Abstract
The citrate carrier from maize (Zea mays) shoot mitochondria was solubilized with Triton X-100 and purified by sequential chromatography on hydroxyapatite and hydroxyapatite/celite in the presence of cardiolipin. SDS-gel electrophoresis of the purified fraction showed a single polypeptide band with an apparent molecular mass of 31 kD. When reconstituted into liposomes, the citrate carrier catalyzed a pyridoxal 5′-P-sensitive citrate/citrate exchange. It was purified 224-fold with a recovery of 50% and a protein yield of 0.22% with respect to the mitochondrial extract. In the reconstituted system the purified citrate carrier catalyzed a first-order reaction of citrate/citrate (0.065 min−1) or citrate/malate exchange (0.075 min−1). Among the various substrates and inhibitors tested, the reconstituted protein transported citrate, cis-aconitate, isocitrate, l-malate, succinate, malonate, glutarate, α-ketoglutarate, oxaloacetate, and α-ketoadipate and was inhibited by pyridoxal 5′-P, phenylisothiocyanate, mersalyl, and p-hydroxymercuribenzoate (but not N-ethylmaleimide), 1,2,3-benzentricarboxylate, benzylmalonate, and butylmalonate. The activation energy of the citrate/citrate exchange was 66.5 kJ/mol between 10°C and 35°C; the half-saturation constant (Km) for citrate was 0.65 ± 0.05 mm and the maximal rate (Vmax) of the citrate/citrate exchange was 13.0 ± 1.0 μmol min−1 mg−1 protein at 25°C.
Metabolite transport occurs in mitochondria via a series of carrier proteins spanning the inner membrane (LaNoue and Schoolwerth, 1979; Day and Wiskich, 1984; Hanson, 1985; Heldt and Flügge, 1987; Douce and Neuburger, 1989). The main properties of all these carriers have been studied in intact mitochondria. However, essential for the identification of a transport protein and for its detailed functional and structural characterization are the purification and the reconstitution of the purified protein in artificial membranes. To date, six of the plant mitochondrial metabolite carriers have been partially purified, reconstituted into liposomes, and kinetically studied, namely the dicarboxylate (Vivekananda et al., 1988), Glu/Asp (Vivekananda and Oliver, 1989), monocarboxylate (Vivekananda and Oliver, 1990), tricarboxylate (McIntosh and Oliver, 1992), and phosphate (McIntosh and Oliver, 1994) carriers from pea seedlings and the α-ketoglutarate from maize (Zea mays) shoots (Genchi et al., 1991). Only the ADP/ATP translocator has been purified to homogeneity from maize shoot mitochondria, characterized, and partially sequenced (Genchi et al., 1996).
The citrate carrier, also known as the tricarboxylate carrier, is an intrinsic protein of the inner mitochondrial membrane, which exchanges cytoplasmic malate for citrate synthesized inside the mitochondrion. The exported citrate is an important source of C skeleton for synthetic processes, especially for Glu biosynthesis that takes place mainly in the chloroplast compartment (Hanning and Heldt, 1993).
In this paper we describe the purification of the citrate carrier from maize cv B 73 shoot mitochondria using functional reconstitution as a monitor of carrier activity during isolation. Upon SDS-PAGE the purified citrate transport protein appears to be a single polypeptide with an apparent molecular mass of 31 kD. The functional properties, as well as the basic kinetic data of the purified carrier incorporated into liposomes, are also described.
MATERIALS AND METHODS
Plant Material and Chemicals
Maize (Zea mays L. cv B 73) kernels, obtained from Maisadour (Mont De Marsan, France), were surface-sterilized for 5 min in 1% (w/v) sodium hypochlorite and rinsed in distilled water. Seeds were allowed to imbibe in water at 25°C overnight, then they were sown on a layer of hydrophilic cotton in plastic boxes and covered by a sheet of thin, wet paper. Seedlings were grown for 4 to 5 d in a dark-controlled environmental chamber at 30°C and 95% RH before harvesting. Hydroxyapatite (Bio-gel HTP) was obtained from Bio-Rad; Triton X-100, celite 535, acrylamide, and N,N′-methylenebisacrylamide were obtained from Serva; Dowex AG1-X8 (100–200 mesh), egg-yolk phospholipids (lecithin from eggs), and Amberlite XAD-2 were obtained from Fluka; [1,5-14C]citrate was obtained from Amersham; cardiolipin was obtained from Avanti-Polar Lipids (Alabaster, AL); and Sephadex G-75 was obtained from Pharmacia. All other reagents were of the highest purity commercially available.
Isolation and Purification of Maize Mitochondria
Maize shoots were disrupted with a Braun mixer in 3 volumes of ice-cold 0.4 m Suc, 20 mm Tris-HCl, pH 8.0, 1 mm EDTA, 0.1% (w/v) BSA, and 0.05% (w/v) Cys three times for approximately 1 min each. The homogenate was filtered through a layer of nylon sheet (80-μm pores; Saacilene 150T, Gaudenzi Tecnica Industriale, Padova, Italy) and centrifuged for 20 min at 10,000g. The pellet was resuspended in a washing medium containing 0.3 m Suc, 5 mm Tris-HCl, pH 7.2, and centrifuged for 5 min at 1,000g. The decanted supernatant was layered onto a discontinuous Suc gradient, and purification of the mitochondria was carried out according to the method of Douce et al. (1972), except that 5 mm Tris-HCl (pH 7.2) was used instead of 10 mm phosphate buffer in all of the purification steps. Purified mitochondria were suspended at a concentration of 15 to 16 mg protein mL−1 washing medium (pH 7.2), frozen in liquid N2, and stored at −80°C.
Purification of the Citrate Carrier
Maize shoot mitochondria were solubilized in 3% Triton X-100 (w/v), 20 mm Na2SO4, 1 mm EDTA, and 10 mm Pipes (1,4-piperazinediethanesulphonic acid), pH 7.0 (buffer A), at a final concentration of 15 mg protein mL−1 buffer. After 10 min at 0°C, the mixture was centrifuged at 105,000g per 15 min. The citrate carrier was purified by hydroxyapatite and hydroxyapatite/celite chromatography as follows: 225 μL of ultracentrifuged supernatant (Triton extract) supplemented with cardiolipin (0.5 mg in 25 μL of buffer A) was applied to a hydroxyapatite column (0.8 cm in diameter, containing 1.0 g of dry material) and eluted with 0.1% Triton X-100 and 10 mm Pipes, pH 7.0 (buffer B). The first 500 μL was collected and 300 μL of this hydroxyapatite eluate was applied to a hydroxyapatite:celite column (7:1; Pasteur pipettes with 300 mg of dry material). The first 300 μL was collected eluting with buffer B. All of the operations were performed in a cold room at 4°C.
Reconstitution of the Citrate Carrier into Liposomes
Liposomes were prepared as described previously (Bisaccia et al., 1985) by sonication of 100 mg/mL egg yolk phospholipids in water for 60 min. Protein eluates were reconstituted by removing the detergent with a hydrophobic ion-exchange column (Palmieri et al., 1995). In this procedure the mixed micelles containing detergent, protein, and phospholipids were repeatedly passed through the same Amberlite XAD-2 column. The composition of the reconstitution mixture was: 200 μL of eluates from the different columns or 20 μL of the Triton extract plus 180 μL of buffer A; 90 μL of egg yolk phospholipids in the form of sonicated liposomes; 90 μL of 10% Triton X-114; 20 mm citrate or other substrates, as indicated in the legends to the tables and figures; 150 μL of 100 mm Pipes (pH 7.0) in the presence of 20 mm KCl in a final volume of 700 μL. After the mixture was vortexed, it was passed 15 times through the Amberlite column (0.5 × 3.6 cm) preequilibrated with a buffer containing 10 mm Pipes, pH 7.0, and 20 mm concentration of the substrate present in the starting mixture. All of the operations were performed at 4°C, except the passage through the column, which was carried out at room temperature.
Transport Measurements
The external substrate was removed by passing 650 μL of the proteoliposomal suspension through a Sephadex G-75 column (0.7 × 15 cm) preequilibrated with 50 mm NaCl and 10 mm Pipes, pH 7.0. The first 600 μL of turbid proteoliposomal eluate was collected and distributed in reaction vessels (180 μL each), incubated at 25°C for 4 min, and used for transport measurements by the inhibitor stop method (Palmieri and Klingenberg, 1979). Transport was initiated by adding 10 μL of [14C]citrate at the final concentrations indicated in the legends to the tables and figures, and after the desired time interval, transport was stopped by adding 10 μL of 350 mm pyridoxal 5′-P. In control samples, the inhibitor was added together with the labeled substrate at time 0. The external radioactivity was removed by passing 180 μL of each sample through an anion-exchange column (Dowex AG1-X8, chloride form, 0.5 × 5 cm). The liposomes eluted with 1 mL of 50 mm NaCl were collected in 4 mL of scintillation mixture, vortexed, and counted. Transport activities were calculated from the experimental values minus the controls. For kinetic measurements, initial transport rates were obtained by measuring transport within 1.5 min.
Other Methods
Polyacrylamide slab-gel electrophoresis of acetone-precipitated samples was performed in the presence of 0.1% SDS according to the method of Laemmli (1970). A minigel system was used: gel size was 8 cm × 10 cm × 1.5 mm (thickness). The stacking gel contained 5% acrylamide, and the separation gel contained 17.5% acrylamide with an acrylamide/bisacrylamide ratio of 30:0.8 to give a high resolution of polypeptides with a molecular mass close to 30 kD. Staining was performed by the silver nitrate method (Morrissey, 1981). Protein was determined by the Lowry method modified for the presence of Triton (Dulley and Grieve, 1975).
RESULTS
Purification of the Citrate Carrier
Maize shoot mitochondria were solubilized in Triton X-100 in the presence of cardiolipin and subjected to chromatography on hydroxyapatite followed by a second chromatography on hydroxyapatite/celite (Table I). The passage of the mitochondrial extract through hydroxyapatite led to a substantial purification of the citrate carrier. About 95% of the proteins present in the extract were bound to this resin. In the hydroxyapatite eluate 51% of the total activity of reconstituted citrate transport was recovered and the specific activity was increased 16-fold. For further purification, the hydroxyapatite pass-through was subjected to chromatography on hydroxyapatite/celite (see Methods). By this purification step, the specific activity of reconstituted citrate transport was increased 14- and 224-fold with respect to that of hydroxyapatite eluate and of mitochondrial extract, respectively. Approximately 50% of the total transport activity was recovered with a protein yield of 0.22%.
Table I.
Purification of the citrate carrier from maize mitochondria
| Purification Step | Protein | Specific Activity | Total Activity | Purification |
|---|---|---|---|---|
| mg | nmol 10 min−1 mg−1 protein | nmol 10 min−1 | fold | |
| Extract | 6.30 | 28 | 176 | 1 |
| Hydroxyapatite | 0.20 | 448 | 90 | 16 |
| HTP/celite | 0.014 | 6279 | 88 | 224 |
The proteoliposomes were loaded with 20 mm citrate and the exchange was started by the addition of 0.1 mm external [14C]citrate.
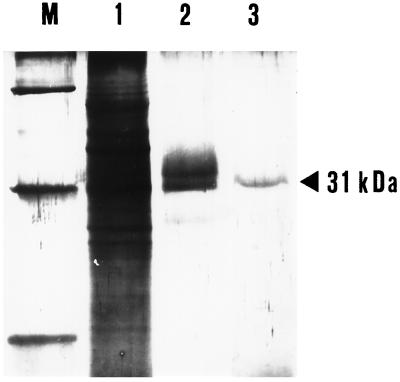
Figure 1 shows a SDS-PAGE of hydroxyapatite pass-through (lane 2) and hydroxyapatite/celite eluate (lane 3) obtained from maize mitochondria solubilized with Triton X-100. Under the conditions described in “Materials and Methods,” the eluate from hydroxyapatite contained five to six protein bands in the region of 29 to 36 kD and several transport activities corresponding to the citrate carrier, the ADP/ATP carrier, the α-ketoglutarate carrier, and porin (voltage-dependent anion channel of the outer mitochondrial membrane). Figure 1, lane 3, shows that a single protein band with an apparent molecular mass of 31 kD was eluted from the hydroxyapatite/celite column.
Figure 1.
Purification of the citrate carrier from maize mitochondria. Results of SDS-gel electrophoresis of fractions obtained by hydroxyapatite and hydroxyapatite/celite columns of maize mitochondria solubilized with Triton X-100 are shown. Lane M, Protein markers (from top to bottom: BSA, carbonic anhydrase, and Cyt c); lane 1, Triton X-100 mitochondrial extract (180 μg in 25 μL); lane 2, hydroxyapatite eluate (20 μg in 100 μL); lane 3, hydroxyapatite/celite eluate (2 μg in 160 μL).
Properties of the Reconstituted Citrate Carrier
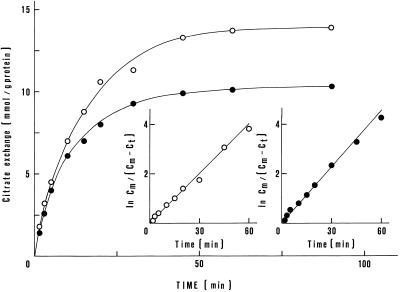
In all of the following experiments, the reconstituted system consists of purified protein eluted from the hydroxyapatite/celite column (Fig. 1, lane 3) and incorporated into liposomes. In Figure 2, the time course of 0.1 mm [14C]citrate uptake in proteoliposomes loaded either with citrate or malate is reported. Citrate uptake increased linearly with time for about 3 min in citrate-loaded liposomes and for about 2 min in malate-loaded liposomes. Furthermore, the total amount of citrate per milligram of protein taken up into the proteoliposomes was different in the two types of vesicles; it was 30% lower with malate-loaded liposomes. These differences can easily be rationalized taking into account the difference in the affinities of citrate and malate to the carrier (see below). There was no activity without incorporation of the carrier protein or with incorporation of heat-denatured carrier protein (2 min at 100°C) into the liposomes, or in the absence of internal citrate or malate.
Figure 2.
Time course of the citrate/citrate and citrate/malate exchanges in reconstituted liposomes. [14C]citrate (0.1 mm) was added at zero time to reconstituted liposomes containing 20 mm citrate (○) or 20 mm malate (•). The insets represent the logarithmic plots of ln citratemax (Cm)/(citratemax − citratet) (Ct), where citratemax is the maximum citrate exchange per mg protein and citratet is the citrate exchange per mg protein at time t, according to the relation ln citratemax/(citratemax − citratet) = kt. The amount of citrate taken up after reaching equilibrium was measured after 90 min; it was 13,920 and 10,310 nmol/mg protein for the citrate/citrate and citrate/malate exchanges, respectively.
The reaction order of the citrate/citrate and citrate/malate exchanges was investigated by plotting the natural logarithm of the fraction of equilibrium citratemax/(citratemax− citratet) against time. As shown in the insets of Figure 2, straight lines were obtained, demonstrating that the two exchange reactions follow a first-order kinetic. First-order rate constants, k, of 0.065 min−1 and t1/2 of 10.0 min for the citrate/citrate exchange, and of 0.075 min−1 and t1/2 of 7.5 min for the citrate/malate exchange were calculated. The initial rates of citrate uptake evaluated by multiplying the first-order constants by the total amounts transported at equilibrium were 904 nmol min−1 mg−1 protein (for the citrate/citrate exchange) and 773 nmol min−1 mg−1 protein (for the citrate/malate exchange), respectively.
The rate of citrate/citrate exchange is temperature dependent. In an Arrhenius plot, a straight line was obtained in the range from 10°C to 35°C (results not shown). The activation energy as derived from the slope was 66.5 kJ/mol.
The substrate specificity of [14C]citrate uptake with respect to intraliposomal counteranions was investigated in proteoliposomes loaded with a variety of substrates. The intraliposomal concentration of the anions used was 20 mm and the exchange time was 10 min. The data reported in Table II show that 0.1 mm [14C]citrate could be taken up in exchange with citrate, cis-aconitate, isocitrate, l-malate, malonate, and succinate. Surprisingly, labeled citrate could also be exchanged for oxaloacetate, α-ketoglutarate, glutarate, and, to a lower extent, α-ketoadipate and adipate. In contrast, [14C]citrate was not significantly taken up in exchange with trans-aconitate, oxalate, pimelate, and α-ketopimelate or with the substrates of other mitochondrial carriers, such as PEP (McIntosh and Oliver, 1992), Asp, Asn, Glu, Gln, Lys, phosphate, pyruvate, and ADP.
Table II.
Dependence of citrate transport in reconstituted liposomes on internal substrates
| Internal Substrate (20 mm) | Citrate Transport |
|---|---|
| nmol 10 min−1 mg−1 protein | |
| None (Cl− present) | 420 |
| Citrate | 6180 |
| cis-Aconitate | 4400 |
| Isocitrate | 3640 |
| trans-Aconitate | 710 |
| 1,2,3-Benzenetricarboxylate | 580 |
| Oxalate | 865 |
| Malonate | 4950 |
| l-Malate | 5300 |
| Succinate | 5070 |
| Glutarate | 3065 |
| Adipate | 1850 |
| Pimelate | 550 |
| Oxaloacetate | 6000 |
| α-Ketoglutarate | 6080 |
| α-Ketoadipate | 2080 |
| α-Ketopimelate | 350 |
| PEP | 865 |
| Asp | 515 |
| Asn | 495 |
| Glu | 490 |
| Gln | 520 |
| Lys | 490 |
| Phosphate | 530 |
| Pyruvate | 805 |
| ADP | 915 |
The proteoliposomes were loaded with the indicated substrates. Transport was initiated by adding 0.1 mm [14C]citrate. The results are the means of three experiments.
The sensitivity of the reconstituted citrate/citrate exchange to externally added substrates and inhibitors was also investigated (Tables III and IV). The citrate/citrate exchange was strongly inhibited by citrate, cis-aconitate, l-malate, succinate, malonate, oxaloacetate, α-ketoglutarate, and less efficiently by isocitrate, glutarate, and α-ketoadipate. In contrast, trans-aconitate, PEP, pyruvate, Glu, phosphate, and ADP had no effect (Table III). In addition, the citrate/citrate exchange was inhibited by the sulfydryl reagents mersalyl and p-hydroxymercuribenzoate (but not N-ethylmaleimide), as well as by the lysyl-specific reagents pyridoxal 5′-P and phenylisothiocyanate. The substrate analog 1,2,3-benzenetricarboxylate and the dicarboxylate analogs benzylmalonate and butylmalonate also inhibited the reconstituted citrate/citrate exchange, although less efficiently than in animal mitochondria, in agreement with previous results (Jung and Laties, 1979; Birnberg et al., 1982). In contrast, atractyloside, phenylglyoxal, and 1,2,4-benzenetricarboxylate had no significant effect (Table IV).
Table III.
Sensitivity of citrate exchange in reconstituted liposomes to externally added substrates
| Addition | Concentration | Inhibition |
|---|---|---|
| mm | % | |
| Citrate | 2.0 | 80 |
| cis-Aconitate | 2.0 | 73 |
| Isocitrate | 2.0 | 46 |
| trans-Aconitate | 2.0 | 10 |
| l-Malate | 2.0 | 70 |
| Succinate | 2.0 | 64 |
| Malonate | 2.0 | 61 |
| Glutarate | 2.0 | 48 |
| Oxaloacetate | 2.0 | 82 |
| α-Ketoglutarate | 2.0 | 85 |
| α-Ketoadipate | 2.0 | 41 |
| PEP | 2.0 | 9 |
| Pyruvate | 2.0 | 8 |
| Glu | 2.0 | 5 |
| Phosphate | 2.0 | 3 |
| ADP | 2.0 | 15 |
The proteoliposomes were loaded with 20 mm citrate and the exchange was started by adding 0.1 mm [14C]citrate. The external substrates were added together with [14C]citrate. The data are the means of three experiments. The control value of uninhibited citrate exchange was 6090 nmol 10 min−1 mg−1 protein.
Table IV.
Sensitivity of citrate exchange in reconstituted liposomes to inhibitors
| Addition | Concentration | Inhibition |
|---|---|---|
| mm | % | |
| Mersalyl | 0.5 | 91 |
| p-Hydroxymercuribenzoate | 0.5 | 85 |
| N-Ethylmaleimide | 2.0 | 4 |
| Benzylmalonate | 2.0 | 55 |
| Butylmalonate | 2.0 | 58 |
| 1,2,3-Benzenetricarboxylate | 2.0 | 45 |
| 1,2,4-Benzenetricarboxylate | 2.0 | 5 |
| Atractyloside | 0.1 | 18 |
| Phenylglyoxal | 10.0 | 15 |
| Phenylisothiocyanate | 10.0 | 97 |
| Pyridoxal 5′-P | 10.0 | 100 |
The proteoliposomes were loaded with 20 mm citrate and the exchange was started by adding 0.1 mm [14C]citrate. The inhibitors were added together with [14C]citrate except the −SH reagents, which were added 2 min before the labeled substrate. The data are the means of three experiments. The control value of uninhibited citrate exchange was 6127 nmol 10 min−1 mg−1 protein.
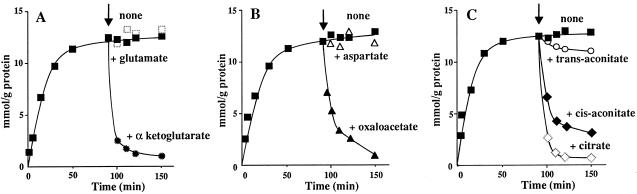
In other experiments the ability of the citrate carrier to transport α-ketoglutarate and oxaloacetate in reconstituted liposomes was further investigated. Figure 3A shows the effect of adding 2.0 mm unlabeled α-ketoglutarate to liposomes that had been incubated with 0.1 mm [14C]citrate in the presence of 10 mm internal cold citrate. The unlabeled α-ketoglutarate was added after a 90-min incubation, when the [14C]citrate taken up by the proteoliposomes had approached equilibrium. The addition of α-ketoglutarate caused an extensive efflux of the intraliposomal [14C]citrate, indicating that the radioactive citrate, taken up by the citrate/citrate homoexchange, is released by exchange for externally added α-ketoglutarate. Similar results were obtained by using oxaloacetate instead of α-ketoglutarate (Fig. 3B), indicating that an exchange between citrate and oxaloacetate had occurred. In contrast to α-ketoglutarate and oxaloacetate, Glu and Asp did not cause any efflux of intraliposomal [14C]citrate from [14C]citrate-loaded liposomes (Fig. 3, A and B). As shown in Figure 3C, the addition of 2.0 mm citrate or cis-aconitate to proteoliposomes incubated with 0.1 mm [14C]α-ketoglutarate (in the presence of 10 mm internal α-ketoglutarate) caused an extensive efflux of radiolabeled α-ketoglutarate. In contrast, trans-aconitate, i.e. a tricarboxylate that is not transported by the reconstituted protein, had virtually no effect on the intraliposomal [14C]α-ketoglutarate content. That the citrate/α-ketoglutarate and the citrate/oxaloacetate exchanges were mediated by the purified and reconstituted citrate carrier protein is demonstrated by the inhibition of these exchanges by the same inhibitors that inhibit the citrate/citrate exchange (data not shown). Furthermore, there was no exchange between citrate and α-ketoglutarate (or oxaloacetate) when using proteoliposomes that had been reconstituted with boiled citrate carrier protein (not shown).
Figure 3.
Substrate-induced efflux of [14C]citrate or [14C]α-ketoglutarate from proteoliposomes prelabeled by carrier-mediated exchange. [14C]citrate (0.1 mm) (A and B) or [14C]α-ketoglutarate (0.1 mM) (C) was added at time 0 to reconstituted liposomes containing 10 mm citrate (A and B) or α-ketoglutarate (C). Where indicated by the arrow, 2 mm nonradioactive α-ketoglutarate or Glu (A), 2 mm nonradioactive oxaloacetate or Asp (B), or 2 mm nonradioactive citrate, cis-aconitate, or trans-aconitate (C) was added. (▪), With no addition; (•), with α-ketoglutarate; (□), with Glu; (▴), with oxaloacetate; (▵), with Asp; (⋄), with citrate; (♦), with cis-aconitate; (○), with trans-aconitate. Transport was stopped by adding pyridoxal 5′-P (see Methods) after the indicated time intervals.
In additional experiments (not shown), we found that the purified preparation of the 31-kD protein, as shown in Figure 1, lane 3, when reconstituted into liposomes, did not catalyze the exchange reactions ADP/ADP (adenine nucleotide carrier), malate/phosphate (dicarboxylate carrier), Asp/Asp (Asp/Glu carrier), Glu/Glu (Glu carrier), pyruvate/pyruvate (monocarboxylate carrier), and phosphate/phosphate (phosphate carrier). Thus, the purified citrate carrier is obviously not contaminated by other mitochondrial carriers.
Km and Vmax Values of Citrate Transport
To obtain the basic kinetic data of the citrate carrier from maize shoot mitochondria the dependence of the exchange rate on substrate concentration was studied by changing the concentration of externally added [14C]citrate at a constant internal concentration of 20 mm citrate. In 12 experiments of this type an average of 0.65 ± 0.05 mm for the Km and 13.0 ± 1.0 μmol min−1 mg−1 protein for the Vmax at 25°C were determined.
Inhibition by Substrates
The inhibition of the reconstituted citrate/citrate exchange by various compounds was analyzed in the presence of different substrate concentrations. α-Ketoglutarate, cis-aconitate, succinate, and malate were all identified as competitive inhibitors, since they were found to increase the apparent Km without changing the Vmax of the citrate exchange. The inhibition constants, Ki, are summarized in Table V.
Table V.
Ki values for substrates competing with citrate for the exchange reaction
| Substrate | Ki | No. of Experiments |
|---|---|---|
| mm | ||
| α-Ketoglutarate | 0.5 ± 0.1 | 3 |
| cis-Aconitate | 1.0 ± 0.1 | 3 |
| l-Malate | 1.2 ± 0.2 | 3 |
| Succinate | 1.6 ± 0.2 | 3 |
The Ki values were calculated from double reciprocal plots of the rate of citrate/citrate exchange versus substrate concentrations. 0.1 to 1.0 mm [14C]citrate was added to proteoliposomes that contained 20 mm citrate and were incubated for 1 min at 25°C. The competing anions were added simultaneously with [14C]citrate at the appropriate concentrations.
DISCUSSION
The data presented in this study demonstrate that we were able to isolate and purify a 31-kD protein from maize mitochondria that catalyzes the transport of citrate. For purification we used a general scheme applied in our laboratory for the isolation of other mitochondrial carriers (Palmieri et al., 1995) with modifications of several experimental conditions. The conclusion that the polypeptide of 31 kD that we have purified from maize mitochondria, is in fact the citrate carrier is supported by the following evidence. Upon reconstitution into liposomes, the purified carrier catalyzes a very active [14C]citrate/citrate exchange. Furthermore, the purified transporter exhibits a substrate specificity (Tables II and III) and an inhibitor sensitivity (Table IV) that partially resemble those observed for the citrate transport system in animal and plant mitochondria (Bisaccia et al., 1989; Claeys and Azzi, 1989; McIntosh and Oliver, 1992). Besides citrate, cis-aconitate, isocitrate, l-malate, succinate, and malonate can be used as counter-anions. However, whereas the citrate transporter partially purified from pea mitochondria (McIntosh and Oliver, 1992) is insensitive to mersalyl, the maize citrate carrier protein is inhibited by sulfydryl reagents (as is the mammalian carrier), suggesting that the latter contain an essential Cys residue. Furthermore, at variance with all previously characterized transport systems we found that the purified citrate antiporter from maize mitochondria can also transport α-ketoglutarate, oxaloacetate, and α-ketoadipate. These findings cannot be explained by contamination of the citrate carrier with the α-ketoglutarate carrier (Genchi et al., 1991) and/or with the oxaloacetate carrier (Ebbigausen et al., 1985) because the latter two transporters do not catalyze citrate/α-ketoglutarate, citrate/oxaloacetate, or citrate/α-ketoadipate exchanges (Ebbigausen et al., 1985; Genchi et al., 1991). During the revision of this paper an interesting article has appeared that describes an exchange of oxaloacetate with citrate, malate, α-ketoglutarate, succinate, and Asp in liposomes reconstituted with Triton X-100-solubilized mitochondria from potato (Hanning et al., 1999). In view of our results and the close similarity in the substrates transported (with the exception of Asp), it is likely that the transport activities observed by Hanning et al. (1999) in reconstituted mitochondrial extracts from potato are catalyzed by a protein homologous to the citrate carrier that we purified from maize.
The citrate transporter protein may play an important role in etiolated maize shoots under various physiological conditions. The citrate exported from the mitochondria to the cytosol in exchange for oxaloacetate can be cleaved by citrate lyase (Kaethner and ap Rees, 1985) to acetyl-CoA and oxaloacetate and used for fatty acid elongation (Ohlrogge and Brause, 1995) and isoprenoid synthesis (McGarvey and Croteau, 1995). The efflux of citrate, isocitrate, or α-ketoglutarate in exchange for malate or oxaloacetate may also be involved in other metabolic processes, such as nitrate assimilation (Hanning and Heldt, 1993) and amino acid biosynthesis, which require production of α-ketoglutarate in the cytosol. Thus, under these conditions citrate and isocitrate exported from the mitochondria by the citrate transporter can be converted to α-ketoglutarate by the cytosolic enzymes aconitase (Wendel et al., 1988) and isocitrate dehydrogenase, and then to Glu (Chen and Gadal, 1990) by Gln synthetase and Glu synthase system (Sukanya et al., 1994; Sakakibara et al., 1998). A further significance for the citrate carrier protein from maize is the possibility that it may transfer reducing equivalents from the mitochondrial matrix to the cytosol by catalyzing a malate/oxaloacetate exchange. It should be noted that a malate-oxaloacetate shuttle has been previously proposed in mammalian and plant mitochondria (Gimpel et al., 1973; Passarella et al., 1977; Krömer and Heldt, 1991).
The purification and characterization of the reconstitutively active citrate carrier from maize shoots represent important first steps toward investigations of this carrier at a molecular level. No N-terminal sequence was detected when samples of the intact carrier protein were subjected to the Edman degradation (results not shown). Therefore, it appears that the protein has a modified α-amino group. By using, in the first instance, complex mixtures of oligonucleotides as primers with sequences based upon partial protein sequences of fragments of the purified protein, we may be able to isolate clones encoding the citrate carrier protein from a maize cDNA library. Experiments in this respect are currently in progress in our laboratory.
Footnotes
This paper is dedicated to the memory of Prof. Giacomino Randazzo.
LITERATURE CITED
- Birnberg PR, Jayroe DL, Hanson JB. Citrate transport in corn mitochondria. Plant Physiol. 1982;70:511–516. doi: 10.1104/pp.70.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaccia F, De Palma A, Palmieri F. Identification and purification of the tricarboxylate carrier from rat liver mitochondria. Biochim Biophys Acta. 1989;977:171–176. doi: 10.1016/s0005-2728(89)80068-4. [DOI] [PubMed] [Google Scholar]
- Bisaccia F, Indiveri C, Palmieri F. Purification of reconstitutively active α-ketoglutarate carrier from pig heart mitochondria. Biochim Biophys Acta. 1985;810:362–369. doi: 10.1016/0005-2728(85)90222-1. [DOI] [PubMed] [Google Scholar]
- Chen RD, Gadal P. Do mitochondria provide the 2-oxoglutarate needed for glutamate synthesis in higher plant chloroplasts? Plant Physiol Biochem. 1990;28:141–145. [Google Scholar]
- Claeys D, Azzi A. Tricarboxylate carrier of bovine liver mitochondria. J Biol Chem. 1989;264:14627–14630. [PubMed] [Google Scholar]
- Day DA, Wiskich JT. Transport processes in isolated plant mitochondria. Physiol Veg. 1984;22:241–261. [Google Scholar]
- Douce R, Christensen EL, Bonner WD., Jr Preparation of intact plant mitochondria. Biochim Biophys Acta. 1972;275:148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M. The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:371–414. [Google Scholar]
- Dulley IR, Grieve PA. A single technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975;64:136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Ebbigausen H, Chen JIA, Heldt HW. Oxaloacetate translocator in plant mitochondria. Biochim Biophys Acta. 1985;810:184–189. [Google Scholar]
- Genchi G, De Santis A, Ponzone C, Palmieri F. Partial purification and reconstitution of the a-ketoglutarate carrier from corn (Zea mays L.) mitochondria. Plant Physiol. 1991;96:1003–1007. doi: 10.1104/pp.96.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzone GC, Genchi F, Bisaccia, De Santis A, Stefanizzi L, Palmieri F. Purification and characterization of the reconstitutively active adenine nucleotide carrier from maize mitochondria. Plant Physiol. 1996;112:845–851. doi: 10.1104/pp.112.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpel JA, De Haan EJ, Tager JM. Permeability of isolated mitochondria to oxaloacetate. Biochim Biophys Acta. 1973;292:582–591. doi: 10.1016/0005-2728(73)90006-6. [DOI] [PubMed] [Google Scholar]
- Hanning I, Baumgarten K, Schott K, Heldt HW. Oxaloacetate transport into plant mitochondria. Plant Physiol. 1999;119:1025–1031. doi: 10.1104/pp.119.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning I, Heldt HW. On the function of mitochondrial metabolism during photosynthesis in spinach leaves (Spinacia oleracea L.). Partitioning between respiration and export of redox equivalents and precursors for nitrate assimilation products. Plant Physiol. 1993;103:1147–1154. doi: 10.1104/pp.103.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JB. Membrane transport systems of plant mitochondria. In: Douce R, Day DA, editors. Encyclopedia of Plant Physiology, Vol 18. Berlin: Springer-Verlag; 1985. pp. 248–280. [Google Scholar]
- Heldt HW, Flügge UI (1987) Subcellular transport of metabolites in plant cells. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants. Academic Press, New York, pp 49–85
- Jung DW, Laties GG. Citrate and succinate uptake by potato mitochondria. Plant Physiol. 1979;63:591–597. doi: 10.1104/pp.63.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaethner TM, ap Rees T. Intracellular location of citrate lyase in leaves of Pisum sativum L. Planta. 1985;163:290–294. doi: 10.1007/BF00393520. [DOI] [PubMed] [Google Scholar]
- Krömer S, Heldt HW. Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta. 1991;1057:42–50. [Google Scholar]
- Laemmli K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaNoue KF, Schoolwerth AC. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh CA, Oliver DJ. Isolation and characterization of the tricarboxylate transporter from pea mitochondria. Plant Physiol. 1992;100:2030–2034. doi: 10.1104/pp.100.4.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh CA, Oliver DJ. The phosphate transporter from pea mitochondria. Isolation and characterization in proteolipid vescicles. Plant Physiol. 1994;105:47–52. doi: 10.1104/pp.105.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Brause J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F, Indiveri C, Bisaccia F, Iacobazzi V. Mitochondrial metabolite carrier proteins: purification, reconstitution, and transport studies. Methods Enzymol. 1995;260:349–369. doi: 10.1016/0076-6879(95)60150-3. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979;56:279–301. doi: 10.1016/0076-6879(79)56029-7. [DOI] [PubMed] [Google Scholar]
- Passarella S, Palmieri F, Quagliariello E. The transport of oxaloacetate in isolated mitochondria. Arch Biochem Biophys. 1977;180:160–168. doi: 10.1016/0003-9861(77)90020-0. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Kawabata S, Takahashi H, Hase T, Sugiyama T. Molecular cloning of the family of glutamine synthetase genes from maize: expression of genes for glutamine synthetase and ferredoxin-dependent glutamate synthase in photosynthetic and non-photosynthetic tissues. Plant Cell Physiol. 1998;33:49–58. [Google Scholar]
- Sukanya R, Li MG, Snustad DP. Root- and shoot-specific responses of individual glutamine synthetase genes of maize to nitrate and ammonium. Plant Mol Biol. 1994;26:1935–1946. doi: 10.1007/BF00019504. [DOI] [PubMed] [Google Scholar]
- Vivekananda J, Beck CF, Oliver DJ. Monoclonal antibodies as tools in membrane biochemistry: identification and partial characterization of the dicarboxylate transporter from pea leaf mitochondria. J Biol Chem. 1988;263:4782–4788. [PubMed] [Google Scholar]
- Vivekananda J, Oliver DJ. Isolation and partial characterization of the glutamate/aspartate transporter from pea leaf mitochondria using a specific monoclonal antibody. Plant Physiol. 1989;91:272–277. doi: 10.1104/pp.91.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekananda J, Oliver DJ. Detection of the monocarboxylate transporter from pea mitochondria by means of a specific monoclonal antibody. FEBS Lett. 1990;260:217–219. [Google Scholar]
- Wendel JF, Goodman MM, Stuber CW, Beckett JB. New isozyme systems for maize (Zea mays L.): aconitate hydratase, adenylate kinase, NADH dehydrogenase and shikimate dehydrogenase. Biochem Genet. 1988;26:421–445. doi: 10.1007/BF02401795. [DOI] [PubMed] [Google Scholar]