Abstract
Introduction
The optimal frequency of intermittent hemodialysis (IHD) in the treatment of acute kidney injury (AKI) remains unclear. Increasing the frequency of IHD, while offering the possible advantage of reduced ultrafiltration requirement and less hemodynamic instability per session, amplifies patient contact with an extracorporeal circuit with possible deleterious cardiovascular and immunological consequences. A recent study suggested that intensive renal replacement therapy (RRT) is associated with a decrease in urine output during AKI. We hypothesized that increased frequency of IHD may be associated with delayed renal recovery.
Methods
This is a post hoc analysis of the Acute Renal Failure Trial Network (ATN) study. The ATN study was a large randomized multicenter trial of intensive versus less-intensive RRT in critically ill patients with AKI. This study used either continuous RRT or IHD, depending on the hemodynamic status of the patient. Of 1124 patients, 246 were treated solely with IHD during the study period and were included in this analysis. The participants were randomized to receive IHD 3 days per week (L-IntRRT) or 6 days per week (IntRRT). The primary outcome of interest was renal recovery at day 28.
Results
L-IntRRT was associated with higher number of RRT-free days through day 28 than IntRRT (mean difference 2.5 days; 95% confidence interval [CI]: −4.79 to −0.27 days; P = 0.028). The likelihood for renal recovery at day 28 was lower in the IntRRT group (OR: 0.49; 95% CI: 0.28–0.87; P = 0.016).
Conclusion
In hemodynamically stable patients with AKI, intensifying the frequency of IHD from 3 to 6 days per week may be associated with impaired renal recovery.
Keywords: acute kidney injury, ATN study, critically ill, intermittent hemodialysis
Acute kidney injury (AKI) is extremely common in critically ill patients and is associated with significant morbidity and mortality.1, 2 Renal replacement therapy (RRT) remains the mainstay of support; available modalities include intermittent hemodialysis (IHD), continuous renal replacement therapy (CRRT), and hybrid therapies known by various terminologies such as sustained low-efficiency dialysis (SLED) or prolonged intermittent renal replacement therapy.3 CRRT and SLED are usually the preferred modalities in hemodynamically unstable critically ill patients and IHD is typically performed in the more stable patients who do not require pharmacologic hemodynamic support. Randomized controlled studies have not demonstrated a survival benefit with CRRT compared with IHD in the management of patients with AKI.4
There are numerous trials evaluating the intensity of CRRT in the treatment of AKI; however, clinical trials evaluating the dose and frequency of intermittent hemodialysis in AKI have been extremely rare. Despite the lack of similarities between AKI and end-stage renal disease with respect to acuity and comorbidities, IHD is most commonly provided 3 times per week to hemodynamically stable patients with AKI, as this is the frequency used most often in the end-stage renal disease population. In a previous single-center trial comparing daily to alternative-day IHD in the management of AKI, the daily IHD group had improved survival at 14 days after discontinuation of RRT and more rapid renal recovery.5 However, despite the prescription of a minimal single pool (sp) Kt/V urea of 1.2 per session, the 2 groups received lower than prescribed dose with delivered spKt/Vurea of 0.92 in the alternate-day IHD group and 0.94 in the daily IHD group per session.
The Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) study (clinicaltrials.gov NCT00076219) enrolled 1124 critically ill patients with AKI and randomized them to strategies that provided intensive RRT (IntRRT) or less-intensive therapy (L-IntRRT). Once randomized, hemodynamically stable patients, as defined by cardiovascular component of the sequential organ failure assessment (CV-SOFA) score of 0 to 2, received IHD as initial mode of RRT, and those with CV-SOFA score of 3 to 4 received CRRT or SLED. Depending on daily changes in the CV-SOFA score, patients were transitioned, per protocol, between CRRT or SLED and IHD.6 In this post hoc analysis, we attempted to elucidate the outcomes of those patients in the ATN study who received only IHD during the entire duration of the study.7 A recent study demonstrated that IntRRT was associated with decreased urine output in patients with AKI.8 In patients with end-stage renal disease, dialysis treatments have been shown to cause cardiac stunning and to induce systemic inflammation.9, 10 We hypothesized that increased frequency of IHD may be detrimental to renal recovery in patients with AKI.
Methods
The ATN study was a multicenter, prospective, randomized controlled trial, comparing 2 strategies of RRT in critically ill patients with AKI. The study was conducted between November 2003 and July 2007 at 27 medical centers in the United States. The design of the study and primary data have been described in previous publications.6, 11 Eligible patients were randomly assigned to the 2 treatment groups by centralized computer-generated randomization, which was stratified based on site, CV-SOFA score (0–2 vs. 3–4), and by the presence and absence of oliguria (defined as an average of less than 20 ml/h urine output for >24 hours). A copy of all the data gathered during the study is stored at the National Institute of Diabetes and Digestive and Kidney Diseases Central Data Repository and is accessible to researchers. After written approval from the Washington University in St. Louis Human Research Protection Office, we obtained the electronic, de-identified data from the repository for analysis.
Patient Population
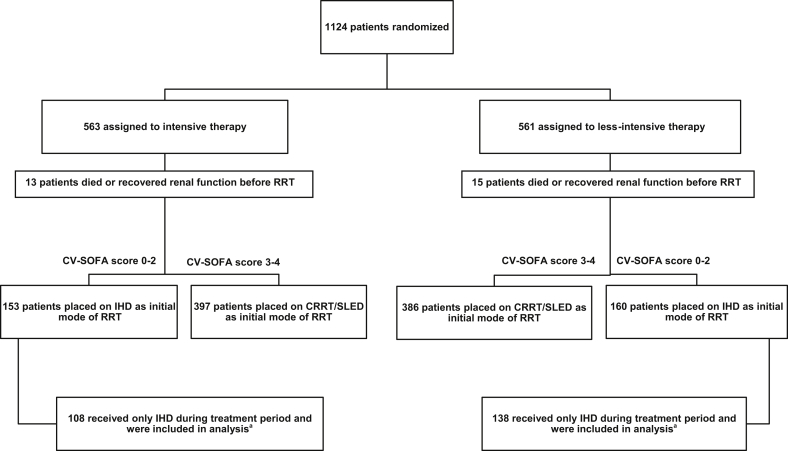
The ATN study enrolled critically ill patients with AKI clinically attributed to acute tubular necrosis who had at least 1 nonrenal organ failure or sepsis in whom the treating team had made the decision to initiate RRT. Patients could receive up to 1 IHD treatment or up to 24 hours of CRRT before enrollment. The cohort of patients we included for this analysis consisted of those individuals in the ATN study who received only IHD during the entire treatment period (Figure 1). We excluded patients who received IHD and then transitioned to CRRT or SLED, and patients who initially received CRRT or SLED and then transitioned to IHD. We included patients who underwent isolated ultrafiltration in addition to IHD, provided they did not receive CRRT or SLED.
Figure 1.
Enrollment, randomization, and modality choices based on initial hemodynamic status. CRRT, continuous renal replacement therapy; CV-SOFA, Cardiovascular Sequential Organ Failure Assessment score; IHD, intermittent hemodialysis; RRT, renal replacement therapy; SLED, sustained low-efficiency dialysis. aFive patients in each group who had a CV-SOFA score of 3 to 4 were started on IHD after given protocol exception by the principal investigator.
Prescription of IHD
Per the study protocol, IHD was prescribed based on a target spKt/Vurea of 1.4 per treatment to achieve a delivered spKt/Vurea of ≥1.2 per treatment, where K is the dialyzer clearance, t is duration of dialysis, and Vurea is the volume of distribution of urea. Pre- and postdialysis blood urea nitrogen was obtained at least 3 times per week during weeks 1 and 2 of protocol RRT and then at least once per week during weeks 3 and 4 of the study, and Kt/Vurea was calculated using the second-generation Daugirdas equation.12 If the measured spKt/Vurea was <1.2, then the IHD prescription for the subsequent treatments was adjusted to increase the delivered dose of dialysis. The adjustments were made at the discretion of the local investigator using 1 of the following 3 options: (i) increasing the duration of dialysis, (ii) using a dialyzer with higher urea clearance, or (iii) increasing prescribed blood flow rates. If spKt/Vurea was >1.4, then subsequent treatment durations were decreased or a dialyzer with lower urea clearance was prescribed. Patients randomized to the intensive RRT arm received IHD treatments 6 times per week (daily treatments, excluding Sunday), whereas patients randomized to the less-intensive RRT strategy received IHD treatments 3 times per week (every other day, excluding Sunday). Isolated ultrafiltration treatments were prescribed as deemed necessary by the treating nephrologist. The prespecified criteria for discontinuation of RRT was urine volume >30 ml/h with measured creatinine clearance >12 ml/min or spontaneous fall in serum creatinine (SCr).
Endpoints
The primary endpoint is renal recovery at day 28 because patients received protocolized hemodialysis for 28 days. Recovery of renal function was predefined as lack of need for continued IHD, with a minimum creatinine clearance of 20 ml/min, and classified as complete, partial, or none. Complete recovery of renal function was defined as SCr ≤0.5 mg/dl above the baseline value. Partial recovery was defined as SCr >0.5 mg/dl from baseline, but not dialysis dependent. Patients who were dialysis dependent at study completion or time of death were categorized as having no renal recovery. Secondary endpoints included all-cause mortality at day 60, in-hospital mortality, and 1-year all-cause mortality. Additional endpoints included duration of renal support and lengths of intensive care unit and hospital stay, as well as dialysis independence at day 60. Duration of renal support was defined as the number of days from initiation of RRT to the final dialysis treatment.
Statistics
We present categorical variables as proportions and continuous variables as mean ± SD. For Tables 1 and 2, we analyzed the differences between the groups using χ2 test for categorical variables or the Fisher exact test for categorical variables and t-test (log transformation applied when necessary) or the Wilcox rank sum test for continuous variables. When outcomes were collected repeatedly through time, we used linear mixed models for continuous outcomes and logistic mixed model for categorical variables and the covariance was modeled as compound symmetric.
Table 1.
Baseline characteristics
| Characteristic | Intensive strategy n = 108 |
Less-intensive strategy n = 138 |
P |
|---|---|---|---|
| Age, yr, mean ± SD | 60.1 ± 15.2 | 60.8 ± 15.1 | 0.70 |
| Sex, n/n with data (%) | |||
| Male | 88/108 (81.5) | 103/138 (74.6) | 0.20 |
| Female | 20/108 (18.5) | 35/138 (25.4) | |
| Race, n/n with data (%) | |||
| White | 76/108 (70.4) | 107/138 (77.5) | 0.42 |
| Black | 19/108 (17.6) | 22/138 (15.9) | |
| Hispanic | 9/108 (8.3) | 7/138 (5.1) | |
| Other | 4/108 (3.7) | 2/138 (1.4) | |
| Cause of AKI, n/n with data (%) | |||
| Ischemia | 71/103 (68.9) | 93/135 (68.9) | 0.99 |
| Nephrotoxins | 39/99 (39.4) | 50/130 (38.5) | 0.89 |
| Sepsis | 44/101 (43.6) | 55/131 (42) | 0.81 |
| Multifactorial | 48/103 (46.6) | 67/133 (50.4) | 0.57 |
| Oliguria, n (%) | |||
| Yes | 65 (60.2) | 98 (71.1) | 0.07 |
| No | 43 (39.8) | 40 (28.9) | |
| Premorbid weight, kg | 86.1 ± 18.2 | 84.8 ± 17.6 | 0.59 |
| Baseline SCr, mg/dl | 1.18 ± 0.4 | 1.19 ± 0.4 | 0.88 |
| Baseline SOFA score before randomization, mean ± SD | |||
| Total | 11.1 ± 3.0 | 10.5 ± 3.1 | 0.18 |
| Cardiovascular | 0.5 ± 0.9 | 0.4 ± 0.8 | 0.54 |
| Respiratory | 2.1 ± 1.1 | 2.1 ± 1.0 | 0.69 |
| Coagulation | 1.1 ± 1.1 | 1.0 ± 1.3 | 0.31 |
| Liver | 1.1 ± 1.3 | 1.0 ± 1.3 | 0.39 |
| CNS | 1.8 ± 1.4 | 1.7 ± 1.4 | 0.46 |
| Age-adjusted Charlson score, mean ± SD | 4.1 ± 2.3 | 4.7 ± 3.0 | 0.09 |
| Baseline APACHE II score, mean ± SD | 23.2 ± 6.2 | 22.2 ± 7.2 | 0.28 |
| Length of stay before randomization, d, mean ± SD | |||
| Hospital | 13.5 ± 17.9 | 11.6 ± 13.2 | 0.06 |
| ICU | 8.8 ± 17.0 | 7.3 ± 9.1 | 0.86 |
| BUN before first RRT, mg/dl, mean ± SD | 75.4 ± 30.2 | 70.7 ± 38.5 | 0.25 |
| RRT before randomization, n/n with data (%) | 61/108 (56.5) | 83/138 (60.1) | 0.56 |
AKI, acute kidney injury; APACHE, acute physiology and chronic health evaluation; Baseline SCr, lowest serum creatinine within 4 days before screening; BUN, blood urea nitrogen; CNS, central nervous system; ICU, intensive care unit; RRT, renal replacement therapy; SOFA, sequential organ failure assessment.
Percentages are based on number of patients without missing data.
Table 2.
Management of intermittent hemodialysis
| Intensive strategy n = 108 |
Less-intensive strategy n = 138 |
P | |
|---|---|---|---|
| No. of treatments provided, n | |||
| HD | 1131 | 829 | |
| Isolated ultrafiltration | 6 | 101 | |
| No. of treatments per patient, mean ± SD | 10.5 ± 8.5 | 6.7 ± 4.9 | 0.0258 |
| Duration of HD sessions, h, mean ± SD | 3.6 ± 1 | 3.6 ± 1 | 0.97 |
| Blood flow rate, ml/min, mean ± SD | 361.4 ± 55.9 | 351.7 ± 65.8 | 0.25 |
| Dialysate flow rate, ml/min, mean ± SD | 734.4 ± 113.1 | 701.7 ± 132.6 | 0.65 |
| Net ultrafiltration, l/treatment, mean ± SD | 1.7 ± 1.3 | 2.1 ± 1.4 | 0.0018 |
| Anticoagulation, n (%) | |||
| None | 732 (65.2) | 524 (62.2) | 0.12 |
| Heparin | 378 (33.7) | 303 (36) | |
| Citrate | 2 (0.2) | 0 (0) | |
| Other | 10 (0.9) | 15 (1.8) | |
| Blood urea nitrogen, mg/dl, mean ± SD | |||
| Predialysis | 44.4 ± 26.7 | 68.1 ± 31 | <0.0001 |
| Postdialysis | 15.7 ± 3.1 | 23.7 ± 13.8 | 0.002 |
| Kt/Vurea | |||
| First treatment, mean ± SD | 1.1 ± 0.26 | 1.14 ± 0.37 | 0.46 |
| Subsequent treatments, mean ± SD | 1.29 ± 0.35 | 1.26 ± 0.25 | 0.5 |
| Average value ≥1.2, n/n with data (%) | 64/101 (63) | 76/132 (67) | 0.37 |
| Kt/Vurea | |||
| Subsequent treatments ≥1.2 n/n with data (%) | 485/726 (67.4) | 368/553 (67) | 0.92 |
| Pre-HD MAP, mm Hg, mean ± SD | 88 ± 15.3 | 88.2 ± 17.4 | 0.75 |
| Lowest Intradialytic MAP, mm Hg, mean ± SD | 77.5 ± 15.7 | 76.7 ± 16.4 | 0.99 |
| Number of HD treatments with lowest MAP <60 mm Hg, n/n with data (%) | 101/909 (11.1) | 96/723 (13.3) | 0.42 |
| Change in MAP (Pre-HD MAP – lowest intradialytic MAP), mm Hg, mean ± SD | 10.5 ± 11.5 | 11.5 ± 13.5 | 0.37 |
| No. of treatments with change in MAP >20 mm Hg, n/n with data (%) | 154/962 (16) | 124/751 (16.5) | 0.73 |
| No. of treatments with change in MAP >30 mm Hg, n/n with data (%) | 45/853 (5.3) | 59/686 (8.6) | 0.02 |
HD, hemodialysis; K, urea clearance of the dialyzer; Kt/Vurea, unitless measurement of dialysis dose; MAP, mean arterial pressure; t, duration of dialysis, V, volume of distribution of urea.
Dichotomous outcome variables (Table 3), including renal function recovery at day 28, mortality at 60 days, in-hospital death, death at 1 year, and dialysis independence by day 60 among survivors, were analyzed using conditional logistic regression, adjusted for the presence of absence of oliguria, with site as the conditioning factor. RRT-free days, hospital-free days, and intensive care unit–free days were analyzed using a linear mixed model, with site treated as a random effect (Table 4). These analyses were also adjusted for the presence or absence of oliguria, age, and sex. We used the Kaplan-Meier method to calculate the cumulative mortality for the patient population (SPSS version 22.0; IBM Corporation, Chicago, IL). The primary exposure of interest was the intensity of intermittent hemodialysis, and we considered a P < 0.05 to be statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC), unless otherwise specified.
Table 3.
Primary and secondary outcomes
| Intensive strategy n = 108 |
Less-intensive strategy n = 138 |
Unadjusted odds ratio (95% CI) | P | Adjusted odds ratioa (95% CI) | P | |
|---|---|---|---|---|---|---|
| Renal recovery (complete and partial) by day 28, n/n with data (%) | 34/106 (32) | 58/136 (43) | 0.64 (0.37–1.08) | 0.09 | 0.49 (0.28–0.87) | 0.016 |
| Renal recovery (complete and partial) by day 28 among survivors (%) | 33/80 (41) | 57/109 (52) | 0.64 (0.36–1.15) | 0.13 | 0.54 (0.28–1.02) | 0.058 |
| Dialysis independence at day 28 | 52/108 (48%) | 76/138 (55%) | 1.32 (0.8–2.19) | 0.28 | 1.66 (0.95–2.87) | 0.073 |
| In-hospital death, n (%) | 27 (25) | 34 (25) | 1.02 (0.57–1.83) | 0.95 | 1.35 (0.7–2.59) | 0.37 |
| Death by day 60, n (%) | 33 (31) | 39 (28) | 1.12 (0.64–1.94) | 0.69 | 1.51 (0.81–2.79) | 0.19 |
| Death by 1 year, n (%) | 47 (44) | 67 (49) | 0.82 (0.49–1.35) | 0.43 | 1.03 (0.58–1.77) | 0.92 |
Renal recovery defined as defined as lack of need for continued hemodialysis with a minimum creatinine clearance of 20 ml/min. Recovery of renal function was considered to be complete if the serum creatinine (SCr) was ≤0.5 mg/dl above the baseline value or partial if the patient was not dialysis dependent, with an SCr >0.5 mg/dl above baseline.
CI, confidence interval.
Adjusted for oliguria.
Table 4.
Duration of renal support, ICU, and hospital length of stay
| Intensive strategy n = 108 |
Less-intensive strategy n = 138 |
Mean difference (95% CI) | P | |
|---|---|---|---|---|
| RRT-free days through day 28, mean ± SDa | 10.42 ± 0.97 | 12.95 ± 0.88 | −2.53 (−4.79 to −0.27) | 0.028 |
| Hospital-free days through day 60, mean ± SDa | 20.28 ± 2.51 | 21.7 ± 2.32 | −1.41 (−6.19 to 3.36) | 0.56 |
| ICU-free days through day 60, mean ± SDa | 31.5 ± 2.92 | 34.81 ± 2.7 | −3.31 (−8.83 to 2.21) | 0.24 |
CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy.
Adjusted for the presence of absence of oliguria, age, and gender.
Results
Between November 3, 2003 and July 2, 2007, 1124 patients were randomized to either IntRRT (n = 563) or L-IntRRT (n = 561). A total of 246 patients received IHD as their only modality of RRT during the entire duration of the study and were included in this analysis (Figure 1). Demographic and baseline clinical characteristics are shown in Table 1 and comparison data for those patients who were excluded from this analysis are shown in Supplementary Table S1. The mean age for the cohort included in this study was 60.5 ± 15.1 years. The population was predominantly male (77.6%) and most of the patients were white (74.4%), with African American individuals accounting for 16.7%. Baseline SCr, defined as the lowest SCr within 4 days before screening, was similar between the 2 groups and etiology of AKI was most commonly attributed to ischemic injury (68.9%). Baseline comorbidities, as defined by Charlson Comorbidity Index and mean Acute Physiology and Chronic Health Evaluation II scores, were not different between the 2 groups. The baseline CV-SOFA score was low and not significantly different (0.5 ± 0.9 vs. 0.4 ± 0.8; P = 0.54). There was no significant difference in the number of patients who received RRT before randomization.
Hemodialysis treatment data are presented in Table 2. As expected, based on the study protocol, patients randomized to the IntRRT group underwent more IHD treatments. Because the protocol permitted isolated ultrafiltration treatments to provide volume management independent of solute management, more isolated ultrafiltration treatments were provided in the L-IntRRT group (101 [0.73/patient] vs. 6 [0.06/patient]). Blood and dialysate flow rates were similar between the 2 groups and there was no difference in the initial or subsequent delivered Kt/Vurea. The net ultrafiltration per session was significantly higher in the L-IntRRT group (2.1 ± 1.4 vs. 1.7 ± 1.3, P = 0.0018), consistent with the longer interdialytic interval. The incidence of hypotension during treatment as determined by change in mean arterial pressure was not different between the 2 groups. The frequency of treatments complicated by decrease in mean arterial pressure by >30 mm Hg was higher in the less-intensive strategy, but noted in only <10% of the treatments. Most of the treatments were performed without any anticoagulation. The mean predialysis serum potassium (mEq/l) was higher in the L-IntRRT group compared with the IntRRT group (4.16 ± 0.7 vs. 4.08 ± 0.6; P < 0.001). Similarly, the mean predialysis serum phosphorus (mg/dl) was higher in the L-IntRRT group compared with the IntRRT group (4.71 ± 1.9 vs. 3.87 ± 1.8; P < 0.001).
Primary and Secondary Outcomes
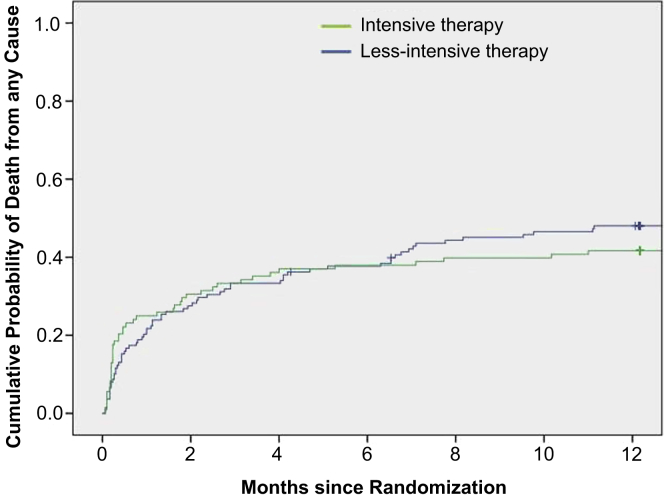
The primary and other outcomes are shown in Tables 3 and 4. Fifty-eight of 136 patients (43%) in the L-IntRRT group had complete recovery of kidney function by day 28, as compared with 34 of the 106 (32%) patients receiving IntRRT (adjusted OR: 0.49; 95% CI: 0.28–0.87; P = 0.016) (Table 3). Mortality at 60 days was 28% in the L-IntRRT group and 31% in the IntRRT group (adjusted OR: 1.51; 95% CI: 0.81–2.79; P = 0.19) (Table 3). There was no significant difference in in-hospital or 1-year mortality (Table 3; Figure 2). Because mortality is a competing risk for recovery of kidney function, we analyzed duration of RRT support on the basis of RRT-free days (alive and dialysis-independent) over the first 28 days. RRT-free days were calculated as the number of days from last IHD treatment to day 28. L-IntRRT was associated with a significantly higher number of RRT-free days (12.95 ± 0.88 days) through day 28 than IntRRT (10.42 ± 0.97 days) (mean difference 2.5 days; 95% CI: −4.79 to −0.27 days; P = 0.028) (Table 4). Dialysis independence at day 28 was 52 of 108 (48%) in the IntRRT arm versus 76 of 138 (55%) in L-IntRRT group (unadjusted OR: 1.32; 95% CI: 0.8–2.19; P = 0.28, and adjusted OR: 1.66; 95% CI: 0.95–2.87; P = 0.073). Overall dialysis independence among survivors at day 60 was 133 of 171 (77.8%) with no difference between the treatment groups (78.1% in IntRRT vs. 77.6% in L-IntRRT, OR: 0.89; 95% CI: 0.4–1.98; P = 0.78).
Figure 2.
Kaplan-Meier plot of cumulative probabilities of death from any cause at 12 months.
Discussion
The Veterans Affairs/National Institutes of Health ATN trial has demonstrated that increasing the intensity of RRT in patients receiving IHD and CRRT did not improve outcomes in patients with AKI. This post hoc subgroup analysis of the study comprises the largest randomized cohort evaluating the use of IHD in the management of critically ill patients with AKI. Increasing the frequency of IHD from 3 to 6 days per week, with an average delivered spKt/Vurea of >1.2 per treatment (after the first treatment), did not improve 60-day or 1-year all-cause mortality. In the primary ATN study, overall 60-day mortality was 53.6%, and in the current IHD-only cohort, it was 29%, reflecting the lower acuity of illness and greater hemodynamic stability in the group that was initiated and maintained on IHD per protocol and did not receive any CRRT. It is important to note that urea kinetics were closely monitored and the prescribed dose of dialysis was delivered to both groups to ensure adequacy of therapy. Patients in the L-IntRRT arm were significantly more likely to recover renal function (using the prespecified definition of creatinine clearance >20 ml/min) by day 28 and had 2.5 more RRT-free days compared with the IntRRT group. However, it is unclear if this is just a perceived delay in renal recovery in the IntRRT group, as the decision to assess for renal recovery was based on increase in urine output and/or spontaneous fall in SCr. It would be difficult to appreciate spontaneous down-trending of SCr when patients are being dialyzed 6 days per week. Also, a counting bias could have led to a difference of 0.5 fewer RRT-free day between the 2 groups, because by design, there is always a skip day between IHD sessions in the L-IntRRT arm.
Even though our analysis did not document a difference in renal independence among the 2 groups, we believe there are possible explanations that can be postulated to account for an actual, albeit modest, delay in recovery of renal function with intensive RRT. Although the IntRRT resulted in lower rates of net ultrafiltration during each IHD session, which we had hypothesized would be associated with less frequent rates of intradialytic hypotension, the rates of intradialytic hypotension per treatment were similar between the 2 groups, resulting in a greater number of treatments complicated by intradialytic hypotension in the IntRRT strategy. Intradialytic hypotension could result in recurrent renal ischemia, which might delay recovery of kidney function. Hemodynamic alterations during hemodialysis have been associated with new ischemic lesions in the kidney late in the course of clinical AKI.13, 14 Renal autoregulation is significantly impaired in an injured kidney (even in the early phases of recovery) and even a slight decrease in blood pressure can potentially affect renal perfusion pressure and blood flow, thus resulting in additional ischemic tubular damage. Alternatively, the more aggressive solute and volume control associated with the more intensive RRT strategy may have diminished the stimulus for an increase in urine output as renal function is beginning to recover. A recent study has shown that intensive RRT is associated with decreased urine output early in the course of AKI.8 Despite differences in the recovery of renal function between the 2 groups, it did not translate to significant differences in dialysis independence at day 28 or 60.
More frequent IHD did not offer any mortality benefit, and this could be due to several factors. Higher dialysis frequency led to more repeated exposure of the patient to an extracorporeal circuit and this can have deleterious effects. Exposure to bioincompatible, cellulose (cuprophane) dialysis membranes have been demonstrated to cause complement activation and is associated with nonrecovery of renal function and overall mortality.15, 16 Even though biocompatible synthetic membranes were recommended for the ATN study, an extremely small number of hemodialysis treatments (<1%) in this subgroup were performed using semisynthetic cellulose triacetate membranes. We do not believe this played a role in the final results of this study; however, new data suggest that even exposure to synthetic dialyzer cartridges and tubing sets can generate toxic hydrocarbons and halocarbons and incite inflammation in the body.17 Long-term exposure to these agents is associated with malignancies, but risks of daily short-term contact remains unclear, especially with regard to recovery of renal function or mortality. Frequent exposure to conventional non-ultrapure dialysate also may trigger inflammatory mediators with resultant adverse effects.9 In addition, acute myocardial dysfunction, which has been demonstrated during hemodialysis in patients with end-stage renal disease, may occur in AKI, thereby negating other putative benefits of more frequent dialysis.10 Increasing the intensity of IHD also affects the pharmacokinetics of various therapeutic agents, especially antimicrobials, and if not carefully monitored and adjusted, underdosing can lead to poor outcomes.
CRRT is associated with more hemodynamic stability during therapy and it is generally the treatment of choice for the severely ill patients with AKI who are in the intensive care unit. Some practitioners believe that the initial modality of RRT may play a role in renal recovery and dialysis independence. A retrospective cohort study demonstrated that patients who initially received CRRT were less likely to be on chronic dialysis as compared with those who received IHD.18 In that study, the acuity of illness, based on SOFA and Acute Physiology and Chronic Health Evaluation II scores, was not defined and therefore may not be comparable to our study population. Also, they did not account for modality changes once RRT was started. Randomized controlled trials and a meta-analysis have not demonstrated any significant difference in outcomes between IHD- and CRRT-treated patients.19, 20, 21 A more recent, large, retrospective study of approximately 4700 patients also did not show any difference in RRTs between IHD and CRRT groups and concluded that either therapy can be provided based on the hemodynamic status.22 In our study, patients with CV-SOFA score of 0 to 2 were assigned to receive IHD as the initial modality of RRT. They remained hemodynamically stable and were therefore continued on IHD for the duration of the study. By design, patients in the ATN study who received only IHD had lower acuity of illness and greater hemodynamic stability, and had lower overall mortality than observed across the entire cohort. Although the ATN study was not designed to compare outcomes across modalities of RRT, rates of recovery of kidney function among surviving patients treated exclusively with IHD (78.1% with IntRRT and 77.6% with L-IntRRT) were similar to those observed across the entire study cohort (74.6% with IntRRT and 76.2% with L-IntRRT). We believe that in hemodynamically stable patients with AKI, provision of IHD 3 times per week, with a target delivered spKt/Vurea of 1.2 to 1.4 per treatment, is a suitable option, and this recommendation has been emphasized in recent guidelines.23, 24
Our study has a few strengths and limitations. The major strength is that patients were carefully randomized in the primary ATN study, with respect to demographics and comorbidities. The IHD treatments were monitored closely to ensure that the prescribed dose of dialysis was delivered. Unlike the previous IHD study,5 the dose of dialysis as measured by spKt/Vurea was adequate in both arms. A major limitation of this study was that even though this constitutes the largest cohort evaluating intensity of IHD in AKI, this was a post hoc subgroup analysis and is likely underpowered. Post hoc analyses carry the risk of imbalance in baseline characteristics despite randomization and therefore increasing the probability of both type I and type II errors. Therefore, the results of this study do not approach the reliability of a prospective prespecified analysis and should be regarded as “hypothesis-generating” for planning future trials.
In conclusion, this subgroup analysis of the ATN study comprises the largest IHD study in AKI. IHD can be safely provided to hemodynamically stable patients with AKI, and if an adequate dose of hemodialysis (spKt/Vurea of 1.2–1.4) is delivered 3 times per week, then increasing the frequency of hemodialysis to 6 days per week does not improve outcomes and may potentially delay renal recovery. Pre– and post–blood urea nitrogen measurements performed during IHD treatments for AKI will aid in ensuring adequate delivered dose of dialysis. Additional IHD treatments can be provided as needed for volume control and management of electrolyte and acid-base disturbances, or if the target dose of dialysis is not achieved.
Disclosure
PMP has been a consultant for Baxter. AV has been a consultant for NxStage, participated in the speaker’s bureau for Sanofi and NxStage, and is on the advisory board of Baxter. The other authors declared no competing interests.
Acknowledgments
The Department of Veterans Affairs (VA)/National Institutes of Health Acute Renal Failure Trial Network study was supported by the Cooperative Studies Program of the VA Office of Research and Development as CSP#530 and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under interagency agreement Y1-DK-3508-01. The analyses presented in this article were not prepared in conjunction with the Acute Renal Failure Trial Network (ATN) Study investigators and does not represent the opinions or views of the ATN study, the VA, or the NIDDK. The authors thank Jane Zhang, PhD, for her guidance in use of the ATN study data.
Footnotes
Table S1. Baseline characteristics of original study participants excluding IHD-only patients.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Baseline characteristics of original study participants excluding IHD-only patients.
References
- 1.Bagshaw S.M., George C., Dinu I. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C., Ricci Z., De Backer D. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care. 2015;19:146. doi: 10.1186/s13054-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himmelfarb J. Continuous dialysis is not superior to intermittent dialysis in acute kidney injury of the critically ill patient. Nat Clin Pract Nephrol. 2007;3:120–121. doi: 10.1038/ncpneph0422. [DOI] [PubMed] [Google Scholar]
- 5.Schiffl H., Lang S.M., Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 6.VA/NIH Acute Renal Failure Trial Network. Palevsky P.M., Zhang J.H. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palevsky P.M., O'Connor T.Z., Chertow G.M. Intensity of renal replacement therapy in acute kidney injury: perspective from within the Acute Renal Failure Trial Network Study. Crit Care. 2009;13:310. doi: 10.1186/cc7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCausland F.R., Asafu-Adjei J., Betensky R.A. Comparison of urine output among patients treated with more intensive versus less intensive RRT: results from the Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2016;11:1335–1342. doi: 10.2215/CJN.10991015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susantitaphong P., Riella C., Jaber B.L. Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: a meta-analysis. Nephrol Dial Transplant. 2013;28:438–446. doi: 10.1093/ndt/gfs514. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre C.W., Burton J.O., Selby N.M. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palevsky P.M., O'Connor T., Zhang J.H. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–435. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daugirdas J.T. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther. 1995;2:295–304. doi: 10.1016/s1073-4449(12)80028-8. [DOI] [PubMed] [Google Scholar]
- 13.Conger J.D. Does hemodialysis delay recovery from acute renal failure? Semin Dial. 1990;3:146–148. [Google Scholar]
- 14.Solez K., Morel-Maroger L., Sraer J.D. The morphology of "acute tubular necrosis" in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979;58:362–376. [PubMed] [Google Scholar]
- 15.Hakim R.M., Wingard R.L., Parker R.A. Effect of the dialysis membrane in the treatment of patients with acute renal failure. N Engl J Med. 1994;331:1338–1342. doi: 10.1056/NEJM199411173312003. [DOI] [PubMed] [Google Scholar]
- 16.Himmelfarb J., Tolkoff Rubin N., Chandran P. A multicenter comparison of dialysis membranes in the treatment of acute renal failure requiring dialysis. J Am Soc Nephrol. 1998;9:257–266. doi: 10.1681/ASN.V92257. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.J., Meinardi S., Pahl M.V. Exposure to potentially toxic hydrocarbons and halocarbons released from the dialyzer and tubing set during hemodialysis. Am J Kidney Dis. 2012;60:609–616. doi: 10.1053/j.ajkd.2012.02.327. [DOI] [PubMed] [Google Scholar]
- 18.Wald R., Shariff S.Z., Adhikari N.K. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury:a retrospective cohort study*. Crit Care Med. 2014;42:868–877. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 19.Vinsonneau C., Camus C., Combes A. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome:a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 20.Mehta R.L., McDonald B., Gabbai F.B. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneider A.G., Bellomo R., Bagshaw S.M. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39:987–997. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 22.Liang K.V., Sileanu F.E., Clermont G. Modality of RRT and recovery of kidney function after AKI in patients surviving to hospital discharge. Clin J Am Soc Nephrol. 2016;11:30–38. doi: 10.2215/CJN.01290215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KDIGO clinical practice guideline for acute kidney injury: section 3: prevention and treatment of AKI. Kidney Int Suppl (2011) 2012;2:37–68. doi: 10.1038/kisup.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palevsky P.M., Liu K.D., Brophy P.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of original study participants excluding IHD-only patients.