Abstract
Introduction
As part of the precision medicine initiative, the National Institutes of Health/National Institute of Diabetes and Digestive Kidney Diseases has proposed collecting human kidney tissue to discover novel therapeutic targets from patients with kidney diseases. Patient attitudes on participating in kidney biopsy−based research are largely unknown.
Methods
We evaluated attitudes toward donating kidney tissue to research among participants who had experienced a clinically indicated kidney biopsy, through a survey conducted 9 months (interquartile range, 5−13 months) after their biopsy.
Results
Of the 177 participants contacted, 117 (66%) participated in the survey. A total of 85 participants (73%) reported that they would allow additional needle passes during a clinically indicated biopsy to donate kidney tissue for research. As reasons for participating in such a study, the participants reported the desire to help others and to contribute to science, and the lack of additional burden while participating in such a study. In a multivariable logistic model, older and African American participants had lower odds of allowing an additional pass for research (odds ratio: age ≥65 years [vs. ≤40], 0.15 [95% confidence interval, 0.03−0.73]; African Americans (vs. all others), 0.15 [95% confidence interval, 0.05−0.44]). However, participants’ self-reported biopsy complications such as pain, anxiety, and hematuria did not affect their willingness to allow additional passes. A total of 23 participants (20%) stated that they would agree to undergo a biopsy for research even if it was not clinically indicated.
Conclusion
Among patients who had experienced a kidney biopsy, a majority were amenable to additional needle passes to donate kidney tissue for research during a future, clinically indicated biopsy, whereas a minority would undergo a biopsy for research purpose only.
Keywords: acute kidney injury, biopsy, chronic kidney disease, kidney diseases, survey
Studying kidney tissue from human participants with kidney diseases will lead to a deeper understanding of the disorder’s phenotypes and can therefore allow for the development of successful treatment therapies in humans. The National Institutes of Health (NIH)/National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) has started working toward this goal by creating the Kidney Precision Medicine Project (KPMP), which proposes to obtain and to evaluate kidney tissue from participants with kidney diseases.1 Although there is a significant interest in obtaining kidney tissue for research purposes among the scientific community, patient perspectives on donating such kidney tissue for research remain unknown. In oncology, studies have looked at patients’ attitudes toward the use of biopsies to acquire tissue for research; however, no one has investigated patient attitudes toward human kidney tissue research.2, 3
To explore patient perspectives on participation in future kidney biopsy−based studies, we conducted a survey of participants who had undergone a clinically indicated kidney biopsy and were part of the Yale acute interstitial nephritis (AIN) cohort. The findings of this study help elucidate patients’ views on donating kidney tissue for research and thus help develop strategies to improve patient enrollment. Availability of human kidney tissue will in turn assist with ongoing investigations that seek to improve kidney disease therapies.
Brief Methods
Detailed methods, survey instrument, and analytic plan are presented in Detailed Methods, in the Supplementary Materials. Briefly, we conducted a 1-time survey of participants of the biopsy-based Yale AIN cohort through phone calls and in-person visits between September 2016 and March 2017. We excluded from the survey participants who were unable to participate because of their mental status or who had died between their kidney biopsy and the survey. All participants provided written informed consent, and the study was approved by the Yale human investigation committee.
Survey
The survey was designed to evaluate attitudes toward kidney biopsy research and was administered to all participants by a single person (B.C.). The survey questions were determined based on prior studies in oncology, adapted to kidney research after input from nephrologists, and pilot-tested in 5 volunteers to ensure comprehension over the phone.
Data Sources
We collected demographic, comorbidity, and objective complication data from review of the electronic health record. We also collected information on patient-reported outcomes during the survey.
Statistical Analysis
We compared continuous and categorical variables using the Wilcoxon rank sum test and χ2 test, respectively. We tested the association of various factors with the outcome of reporting willingness to allow an additional pass during a clinically indicated biopsy using a multivariable logistic regression model.
Results
Demographics of Survey Participants
Of the 199 patients enrolled in the Yale AIN cohort from January 2015 to October 2016, 177 were alive at the time of survey (Supplementary Figure S1). Among these 177 patients, 117 completed the survey. Patients who completed the survey were more likely to have baseline chronic kidney disease, to have have progressive chronic kidney disease as a biopsy indication, and to be outpatients at initial biopsy (Supplementary Table S1).
Participation in Biopsy-based Research
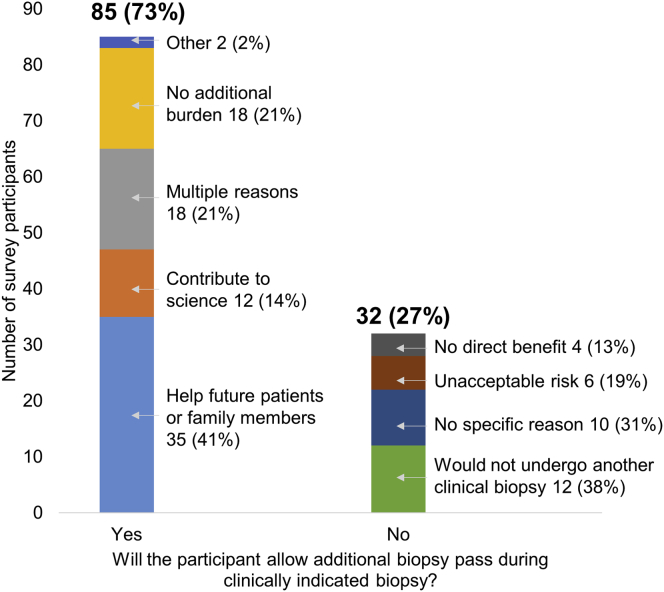
In all, 85 participants (73%) reported that they would allow additional passes to donate tissue to research during a clinically indicated biopsy (Figure 1). The common reasons for agreeing to participation in such a study were to help others (35 [41%]), lack of additional burden (18 [21%]), and contributing to science (12 [14%]). Thirty-two participants (27%) would not allow an extra pass to donate kidney tissue to research. Twenty-three participants (20%) reported that they would allow a research biopsy even if another clinically indicated biopsy was not needed.
Figure 1.
Participants’ willingness to allow an additional biopsy pass during a clinically indicated biopsy.
Determinants of Participation
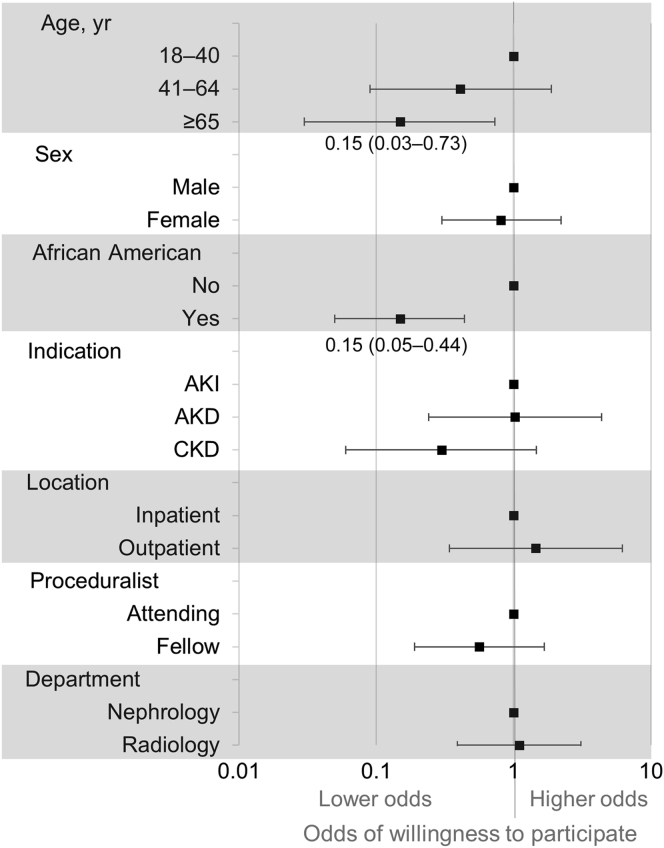
Participants who were willing to allow an additional pass during a clinically indicated biopsy were more likely to be younger (54 [43−65] vs. 64 [51−72] years, P = 0.02;) (Table 1). Participant responses also differed based on their racial/ethnic background; 17 (52%) of 33 African Americans and 68 (81%) of 84 participants of the other racial/ethnic groups reported willingness to participate (P = 0.008). However, participants did not differ on self-reported biopsy complications such as pain, anxiety, and hematuria. The 1 participant who required a blood transfusion after the biopsy reported willingness to allow an extra biopsy pass. No survey participant had required intervention to stop bleeding. In a multivariable logistic regression model controlling for various pre- and postbiopsy factors, we found that older and African American participants both had 85% lower odds of reporting willingness to allow an additional pass (Figure 2).
Table 1.
Determinants of participation in biopsy-based kidney research
| Characteristic | Additional pass to donate tissue to researcha |
P valueb | |
|---|---|---|---|
| Yes (n = 85) | No (n = 32) | ||
| Demographics and comorbidities | |||
| Age, yr | 54 (43, 65) | 64 (51, 72) | 0.02 |
| Female sex | 42 (49%) | 17 (53%) | 0.72 |
| Race/ethnicity | 0.008 | ||
| White, non-Hispanic | 50 (59%) | 14 (44%) | |
| Black, non-Hispanic | 17 (20%) | 16 (50%) | |
| Hispanic | 12 (14%) | 2 (6%) | |
| Other or unknown | 6 (7%) | 0 (0%) | |
| Site | 0.71 | ||
| York Street | 64 (75%) | 23 (72%) | |
| St. Raphael’s | 21 (25%) | 9 (28%) | |
| Comorbidities | |||
| Diabetes | 25 (29%) | 8 (25%) | 0.64 |
| Hypertension | 63 (74%) | 26 (81%) | 0.42 |
| Chronic kidney disease | 49 (58%) | 19 (59%) | 0.87 |
| Congestive heart failure | 9 (11%) | 6 (19%) | 0.24 |
| Biopsy-related factors | |||
| Indication for biopsy | |||
| Acute kidney injury | 26 (31%) | 11 (34%) | 0.19 |
| Acute kidney disease | 41 (48%) | 10 (31%) | |
| Progressive chronic kidney disease | 18 (21%) | 11 (34%) | |
| Location of biopsy | |||
| Inpatient | 31 (36%) | 14 (44%) | 0.47 |
| Outpatient | 54 (64%) | 18 (56%) | |
| Type of imaging guidance | 0.67 | ||
| Computed tomography | 28 (33%) | 13 (41%) | |
| Ultrasonography | 54 (64%) | 19 (59%) | |
| Fluoroscopy | 1 (1%) | 0 (0%) | |
| Level of training of operator | 0.08 | ||
| Attending physician | 57 (67%) | 16 (50%) | |
| Fellow or resident | 27 (32%) | 16 (50%) | |
| Department of operator | 0.90 | ||
| Nephrology | 51 (60%) | 19 (59%) | |
| Radiology | 33 (39%) | 13 (41%) | |
| Number of biopsy passes | 3 (2, 3) | 3 (2, 3) | 0.63 |
| Number of cores obtained | 2 (2, 3) | 2 (2, 3) | 0.91 |
| Survey-related factors | |||
| Time from biopsy to survey, mo | 9 (5, 13) | 8 (5, 13) | 0.75 |
| <6 mo | 24 (28%) | 13 (41%) | 0.24 |
| 6−12 mo | 35 (41%) | 8 (25%) | |
| >12 mo | 26 (31%) | 11 (34%) | |
| Number of times contacted for survey | 2 (1, 2) | 2 (1, 2.5) | 0.76 |
| Type of contact | 0.26 | ||
| In-person | 8 (9%) | 1 (3%) | |
| Telephone call | 77 (91%) | 31 (97%) | |
| Postbiopsy complications | |||
| Pain during biopsy | 2 (1, 4) | 1 (1, 3.5) | 0.28 |
| Anxiety at the time of biopsy | 2 (1, 6) | 3 (1, 7) | 0.43 |
| Hematuria after biopsy | 8 (9%) | 1 (3%) | 0.27 |
n (%) or Median (interquartile range) reported.
Wilcoxon rank-sum test or χ2 test.
Figure 2.
Association of prebiopsy factors with willingness to participate in biopsy-based kidney research. Multivariable logistic model showing association of pre-biopsy factors with willingness to allow additional pass during clinically indicated biopsy to donate kidney tissue to research. Hosmer−Lemeshow goodness-of-fit P value (with 10 groups) = 0.94; area under the curve of the model = 0.78 (0.67−0.88). Adjusted odds ratios (square boxes) and 95% confidence intervals (whiskers) are shown. AKI, acute kidney injury; AKD, acute kidney disease; CKD, chronic kidney disease.
Discussion
Human kidney tissue−based research holds the promise of discovery of therapeutic targets. In our survey of participants who had experienced a kidney biopsy, we noted that 73% of participants would allow an additional pass during a clinically indicated biopsy to donate tissue to research, whereas only 20% would allow a research protocol−only biopsy. A majority were motivated by altruistic intentions, whereas a minority reported that they would participate because there was no additional burden. We also noted that African American and older participants were less likely to participate. Postbiopsy complications did not affect responses.
Although there is no study evaluating patient attitudes toward donating kidney tissue to research, such studies were conducted in oncologic conditions. In breast cancer patients, Seah et al. reported that 72% of participants would allow an additional pass during a clinically indicated breast biopsy to donate tissue to research, whereas only 44% and 18% would allow research protocol−only biopsies of the breast and liver, respectively.2, 3 Our results are consistent with this study. We report that 73% of surveyed participants would allow an additional pass, whereas only 20% would participate in a study that conducted research protocol−only biopsies. Thus, researchers will have higher success in obtaining tissue by performing an additional pass during a clinically indicated kidney biopsy.
In addition, we noted that African American persons were less likely to express willingness to participate in medical research, which is consistent with prior studies. One survey indicated that African Americans were less likely to express willingness to participate in medical research, particularly if they were older or had knowledge of the Tuskegee syphilis study,4 which withheld antibiotics from African Americans enrolled in the study for decades after it became clinically available.5 Other studies described similar results.6, 7 Because our survey was not designed to specifically evaluate the effect of race on research participation, a future, qualitative study is needed to further evaluate this. Importantly, researchers will need to make special efforts to ensure that African American participants are appropriately represented in the Kidney Precision Medicine Project, especially since kidney disease disproportionately affects this racial/ethnic group. Increased participation could be achieved through community engagement and outreach, and rebuilding trust through timely sharing of results with the community.
Our study has several strengths. First, we surveyed 117 (66%) of the 177 eligible participants across broad ranges of age, sex, and race, and the reason for nonparticipation in 40 (67%) of the 60 participants was logistical. Second, we asked open-ended questions, allowing participants to express their opinion toward the kidney biopsy procedure or research in general. Our study also has some limitations. First, we did not survey patients before their kidney biopsy. Instead we surveyed individuals who had already experienced a kidney biopsy. Although it is possible that the responses may have varied based on the timing of the survey in relation to the biopsy, we chose to survey the group that had already experienced a kidney biopsy as they were familiar with the process, possible complications, and associated care. Moreover, this group would also be least likely to be pressured into providing a favorable response, as the survey responses could have no conceivable impact on a kidney biopsy procedure that has already occurred. Second, we did not evaluate in detail the factors that led to lower willingness to participate in research among African Americans. In addition, we did not collect data on socioeconomic status or level of education, which could have modified the association of race on survey responses. Third, we did not reliably collect information on participants’ knowledge of renal biopsy−related complications and could not evaluate how this knowledge might have affected their responses to survey questions. Finally, all participants were from 2 Yale-affiliated hospitals and may not be representative of the attitudes across the country. However, at the St. Raphael’s campus, all nephrology patients are cared for by nephrologists who are not members of the Yale full-time faculty.
In conclusion, our findings indicate that most patients would participate in a study conducting an additional pass during a clinically indicated kidney biopsy to donate tissue to research, whereas few would be willing to undergo a research protocol−only biopsy. We also note that African American participants are less likely to participate in such a study, and researchers will need to make special efforts to include this group to ensure generalizability of study findings.
Disclosure
All the authors declared no competing financial interests. The authors (CRP, DGM, AJP, RLL, SA, FPW) are members of the NIH/NIDDK-sponsored Kidney Precision Medicine Program (UG3DK114866), which plans to collect human kidney tissue for research.
Acknowledgments
We wish to thank the participants of the Yale AIN study, without whom this study would not have been possible. This work was supported by the National Institutes of Health awards K24DK090203 (CRP), K23DK097201 (FPW), and T32DK007276 (DGM). Additional support was provided by the Robert E. Leet and Clara Guthrie Patterson Trust Mentored Clinical Research Award (DGM). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. This study was presented at the American Society of Nephrology meeting at New Orleans, LA, October 31–November 5, 2017.
Footnotes
Detailed Methods.
Appendix 1. Yale acute interstitial nephritis (AIN) study–postbiopsy questionnaire.
Table S1. Baseline characteristics of survey participants as compared with those who were alive at discharge but did not participate.
Figure S1. Flow diagram of survey participants.
Supplementary materials are linked to the online version of the paper at www.kireports.org.
Supplementary Material
Yale acute interstitial nephritis (AIN) study–postbiopsy questionnaire.
Baseline characteristics of survey participants as compared with those who were alive at discharge but did not participate.
Flow diagram of survey participants.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases: Kidney Precision Medicine Project. Available at: https://www.niddk.nih.gov/research-funding/research-programs/kidney-precision-medicine-project-kpmp. Accessed July 24, 2017.
- 2.Naim F., Ballinger R., Rombach I. Patient attitudes toward undergoing additional breast biopsy for research. Breast. 2013;22:850–855. doi: 10.1016/j.breast.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Seah D.S., Scott S.M., Najita J. Attitudes of patients with metastatic breast cancer toward research biopsies. Ann Oncol. 2013;24:1853–1859. doi: 10.1093/annonc/mdt067. [DOI] [PubMed] [Google Scholar]
- 4.Brandt A.M. Racism and research: the case of the Tuskegee syphilis study. Hastings Cent Rep. 1978;8:21–29. [PubMed] [Google Scholar]
- 5.Shavers V.L., Lynch C.F., Burmeister L.F. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 6.Shavers V.L., Lynch C.F., Burmeister L.F. Factors that influence African-Americans' willingness to participate in medical research studies. Cancer. 2001;91:233–236. doi: 10.1002/1097-0142(20010101)91:1+<233::aid-cncr10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Corbie-Smith G., Thomas S.B., Williams M.V., Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Yale acute interstitial nephritis (AIN) study–postbiopsy questionnaire.
Baseline characteristics of survey participants as compared with those who were alive at discharge but did not participate.
Flow diagram of survey participants.