Chronic kidney disease (CKD) affects approximately 10% of the world’s adult population: it is within the top 20 causes of death worldwide,1 and its impact on patients and their families can be devastating. World Kidney Day and International Women’s Day in 2018 coincide, thus offering an opportunity to reflect on the importance of women’s health and specifically their kidney health, on the community, and the next generations; as well as to strive to be more curious about the unique aspects of kidney disease in women, so that we may apply those learnings more broadly.
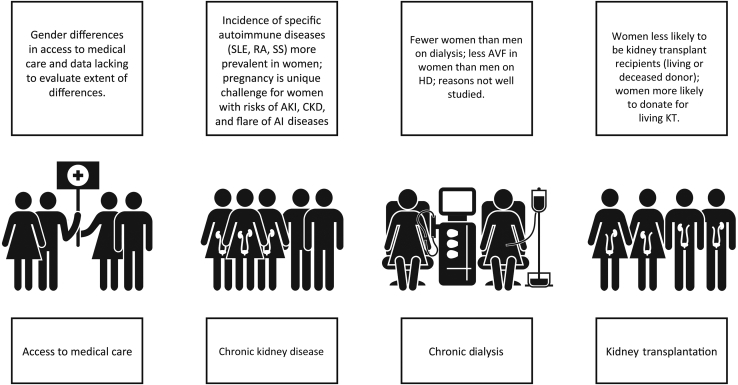
Girls and women, who make up approximately 50% of the world’s population, are important contributors to society and their families. Besides childbearing, women are essential in childrearing and contribute to sustaining family and community health. Women in the 21st century continue to strive for equity in business, commerce, and professional endeavors, while recognizing that in many situations, equality does not exist. In various locations around the world, access to education and medical care is not equitable among men and women; women remain underrepresented in many clinical research studies, thus limiting the evidence base on which to make recommendations to ensure the best outcomes (Figure 1).
Figure 1.
Sex differences throughout the continuum of CKD care. AI, autoimmune; AKI, acute kidney injury; AVF, arteriovenous fistula; CKD, chronic kidney disease; HD, hemodialysis; KT, kidney transplant; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, systemic scleroderma.
In this editorial, we focus on what we do and do not know about women’s kidney health and kidney disease, and what we might learn in the future to improve outcomes for all.
Kidney Health and Women’s Health: A Case for Optimizing Outcomes for Present and Future Generations
What We Know and Do Not Know
Pregnancy is a unique challenge and is a major cause of acute kidney injury (AKI) in women of childbearing age; AKI and preeclampsia (PE) may lead to subsequent CKD, but the entity of the risk is not completely known.2, 3, 4, 5 CKD has a negative effect on pregnancy even at very early stages.6, 7 The risks increase with CKD progression, thus posing potentially challenging ethical issues around conception and maintaining of pregnancies.6, 7, 8 We do know that PE increases the probability of hypertension and CKD in later years, but we have not evaluated a surveillance or reno-protective strategy to determine whether progressive loss of kidney function can be attenuated.9, 10, 11, 12
Specific systemic conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and systemic scleroderma (SS) are more likely to affect women than men. We do not know the relative contribution of these acute and chronic conditions on progression to end-stage renal disease (ESRD) in women.
In CKD cohorts, the prevalence in women is always less than in men, and they have slower progression to ESRD.13, 14, 15 We do not know why and how much of this is due to differences in identification of kidney impairment, different access to care, or true difference in disease severity and prevalence.
Women with CKD have a higher cardiovascular risk than women without CKD,16 but their risk is still lower than that of men with similar degrees of kidney impairment. In hemodialysis cohorts, there are differences in vascular access types in women versus men, which may be due to biological or systemic factors. In some locations, there is differential use of peritoneal and hemodialysis in women and men.
Women are more likely to donate kidneys for transplantation than to receive them. We do not know whether this is because of the differential incidence of CKD in men versus women, cultural factors, or other reasons.
There remain gender differences in access to care in different regions of the world, and we do not have data to directly evaluate the extent of these differences, in the poorest parts of the world in particular.
Pregnancy, Preeclampsia, Pregnancy-Induced Hypertensive Disorders, and Fetal Health: The Importance of Women’s Health to Present and Future Kidney Health
What We Know
PE is the principal cause of AKI and maternal death, particularly in developing countries.2, 17 Pregnancy is the most common cause of AKI in women of childbearing age.10, 18, 19 Several diseases and conditions, besides PE, hypertensive disorders of pregnancy, and CKD, can lead to pregnancy-related AKI. Causes vary in different regions. Septic abortion after an illegal procedure is the leading cause of early AKI in countries where legal abortions are not available, whereas PE after assisted fertilization is becoming a leading cause in developed countries.12, 20, 21, 22
PE and hypertensive disorders of pregnancy occur in 3% to 10% of all pregnancies2, 3, 18; in these disorders, the kidney is the main target of an unbalanced pro-angiogenic and anti-angiogenic derangement, leading to hypertension, proteinuria, and widespread endothelial damage. The incidence of PE, higher in low-to-middle−income countries (possibly reflecting undiagnosed predisposing diseases), peaks at the extremes of reproductive age for reasons mentioned above.12, 20, 21, 22
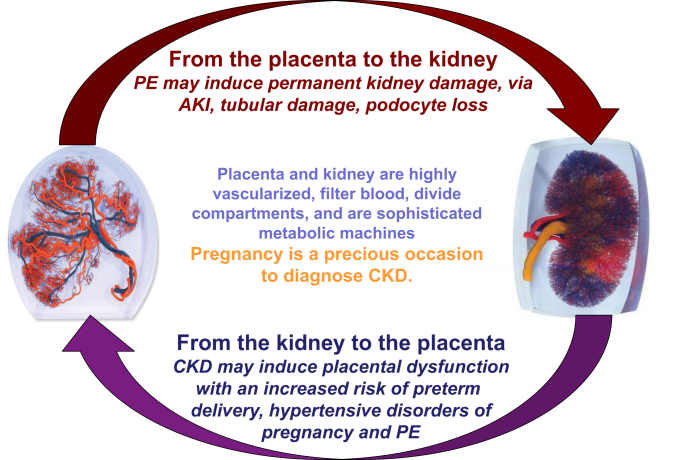
The relationship between the kidney and placenta is biunivocal, and the presence of CKD is a risk factor for PE and hypertensive disorders of pregnancy (Figure 2). Besides CKD, other conditions cited as risk factors for PE (diabetes, immunologic diseases, baseline hypertension, obesity, and metabolic syndrome), are also risk factors for CKD. Given that even minor alterations of kidney function are present in many of these disorders, the importance of kidney function is indirectly recognized in the development of PE. Newer definitions of PE recognize differences between “placental” and “maternal” causes of PE, based on novel angiogenic-antiangiogenic markers,23, 24 which may be important for management during and after pregnancy.
Figure 2.
Pregnancy and kidney function: complex interactions between 2 organs, the kidney and the placenta. AKI, acute kidney injury; CKD, chronic kidney disease; PE, preeclampsia.
There are long-term effects of PE on both maternal and fetal health, but this remains an area of active research with many unknowns.
PE is a risk factor for the future development of CKD and ESRD in the mother.3, 4, 5 The reasons are not fully understood; podocyte loss is a hallmark of PE, suggesting permanent glomerular damage.25 Endotheliosis, associated with PE but also found in normal pregnancies, may herald glomerulosclerosis; tubular and vascular damage may also be present, and all these alterations may coexist.26, 27
Besides maternal risks, PE is associated with intrauterine and perinatal death, preterm delivery, and restricted intrauterine growth; the latter 2 are linked to “small babies.”2, 3, 5 Small babies and preterm babies have highly increased risks of neurological deficits and postnatal complications, especially sepsis.28, 29, 30, 31, 32 The risks may be higher in low-income countries, as survival and deficit-free survival depend on the provision of postnatal intensive care.20, 21 In the long term, small babies are at risk for the development of diabetes, metabolic syndrome, cardiovascular disease (CVD), and CKD in adulthood.33, 34, 35, 36, 37 Because kidney development is completed in the last phases of pregnancy, delayed, insufficient kidney growth, resulting in low nephron number, is probably the basis of the increased risk of CKD and hypertension in small-for−gestational age and preterm babies.33, 34, 35, 36, 37
Pregnancy in CKD, Dialysis, and Transplantation
What We Know
Chronic Kidney Disease
CKD is a risk factor for adverse pregnancy outcomes from its early stages6, 38, 39 (Table 1). The risks increase from CKD stage 1 to CKD stage 5, and may be higher in glomerular nephropathies, autoimmune diseases, and diabetic nephropathy.6, 7, 38, 39, 40, 41 Results of pregnancy after kidney donation suggest that reduction of kidney parenchyma may be associated with a higher risk of PE and hypertensive disorders of pregnancy.42, 43
Table 1.
Adverse pregnancy outcomes in patients with chronic kidney disease and in their offspring
| Term | Definition | Main issues |
|---|---|---|
| Maternal death | Death in pregnancy or within 1 wk to 1 mo postpartum | Too rare to be quantified, at least in highly resourced settings, where cases are in the setting of severe flares of immunologic diseases (SLE in premature infants). Still an issue in AKI and in low resourced countries; not quantified in low-resourced countries, where it merges with dialysis need. |
| CKD progression | Decrease in GFR, rise in sCr, shift to a higher CKD stage | Differently assessed and estimated; may be linked to obstetric policy (anticipating delivery in the case of worsening of the kidney function); between 20% and 80% in advanced CKD. Probably not increased in early CKD stages. |
| Immunologic flares and neonatal SLE | Flares of immunologic diseases in pregnancy | Once thought to be increased in pregnancy, in particular in SLE; probably a risk in patients who start pregnancy with an active disease, or with a recent flare-up. Definition of a “safe” zone is not uniformly agreed; in quiescent, well-controlled diseases, do not appear to be increased with respect to nonpregnant, carefully matched controls. |
| Transplant rejection | Acute rejection in pregnancy | Similar to SLE, rejection episodes are not increased with respect to matched controls; may be an issue in unplanned pregnancies, in unstable patients. |
| Abortion | Fetal loss, before 21−24 gestational wk | May be increased in CKD, but data are scant. An issue in immunologic diseases (eventually, but not exclusively linked to the presence of LLAC) and in diabetic nephropathy. |
| Stillbirth | Delivery of a nonviable infant after 21−24 gestational wk | Probably not increased in early CKD; may be an issue in dialysis patients; when not linked to extreme prematurity, may specifically linked to SLE, immunologic diseases and diabetic nephropathy. |
| Perinatal death | Death within 1 wk to 1 mo after delivery | Usually a result of extreme prematurity, which bears a risk of respiratory distress, neonatal sepsis, cerebral hemorrhage. |
| Small, very small baby | Infant weighting <2500−1500 g at birth | Has to be analyzed with respect to gestational age. |
| Preterm, early preterm, extremely preterm | Delivery before 37–34 or 28 completed gestational wk | Increase in risk of preterm and early preterm delivery across CKD stages; extremely preterm may be an important issue in undiagnosed or late referred CKD and PE-AKI. |
| SGA (IUGR) | <5th or <10th centile for gestational age | Strictly and inversely related to preterm delivery; SGA and IUGR are probably related to risk for hypertension, metabolic syndrome, and CKD in adulthood. |
| Malformations | Any kind of malformations | Malformations are not increased in CKD patients not treated by teratogen drugs (MMF, mTor inhibitors, ACEis, ARBs); exception: diabetic nephropathy (attributed to diabetes); hereditary diseases, such as PKD, reflux nephropathy, CAKUT may be evident at birth. |
| Hereditary kidney diseases | Any kind of CKD | Several forms of CKD recognize a hereditary pattern or predisposition; besides PKD, reflux, and CAKUT, Alport’s disease, IgA, kidney tubular disorders and mitochondrial diseases have a genetic background, usually evident in adulthood and not always clearly elucidated. |
| CKD−hypertension | Higher risk of hypertension and CKD in adulthood | Late maturation of nephrons results in a lower nephron number in preterm infants; the risks are probably higher in SGA-IUGR than in preterm infants adequate for gestational age. |
| Other long-term issues | Developmental disorders | Mainly due to prematurity, cerebral hemorrhage, or neonatal sepsis; are not specific to CKD, but are a threat in all preterm infants. |
ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; CAKUT, congenital anomalies of the kidney and urinary tract; ARBs, angiotensin II receptor blockers; CKD, chronic kidney disease; GFR, glomerular filtration rate; IUGR, intrauterine growth restriction; LLAC, lupus-like anticoagulant; MMF, mycophenolate mofetil; mTor, mechanistic target of rapamycin; PE-AKI, preeclampsia acute kidney injury; PKD, polycystic kidney disease; sCR, serum creatinine; SGA, small for gestational age; SLE, systemic lupus erythematosus.
Hypertension and proteinuria at baseline are important modulators of pregnancy-related risks. Among the risks, we know that malformations are not increased with respect to the overall population (out of the context of inherited diseases, such as reflux nephropathy, polycystic kidney disease, or congenital anomalies of the kidney and urinary tract). Maternal death is unusual in highly resourced countries, whereas the incidence of preterm delivery and of small-for−gestational age babies, intrinsically linked, is increased in stage 1 CKD patients, and rises with the worsening of kidney function. Likewise, the effect of pregnancy on CKD progression is not fully understood because of different study designs, obstetric policies, and duration of follow-up. Overall, short- and long-term decrease in kidney function is unusual in early CKD, but the risk increases as CKD severity increases.6, 7, 38, 39, 40, 41, 44, 45, 46, 47, 48 Pregnancy is a potential occasion for the initial diagnosis of CKD. In poorly or unevenly resourced countries, advanced CKD may be discovered only during pregnancy. The implications of dialysis initiation may present important clinical and ethical issues; in highly resourced countries with established prenatal care, the diagnosis of earlier stages of CKD may lead to more intensive therapy and surveillance.49, 50, 51
Dialysis and Transplantation
Fertility is reduced in ESRD; Australian and European data suggest a 1:10 ratio from the general population to transplantation and from transplantation to dialysis (1:100 probability as compared to the general population).52, 53 The first sporadic cases of successful pregnancy on dialysis were described in the 1970s, but in the new millennium, this became an acknowledged real clinical possibility.8, 54, 55
More than 1000 pregnancies have been reported in dialysis patients.55 The most important advance has been the demonstration of a strong relationship between the intensity (frequency and duration) of the dialysis sessions and positive pregnancy results: thus, intensifying dialysis up to daily is the current standard of care.8, 54 Changing attitudes toward counselling women with advanced CKD may be affected, with the knowledge of positive outcomes on dialysis for women and their offspring.
Fertility is partly restored after kidney transplantation.56, 57, 58, 59, 60 However, even in an ideal situation (normal kidney function, no hypertension or proteinuria, at least 2 years after transplantation, without recent rejection episodes), the risk of complications is higher in women with transplanted kidneys than in the general population. However, if teratogen drugs (mycophenolic acid and rapamycine) are avoided, the outcomes of pregnancy after kidney transplantation share the same risk factors as CKD (kidney function, hypertension, and proteinuria).59
Experience with pregnancy in patients with a reduced renal function or failing kidney graft is limited, and counseling is still necessarily based on personal experience or indirect evidence.61, 62 Assisted fertilization techniques are increasingly popular in some settings, but dedicated studies in CKD patients are few; multiple pregnancies may bear an added risk in CKD patients, with both native and transplanted kidneys.
Autoimmune Diseases, Women, and Kidney Disease
What We Know
Autoimmune diseases such as SLE, RA, and SS preferentially affect women and are characterized by systemic inflammation leading to target organ dysfunction, including kidneys. Sex differences in the incidence and severity of these diseases result from a complex interaction of hormonal, genetic, and epigenetic factors (Table 2). The public health burden of autoimmune diseases, which collectively represent a leading cause of morbidity and mortality among women throughout adulthood, is substantial.63, 64, 65
Table 2.
Sex differences in the incidence and severity of autoimmune diseases
| Characteristic | SLE | RA | SS |
|---|---|---|---|
| Peak incidence | Reproductive age | Perimenopausal | After 50−60 yr |
| Female/male ratio | Peak 15:1 | Peak 4:1 | Peak 14:1 |
| Total 9:1 | After 60 yr 1:1 | Total 3:1 | |
| Influence of estrogen | |||
| High levels | Negative | Positive | Unknown |
| Low levels | Unknown | Negative | Negative |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, systemic scleroderma.
SLE is an autoimmune disease with multiple organ involvement, affecting approximately 5 million people worldwide; it is disproportionately predominant in women (9:1 female-to-male ratio) and individuals of non-European ancestry. The highest female predominance (up to 15:1) is in the peak reproductive years. The biology of these differences has been explored: 1 explanation is the number of X chromosomes and genetic variants on the X chromosome66, 67, 68; another important etiological explanation is the role of estrogen in SLE. Estrogen’s primary effects are mediated by transcription activity of the intracellular estrogen receptors, the profile of which is altered in T cells from female SLE patients.69, 70 Cathepsin S protein has recently been identified as a potential cause of lupus, triggering the immune system to attack healthy cells, particularly in females.71 Numerous non-HLA genetic markers may predispose individuals of European, Hispanic, and African American ancestry to lupus.72 Susceptibility to SLE during pregnancy is also multifactorial, 1 factor being upregulation of interferon-α. Elevated interferon-α, expressed by the placenta, plays a pathogenic role in SLE, contributing both to the success of placental reproduction and to increased susceptibility to SLE.73 Regulatory T cells (which may be the key to cell-modulating feto-maternal tolerance) have abnormalities of structure and function, and may contribute to pregnancy pathology in women with SLE and to challenges of managing these patients during pregnancy.74 SLE affects kidneys in about 50% of patients, including glomerular, interstitial, and vascular lesions. Lupus nephritis is a major risk factor for overall morbidity and mortality in SLE, and, despite potent therapies, still leads to significant impairment of kidney function for many patients.75 Kidney disease is a critical concern in counseling women with lupus who are considering pregnancy, with previous kidney involvement and lower C4 levels conferring high risk of active nephritis occurring in pregnancy.76 Socioeconomic disparities are also linked to the health of patients with lupus. Poverty is associated with an increased long-term level of accumulated disease-associated damage and a 1.67-fold increased likelihood of experiencing a clinically meaningful increase in damage. Frequency of adverse pregnancy outcomes in women with lupus is 2-fold higher in women of black and Hispanic ethnicity than in women of white ethnicity. In black women, socioeconomic status was a determinant of pregnancy outcomes and a key contributor to adverse pregnancy outcomes.77, 78
Rheumatoid arthritis also preferentially affects women (4:1 ratio to men), with a peak incidence at age 45 to 55 years, coinciding with the perimenopausal years. This suggests a possible association between estrogen deficiency and disease onset. The female-to-male incidence ratio after age 60 years is approximately 1:1, potentially implicating changes in sex hormones in the development of RA, and a pattern of RA symptom improvement or even remission during pregnancy is well recognized.79, 80, 81 Renal involvement in RA is relatively common and multifactorial and is a predictor of mortality in RA patients. The risk of CKD is significantly higher in patients with RA than in the general population. The development of CKD may result from several ongoing processes, including specific renal involvement associated with RA (e.g., glomerulonephritis, interstitial nephritis), chronic inflammation, comorbidities, and nephrotoxic antirheumatic drugs). The strong association between RA activity and amyloid A (AA) amyloidosis increases morbidity and is the main cause of ESRD with RA and nephropathy. Importantly, some of the lifelong and combined RA pharmacotherapy can lead to various renal side effects.82, 83, 84
Systemic scleroderma predominantly affects women (female-to-male ratios ranging from 3:1 to 14:1), with the peak incidence in the fifth and sixth decades. Estrogen may play a role in scleroderma pathogenesis through its stimulatory effect on transforming growth factor−β1 receptor and platelet-derived growth factor receptor.85 Vasculopathy is an important disease-related manifestation in SS, and the low estrogenic state associated with menopause has been suggested to aggravate vascular manifestations in affected women.86 SS can also be complicated by a number of different forms of kidney disease, including scleroderma renal crisis, which represents a form of malignant hypertension with acute renal failure, or, more commonly, ischemic nephropathy leading to slowly progressive CKD, accompanied by hypertension and albuminuria.78 Normotensive acute renal failure in patients with SS may be caused by interstitial nephritis or antineutrophil cytoplasmic antibodies (ANCA) vasculitis, a separate entity in scleroderma with poor outcomes.87, 88, 89
Women, CKD, and Access to Renal Replacement Therapies
What We Know
Although renal replacement therapy (RRT), including dialysis and transplantation, is life sustaining, not all patients receive RRT. The rate of ESRD treated by RRT differs greatly between countries and regions, and intricately depends on the economy of a country and health care system.90, 91 Worldwide, only 50% of patients requiring RRT receive treatment,92 and in low- and middle-income countries and regions, even less; in large parts of sub-Saharan Africa, less than 2% of ESRD patients are treated by RRT.93 The equality of access to RRT for women and girls is of particular concern because, in many societies, they are disadvantaged by discrimination rooted in sociocultural factors.94, 95
Sex Differences in Access to Dialysis
At least 2.284 million persons may have died prematurely due to lack of access to RRT, with treatment gaps being much larger in low-income countries, with conservative estimates in Asia and Africa of 1.907 million and 432,000 persons not receiving RRT. By 2030, the estimated number of RRT should be more than double, to 5.439 million (3.899–7.640 million), with the most growth in Asia (0.968 million to a projected 2.162 million [1.71–3.14 million]).92 These numbers are derived from an extensive systematic review.
There are few data to compare the gender difference for the treatment gaps. Studies in Africa show that men have been more likely to receive RRT than women.96, 97 In Japan, the incidence of treated ESRD in female patients was less than half of that in male patients (3287 in men vs. 1764 women per million population treated)91; no explanations are given for this finding. One US study reports women having a significantly higher odds ratio of 1.70 for late initiation of dialysis compared to men.98 Levels of awareness of previous kidney disease in women were reported to be much lower than in men (2.9% ± 1.6% in women vs. 17.9% ± 5.9% in men), which may contribute to later initiation of RRT.99
Mortality rates are similar in men and women on dialysis, but the incidence rates of some dialysis-associated complications and morbidity are higher in women. A US report of hospitalizations in 111,653 patients undergoing maintenance hemodialysis describes higher hospitalization rates in women and higher risk for 30-day readmissions.100
In addition, the prevalent use of arteriovenous fistula, which is associated with reduced mortality, complication, and costs, is lower among female than among male hemodialysis patients.101 This may be due to a number of different factors, including anatomical/surgical issues relating to vessel size, timing of referral, and attitudinal differences. This has not been systematically studied.
Dialysis dose, which is evaluated by Kt/V, may result in underdialysis in women, who have an average smaller volume of urea distribution or total body water than men.102 Women receiving dialysis have also been reported to have worse clinical parameters, including anemia, nutrition, and quality of life.103 The reasons for this are not certain.
Sex Differences in Access to Kidney Transplantation
Transplantation represents the best form of RRT in patients without contraindications. Worldwide data indicate that women are less likely than men to be recipients of kidney transplants, from either a cadaveric or living donor, but are more likely to serve as living donors for kidney transplantation.104 Data from different countries, including the United States, France, China, and India, confirm differential kidney transplant rates (lower in women than men), less likelihood of women being registered on national transplant waiting lists, and longer time from dialysis initiation to listing. Mothers are more likely to be donors, as are female spouses.91, 105, 106, 107, 108 Sex inequality also exists in the pediatric population. A survey from 35 countries participating in the European Society for Pediatric Nephrology/European Renal Association−European Dialysis and Transplant Association Registry reported that girls had lower access to renal transplantation than boys.109
Socioeconomic factors undoubtedly play a role in the inequality of transplantation between sexes, especially in low- and middle-income countries and regions. Generally, men provide the major income for their family, which may discourage them from donating kidneys. Different employment status and incomes between the sexes may contribute to sex differences in transplantation because employment and income status are usually associated with better health care insurance that cover the costs for transplantation. Psychosocial factors and education of women have been suggested as a contribution to the sex disparity. US data showed that black women were less likely to want living donor kidney transplantation compared with men, despite being twice as likely as men to receive unsolicited offers for kidneys. They were also less likely to have been evaluated for a kidney transplant.110 Other reports describe disparities in age and sex in access to kidney transplantation, which originate at the time of pre-referral discussions about kidney transplantation; irrespective of age, women were more likely not to have had discussions with medical professionals. This result may imply that there is a need for better clinical guidelines and education for women, their social network, and their providers.111
Present and Future: What We Do Not Know
Given the data presented above with respect to pregnancy, AKI, autoimmune diseases, CKD, dialysis, and transplantation, there are many unanswered questions. In high-income countries with increasing maternal age and assisted fertilization, there may be an increase in PE that may affect future generations if associated with adverse fetal outcomes. The increase in in vitro fertilization techniques for women of advanced maternal age may lead to multiple pregnancies, which may predispose to PE, intrauterine growth restriction, or both. Will this lead to an increase in CKD and CVD for women in the future?
Because of the high heterogeneity of CKD, we do not know whether and how pregnancy outcomes are modulated by the different nephropathies, as, apart from the most common ones such as IgA or lupus nephropathy, diabetic nephropathy, and reflux nephropathy, evidence is scant.44, 45, 112, 113, 114 How should we define preconception risks of pregnancy with respect to current proteinuria cut-offs? Indications on when to start dialysis in pregnancy are not well established, nor is the specific role of frequency and duration. In women with kidney transplants, given the changing expanded donor policies, higher age at transplantation, and reduced fertility in older women, there may be changes in attitudes toward pregnancy with less than optimal kidney function.56, 60 How this will affect short- and long-term outcomes of mothers and their infants is not clear.
Teen pregnancies are very common in some parts of the world, and are often associated with low income and cultural levels. The uneven legal rules for assisted fertilization and the lack of systematic assessment of the kidney function point to the need for further research.
Despite elegant demonstrations for the role of sex hormones in vascular health and immunoregulation, the striking predominance of SLE, RA, and SS in women remains unexplained relative to other systemic diseases such as ANCA vasculitis and hemolytic-uremic syndrome. Thrombotic thrombocytopenic purpura has a higher incidence in women, although this is likely due to the association with other conditions that are more common in women. The incidence of kidney involvement in SLE during pregnancy and similarities and differences in women with PE have not been well studied. The role of different medications and responses to medications for autoimmune diseases relative to sex has also not been well studied.
More attention to similarities between conditions, the importance of sex hormones in inflammation, immune-modulation, and vascular health, may lead to important insights and clinical breakthroughs over time. If women are more likely to be living donors, at differential ages, does this affect both CVD risk and risk for ESKD? Have we studied this well enough, in the current era, with modern diagnostic criteria for CKD and sophisticated tools to understand renal reserve? Are the additional exposures that women have after living donation compounded by hormonal changes in vasculature as they age? Are the risks of CKD and PE increased in the younger female kidney living donor?
In the context of specific therapies for the treatment or delay of CKD progression, do we know whether there are sex differences in therapeutic responses to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers? Should we look at dose finding/adjustments by sex? If vascular biology and immune biology are affected by sex hormones as described earlier, do we know the impact of various therapies by level or ratio of sex hormones? In low-to-middle−income countries, how do changing economic and social cultures affect women’s health, and what is the nutritional impact on CKD of the increasing predominance of obesity, diabetes, and hypertension?
Summary
Women have unique risks for kidney diseases; and kidney diseases, as well as issues related to access to care, have a profound impact on both the current and next generations. Advocating for improved access to care for women is critical to maintain the health of families, communities, and populations.
Focused studies on the unique contribution of sex hormones, or the interaction of sex hormones and other physiology, is important to improve our understanding of the progression of kidney diseases. Immunological conditions such as pregnancy (viewed as a state of tolerance to nonself) as well as SLE and other autoimmune and systemic conditions common in women, if better studied, may also lead to breakthroughs in understanding and care paradigms.
There is a clear need for higher awareness, timely diagnosis, and proper follow-up of CKD in pregnancy. In turn, pregnancy may also be a valuable occasion for early diagnosis of CKD, allowing planning of therapeutic interventions.
On this occasion, World Kidney Day and the International Women’s Day 2018 are commemorated on the same day, offering us the opportunity to highlight the importance of women’s health and particularly their kidney health. On its 13th anniversary, World Kidney Day promotes affordable and equitable access to health education, health care, and prevention for all women and girls in the world.
The coinciding of World Kidney Day and International Women’s Day offers an opportunity to develop and to define best practices and future research agendas, and, ultimately, to optimize the outcomes of all people living with or at risk for kidney disease.
Disclosure
All authors have contributed to the manuscript equally. All the authors declared no competing interests.
Footnotes
This article is being published in Kidney International Reports and reprinted concurrently in several journals. The articles cover identical concepts and wording, but vary in minor stylistic and spelling changes, detail, and length of manuscript in keeping with each journal’s style. Any of these versions may be used in citing this article.
Note that all authors contributed equally to the conception, preparation, and editing of the manuscript.
Contributor Information
Adeera Levin, Email: alevin@providencehealth.bc.ca.
World Kidney Day Steering Committee:
Philip Kam, Tao Li, Guillermo Garcia-Garcia, Mohammed Benghanem-Gharbi, Kamyar Kalantar-Zadeh, Charles Kernahan, Latha Kumaraswami, Giorgina Barbara Piccoli, Gamal Saadi, Louise Fox, Elena Zakharova, and Sharon Andreoli
Appendix
World Kidney Day Steering Committee Members
Members of the World Kidney Day Steering Committee are Philip Kam Tao Li, Guillermo Garcia-Garcia, Mohammed Benghanem-Gharbi, Kamyar Kalantar-Zadeh, Charles Kernahan, Latha Kumaraswami, Giorgina Barbara Piccoli, Gamal Saadi, Louise Fox, Elena Zakharova, and Sharon Andreoli.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 08;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Dadelszen P., Payne B., Li J. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7. [DOI] [PubMed] [Google Scholar]
- 3.Mol B.W.J., Roberts C.T., Thangaratinam S. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 4.Vikse B.E., Irgens L.M., Leivestad T. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 5.Theilen L.H., Fraser A., Hollingshaus M.S. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–244. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccoli G.B., Cabiddu G., Attini R. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015;26:2011–2022. doi: 10.1681/ASN.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J.-J., Ma X.-X., Hao L., Liu L.-J. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol. 2015;10:1964–1978. doi: 10.2215/CJN.09250914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhunaizi A., Melamed N., Hladunewich M.A. Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2015;24:252–259. doi: 10.1097/MNH.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 9.Piccoli G.B., Cabiddu G., Castellino S. A best practice position statement on the role of the nephrologist in the prevention and follow-up of preeclampsia: the Italian Study Group on Kidney and Pregnancy. J Nephrol. 2017;30:307–317. doi: 10.1007/s40620-017-0390-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Ma X., Zheng J. Pregnancy outcomes in patients with acute kidney injury during pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:235. doi: 10.1186/s12884-017-1402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jim B., Garovic V.D. Acute kidney injury in pregnancy. Semin Nephrol. 2017;37:378–385. doi: 10.1016/j.semnephrol.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acharya A. Management of acute kidney injury in pregnancy for the obstetrician. Obstet Gynecol Clin North Am. 2016;43:747–765. doi: 10.1016/j.ogc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Iseki K. Gender differences in chronic kidney disease. Kidney Int. 2008;74:415–417. doi: 10.1038/ki.2008.261. [DOI] [PubMed] [Google Scholar]
- 14.Nitsch D., Grams M., Sang Y. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin A., Djurdjev O., Beaulieu M., Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52:661–671. doi: 10.1053/j.ajkd.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Weiner D.E., Tighiouart H., Elsayed E.F. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Oladapo O.T., Adetoro O.O., Ekele B.A. When getting there is not enough: a nationwide cross-sectional study of 998 maternal deaths and 1451 near-misses in public tertiary hospitals in a low-income country. Br J Obstet Gynaecol. 2016;123:928–938. doi: 10.1111/1471-0528.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tranquilli A.L., Dekker G., Magee L. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Bao H., Jiang Z. Pregnancy-related acute kidney injury and a review of the literature in China. Intern Med. 2015;54:1695–1703. doi: 10.2169/internalmedicine.54.3870. [DOI] [PubMed] [Google Scholar]
- 20.Prakash J., Pant P., Prakash S., Sivasankar M. Changing picture of acute kidney injury in pregnancy: study of 259 cases over a period of 33 years. Indian J Nephrol. 2016;26:262–267. doi: 10.4103/0971-4065.161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibarra-Hernández M., Orozco-Guillén O.A., de la Alcantar-Vallín M.L. Acute kidney injury in pregnancy and the role of underlying CKD: a point of view from México. J Nephrol. 2017;30:773–780. doi: 10.1007/s40620-017-0444-4. [DOI] [PubMed] [Google Scholar]
- 22.Blázquez A., García D., Rodríguez A. Is oocyte donation a risk factor for preeclampsia? A systematic review and meta-analysis. J Assist Reprod Genet. 2016;33:855–863. doi: 10.1007/s10815-016-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Gorman N., Wright D., Poon L.C. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49:756–760. doi: 10.1002/uog.17455. [DOI] [PubMed] [Google Scholar]
- 24.Zeisler H., Llurba E., Chantraine F. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 25.Garovic V.D. The role of the podocyte in preeclampsia. Clin J Am Soc Nephrol. 2014;9:1337–1340. doi: 10.2215/CJN.05940614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wide-Swensson D., Strevens H., Willner J. Antepartum percutaneous renal biopsy. Int J Gynaecol Obstet. 2007;98:88–92. doi: 10.1016/j.ijgo.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Shiiki H., Dohi K., Hanatani M. Focal and segmental glomerulosclerosis in preeclamptic patients with nephrotic syndrome. Am J Nephrol. 1990;10:205–212. doi: 10.1159/000168082. [DOI] [PubMed] [Google Scholar]
- 28.Linsell L., Malouf R., Morris J. Risk factor models for neurodevelopmental outcomes in children born very preterm or with very low birth weight: a systematic review of methodology and reporting. Am J Epidemiol. 2017;185:601–612. doi: 10.1093/aje/kww135. [DOI] [PubMed] [Google Scholar]
- 29.Guellec I., Lapillonne A., Marret S. Effect of intra- and extrauterine growth on long-term neurologic outcomes of very preterm infants. J Pediatr. 2016;175:93–99. doi: 10.1016/j.jpeds.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Moore T., Hennessy E.M., Myles J. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillén Ú., DeMauro S., Ma L., Zupancic J. Relationship between attrition and neurodevelopmental impairment rates in extremely preterm infants at 18 to 24 months: a systematic review. Arch Pediatr Adolesc Med. 2012;166:178–184. doi: 10.1001/archpediatrics.2011.616. [DOI] [PubMed] [Google Scholar]
- 32.Ranke M.B., Schweizer R., Rodemann S.M. Schoolchildren born VLBW or VLGA show height-related changes in body composition and muscle function but no evidence of metabolic syndrome risk factors. Results from the NEOLONG study. J Pediatr Endocrinol Metab. 2015;29:163–172. doi: 10.1515/jpem-2015-0266. [DOI] [PubMed] [Google Scholar]
- 33.Castanys-Muñoz E., Kennedy K., Castañeda-Gutiérrez E. Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr. 2017;106:1230–1238. doi: 10.1111/apa.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong K.K., Kennedy K., Castañeda-Gutiérrez E. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr. 2015;104:974–986. doi: 10.1111/apa.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low Birth Weight and Nephron Number Working Group The impact of kidney development on the life course: a consensus document for action. Nephron. 2017;136:3–49. doi: 10.1159/000457967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luyckx V.A., Bertram J.F., Brenner B.M. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 37.Luyckx V.A., Brenner B.M. Birth weight, malnutrition and kidney-associated outcomes—a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 38.Davison J.M., Lindheimer M.D. Pregnancy and chronic kidney disease. Semin Nephrol. 2011;31:86–99. doi: 10.1016/j.semnephrol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Hall M. Pregnancy in women with CKD: a success story. Am J Kidney Dis. 2016;68:633–639. doi: 10.1053/j.ajkd.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Nevis I.F., Reitsma A., Dominic A. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabiddu G., Castellino S., Gernone G. A best practice position statement on pregnancy in chronic kidney disease: the Italian Study Group on Kidney and Pregnancy. J Nephrol. 2016;29:277–303. doi: 10.1007/s40620-016-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg A.X., Nevis I.F., McArthur E. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124–133. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josephson M.A. Transplantation: pregnancy after kidney donation: more questions than answers. Nat Rev Nephrol. 2009;5:495–497. doi: 10.1038/nrneph.2009.129. [DOI] [PubMed] [Google Scholar]
- 44.Gianfreda D., Quaglini S., Frontini G. Does pregnancy have any impact on long term damage accrual and on the outcome of lupus nephritis? J Autoimmun. 2017;84:46–54. doi: 10.1016/j.jaut.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Blom K., Odutayo A., Bramham K., Hladunewich M.A. Pregnancy and glomerular disease: a systematic review of the literature with management guidelines. Clin J Am Soc Nephrol. 2017;12:1862–1872. doi: 10.2215/CJN.00130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imbasciati E., Gregorini G., Cabiddu G. Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis. 2007;49:753–762. doi: 10.1053/j.ajkd.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Fischer M.J. Chronic kidney disease and pregnancy: maternal and fetal outcomes. Adv Chron Kidney Dis. 2007;14:132–145. doi: 10.1053/j.ackd.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Bramham K. Diabetic nephropathy and pregnancy. Semin Nephrol. 2017;37:362–369. doi: 10.1016/j.semnephrol.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Eswarappa M., Rakesh M., Sonika P. Spectrum of renal injury in pregnancy-induced hypertension: experience from a single center in India. Saudi J Kidney Dis Transplant. 2017;28:279. doi: 10.4103/1319-2442.202790. [DOI] [PubMed] [Google Scholar]
- 50.Prakash J. The kidney in pregnancy: a journey of three decades. Indian J Nephrol. 2012;22:159–167. doi: 10.4103/0971-4065.98750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccoli G.B., Fassio F., Attini R. Pregnancy in CKD: whom should we follow and why? Nephrol Dial Transplant. 2012;27(suppl 3):iii111–iii118. doi: 10.1093/ndt/gfs302. [DOI] [PubMed] [Google Scholar]
- 52.Piccoli G.B., Cabiddu G., Daidone G. The children of dialysis: live-born babies from on-dialysis mothers in Italy—an epidemiological perspective comparing dialysis, kidney transplantation and the overall population. Nephrol Dial Transplant. 2014;29:1578–1586. doi: 10.1093/ndt/gfu092. [DOI] [PubMed] [Google Scholar]
- 53.Jesudason S., Grace B.S., McDonald S.P. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol. 2014;9:143–149. doi: 10.2215/CJN.03560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hladunewich M.A., Hou S., Odutayo A. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol. 2014;25:1103–1109. doi: 10.1681/ASN.2013080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piccoli G.B., Minelli F., Versino E. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant. 2016;31:1915–1934. doi: 10.1093/ndt/gfv395. [DOI] [PubMed] [Google Scholar]
- 56.Deshpande N.A., James N.T., Kucirka L.M. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388–2404. doi: 10.1111/j.1600-6143.2011.03656.x. [DOI] [PubMed] [Google Scholar]
- 57.Deshpande N.A., Coscia L.A., Gomez-Lobo V. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol. 2013;6:116–125. [PMC free article] [PubMed] [Google Scholar]
- 58.Bramham K., Nelson-Piercy C., Gao H. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol. 2013;8:290–298. doi: 10.2215/CJN.06170612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccoli G.B., Cabiddu G., Attini R. Outcomes of pregnancies after kidney transplantation: lessons learned from CKD. A comparison of transplanted, nontransplanted chronic kidney disease patients and low-risk pregnancies: a multicenter nationwide analysis. Transplantation. 2017;101:2536–2544. doi: 10.1097/TP.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 60.Webster P., Lightstone L., McKay D.B., Josephson M.A. Pregnancy in chronic kidney disease and kidney transplantation. Kidney Int. 2017;91:1047–1056. doi: 10.1016/j.kint.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 61.Pietrzak B., Mazanowska N., Kociszewska-Najman B. Successful pregnancy outcome after in vitro fertilization in a kidney graft recipient: a case report and literature review. Ann Transplant. 2015;20:338–341. doi: 10.12659/AOT.893735. [DOI] [PubMed] [Google Scholar]
- 62.Norrman E., Bergh C., Wennerholm U.-B. Pregnancy outcome and long-term follow-up after in vitro fertilization in women with renal transplantation. Hum Reprod. 2015;30:205–213. doi: 10.1093/humrep/deu293. [DOI] [PubMed] [Google Scholar]
- 63.Tedeschi S.K., Bermas B., Costenbader K.H. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149:211–218. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Marder W, Vinet É, Somers EC. Rheumatic autoimmune diseases in women and midlife health. Womens Midlife Health. 2015. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5444314/. Accessed October 19, 2017. [DOI] [PMC free article] [PubMed]
- 65.Ortona E., Pierdominici M., Maselli A. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016;52:205–212. doi: 10.4415/ANN_16_02_12. [DOI] [PubMed] [Google Scholar]
- 66.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2002;16:847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 67.Weckerle C.E., Niewold T.B. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011;40:42–49. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scofield R.H., Bruner G.R., Namjou B. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierdominici M., Ortona E. Estrogen impact on autoimmunity onset and progression: the paradigm of systemic lupus erythematosus. Int Trends Immun. 2013;1:24–34. [Google Scholar]
- 70.Maselli A., Conti F., Alessandri C. Low expression of estrogen receptor β in T lymphocytes and high serum levels of anti-estrogen receptor α antibodies impact disease activity in female patients with systemic lupus erythematosus. Biol Sex Differ. 2016;7:3. doi: 10.1186/s13293-016-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S.J., Schätzle S., Ahmed S.S. Increased cathepsin S in Prdm1(-/-) dendritic cells alters the TFH cell repertoire and contributes to lupus. Nat Immunol. 2017;18:1016–1024. doi: 10.1038/ni.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langefeld C.D., Ainsworth H.C., Cunninghame Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun. 2017;17:16021. doi: 10.1038/ncomms16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niewold T.B., Hua J., Lehman T.J.A. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tower C., Mathen S., Crocker I., Bruce I.N. Regulatory T cells in systemic lupus erythematosus and pregnancy. Am J Reprod Immunol. 2013;69:588–595. doi: 10.1111/aji.12081. [DOI] [PubMed] [Google Scholar]
- 75.Almaani S., Meara A., Rovin B.H. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12:825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buyon J.P., Kim M.Y., Guerra M.M. Kidney outcomes and risk factors for nephritis (flare/de novo) in a multiethnic cohort of pregnant patients with lupus. Clin J Am Soc Nephrol. 2017;12:940–946. doi: 10.2215/CJN.11431116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yelin E., Yazdany J., Trupin L. Relationship between process of care and a subsequent increase in damage in systemic lupus erythematosus. Arthritis Care Res. 2017;69:927–932. doi: 10.1002/acr.22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaplowitz E.T., Ferguson S., Guerra M. Socioeconomic status contributes to racial/ethnic disparities in adverse pregnancy outcomes among women with systemic lupus erythematosus. Arthritis Care Res [Hoboken] 2017 May 8 doi: 10.1002/acr.23263. [DOI] [PubMed] [Google Scholar]
- 79.Myasoedova E., Crowson C.S., Kremers H.M. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goemaere S., Ackerman C., Goethals K. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J Rheumatol. 1990;17:1620–1622. [PubMed] [Google Scholar]
- 81.de Man Y.A., Dolhain R.J.E.M., van de Geijn F.E. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- 82.Icardi A., Araghi P., Ciabattoni M. [Kidney involvement in rheumatoid arthritis] Reumatismo. 2003;55:76–85. doi: 10.4081/reumatismo.2003.76. [DOI] [PubMed] [Google Scholar]
- 83.Anders H.-J., Vielhauer V. Renal co-morbidity in patients with rheumatic diseases. Arthritis Res Ther. 2011;13:222. doi: 10.1186/ar3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiu H.-Y., Huang H.-L., Li C.-H. Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications—a national population-based cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vinet É., Bernatsky S., Hudson M. Effect of menopause on the modified Rodnan skin score in systemic sclerosis. Arthritis Res Ther. 2014;16:R130. doi: 10.1186/ar4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sammaritano L.R. Menopause in patients with autoimmune diseases. Autoimmun Rev. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Penn H., Denton C.P. Diagnosis, management and prevention of scleroderma renal disease. Curr Opin Rheumatol. 2008;20:692–696. doi: 10.1097/BOR.0b013e3283108df7. [DOI] [PubMed] [Google Scholar]
- 88.Anders H.J., Wiebecke B., Haedecke C. MPO-ANCA-positive crescentic glomerulonephritis: a distinct entity of scleroderma renal disease? Am J Kidney Dis. 1999;33:e3. doi: 10.1016/s0272-6386(99)70244-1. [DOI] [PubMed] [Google Scholar]
- 89.Zakharova EV, Makarova TA, Stolyarevich ES. ANCA-associated vasculitis in patient with CREST-syndrome—case report. Available at: https://www.peertechz.com/Clinical-Nephrology/ACN-2-115.php. Accessed October 19, 2017.
- 90.Eckardt K.-U., Coresh J., Devuyst O. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 91.Saran R., Robinson B., Abbott K.C. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 93.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125:229–243. discussion 243–246. [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization. Addressing gender within primary health care reforms. In: Gender, women and primary health care renewal: a discussion paper. Available at: http://apps.who.int/iris/bitstream/10665/44430/1/9789241564038_eng.pdf. Accessed October 19, 2017.
- 95.Eguavoen A.N.T., Odiagbe S.O., Obetoh G.I. The status of women, sex preference, decision-making and fertility control in Ekpoma community of Nigeria. J Soc Sci. 2007;15:43–49. [Google Scholar]
- 96.Halle M.P., Takongue C., Kengne A.P. Epidemiological profile of patients with end stage renal disease in a referral hospital in Cameroon. BMC Nephrol. 2015;16:59. doi: 10.1186/s12882-015-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ajayi S., Raji Y., Bello T. Unaffordability of renal replacement therapy in Nigeria. Hong Kong J Nephrol. 2016;18(suppl C):15–19. [Google Scholar]
- 98.Kausz A.T., Obrador G.T., Arora P. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000;11:2351–2357. doi: 10.1681/ASN.V11122351. [DOI] [PubMed] [Google Scholar]
- 99.Coresh J., Byrd-Holt D., Astor B.C. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 100.Adams S.V., Rivara M., Streja E. Sex differences in hospitalizations with maintenance hemodialysis. J Am Soc Nephrol. 2017;28:2721–2728. doi: 10.1681/ASN.2016090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ethier J., Mendelssohn D.C., Elder S.J. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23:3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Depner T.A. Prescribing hemodialysis: the role of gender. Advanc Renal Replace Ther. 2003;10:71–77. doi: 10.1053/jarr.2003.50007. [DOI] [PubMed] [Google Scholar]
- 103.Sehgal A.R. Outcomes of renal replacement therapy among blacks and women. Am J Kidney Dis. 2000;35:S148–S152. doi: 10.1016/s0272-6386(00)70242-3. [DOI] [PubMed] [Google Scholar]
- 104.Jindal R.M., Ryan J.J., Sajjad I., Murthy M.H., Baines L.S. Kidney transplantation and gender disparity. Am J Nephrol. 2005;25:474–483. doi: 10.1159/000087920. [DOI] [PubMed] [Google Scholar]
- 105.Couchoud C., Bayat S., Villar E. REIN registry. A new approach for measuring gender disparity in access to renal transplantation waiting lists. Transplantation. 2012;94:513–519. doi: 10.1097/TP.0b013e31825d156a. [DOI] [PubMed] [Google Scholar]
- 106.Liu G., Li X., Liu T. Gender disparity of living donor renal transplantation in East China. Clin Transplant. 2013;27:98–103. doi: 10.1111/ctr.12003. [DOI] [PubMed] [Google Scholar]
- 107.Naghibi O., Naghibi M., Nazemian F. Gender disparity in kidney transplantation. Saudi J Kidney Dis Transpl. 2008;19:545–550. [PubMed] [Google Scholar]
- 108.Bal M.M., Saikia B. Gender bias in renal transplantation: are women alone donating kidneys in India? Transplant Proc. 2007;39:2961–2963. doi: 10.1016/j.transproceed.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 109.Hogan J., Couchoud C., Bonthuis M. Gender disparities in access to pediatric renal transplantation in Europe: data from the ESPN/ERA-EDTA Registry. Am J Transplant. 2016;16:2097–2105. doi: 10.1111/ajt.13723. [DOI] [PubMed] [Google Scholar]
- 110.Gillespie A., Hammer H., Kolenikov S. Sex differences and attitudes toward living donor kidney transplantation among urban black patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9:1764–1772. doi: 10.2215/CJN.12531213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salter M.L., McAdams-Demarco M.A., Law A. Age and sex disparities in discussions about kidney transplantation in adults undergoing dialysis. J Am Geriatr Soc. 2014;62:843–849. doi: 10.1111/jgs.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piccoli G.B., Attini R., Cabiddu G. Maternal-foetal outcomes in pregnant women with glomerulonephritides. Are all glomerulonephritides alike in pregnancy? J Autoimmun. 2017;79:91–98. doi: 10.1016/j.jaut.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Seeger H., Salfeld P., Eisel R. Complicated pregnancies in inherited distal renal tubular acidosis: importance of acid-base balance. J Nephrol. 2017;30:455–460. doi: 10.1007/s40620-016-0370-x. [DOI] [PubMed] [Google Scholar]
- 114.Yefet E., Tovbin D., Nachum Z. Pregnancy outcomes in patients with Alport syndrome. Arch Gynecol Obstet. 2016;293:739–747. doi: 10.1007/s00404-015-3893-9. [DOI] [PubMed] [Google Scholar]