Abstract
Introduction
Alterations in glomerular filtration can considerably influence the dynamics and functions of the Bowman capsule. Despite the potentially important role in maintaining normal renal functions, few studies have focused on Bowman capsule volume in normal human kidneys.
Methods
We analyzed specimens from biopsies performed 1 hour after kidney transplantation from living donors without apparent renal disease. The measurements of all cross-sectional areas of the Bowman capsules and glomerular capillaries were used to estimate the mean Bowman capsule volume (BV) and glomerular capillary volume (GV) in each subject. The G/B ratio was defined as the ratio of GV to BV. The morphometric findings were examined in relation to the clinical findings in donors just before kidney transplantation.
Results
We analyzed 37 adults with a mean creatinine clearance of 111 ml/min. The mean BV and GV of these subjects were 6.10 ± 2.46 × 106 μm3 and 3.83 ± 1.52 × 106 μm3, respectively. Both the BV and GV varied up to 6-fold and were significantly higher in elderly, obese, or hypertensive subjects in comparison to nonelderly, nonobese, or normotensive subjects, whereas the renal function of each subgroup was similar. The G/B ratio (0.63 ± 0.05) was unaffected, and BV and GV were strongly correlated regardless of these clinical factors (r = 0.980 [95% confidence interval = 0.961−0.990], P < 0.001).
Conclusion
In the normal adult kidney, there may be an optimal BV to GV ratio for maintaining effective filtration in a variety of clinical situations, including advanced age, obesity, and hypertension.
Keywords: Bowman capsule, glomerular capillary, hypertension, obesity, renal biopsy
The Bowman capsule is a component of the renal corpuscle, which is an origin of the urinary tubules that constitute the nephrons of the kidney.1, 2 It is composed of the Bowman cavity, a space surrounded by parietal epithelial cell layers and visceral podocytes.3, 4 Physiologically, Bowman capsules are continuously exposed to a large amount of primary urine, which is produced by glomerular capillary filtration. Indeed, approximately 150 L per day of the primary urine are filtered through the glomerular capillaries, and pass through the urinary tubules via the Bowman capsules.5 It is therefore hypothesized that changes in glomerular filtration can considerably influence the dynamics and functions of the Bowman capsule.
Glomerular filtration is maintained by ultrafiltration, which is defined by glomerular capillary pressure, plasma osmotic pressure, and Bowman capsule pressure.6, 7 Despite dramatic changes in systemic blood pressure, glomerular capillary pressure is tightly controlled by an automatic regulation system of renal blood flow, including the vasoconstriction of the glomerular afferent and efferent arteries.8, 9, 10, 11 However, it is known that conditions such as obesity, diabetes, or chronic renal failure can impair this regulation system through several mechanisms.12, 13, 14 As a result, these clinical situations may cause glomerular hyperfiltration, a state of overwork in the glomeruli.
The chronic rise in glomerular filtration pressure can be reflected in the enlargement of the glomerular capillaries, such as an increase in the glomerular tuft volume or glomerular diameter. Previous human and animal studies have demonstrated a close link between glomerular hyperfiltration and glomerular hypertrophy.15 Of note, previous studies have shown the significance of glomerular hypertrophy as a predictor of a subsequent loss of renal function in many types of progressive renal disease.16, 17, 18, 19 However, despite the potential importance in the normal renal function, few previous morphometric studies have examined the size of Bowman capsules in normal human kidneys. Thus, the significance in Bowman capsule size differences between individuals remains largely unknown. This study therefore aimed to estimate Bowman capsule volume and to investigate the relationship between Bowman capsule volume and the glomerular capillary volume in subjects with various clinical conditions, including advanced age, obesity, and hypertension, but without apparent renal disease.
Methods
Patients
In the present study, we investigated biopsy specimens from donor kidneys in living kidney transplantation performed at Jikei Hospital, Tokyo, from 2005 to 2014. The kidney donors were selected according to the Amsterdam Forum guidelines.20 Subjects with renal manifestations (including apparent renal morphological or functional impairment or urinalysis abnormalities) were excluded at the time of donor selection. To evaluate the renal function, 24-hour urine was collected to investigate the amount of proteinuria, creatinine excretion, and creatinine clearance (CCr). Subjects with a urinary protein excretion of ≥300 mg/d and those with a moderately impaired renal function, defined as a CCr of <80 ml/min, were excluded from the study. Impaired glucose tolerance, defined as hemoglobin A1c (HbA1c) > 6.2% (National Glycohemoglobin Standardization Program [NGSP]), was a criterion for exclusion. The presence or a history of hypertension was not an exclusion criterion if it was controlled (systolic blood pressure < 130 mm Hg, diastolic blood pressure < 80 mm Hg) by diet or by the use of antihypertensive medications. Renal tissue specimens with < 5 nonsclerotic glomeruli were also excluded based on the results of a previous study.21
A total of 59 kidney transplant donors were recruited from the renal biopsy archives during this period. Of these samples, 22 contained <5 nonsclerotic glomeruli and were thus excluded. Finally, 37 biopsy samples from 37 donors were included in the present study.
Definitions
“Elderly” was defined as ≥60 years of age.22 According to the criteria proposed by the Japan Society for the Study of Obesity, a BMI of ≥25 kg/m2 signified obesity.23 Hypertension was defined as a systolic blood pressure of >140 mm Hg and/or a diastolic blood pressure of >90 mm Hg, or the use of antihypertensive medications. The estimated glomerular filtration rate (eGFR) was calculated from the serum creatinine (sCr) level using a modified equation for estimating the GFR in Japanese individuals: eGFR = 194 × age–0.287 × sCr–1.094 (× 0.739 if female).24
Pathological Analysis
The renal biopsies of the kidney transplant donors were performed under direct vision using a needle biopsy gun. All of the biopsy specimens used in this study were obtained after transplantation, at 1 hour after the initiation of blood reperfusion. An 18-gauge biopsy needle was used in all cases. The tissues were embedded in paraffin, cut into 3- to 4-μm sections, and stained with hematoxylin–eosin, periodic acid–Schiff, Masson trichrome, and periodic acid–methenamine silver. The total number of glomeruli identified in the specimens and the percentage of glomeruli affected by global sclerosis were assessed. The area of interstitial fibrosis/tubular atrophy was semiquantitatively evaluated according to the proportion of cortical area involvement.
Morphological Measurements
The areas of all Bowman capsules and glomerular capillaries were measured using a computerized image analyzer (Leica IM500, Leica Microsystems, Wetzlar, Germany). Periodic acid–methenamine silver staining was basically used for the measurements. The Bowman capsule area was defined as the area of the inner side of the glomerular parietal epithelial cell layers. Likewise, the glomerular area was defined as the area of the outer capillary loops of the tuft. Glomeruli that were affected by global glomerulosclerosis were excluded from the analyses. The mean Bowman capsule area (BA) and mean glomerular capillary area (GA) were calculated by averaging all of the measured areas of the Bowman capsules and glomerular capillary loops. The mean Bowman capsule volume (BV) and the mean glomerular capillary volume (GV) were calculated from the measured BA or GA, as follows: BV = (BA)3/2 × β/d × (f)-3, GV= (GA)3/2 × β/d × (f)−3, where β is a dimensionless shape coefficient (β = 1.38 for spheres), d is a size distribution coefficient used to adjust for variations in glomerular size (d = 1.01), and f is a correction factor used to adjust for the volume shrinkage associated with paraffin fixation (f = 0.85).25, 26, 27 The ratio of GV to BV was defined as the G/B ratio and was analyzed in relation to the clinical variables.
Statistical Analysis
Continuous variables were expressed as the mean ± SD. The variables were assessed for normality both visually (normal probability plot) and by inferential statistics (Shapiro–Wilk W and Kolmogorov–Smirnov tests). Continuous variables were compared by a t test or Wilcoxon rank-sum test, as appropriate. The clinical parameters were subjected to a univariate or multivariate linear regression analysis to determine their relationships with morphometric variables. P values of < 0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using the R software package, EZR (Saitama Medical Center, Jichi University, Saitama, Japan), which is based on the R software package (The R Foundation for Statistical Computing, version 3.2.2).
Results
The Clinical and Histopathological Characteristics of Kidney Transplant Donors
Clinical and histopathological characteristics of the kidney transplant donors included in this study are shown in Table 1. The mean age of the donors was 56 years, and there was a female predominance. Twelve (32%) of these donors were obese and 7 (19%) had hypertension. Among the 7 donors with hypertension, 5 received antihypertensive medications (calcium antagonists in 3 cases and angiotensin type 1 receptor blockers in 2). All of the donors had a preserved renal function; their mean CCr value was 111 ml/min. A minority of the donors showed mild to moderate levels of chronic renal histopathological injury, including >25% global glomerulosclerosis and/or interstitial fibrosis/tubular atrophy.
Table 1.
Clinical and histopathological characteristics at the time of biopsy (N = 37)
| Variable | Mean ± SD (range) or % |
|---|---|
| Clinical | |
| Age, yr | 56 ± 9 (36–70) |
| Gender, % male | 29.7 |
| BMI, kg/m2 | 23.3 ± 3.3 (17.3–31.1) |
| Hypertension, % | 18.9 |
| Serum creatinine, mg/dl | 0.70 ± 0.14 (0.40–1.00) |
| eGFR, ml/min per 1.73 m2 | 76 ± 16 (58–119) |
| Creatinine clearance, ml/min | 111 ± 22 (80–171) |
| Urinary protein excretion, mg/d | 35 ± 36 (0–134) |
| Serum albumin, g/dl | 4.2 ± 0.3 (3.6–5.1) |
| Serum uric acid, mg/dl | 4.8 ± 1.0 (2.2–7.2) |
| Serum total cholesterol, mg/dl | 217 ± 32 (159–294) |
| Serum triglyceride, mg/dl | 120 ± 59 (39–330) |
| Hemoglobin A1c, % | 5.2 ± 0.3 (4.5–5.9) |
| Histopathological | |
| Number of glomeruli identified in specimens | 19 ± 8 (6–34) |
| Number of glomeruli included in morphometric analysis | 18 ± 8 (5–34) |
| Glomeruli affected by global glomerulosclerosis, % | 5.0 ± 7.0 (5.0–29.0) |
| Interstitial fibrosis and/or tubular atrophy, % | 6.5 ± 5.6 (0–30.0) |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Morphological Measurements
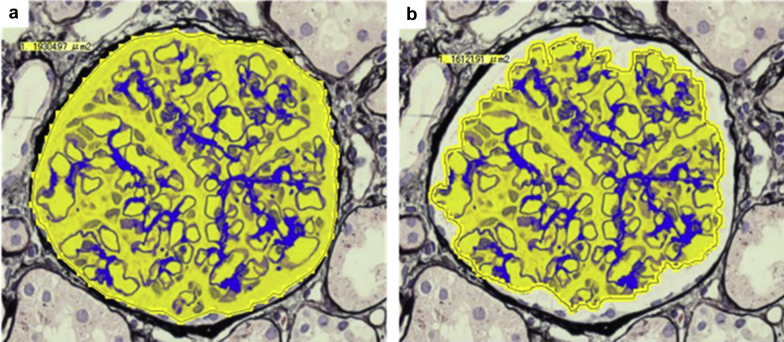
Representative examples of the areal measurements of the Bowman capsules and glomerular capillaries are shown in Figure 1. The results of the morphometric analyses are summarized in Table 2. Bowman capsule and glomerular capillary volumes were separately analyzed. In this cohort of living-donor kidneys without apparent renal disease, both the BV and GV varied by up to 6-fold among the individuals. The mean G/B ratio was 0.63 ± 0.05. Similar results were obtained when the donors who were treated with antihypertensive medications were excluded (n = 32, data not shown).
Figure 1.
Representative examples of the measurements of the Bowman capsule and glomerular capillary areas. The Bowman capsule area and glomerular capillary area were measured using a computerized image analyzer. (a) The Bowman capsule area was defined as the area of the inner side of the glomerular parietal epithelial cell layers. (b) The glomerular area was defined as the area of the outer capillary loops of the tuft. Periodic acid–methenamine silver staining (original magnification ×400).
Table 2.
Comparisons of morphological parameters between the groups categorized by clinical valuables in relation to renal function
| Parameter | All (N = 37) | Female (n = 26) | Male (n = 11) | P | Nonelderly (n = 23) | Elderly (n = 14) | P | Nonobese (n = 25) | Obese (n = 12) | P | Normotensive (n = 30) | Hypertensive (n = 7) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine clearance, ml/min | 111 ± 20 | 113 ± 22 | 108 ± 23 | 0.531 | 111 ± 18 | 111 ± 27 | 0.970 | 111 ± 23 | 112 ± 20 | 0.907 | 112 ± 22 | 109 ± 21 | 0.724 |
| Bowman capsule volume, × 106 μm3 | 6.10 ± 2.46 | 5.85 ± 2.09 | 6.65 ± 3.23 | 0.375 | 5.51 ± 2.48 | 7.06 ± 2.18 | 0.063 | 5.46 ± 1.99 | 7.42 ± 2.90 | 0.021 | 5.66 ± 2.28 | 7.92 ± 2.58 | 0.027 |
| Glomerular capillary volume, × 106 μm3 | 3.83 ± 1.52 | 3.70 ± 1.34 | 4.09 ± 1.92 | 0.487 | 3.44 ± 1.52 | 4.47 ± 1.32 | 0.045 | 3.39 ± 1.24 | 4.71 ± 1.70 | 0.012 | 3.54 ± 1.37 | 5.05 ± 1.63 | 0.016 |
| Glomerular capillary volume/Bowman capsule volume ratio | 0.63 ± 0.05 | 0.63 ± 0.05 | 0.62 ± 0.06 | 0.500 | 0.63 ± 0.06 | 0.64 ± 0.05 | 0.548 | 0.62 ± 0.05 | 0.64 ± 0.05 | 0.317 | 0.63 ± 0.06 | 0.64 ± 0.05 | 0.732 |
The donors were divided into subgroups based on the clinically available categorical variables, including gender, age, obesity, or hypertension. The morphometric variables were then compared between the subgroups with reference to the CCr value, which reflected the renal function. Renal function did not differ among the subgroups. The volume metrics for the Bowman capsules and glomerular capillaries were both significantly larger in elderly, obese, and hypertensive individuals. However, the G/B ratio was not affected among the subgroups.
Multivariate Analyses of Factors Associated With BV and GV
The BV and GV were separately subjected to univariate and multivariate analyses, which investigated their association with continuous clinical variables (Table 3). In the univariate analyses, age, BMI, and mean arterial pressure (MAP) were significant factors for both the BV and GV. In the multivariate analyses, age, BMI, but not MAP, were identified as factors that were associated with both the BV and GV. Similar results were obtained when we excluded the individuals who were treated with antihypertensive medications (n = 32, data not shown).
Table 3.
Factors influencing Bowman capsule volume and glomerular capillary volume by univariate and multivariate regression analyses
| Variable | Bowman capsule volume |
Glomerular capillary volume |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| r | P | t | P | r | P | t | P | |
| Age, yr | 0.339 | 0.040 | 2.42 | 0.004 | 0.371 | 0.024 | 2.74 | 0.010 |
| BMI, kg/m2 | 0.553 | <0.001 | 3.68 | <0.001 | 0.553 | <0.001 | 3.81 | <0.001 |
| MAP, mm Hg | 0.364 | 0.027 | 1.14 | 0.262 | 0.340 | 0.040 | 0.92 | 0.366 |
BMI, body mass index; MAP, mean arterial pressure.
Correlation Between BV and GV
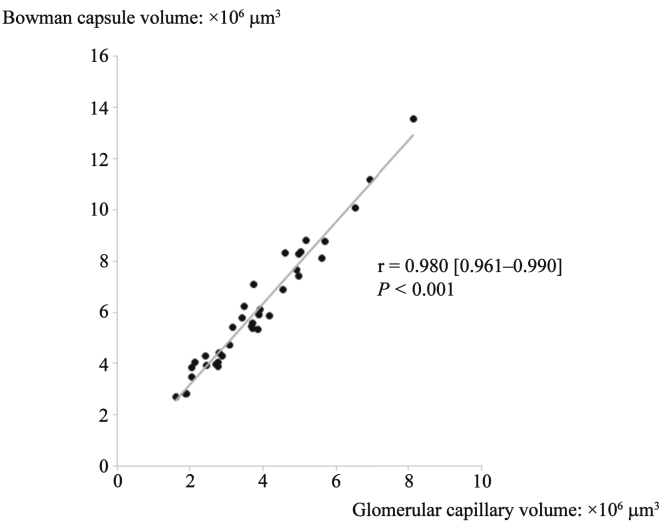
The BV and GV both showed wide variation of up to 6-fold among individuals, and the values were tightly correlated (r = 0.980 [95% confidence interval = 0.961−0.990], P < 0.001; Figure 2). Although the BV and GV in elderly, obese, or hypertensive subjects were significantly higher than those in nonelderly, nonobese, or normotensive subjects, the tight correlation between BV and GV was not affected by these clinical factors (Table 4).
Figure 2.
Correlation between the mean Bowman capsule volume and mean glomerular capillary volume. Although both metrics showed wide variations between individuals, the mean Bowman capsule volume and mean glomerular capillary volume were tightly correlated. Solid line indicates an approximately straight line.
Table 4.
Comparison of correlations between Bowman capsule volume and glomerular capillary volume in subgroups with or without categorical variables
| Subjects | r | 95% CI | P |
|---|---|---|---|
| All (N = 37) | 0.980 | 0.961–0.990 | < 0.001 |
| Nonelderly (n = 23) | 0.981 | 0.954–0.992 | < 0.001 |
| Elderly (n = 14) | 0.971 | 0.910–0.991 | < 0.001 |
| Nonobese (n = 25) | 0.974 | 0.943–0.989 | < 0.001 |
| Obese (n = 12) | 0.983 | 0.939–0.995 | < 0.001 |
| Normotensive (n = 30) | 0.978 | 0.954–0.989 | < 0.001 |
| Hypertensive (n = 7) | 0.980 | 0.866–0.997 | < 0.001 |
CI, confidence interval.
Discussion
In the present study, we morphometrically assessed the Bowman capsule size in adults. The subjects were individuals with relatively healthy kidneys, as they all fulfilled the donor criteria for living kidney transplantation. Even among these subjects without apparent renal disease, there were considerably wide variations in the Bowman capsule size. Our finding, that the Bowman capsule volume varied by up to 6-fold among individuals, is fairly consistent with a previous report by Hoy et al., who showed that the renal corpuscle volume varied by up to 5.6-fold in an adult autopsy series that included Australian Aborigines, Australian whites, African Americans and U.S. whites.28 Among the factors that were analyzed, age and BMI were identified as being closely associated with both the BV and GV. Of note, the BV and GV showed a tight correlation, regardless of these factors.
Obesity is known to cause various metabolic, hemodynamic, and structural alterations in the kidneys.29, 30, 31 A few previous studies have suggested an association between obesity and Bowman capsule size. Henegar et al. reported that the Bowman cavity size was increased in a canine model of obesity-induced renal injury.32 A similar result was reported by Tobar et al., who morphometrically analyzed the Bowman capsule size in biopsy specimens from patients with persistent proteinuria.33 That study showed that both the Bowman cavity size and the proximal tubular size in obese patients were larger than those in lean patients, implying that the imbalanced volume ratio between Bowman capsules and glomerular capillaries in obese individuals may cause an additional pathological situation and lead to greater urinary protein excretion. Among the clinical factors that were examined in the present study, obesity was considered to have the strongest association with an enlarged Bowman capsule size. However, our results did not show an imbalance between the sizes of Bowman capsules and glomerular capillaries, even in the presence of obesity. This was probably because none of our patients showed any renal manifestations, including persistent proteinuria, and the BMI values were relatively low in the obese subjects included in this study.
In our study, aging and hypertension were factors that were likely to affect the sizes of both the glomerular capillaries and Bowman capsules; however, blood pressure was not a statistically significant factor in the multivariate analyses. Each factor was closely related to the pathogenesis of nephrosclerosis, in which both glomerular hypertension and ischemic glomerular collapse may occur.34, 35 In nephrosclerosis, these hemodynamic alterations can be caused by both the breakdown of the renal arterial auto-regulatory system and the narrowing of the intrarenal arterial lumens.34, 35 In fact, the concomitant appearance of histopathological changes such as glomerular hypertrophy and glomerular collapse is often found within the kidney of the same subject in patients with advanced nephrosclerosis.36 Assessing the sizes of the glomerular capillaries and Bowman capsules might help to elucidate complex pathological situations such as those that exist in patients with advanced nephrosclerosis.
Despite the wide variation in each metric, the mechanisms underlying the balanced volume ratio between Bowman capsules and glomerular capillaries remain largely unknown. Recent studies suggest the pathological importance of parietal epithelial cells, which are located outside the layers of Bowman capsules. Activated parietal epithelial cells can invade the glomerular tuft at the site of adhesion.37, 38 Parietal epithelial cells are not a simple set of cell layers; rather, they are an important source that is responsible for the repair of glomerular injuries and possibly for the maintenance of the normal filtration function. Thus, the volume ratio of the Bowman capsule and glomerulus may be extremely important in the sense that it defines the spatial distance between the parietal epithelial cells and the podocytes covering the glomerular capillaries. It is noteworthy that temporal changes in the ratio of the glomerular capillary area to the Bowman capsule area were demonstrated in a rat model of chronic Masugi nephritis.39 That study clearly showed a transient increase in the ratio at the initial phase of glomerular injury, followed by the paralleled restoration of the ratio. A study using a rat model of diabetic nephropathy also showed a similar trend, suggesting that such dynamic changes are commonly involved in the glomerular response to injury, regardless of the trigger.40 Future studies should be extended to include pathological conditions in humans that may lead to such an imbalance within the renal corpuscles.
Our study does have some limitations. First, the current study included a limited number of subjects; thus, the small sample size might have influenced the results of the statistical analyses. Second, in our measurement method, we averaged all of the cross-sectional areas that were observed on the specimens, and thus could not recognize the variations among renal corpuscles within the individuals, as was previously reported in the measurement of the glomerular tuft size.41 Third, although the kidney specimens were obtained from individuals with normal renal function, the kidneys were reperfused after the shutdown of the blood flow during transplantation surgery, and they may not necessarily reflect the physiological situation. In addition, it should be noted that morphological changes occur in the processing and fixation process of the specimens. For these reasons, we are not able to define the actual “golden ratio” between the Bowman capsule volume and the glomerular capillary volume based on our current results.
In conclusion, we used morphometric approaches to analyze the volume of Bowman capsules and investigated the related factors in adults with normal renal function. Although our results showed that Bowman capsule volume is significantly influenced by age and BMI, the volume ratio of Bowman capsules and glomerular capillaries was not affected by these factors. Thus, these results suggest that there may be an optimal volume ratio that is physiologically suitable, or that achieves the most effective glomerular filtration.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Parts of this study were presented at the 60th Annual Meeting of Japanese Society of Nephrology, May 27, 2017, Sendai, Japan.
References
- 1.Jacobson H.R. Functional segmentation of the mammalian nephron. Am J Physiol. 1981;241:F203–F218. doi: 10.1152/ajprenal.1981.241.3.F203. [DOI] [PubMed] [Google Scholar]
- 2.Webber W.A., Lee J. The ciliary pattern of the parietal layer of Bowman capsule. Anat Rec. 1974;180:449–455. doi: 10.1002/ar.1091800304. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen F., Bentzon M.W. The ultastructure of the normal human glomerulus. Thickness of glomerular basement membrane. Lab Invest. 1968;18:42–48. [PubMed] [Google Scholar]
- 4.Arakawa M., Tokunaga J. A scanning electron microscope study of the human Bowman’s epithelium. Contrib Nephrol. 1977;6:73–78. doi: 10.1159/000399754. [DOI] [PubMed] [Google Scholar]
- 5.Shannon J.A., Smith H.W. The excretion of inurin, xulose and urea by normal and phlorizinized men. J Clin Invest. 1935;14:393–401. doi: 10.1172/JCI100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriz W., Elger M., Mundel P., Lemley K.V. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995;5:1731–1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- 7.Scott R.P., Quaggin S.E. Review series: the cell biology of renal filtration. J Cell Biol. 2015;209:199–210. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster R.P., Maes J.P. Effect of experimental neurogenic hypertension on renal blood flow and glomerular filtration rates in intact denervated kidneys of unanesthetized rabbits with adrenal glands demedullated. Am J Physiol. 1947;150:534–540. doi: 10.1152/ajplegacy.1947.150.4.534. [DOI] [PubMed] [Google Scholar]
- 9.Robertson C.R., Deen W.M., Troy J.L., Brenner B.M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972;223:1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- 10.Navar L.G. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978;234:F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 11.Navar L.G., Bell P.D., Burke T.J. Role of a macula densa feedback mechanism as a mediator of renal autoregulation. Kidney Int Suppl. 1982;12:S157–S164. [PubMed] [Google Scholar]
- 12.Chagnac A., Weinstein T., Korzets A. Glomerular hemodynamics in severe obesity. Am J Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 13.Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int. 1991;40:792–807. doi: 10.1038/ki.1991.277. [DOI] [PubMed] [Google Scholar]
- 14.Brenner B.M., Lawler E.V., Mackenzie H.S. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 15.Fogo A., Ichikawa I. Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am J Kidney Dis. 1991;17:666–669. doi: 10.1016/s0272-6386(12)80347-7. [DOI] [PubMed] [Google Scholar]
- 16.Fogo A., Hawkins E.P., Berry P.L. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 17.Nishimoto K., Shiiki H., Nishino T. Reversible glomerular hypertrophy in adult patients with primary focal segmental glomerulosclerosis. J Am Soc Nephrol. 1997;8:1668–1678. doi: 10.1681/ASN.V8111668. [DOI] [PubMed] [Google Scholar]
- 18.Nochy D., Heudes D., Glotz D. Preeclampsia associated focal and segmental glomerulosclerosis and glomerular hypertrophy: a morphometric analysis. Clin Nephrol. 1994;42:9–17. [PubMed] [Google Scholar]
- 19.Tóth T., Takebayashi S. Glomerular hypertrophy as a prognostic marker in childhood IgA nephropathy. Nephron. 1998;80:285–291. doi: 10.1159/000045188. [DOI] [PubMed] [Google Scholar]
- 20.Delmonico F. A report of the Amsterdam Forum on the Care of the Live Kidney Donor data and medical guidelines. Transplantation. 2005;79:S53–S66. [PubMed] [Google Scholar]
- 21.Hoy W.E., Samuel T., Hughson M.D. How many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies? J Am Soc Nephrol. 2006;17:556–563. doi: 10.1681/ASN.2005070772. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Good Health Adds Life to Years. Global Brief for World Health Day 2012. Geneva, Switzerland: World Health Organization; 2012.
- 23.Japan Society for the Study of Obesity New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo S., Imai E., Horio M., Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Weibel E.R. Academic Press; London, UK: 1979. Sterological Methods. Vol. 1: Practical Methods of Biological Morphometry; pp. 44–45. 131–134. [Google Scholar]
- 26.Fulladosa X., Moreso F., Narvaez J.A. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003;14:2662–2668. doi: 10.1097/01.asn.0000088025.33462.b0. [DOI] [PubMed] [Google Scholar]
- 27.Miller P.L., Meyer T.W. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990;63:862–866. [PubMed] [Google Scholar]
- 28.Hoy W.E., Douglas-Denton R.N., Hughson M.D. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003;83:S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 29.Wahba I.M., Mak R.H. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 30.Griffin K.A., Kramer H., Bidani A.K. Adverse renal consequence of obesity. Am J Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep., in press. [DOI] [PMC free article] [PubMed]
- 32.Henegar J.R., Bigler S.A., Henegar L.K. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 33.Tobar A., Ori Y., Benchetrit S. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;25:8e75547. doi: 10.1371/journal.pone.0075547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidani A.K., Griffin K.A. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 35.Denic A., Glassock R.J., Rule A.D. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill G.S., Heudes D., Jacquot C. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int. 2006;69:823–831. doi: 10.1038/sj.ki.5000163. [DOI] [PubMed] [Google Scholar]
- 37.Smeets B., Kuppe C., Sicking E.M. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1262–1274. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smeets B., Stucker F., Wetzels J. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol. 2014;184:3239–3248. doi: 10.1016/j.ajpath.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirato I., Hosser H., Kimura K. The development of focal segmental glomerulosclerosis in Masugi nephritis is based on progressive podocyte damage. Virchows Arch. 1996;429:255–273. doi: 10.1007/BF00198342. [DOI] [PubMed] [Google Scholar]
- 40.Gao Q., Shen W., Qin W. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 41.Puelles V.G., Douglas-Denton R.N., Cullen-McEwen L.A. Podocyte number in children and adults: associations with glomerular size and numbers of other glomerular resident cells. J Am Soc Nephrol. 2015;26:2277–2288. doi: 10.1681/ASN.2014070641. [DOI] [PMC free article] [PubMed] [Google Scholar]