistance with computer programming, Joel Hart, Sarah Hicks, Kurt White, Tet Lu, James Hays, Lydia Fischer, James Millward, David Haines, and Shreya Velamakanni for recruitment, subject management, and data reduction assistance. Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, through the Indiana Alcohol Research Center (P60AA07611). Additional support was provided by Research Facilities Improvement Program grant number C06 RR020128-01 from the National Center for Research Resources, National Institutes of Health, and the Indiana Clinical and Translational Sciences Institute, funded in part by grant number TR000006 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological psychiatry. 2011;69:e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GP, Ridderinkhof KR, van der Molen MW. Speed-accuracy modulation in case of conflict: the roles of activation and inhibition. Psychol Res. 2003;67:266–279. doi: 10.1007/s00426-002-0127-0. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Hoeksma MR, Talsma D, Verbaten MN. The pure electrophysiology of stopping. Int J Psychophysiol. 2005;55:191–198. doi: 10.1016/j.ijpsycho.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Rigal F, Coppola R, Cappelletti J, King C, Johnson J. A new system for gray-level surface distribution maps of electrical activity. Electroencephalogr Clin Neurophysiol. 1982;53:237–242. doi: 10.1016/0013-4694(82)90029-3. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological Correlates of Response Production and Inhibition in Alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:1398–1406. [PubMed] [Google Scholar]
- Cyders MA, VanderVeen JD, Plawecki M, Millward JB, Hays J, Kareken DA, O’Connor S. Gender-Specific Effects of Mood on Alcohol-Seeking Behaviors: Preliminary Findings Using Intravenous Alcohol Self-Administration. Alcoholism, clinical and experimental research. 2016;40:393–400. doi: 10.1111/acer.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: The control of response processes. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio LM, Velasques BB, Ribeiro P, Nardi AE, de Carvalho MR. Evoked Potential in Panic Disorder Patients: A Systematic Review. CNS Neurol Disord Drug Targets. 2015;14:863–871. doi: 10.2174/1871527314666150303164539. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimoska A, Johnstone S, Barry R, Clarke A. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biological psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and alcohol dependence. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M, Bathelt J, De Haan M. Event-related potential measures of executive functioning from preschool to adolescence. Dev Med Child Neurol. 2017;59:581–590. doi: 10.1111/dmcn.13395. [DOI] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res Cogn Brain Res. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neuroscience and biobehavioral reviews. 2012;36:572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison EL. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug and alcohol dependence. 2008;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biological psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Wessel JR. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology. 2013;50:900–908. doi: 10.1111/psyp.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke M, Schrobsdorff H, Herrmann JM. Coordinated Activity in the Brain, Coordinated Activity in the Brain. New York, NY: 2009. Denoising and Averaging Techniques for Electrophysiological Data; pp. 65–189. [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Field M, Christiansen P, Stancak A. P300 during response inhibition is associated with ad-lib alcohol consumption in social drinkers. Journal of psychopharmacology. 2013;27:507–514. doi: 10.1177/0269881113485142. [DOI] [PubMed] [Google Scholar]
- Junger E, Gan G, Mick I, Seipt C, Markovic A, Sommer C, Plawecki MH, O’Connor S, Smolka MN, Zimmermann US. Adolescent Women Induce Lower Blood Alcohol Levels Than Men in a Laboratory Alcohol Self-Administration Experiment. Alcoholism, clinical and experimental research. 2016;40:1769–1778. doi: 10.1111/acer.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, Disinhibited Personality, and Early-Onset Alcohol Problems. Alcoholism: Clinical and Experimental Research. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W, Oberlin BG, Harezlak J, Wang Y, O’Connor SJ. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O’Connor SJ, Foroud T. A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcoholism, clinical and experimental research. 2010;34:2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AEK, Wetherill L, Plawecki MH, Kareken DA, Liang T, Nurnberger JL, Windisch K, Xuei X, Edenberg HJ, Foroud TM, O’Connor SJ. Adaptation of Subjective Responses to Alcohol is Affected by an Interaction of GABRA2 Genotype and Recent Drinking. Alcoholism: Clinical and Experimental Research. 2015;39:1148–1157. doi: 10.1111/acer.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcoholism, clinical and experimental research. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Gilbertson R, Prather R, Nixon SJ. Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcoholism, clinical and experimental research. 2013;37:941–951. doi: 10.1111/acer.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Kouri E, Bolduc M, Amass L. Ethanol-induced alterations in EEG alpha activity and apparent source of the auditory P300 evoked response potential. Alcohol. 1990;7:471–477. doi: 10.1016/0741-8329(90)90034-a. [DOI] [PubMed] [Google Scholar]
- Martin FH, Garfield J. Combined effects of alcohol and caffeine on the late components of the event-related potential and on reaction time. Biol Psychol. 2006;71:63–73. doi: 10.1016/j.biopsycho.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcoholism, clinical and experimental research. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Motlagh F, Ibrahim F, Menke JM, Rashid R, Seghatoleslam T, Habil H. Neuroelectrophysiological approaches in heroin addiction research: A review of literatures. Journal of neuroscience research. 2016;94:297–309. doi: 10.1002/jnr.23703. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping Breath Alcohol Concentration Reduces Experimental Variance: Application to the Study of Acute Tolerance to Alcohol and Alcohol Elimination Rate. Alcoholism: Clinical and Experimental Research. 1998;22:202–210. [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O’Connor SJ, Yoder KK, Kareken DA. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology. 2015;232:861–870. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner CNM, MacDonald TK, Olmstead MC. Alcohol Intoxication Reduces Impulsivity in the Delay-Discounting Paradigm. Alcohol and Alcoholism. 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Perlman G, Markin A, Iacono WG. P300 amplitude reduction is associated with early-onset and late-onset pathological substance use in a prospectively studied cohort of 14-year-old adolescents. Psychophysiology. 2013;50:974–982. doi: 10.1111/psyp.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O’Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55:2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ramchandani V, Bolane J, Li T-K, O’Connor S. A Physiologically-Based Pharmacokinetic (PBPK) Model for Alcohol Facilitates Rapid BrAC Clamping. Alcoholism: Clinical and Experimental Research. 1999b;23:617–623. [PubMed] [Google Scholar]
- Ramchandani V, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcoholism, clinical and experimental research. 1999a;23:1320–1330. [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. Alcohol increases impulsivity and abuse liability in heavy drinking women. Exp Clin Psychopharmacol. 2012;20:454–465. doi: 10.1037/a0029087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacology, biochemistry, and behavior. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Stephens DN, Duka T. Heightened Impulsivity: Associated with Family History of Alcohol Misuse, and a Consequence of Alcohol Intake. Alcoholism, clinical and experimental research. 2016;40:2208–2217. doi: 10.1111/acer.13184. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Wintink AJ, Cudmore LJ. P3 topographical change with task familiarization and task complexity. Cognitive Brain Research. 2001;12:451–457. doi: 10.1016/s0926-6410(01)00082-9. [DOI] [PubMed] [Google Scholar]
- Shepard D. A two-dimensional interpolation function for irregularly-spaced data, in Series A two-dimensional interpolation function for irregularly-spaced data. Proceedings of the 1968 23rd ACM national conference; ACM; 1968. pp. 517–524. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a Timeline Method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, Ramchandani VA. Exposure-Response Relationships during Free-Access Intravenous Alcohol Self-Administration in Nondependent Drinkers: Influence of Alcohol Expectancies and Impulsivity. Int J Neuropsychopharmacol. 2017;20:31–39. doi: 10.1093/ijnp/pyw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol. 2001;58:229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, McLaren R. Effects of reward and punishment on the interaction between going and stopping in a selective stop-change task. Psychol Res. 2016 doi: 10.1007/s00426-016-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and biobehavioral reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Weafer J, Dzemidzic M, Eiler W, 2nd, Oberlin BG, Wang Y, Kareken DA. Associations between regional brain physiology and trait impulsivity, motor inhibition, and impaired control over drinking. Psychiatry Res. 2015;233:81–87. doi: 10.1016/j.pscychresns.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, Kareken DA. When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcoholism, clinical and experimental research. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcoholism, clinical and experimental research. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of SSD Density for All Sessions and Blocks and the Session 1 Baseline (S1B0) 2 Component Gaussian Mixture Model. The model, defined by the first exposure to the task independent of infusion type, closely approximates the overall SSD distribution.
Supplemental Figure 2. Go-Stop Stimulus ERP Overlap Decontamination. Session 1 Baseline Grand Mean Stop ERPs before (dashed) after (solid) applying the modified ADJAR decontamination procedure to individual data. The impact of decontamination is displayed for electrode Cz across the Fast (Top), Intermediate (Middle), and Slow (Bottom) Stop Signal Delay groups. As applied to this data, the modified ADJAR decontamination procedure attempts to remove any overlap between the STOP stimulus response and the preceding GO stimulus response. Consequently, it is expected that as the amount of time between those stimuli decreases (alternatively, as Stop Signal Delay decreases), the effect of decontamination on the STOP signal ERP will become more pronounced. In agreement with those expectations, the Fast group (top) demonstrates the greatest change, the Intermediate group a moderate change, and the Slow group the least change in STOP signal ERP morphology.
Supplemental Figure 3. P3 Amplitude Mask Generation. Scalp distribution of Session 1 Baseline decontaminated P3 Amplitude Voltages at (300, 350, 400) and (450, 500, 550) msec of the Intermediate Stop Signal Delay Group showing a midline distribution. The set of comprising the 5 midline leads {Fz – Pz}, their nearest 10 off-midline neighbors, and posterior lead POz was chosen to reduce data dimensionality. The moment when the average voltages in the mask peaked was defined as the single P3 latency in all leads.
Supplemental Figure 4. Baseline Grand Mean of PURE GO ERPs (no STOP signal) at electrode locations Fz (Top), Cz (Middle), and Pz (Bottom) are displayed for each of the 3 SSD groups for Session 1 (Left) and Session 2 (Right). The timing of the substantial P3 components indicated the need for removing their influence on the assessment of the brain’s response to the subsequent STOP stimuli.