Abstract
Objective
The aim of this study was to evaluate the mechanical and biological properties of orthodontic bonding agents containing silver- or zinc-doped bioactive glass (BAG) and determine the antibacterial and remineralization effects of these agents.
Methods
BAG was synthesized using the alkali-mediated solgel method. Orthodontic bonding agents containing BAG were prepared by mixing BAG with flowable resin. Transbond™ XT (TXT) and Charmfil™ Flow (CF) were used as controls. Ion release, cytotoxicity, antibacterial properties, the shear bond strength, and the adhesive remnant index were evaluated. To assess the remineralization properties of BAG, micro-computed tomography was performed after pH cycling.
Results
The BAG-containing bonding agents showed no noticeable cytotoxicity and suppressed bacterial growth. When these bonding agents were used, demineralization after pH cycling began approximately 200 to 300 µm away from the bracket. On the other hand, when CF and TXT were used, all surfaces that were not covered by the adhesive were demineralized after pH cycling.
Conclusions
Our findings suggest that orthodontic bonding agents containing silver- or zinc-doped BAG have stronger antibacterial and remineralization effects compared with conventional orthodontic adhesives; thus, they are suitable for use in orthodontic practice.
Keywords: Bioactive glass, Adhesive, Antibacterial, Remineralization
INTRODUCTION
White spot lesions (WSLs) are a common, undesirable side effect of orthodontic treatment. In particular, WSLs on the anterior teeth result in poor esthetics and should be prevented. Primary measures for WSL prevention include appropriate tooth brushing using fluoride toothpaste, the use of a fluoride mouthwash, and the application of fluoride varnish. However, these preventive measures depend entirely on patient compliance, considering they require patients to visit the hospital or clinic and involve additional costs. Therefore, they are not effective for noncompliant patients. Accordingly, measures that do not require co-operation by the patient are preferred.1
Several research groups have attempted to prevent WSLs with the use of fluoride-containing sealants, primers, and adhesives at the time of bracket bonding, so that fluoride ions are released during orthodontic treatment. However, the bond strength of these products is weaker than that of existing resin adhesives, and the release of fluoride ions decreases over time.1
Recently, efforts have been made to prevent dental caries by the addition of bioactive glass (BAG), which exhibits antibacterial and remineralizing properties, to resin adhesives and restorative materials.2,3,4,5 Various products that contain BAG are already used in medicine and dentistry; they take advantage of the ability of BAG to bind with bone and soft tissue.5,6,7 Meanwhile, in several studies, BAG was doped with metal ions for various purposes. For instance, Bellantone et al.8 reported that silver-doped BAG prevented the growth of both gram-negative and gram-positive bacteria. A study by Wang et al.9 demonstrated that silver-doped BAG not only exhibits antibacterial activities but also promotes stem cell differentiation within the dental pulp. Similarly, Balasubramanian et al.10 reported that zinc-doped BAG exhibits antibacterial and anti-inflammatory effects and influences bone formation. Goh et al.11 evaluated the antibacterial activity of silver- and copper-doped BAG and found that both were effective. They reported that silver-doped BAG may quickly kill bacteria through the rapid release of silver ions, while copper-doped BAG, which slowly releases copper ions, may function as a long-term antibacterial agent.
The aim of the present study was to investigate the mechanical and biological properties of orthodontic bonding agents containing silver- and zinc-doped BAG and determine the antibacterial and remineralization effects of these agents.
MATERIALS AND METHODS
Synthesis of BAG
We used the quick alkali-mediated sol-gel method12 to synthesize silver- and zinc-doped BAG. Tetraethyl orthosilicate, triethyl phosphate, Ca(NO3)2•4H2O, AgNO3, and Zn(NO3)2•6H2O were used as precursors. The composition of the synthesized BAG was as follows.
A0: 58-SiO, 33-CaO, 9-P2O5
A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O
A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO
Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO
Synthesis of BAG-containing orthodontic bonding agents
For evaluation of the mechanical and biological properties of BAG-containing orthodontic bonding agents, resin disks measuring 5 mm in diameter and 2 mm in height and containing 10% to 15% (wt/wt) BAG in flowable resin (CharmFil™ Flow [CF]; Denkist, Seoul, Korea) were prepared (Table 1). Specifically, 10% to 15% (wt/wt) BAG and flowable resin were manually blended, followed by mixing in an amalgamator (ProMix™; Dentsply Caulk, York, PA, USA). This mixture was then placed in a resin dispenser and injected into a brass mold. The upper surface was covered with a slide, and the disk was cured using light emitting diode (LED) curing units (DEMI, Kerr, CA, USA; > 1,000 mW/cm2) for 20 seconds on each side as well as the upper and lower surfaces. Each resin disk, which would be used in the MTT assay and in evaluations of antibacterial properties and ion dissolution, was sterilized using ethylene oxide gas.
Table 1. The orthodontic bonding agents tested in the present study.
Control (CF and TXT) and bioactive-glass containing agents.
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
Ion dissolution
To evaluate the ion-releasing properties of the orthodontic bonding agents, the sterilized resin disks were immersed in simulated body fluid (pH, 7.4; Biosesang, Seongnam, Korea) for 1, 6, 24, 72, 168, or 840 hours. Then, inductively coupled plasma optical emission spectrometry (Optima 8300; Perkin Elmer, Waltham, MA, USA) was performed.
Microhardness
Microhardness was measured by the application of a 200-gf load to the upper surface of the disk using a microhardness tester (MVK-H1; Akashi Co. Ltd., Tokyo, Japan). Three samples from each group were tested, and each specimen was measured three times.
Cytotoxicity
For ion release, the sterilized resin disks were placed in Dulbecco's Modified Eagle's Medium (DMEM; LM001-01; WELGENE, Gyeongsan, Korea) for 24 hours in an incubator at 37℃ and containing 5% CO2. The original extract was diluted in ratios of 1:2, 1:4, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, and 1:1,024. Human gingival fibroblasts (HGFs; ATCC, Manassas, VA, USA) were cultured in DMEM containing 2% fetal bovine serum, 100 IU/mL penicillin, and 100 IU/mL streptomycin. The diluted bonding agent was mixed with HGFs (2 × 104 cells) in a 96-well plate, and the mixture was cultured for 24 hours. Then, the MTT assay was performed.
Antibacterial properties
Streptococcus mutans (Ingbritt) was used to evaluate the antibacterial properties of the orthodontic bonding agents. Brain–heart infusion agar, a sterilized resin disk (n = 21; 3 samples for each groups), and S. mutans (1.0 × 104 CFU/mL) were placed in a 1.5-mL e-tube. This preparation was cultured for 24 hours in an incubator at 37℃ and containing 5% CO2. Following culture, the tube was stirred on a vortex mixer to shake off the bacteria attached to the resin disk. Next, 100 µL of the culture medium was transferred to a 96-well plate, and the absorbance was measured at 650 nm (Sunrise™; TECAN, Männedorf, Switzerland). The assays were independently performed in triplicate.
Shear bond strength (SBS) and adhesive remnant index (ARI)
Sixty human premolars were divided into six groups (n = 10 each): CF, Transbond™ XT (TXT; 3M, Monrovia, CA, USA), A0-10, A1-10, A1Z5-10, and Z5-10 groups. The teeth were etched with 35% phosphoric acid gel (Ultra Etch; Ultradent, South Jordan, UT, USA) for 30 seconds, rinsed for 10 seconds, and dried. An adhesive (Adper™ Scotchbond™ Multi-Purpose; 3M) was applied according to the manufacturer's instructions. The respective orthodontic bonding agent was applied to a premolar bracket (K-smart; Daeseung Medical Co., Seoul, Korea). Then, the bracket was placed on the buccal surface of the tooth, any excess bonding agent was carefully removed, and light curing was performed for 5 seconds on each side of the bracket. Subsequently, the specimens were stored in distilled water at room temperature for 24 hours before SBS measurement. SBS was measured using a universal testing machine (Instron Co., Canton, MA, USA) at a crosshead speed of 1 mm/min. For the evaluation of bond failure, the ARI score was calculated as follows using a stereoscopic microscope (S-645 e-scope; DAWINBIO, Seoul, Korea): 1, all adhesive remaining on the tooth; 2, more than 90% adhesive remaining on the tooth; 3, 10% to 90% adhesive remaining on the tooth; 4, less than 10% adhesive remaining on the tooth; and 5, no adhesive remaining on the tooth.
The study was approved by the Ethics Committee of Pusan National University Dental Hospital (PNUDH-2016-025).
Remineralization properties
To evaluate the remineralization effects of the orthodontic bonding agents, we used the pH cycling protocol described by Stookey et al.13
All tooth surfaces except that used for bracket bonding were covered with nail varnish for protection from etching.
Conventional etching was performed for 30 seconds with 35% phosphoric acid gel (Ultra Etch; Ultradent). The teeth were subsequently rinsed for 10 seconds and dried.
Adhesive was applied according to the manufacturer's instructions. Following application of the orthodontic bonding agent to the premolar bracket, the bracket was placed on the buccal surface of the tooth and any excess bonding agent was carefully removed. Each side of the bracket was light cured for 5 seconds.
After bonding, the specimens were stored in distilled water at room temperature for 24 hours before pH cycling. The specimens were immersed in demineralizing solution (2.0 mmol/L calcium nitrate tetrahydrate, 2.0 mmol/L potassium dihydrogen phosphate, and 75.0 mmol/L acetate at a pH of 4.4) for 6 hours and remineralizing solution (20.2 mmol/L sodium cacodylate, 1.5 mmol/L calcium nitrate tetrahydrate, 0.9 mmol/L potassium dihydrogen phosphate, and 130 mmol/L calcium chloride at a pH of 6.8) for 18 hours; this cycle was repeated for 14 days. Between the demineralization and remineralization cycles, the specimen was rinsed using deionized water for 1 minutes and dried. The demineralizing and remineralizing solutions were changed every week.
After pH cycling, each sample was scanned using micro-computed tomography (CT; InspeXio SMX-90CT; Shimadzu, Tokyo, Japan) with the following parameters: voxel size, 10 × 10 × 10 µm3; voltage, 90 kVp; and current, 110 mA. Using image analysis software (ImageJ; NIH, Bethesda, MA, USA), intensity histograms were generated in the center of the lesion, perpendicular to the enamel surface. Mineral loss in the lesion was calculated in accordance with the method proposed by Paschos et al.,14 and the width of the remineralization zone was determined from the bracket base to the starting point of the lesion. The lesion depth, remineralization zone width, and mineral loss in the lesion were compared among the six groups.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine significant differences in antibacterial properties, microhardness, SBS, and remineralization effects among groups. A nonparametric Kruskal–Wallis test was used for the comparison of ARI scores (p = 0.05).
All statistical analyses were performed using the R language program (version 3.3.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
In vitro ion dissolution
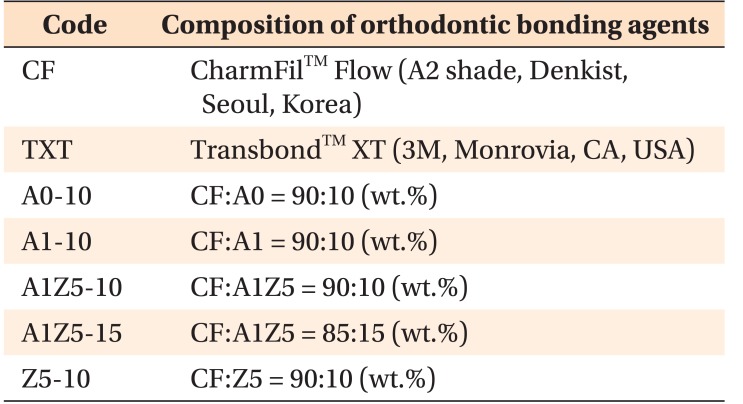
Figure 1 shows the ion-release properties of the BAG-containing orthodontic bonding agents. The calcium and phosphate concentrations decreased in all samples after 72 hours. Silver was first detected at 6 hours in the A1-10 and A1Z5-10 groups, and its concentration continuously increased up to 840 hours. A very small amount of zinc was detected after 840 hours. No pH changes were observed with the passage of time (Figure 2).
Figure 1. Change in calcium (A), phosphate (B), silver (C), and zinc (D) concentrations over time in ion dissolution tests for the orthodontic bonding agents tested in the present study (control [CF and TXT] and bioactive-glass containing agents). The calcium and phosphate concentrations have decreased in all samples after 72 hours. Silver is first detected at 6 hours in the A1-10 and A1Z5-10 groups, and it exhibits a continuous increase in concentration up to 840 hours. A very small amount of zinc is detected after 840 hours.
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
Figure 2. Mean changes in pH over time in ion dissolution tests for the orthodontic bonding agents tested in the present study (control [CF and TXT] and bioactive-glass containing agents). No pH changes can be observed over time.
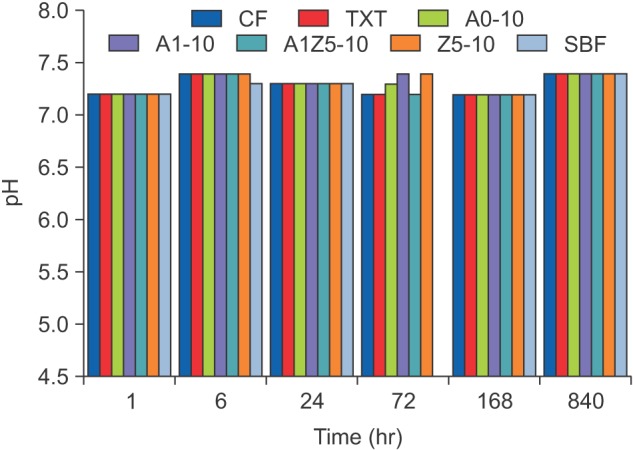
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA); SBF, simulated body fluid.
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
Microhardness
The microhardness values for CF and TXT were 35.000 ± 1.166 and 55.267 ± 2.982 Hv, respectively. When BAG was added to CF, the microhardness increased significantly. In particular, the hardness of CF mixed with 15% (wt/wt) A1Z5 was similar to that of TXT (Figure 3).
Figure 3. Microhardness of bioactive-glass (BAG)-containing orthodontic bonding agents tested in the present study. When BAG was added to CF, the microhardness increased significantly. In particular, the hardness of CF mixed with 15% (wt/wt) A1Z5 was similar to that of TXT. CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, USA).
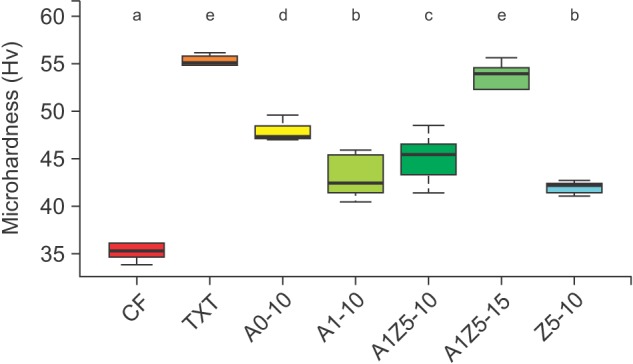
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
a-eDifferent superscripts (3M, Monrovia, CA, USA) indicate significant differences (p < 0.05). The error bars represent the standard deviation in each group.
Cytotoxicity
According to the results of the MTT assay, the HGF viability was 37% when the original CF and TXT extracts were used and 60% to 80% when the BAG-containing orthodontic bonding agents were used. The HGF viability increased with an increase in the dilution of the solutions; it exhibited a gradual increase from the original extracts to the 1:64 dilution.
Antibacterial properties
Figure 4 shows the antibacterial properties of the BAG-containing orthodontic bonding agents. The control, which was a culture containing only S. mutans, exhibited the highest absorbance (0.41), indicating that bacterial growth was most prolific in this group. TXT and CF exhibited absorbance values of 0.38 and 0.35, respectively, which were not significantly different from the control value (p > 0.05). On the other hand, A0-10, A1-10, A1Z5-10, A1Z5-15, and Z5-10 exhibited absorbance values of 0.22, 0.30, 0.22, 0.29, and 0.28, respectively, which were significantly lower than the control values (p < 0.05 for all).
Figure 4. Antibacterial properties of bioactive-glass containing orthodontic bonding agents tested in the present study (n = 21, 3 samples for each group).
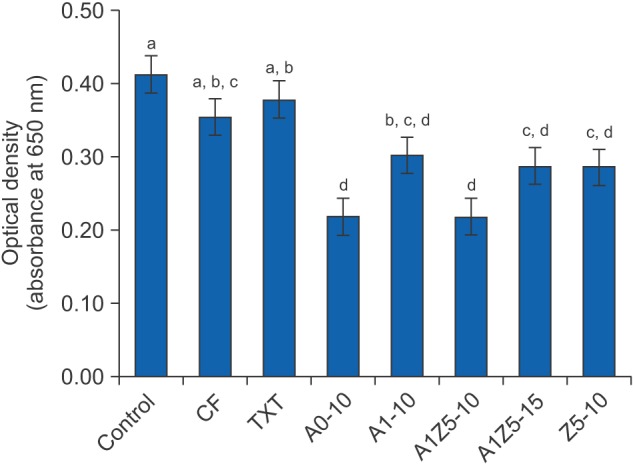
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
a-dDifferent superscripts indicate significant differences (p < 0.05). The error bars represent the standard deviation in each group.
SBS
A1-10 exhibited the highest SBS value of 15.17 ± 3.23 MPa, while CF exhibited the lowest value of 12.90 ± 7.56 MPa. However, there were no significant differences between groups (Table 2).
Table 2. Shear bond strength values for the orthodontic bonding agents tested in the present study.
Control (CF and TXT) and bioactive-glass containing agents.
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
ARI score
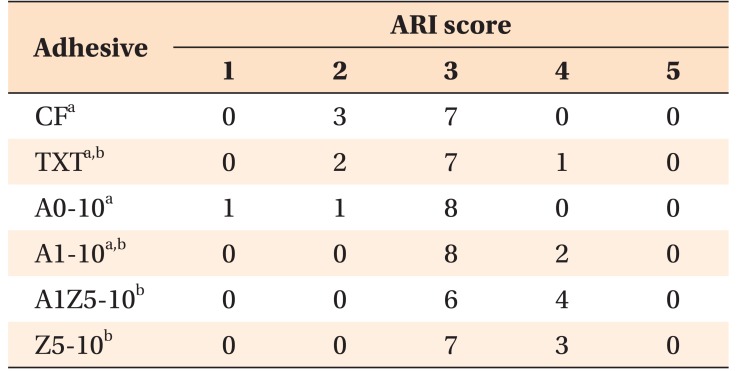
Table 3 shows the ARI scores, which were comparable for CF, TXT, A0-10, and A1-10 and for TXT, A1-10, A1Z5-10, and Z5-10.
Table 3. Adhesive remnant index (ARI) scores for the orthodontic bonding agents tested in the present study.
Control (CF and TXT) and bioactive-glass containing agents. CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
a,bValues with the same superscripts are not significantly different (Kruskal–Wallis test; α = 0.05, n = 10).
ARI scores were as follows: 1, all adhesive remaining on the tooth; 2, more than 90% adhesive remaining on the tooth; 3, 10% to 90% adhesive remaining on the tooth; 4, less than 10% adhesive remaining on the tooth; and 5, no adhesive remaining on the tooth.
Remineralization effects
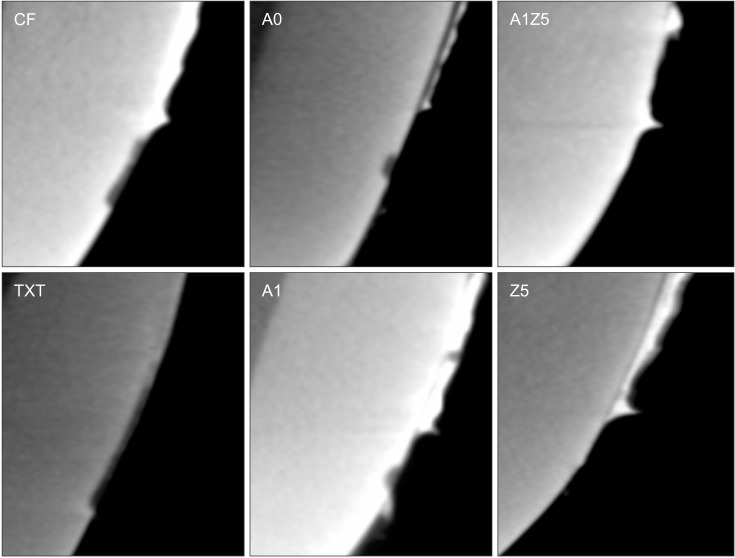
The remineralization effects of the bonding agents are shown in Figure 5. Table 4 shows that there were significant differences between groups with regard to the lesion depth, mineral loss, and remineralization zone width. When the BAG-containing orthodontic bonding agents were used, demineralization after pH cycling began approximately 200 to 300 µm away from the bracket edge. On the other hand, when CF and TXT were used, all surfaces that were not covered by the adhesive were demineralized after pH cycling. Z5-10 exhibited the least mineral loss and smallest lesion depth among all specimens (Table 4).
Figure 5. Representative micro-computed tomography slices obtained after pH cycling for the orthodontic bonding agents tested in the present study (control [CF and TXT] and bioactive-glass [BAG]-containing agents). There are significant differences in the lesion depth, mineral loss in the lesion, and remineralization zone width among groups. The BAG-containing agents have caused demineralization from an area approximately 200 to 300 µm away from the bracket edge. Z5-10 exhibits the least mineral loss and smallest lesion depth.
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
Table 4. Lesion depth and mineral loss in the lesion in each group of orthodontic bonding agents tested in the present study.
Values are presented as mean ± standard deviation.
Control (CF and TXT) and bioactive-glass containing agents.
CF, CharmfilTM Flow (A2 shade, Denkist, Seoul, Korea); TXT, TransbondTM XT (3M, Monrovia, CA, USA).
A0: 58-SiO, 33-CaO, 9-P2O5; A1: 58-SiO, 32-CaO, 9-P2O5, 1-Ag2O; A1Z5: 58-SiO, 27-CaO, 9-P2O5, 1-Ag2O, 5-ZnO; Z5: 58-SiO, 28-CaO, 9-P2O5, 5-ZnO.
*By ANOVA; a-cDifferent superscripts indicate significantly different value (p < 0.05).
DISCUSSION
In the present study, we evaluated the mechanical and biological properties of orthodontic bonding agents containing silver- or zinc-doped BAG and determined the antibacterial and remineralization effects of these agents. Recently, on the basis of the dentin remineralizing effects of BAG, researchers have attempted to use BAG as a filler in resin composites.2,3,4 In this regard, we synthesized silver- and zinc-doped BAG using the quick alkali-mediated sol-gel method12 and mixed it with flowable resin to prepare BAG-containing orthodontic bonding agents. Silver and zinc were selected as the dopants because they possess antibacterial properties.15 These metal ions act on the proteins, cell wall, cytoplasm, and cell envelope of microorganisms to inhibit their growth.16 Bellantone et al.8 studied the antibacterial effects of silver-doped BAG and undoped BAG on gram-negative and grampositive bacteria and reported that silver-doped BAG exhibited stronger antibacterial effects. Another study17 reported that silver-free BAG also exhibited antibacterial activity via a pH increase and osmotic pressure changes with the release of cations (Ca2+, Si4+, Na+). Zinc is also known to suppress demineralization, promote dentin remineralization, and prevent dental caries by inhibiting the growth of intraoral microorganisms.18,19
In the present study, we evaluated the antibacterial activity of BAG-containing orthodontic bonding agents against S. mutans. A0-10, A1-10, A1Z5-10, A1Z5-15, and Z5-10 were significantly more effective in inhibiting bacterial growth compared with CF and TXT (p < 0.05). These results are similar to those in a study by Chatzistavrou et al.4
After curing the BAG-containing orthodontic bonding agents, we performed an ion dissolution test to examine the elution of ions. The eluted volume of silver increased over time, while the concentrations of calcium and phosphate decreased. Zinc was barely detected. Brown et al.20 reported a continuous increase in the calcium concentration and a decrease in the phosphate concentration; the latter was attributed to the deposition of calcium phosphate. In the present study, both calcium and phosphate ions decreased not only with the BAG-containing orthodontic bonding agents but also with CF and TXT. Thus, additional analysis is required to validate our findings.
We assessed the cytotoxicity of BAG-containing orthodontic bonding agents against HGFs and found that the toxicity was similar to or lesser than that of CF and TXT. This finding is consistent with the results of previous studies regarding the cytotoxicity of silver in BAG.21,22 The cytotoxicity of dental composites is generally attributed to the release of unreacted monomer. In this regard, small amounts of monomer do not exhibit intraoral toxicity.23 That is, the cytotoxicity of BAG-containing orthodontic bonding agents is probably similar to that of existing resin bonding agents after introduction to the intraoral environment.
With regard to physical properties, the addition of 10% (wt/wt) A1Z5 to CF increased the microhardness value by 29%, while the addition of 15% (wt/wt) A1Z5 increased the value by 53% to achieve a microhardness similar to that of TXT. Therefore, we believe that these BAG-containing bonding agents can be used as restorative materials for small cavities or on the adjacent surfaces of anterior teeth. Moreover, they can be used in high-stress regions if the BAG content is increased.
For the use of BAG-containing orthodontic bonding agents as bracket bonding materials, the bond strength must be at least 6 to 8 MPa.24 In the present study, SBS of TXT was 13.7 MPa, while the average SBS of the BAG-containing orthodontic bonding agents was 13.0 MPa. SBS of CF was 12.9 MPa, which was not significantly different from that of TXT. A previous study using flowable resin for bracket bonding25 reported similar findings, whereas another study showed that the bond strength of CF was slightly lesser than that of TXT.26 However, even in that study, the bond strength was greater than 7 to 8 MPa. In other words, the bond strength of flowable resin is not a major obstacle to its clinical use for bracket bonding; however, it cannot be applied in all cases because of its limited workability. The addition of BAG imparts a certain level of workability to resins and enables their use as bracket bonding agents. In the present study, the ARI score indicated similar bond failure patterns for the BAG-containing orthodontic bonding agents and CF and TXT.
We evaluated the remineralization effects of BAG-containing orthodontic bonding agents using the Featherstone laboratory pH cycling model.13 The tooth specimens were examined by micro-CT after pH cycling, and the extent of enamel demineralization was measured using three-dimensional image analysis software. The results showed that CF and TXT resulted in the demineralization of all surfaces that had not been coated with the bonding agent, whereas the BAG-containing orthodontic bonding agents caused demineralization from a point approximately 200 to 300 µm away from the bracket edge. This finding indicates that the latter prevented demineralization not only at the bracket base but also in the surrounding area and is consistent with the results of a study by Manfred et al.,3 who examined—on the basis of microhardness—the remineralization effects of BAG-containing orthodontic bonding agents and found that synthesized bonding agents prevented demineralization.
CONCLUSION
The results of the present study suggest that BAG-containing orthodontic bonding agents have stronger antibacterial and remineralization effects compared with conventional orthodontic adhesives; thus, they are suitable for use as bracket bonding materials in orthodontic practice.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1C1A1A01051832).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Bishara SE, Ostby AW. White spot lesions: formation, prevention, and treatment. Semin Orthod. 2008;14:174–182. [Google Scholar]
- 2.Hashimoto M, Iijima M, Nagano F, Ohno H, Endo K. Effect of monomer composition on crystal growth by resin containing bioglass. J Biomed Mater Res B Appl Biomater. 2010;94:127–133. doi: 10.1002/jbm.b.31632. [DOI] [PubMed] [Google Scholar]
- 3.Manfred L, Covell DA, Crowe JJ, Tufekci E, Mitchell JC. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle Orthod. 2013;83:97–103. doi: 10.2319/110811-689.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzistavrou X, Velamakanni S, DiRenzo K, Lefkelidou A, Fenno JC, Kasuga T, et al. Designing dental composites with bioactive and bactericidal properties. Mater Sci Eng C Mater Biol Appl. 2015;52:267–272. doi: 10.1016/j.msec.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 6.Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi Z, Bahrololoom ME, Shariat MH, Bagheri R. Bioactive glasses in dentistry: a review. J Dent Biomater. 2015;2:1–9. [Google Scholar]
- 8.Bellantone M, Williams HD, Hench LL. Broad-spectrum bactericidal activity of Ag(2)O-doped bioactive glass. Antimicrob Agents Chemother. 2002;46:1940–1945. doi: 10.1128/AAC.46.6.1940-1945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YY, Chatzistavrou X, Faulk D, Badylak S, Zheng L, Papagerakis S, et al. Biological and bactericidal properties of Ag-doped bioactive glass in a natural extracellular matrix hydrogel with potential application in dentistry. Eur Cell Mater. 2015;29:342–355. doi: 10.22203/ecm.v029a26. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanian P, Strobel LA, Kneser U, Boccaccini AR. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed Glasses. 2015;1:51–69. [Google Scholar]
- 11.Goh YF, Alshemary AZ, Akram M, Kadir MRA, Hussian R. Bioactive glass: an in-vitro comparative study of doping with nanoscale copper and silver particles. Int J Appl Glass Sci. 2014;5:255–266. [Google Scholar]
- 12.Xia W, Chang J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkalimediated sol-gel method. Mater Lett. 2007;61:3251–3253. [Google Scholar]
- 13.Stookey GK, Featherstone JD, Rapozo-Hilo M, Schemehorn BR, Williams RA, Baker RA, et al. The featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent. 2011;24:322–328. [PubMed] [Google Scholar]
- 14.Paschos E, Kleinschrodt T, Clementino-Luedemann T, Huth KC, Hickel R, Kunzelmann KH, et al. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2009;135:603–612. doi: 10.1016/j.ajodo.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Venugopal A, Muthuchamy N, Tejani H, Gopalan AI, Lee KP, Lee HJ, et al. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J Orthod. 2017;47:3–10. doi: 10.4041/kjod.2017.47.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Stoor P, Söderling E, Salonen JI. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol Scand. 1998;5:161–165. doi: 10.1080/000163598422901. [DOI] [PubMed] [Google Scholar]
- 18.Boyd D, Li H, Tanner DA, Towler MR, Wall JG. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J Mater Sci Mater Med. 2006;17:489–494. doi: 10.1007/s10856-006-8930-6. [DOI] [PubMed] [Google Scholar]
- 19.Osorio R, Cabello I, Toledano M. Bioactivity of zincdoped dental adhesives. J Dent. 2014;42:403–412. doi: 10.1016/j.jdent.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Brown ML, Davis HB, Tufekci E, Crowe JJ, Covell DA, Mitchell JC. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. Angle Orthod. 2011;81:1014–1020. doi: 10.2319/120710-708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi S, Gwinner F, Mitchell JC, Pfeifer C, Ferracane JL. Cytotoxicity of resin composites containing bioactive glass fillers. Dent Mater. 2015;31:195–203. doi: 10.1016/j.dental.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silvercontaining adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29:450–461. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YG, Kim JY, Kim J, Won PJ, Nam JH. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop. 2011;140:779–789. doi: 10.1016/j.ajodo.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds IR, von Fraunhofer JA. Direct bonding of orthodontic brackets--a comparative study of adhesives. Br J Orthod. 1976;3:143–146. doi: 10.1179/bjo.3.3.143. [DOI] [PubMed] [Google Scholar]
- 25.Park SB, Son WS, Ko CC, García-Godoy F, Park MG, Kim HI, et al. Influence of flowable resins on the shear bond strength of orthodontic brackets. Dent Mater J. 2009;28:730–734. doi: 10.4012/dmj.28.730. [DOI] [PubMed] [Google Scholar]
- 26.Ryou DB, Park HS, Kim KH, Kwon TY. Use of flowable composites for orthodontic bracket bonding. Angle Orthod. 2008;78:1105–1109. doi: 10.2319/013008-51.1. [DOI] [PubMed] [Google Scholar]