Abstract
We have assessed how varying CO2 (180, 380, and 720 μatm) and growth light intensity (40 and 400 μmol photons m−2 s−1) affected Trichodesmium erythraeum IMS101 growth and photophysiology over free iron (Fe′) concentrations between 20 and 9,600 pM. We found significant iron dependencies of growth rate and the initial slope and maximal relative PSII electron transport rates (rPm). Under iron-limiting concentrations, high-light increased growth rates and rPm; possibly indicating a lower allocation of resources to iron-containing photosynthetic proteins. Higher CO2 increased growth rates across all iron concentrations, enabled growth to occur at lower Fe′ concentrations, increased rPm and lowered the iron half saturation constants for growth (Km). We attribute these CO2 responses to the operation of the CCM and the ATP spent/saved for CO2 uptake and transport at low and high CO2, respectively. It seems reasonable to conclude that T. erythraeum IMS101 can exhibit a high degree of phenotypic plasticity in response to CO2, light intensity and iron-limitation. These results are important given predictions of increased dissolved CO2 and water column stratification (i.e., higher light exposures) over the coming decades.
Keywords: Trichodesmium erythraeum, Cyanobacteria, ocean acidification, CO2, iron limitation, light intensity, fluorescence light curves, electron transport rates
Introduction
In vast regions of the oligotrophic tropical and sub-tropical open oceans, input of new nitrogen is primarily dependent on the N2-fixing capabilities of diazotrophic cyanobacteria, including unicellular cyanobacteria such as UCYN-A (Martinez-Perez et al., 2016) and filamentous cyanobacteria such as Trichodesmium spp. (Carpenter and Capone, 1992; Capone et al., 1997; Campbell et al., 2005). Trichodesmium spp. are a fundamentally important organism as they represent up to 50% of new nitrogen in some regions (Karl et al., 1997; Capone et al., 2005), and contribute between 80 and 110 Tg of fixed N2 to the open ocean ecosystems per year (Capone et al., 1997).
Diazotrophy is performed by the two-component enzyme nitrogenase, which comprises of an iron-molybdenum protein (dinitrogen reductase) and an iron protein (nitrogenase reductase). The former reduces the latter using a low-potential electron donor (ferredoxin) and consumes two molecules of ATP per electron. Nitrogenase contains 19 iron atoms per heterodimeric protein molecule (Shi et al., 2007). This is important because iron is a cofactor for a whole range of enzymes involved in photosynthetic and respiratory electron transport, nitrate and nitrite reduction, chlorophyll synthesis and other biosynthetic or degradative reactions (Geider and La Roche, 1994). Trichodesmium's dependence on diazotrophy means the genus has a relatively high metalloenzyme inventory. As a result, iron availability may be critical in controlling rates of nitrogen fixation in large areas of the open ocean (Rueter, 1988; Rueter et al., 1992; Falkowski and Raven, 1997; Wu et al., 2000).
The majority of Fe(III) in the open ocean is chelated by organic compounds (Shi et al., 2010) with the remaining fraction present as hydrolysed species [Fe(OH]. The neutral tri-hydrolysed species [Fe(OH)3] has very low solubility. As ocean pH decreases, so too does the hydroxide concentration, which slightly increases the solubility of iron in seawater (Liu and Millero, 1999). As hydroxide ions and organic chelators compete for the binding of Fe(III), ocean acidification will alter the organic chelation of iron, the degree of which is subject to the pKa of the binding site (Shi et al., 2007). This may therefore act to limit the bioavailability of iron. This is particularly important for areas of the ocean where a significant fraction of new iron comes from dissolved iron in deep waters. In areas where the major source is particulate iron, this may be partially compensated for by an increased ability of some chelators to dissolve iron from oxyhydroxides, arial dust, and siderophores (Shi et al., 2007; Rubin et al., 2011), and/or by enhanced photo-induced redox cycling (Croot and Heller, 2012).
The exceedingly low solubility of ferric iron (Fe3+) (10−18 M at pH 7.0 and effectively insoluble at higher pH), coupled with the fact that a major source of iron flux to the open ocean gyres is from atmospheric dust deposition (Gao et al., 2001) has led cyanobacteria to employ various mechanisms to (i), increase iron acquisition from the environment (i.e., outer membrane receptor proteins, ligand complexes, periplasmic and cytoplasmic iron transport proteins) (Braun and Killmann, 1999) (ii), decrease the cellular iron requirement by regulating the expression of genes encoding for cellular iron homeostasis (i.e., IsiA, IsiB, Fur) (Webb et al., 2001) and (iii), intracellular recycling of iron (Saito et al., 2011). These mechanisms allow increased production of high-affinity iron transporters and down-regulation of membrane-bound photosynthetic electron transport (PET) components in proportion to their iron requirement (Ivanov et al., 2000). For example, the Cu-containing plastocyanin in place of cytochrome c553 and flavodoxin in place of ferredoxin (Geider and La Roche, 1994).
There is still a dearth of knowledge regarding Trichodesmium's growth and photo-physiological response to iron-limitation; especially in combination with the effects of ocean acidification (Berman-Frank et al., 2001, 2007; Shi et al., 2007, 2010). Given the significant role that Trichodesmium plays in biogeochemical cycles, it would be extremely useful for future climate models if such responses were better understood.
Recent studies have considered integrated responses of CO2 and light (Kranz et al., 2010a), CO2 and iron (Shi et al., 2012), CO2 and (Eichner et al., 2014), and CO2, temperature and light (Boatman et al., 2017). These studies illustrate how Trichodesmium's productivity and growth is modulated by numerous environmental factors, highlighting the need for more systematic, multivariable experiments under co-limiting conditions. This requires that fully-acclimated balanced growth is established by culturing Trichodesmium for long time periods, under controlled and defined conditions.
Like the majority of experiments investigating physiological effects of iron-limitation, most research involving Trichodesmium has used a single independent variable (e.g., iron) whilst keeping CO2 and light intensity constant (Berman-Frank et al., 2001, 2007; Chappell and Webb, 2010). Most of these studies incorporate 5–8 treatments and culturing periods that may not have been long enough to establish balanced growth. In addition, growth conditions were frequently undefined.
Notable exceptions were the studies by Shi et al. (2012), who investigated iron-limitation at three CO2 concentrations at constant light intensity and temperature, and Hong et al. (2017), who investigated two CO2 concentrations at constant light intensity and temperature. Our approach comprised a systematic, multivariable experiment; where numerous T. erythraeum IMS101 treatments were grown over long durations with controlled and well-defined growth conditions. Our aim was to assess the response of T. erythraeum IMS101 growth, relative photosystem II (PSII) electron transport rates and photo-physiology to free iron (Fe′), and investigate how the integrated effect of CO2 and light intensity influence this response.
Materials and methods
Prior to the experiment, T. erythraeum IMS101 was maintained in the exponential growth phase in semi-continuous cultures at two light intensities (40 and 400 μmol photons m−2 s−1) on a 12:12 light:dark cycle at three targeted CO2 concentrations (180, 380, and 720 μatm) and optimal growth temperature (26°C ± 0.2) for a period of ~10 months. Each culture was used to inoculate T. erythraeum IMS101 across a range (20–13,000 pM) of Fe′ concentrations (4 treatments at LL and 5 treatments at HL), generating 27 experimental treatments in total. All 27 treatments were maintained in exponential growth phase in semi-continuous cultures at the specific growth conditions (i.e., light intensity, temperature, CO2 and Fe′) for ~6 months (~14 generations for the slowest growing cultures and 95 generations for the faster growing cultures) to ensure fully acclimated balanced growth was achieved.
Experimental setup
Cultures were grown at low volume (5 mL) in 12 mL polypropylene (PP) screw cap test tubes, incubated in a custom-made, water-jacketed aluminium temperature block illuminated from below. Sampling methodology and analytical techniques followed those described in Boatman et al. (2017); and involved median growth rates being determined from a minimum of three replicate growth curves. Experimental setup was slightly different as we employed trace metal-clean culturing techniques and used a modified YBCII growth medium, polypropylene growth tubes, and a modified approach to target a CO2 concentration as described below.
Growth rates were quantified from the linear regression of the slope of ln minimum fluorescence (Fo) measured daily (between 09:00 to 10:30) on dark-acclimated cultures (20 min) using a FRRfII FastAct Fluorometer system (Chelsea Technologies Group Ltd, UK). Cultures were kept within the early part of the exponential growth phase and optically thin to avoid nutrient limitation and self-shading as well as to minimise CO2 drift, as described in Boatman et al. (2017).
Culture medium preparation
Single batches (4 L each) of filter-sterilised (0.25 μm pore) YBCII media (Chen et al., 1996) were made at each total iron (FeT) concentration (i.e., 400, 200, 100, 40, 4 nM) in acid washed plastic containers. Hydrated and anhydrous salts were added with Milli-Q water (Millipore Milli-Q Biocel, ZMQS60FOI) and the pH adjusted to ~8.2 using filter-sterilised NaOH (pH checked by taking 5 mL aliquots). Trace metal contaminants were removed by filtering through a Chelex column setup within a trace metal-clean laminar flow cabinet (Class II). Trace metals and f/2 vitamins were added to each 4 L container using a revised EDTA concentration (20 μM) (Shi et al., 2012). Varying amounts of a 40 μM Fe stock (FeCl3:Na2EDTA) were added to give the range of FeT concentrations. Each container was filter-sterilised (0.2 μm pore) into 1 L (sterile) plastic stericups (no headspace) (Fisher Scientific 10518822, UK), and stored within double zip-locked polyethylene (PE) bags. Prior to use, growth tubes were acid washed (2 weeks in 10% HCl), rinsed with Milli-Q water (Millipore Milli-Q Biocel, ZMQS60FOI), microwave sterilised, air dried in a laminar flow cabinet (Class II), and stored within double zip-locked PE bags (Anderson and Morel, 1982). Each dilution was made into a new tube to avoid the build-up of contaminants.
Calculating iron speciation
The speciation program visual MINTEQ (Gustafsson, 2012) was used to calculate the solubility and organic complexation of iron, as well as determine the chemical speciation as a function of pH (Gledhill et al., 2015). Modelled concentrations (M) of Fe2(OH)2(EDTA, Fe(EDTA)−, FeH(EDTA) (aq) and FeOH(EDTA)−2 were summed and used in the calculation of the photo-redox disassociation of Fe(EDTA), which made up the dominant source of Fe′ in the media. The photo-redox calculation was based upon a set of rate constant equations defined by Sunda et al. (2005), giving a diurnally averaged Fe′ concentration in the growth media (Supplementary Table 1);
| (1) |
| (2) |
| (3) |
where Fe(III)′ is the free iron (Fe′) concentration (M); Fe(EDTA) is the total iron (FeT) concentration (M); [EDTA*] is the total EDTA concentration (M); Kd′ (dark) is the constant in the dark (= kd/kf), which is the ratio of the rate constants for the disassociation and formation of Fe(EDTA) chelates; Khv is the rate constant (= khv/kf) for Fe(EDTA) photolysis at a specific light intensity; Ehv is the light intensity (μmol photons m−2 s−1) relative to that at which Khv was measured (i.e., 500 μmol photons m−2 s−1); LP is the light period of the culture treatment (h−1), and pH was a post-culturing measurement on the NBS scale.
Inorganic carbon chemistry
The inorganic carbon chemistry (Ci) of each media bottle was determined from a 15 mL sample for total dissolved inorganic carbon (TIC) analysis (Shimadzu TOC-V Analyser & ASI-V Autosampler), and a 10 mL sample for pH (Thermo Scientific Orion Ross Ultra pH Electrode EW-05718-75, UK). All carbon chemistry calculations were made in CO2SYS (Lewis and Wallace, 1998), using the 1st and 2nd equilibrium constants (K1 and K2) for carbonic acid (Millero, 2010), the dissociation constant for KSO4 (Dickson, 1990), the boric acid constant (KB) (Lee et al., 2010), and the total pH scale. The pH probes were rinsed and calibrated with fresh (<2 weeks) artificial seawater buffers (TRIS and AMP) prior to use (Dickson, 1993).
Once a culture reached a pre-determined Fo, it was diluted (0.5 mL culture to 4.5 mL media) with filter-sterilised (0.2 μm pore) YBCII media to return the culture to a starting Fo value. To obtain a target CO2 concentration in the YBCII media, medium was bubbled with a CO2-air mixture (BOC Industrial Gases, UK) using an acid washed (10% HCl), microwave sterilised section of PTFE tubing. A series of 5 ml aliquots were taken to measure the pH (precision ± 0.002) until the target pH (and thus the target CO2) had been achieved. Once at the target CO2 concentration (±1%), the medium was immediately distributed into the test tubes, already containing the 0.5 mL of culture. The gaseous headspace was flushed with a filtered (0.2 μm pore) standard gas mixture at the target CO2 concentration (BOC Industrial Gases, UK) and the screw caps tightened. Parafilm was wrapped around the caps before the tubes were removed from the laminar flow cabinet.
Prior to every dilution, a 2 mL sample of culture and a 2 mL sample of filtrate were collected within 5 mL plastic cryogenic vials (Sigma-Aldrich V5257-250EA). Filtrate was used to measure the post-culturing pH. Assuming a constant alkalinity throughout the entire growth phase (Kranz et al., 2010b), the post-culturing CO2 was calculated from the post-culturing pH and initial alkalinity. The second 2 mL aliquot of culture was pipetted into a new growth tube to run a fluorescence light curve (FLC).
Fluorescence light curves
Triplicate FLCs were performed for each treatment using an FRRfII FastAct Fluorometer system (Chelsea Technologies Group Ltd, UK) on dark-acclimated cultures (20 min). The FLCs lasted ~1 h and consisted of 12 light steps ranging between 6 and 1,400 μmol photons m−2 s−1, each lasting 5 min in duration.
The operating efficiency of PSII (Fq′/Fm′) was calculated as follows;
| (4) |
where Fm′ is the maximum fluorescence in the light-acclimated state and F′ is the steady-state fluorescence at any point.
Baseline fluorescence (Fb) originating from sources other than functional, photosynthetically active PSII was determined using the following equation (Oxborough, 2012);
| (5) |
where Fm is the maximum fluorescence in the dark-acclimated state, Fv (= Fm − Fo) is the variable fluorescence in the dark-acclimated state and Fv/Fm* is the assumed Fv/Fm from functional PSII. In this study, the value of Fv/Fm* was set to the highest measured value from all treatments under iron-replete conditions. This method assumes that across environmental gradients, all photosynthetically active PSII operate with the same intrinsic photochemical efficiency in the dark-acclimated state. The impact of free phycobilisome complexes within the cell is assumed negligible, as their peak emission wavelengths are too short for detection by the FRRfII.
When applicable (i.e., Fv/Fm < Fv/Fm*), Fb was subtracted from F′ and Fm′, and Fq′/Fm′ recalculated.
Relative PSII electron transport rates (rP) were calculated as follows;
| (6) |
where Fq′/Fm′ is the operating efficiency of PSII (baseline corrected if required) and E is the actinic light intensity (μmol photons m−2 s−1).
Curve fitting of growth rate data
Additional growth rate and FLC data points were incorporated from the temperature and light response curves reported in Boatman et al. (2017). These experiments shared identical growth light intensities (low light = 40 μmol photons m−2 s−1, high light = 400 μmol photons m−2 s−1), light:dark cycle (12:12), CO2 concentrations (low-CO2 = 180 μatm, mid-CO2 = 380 μatm and high-CO2 = 720 μatm), and growth temperature (26°C); only differing in the use of non-chelated hydrated and anhydrous salts as well as the YBCII EDTA concentration (i.e., 2 μM).
Growth rate-Fe (μ-Fe) curves were modelled using a Michaelis-Menten equation (Michaelis and Menten, 1913);
| (7) |
where μm is the maximum growth rate (d−1); Fe′ is the free Fe concentration of the media (pM) and Km is the half saturation concentration (pM).
Curve fitting was performed on the median growth rate for each CO2 and light treatment, using a non-linear least squares algorithm to produce curves of best fit (r2 > 0.817). Statistical analysis was performed using F-tests; analysing the variance of separate and combined CO2 curve fits by comparing a calculated F-statistic to an F-value at a 0.05 alpha level.
Curve fitting of FLC data
Relative electron transport rates (rP) were modelled using a P-E equation (Platt and Jassby, 1976), and were performed on each replicate using a Marquardt–Levenberg least squares algorithm to generate the best fit (r2 > 0.993);
| (8) |
where rPm′ is the maximum relative PSII electron transport rate (unitless); α is the initial slope of the rP-light curve (dimensionless); β is the parameter that accounts for downregulation and/or photoinhibition at supra-optimal light intensities (dimensionless); and E is the light intensity (μmol photons m−2 s−1).
The achieved maximum relative PSII electron transport rate (rPm), light intensity at which rP was maximal (Eopt) and light-saturation parameter (Ek) were calculated from the fitted parameters as follows:
| (9) |
| (10) |
| (11) |
Results
Overall, CO2 concentrations in the cultures at the time of sampling were between 55 and 75 μatm lower than the target pCO2 concentrations (i.e., 180, 380 and 720 μatm). This was due to drawdown of TIC associated with biomass production and was similar across all iron and light treatments (Table 1).
Table 1.
The growth conditions (± SE) of T. erythraeum IMS101 cultures.
| Variables | Units | Low CO2 | Mid CO2 | High CO2 | |||
|---|---|---|---|---|---|---|---|
| LL | HL | LL | HL | LL | HL | ||
| pH | Total | 8.426 | 8.426 | 8.145 | 8.135 | 7.853 | 7.887 |
| H+ | nM | 3.8 (0.2) | 3.8 (0.1) | 7.2 (0.1) | 7.3 (0.1) | 14.0 (0.4) | 13.0 (0.4) |
| AT | μM | 2,396 (155) | 2,399 (77) | 2,453 (82) | 2,324 (41) | 2,256 (54) | 2,427 (87) |
| TIC | μM | 1,740 (106) | 1,751 (54) | 2,009 (66) | 1,906 (35) | 2,001 (45) | 2,138 (72) |
| μM | 1,326 (66) | 1,344 (36) | 1,729 (53) | 1,647 (30) | 1,835 (39) | 1,949 (62) | |
| μM | 410 (41) | 403 (19) | 271 (13) | 250 (5) | 147 (7) | 171 (10) | |
| CO2 | μM | 3.4 (0.1) | 3.5 (0.1) | 8.6 (0.2) | 8.4 (0.2) | 17.8 (0.3) | 17.4 (0.1) |
| pCO2 | μatm | 126 (4) | 129 (2) | 318 (6) | 312 (7) | 662 (12) | 644 (3) |
| Chl a | μg L−1 | 12.3 (1.8) | 17.9 (3.1) | 19.9 (3.1) | 35.0 (5.5) | 21.4 (3.3) | 33.7 (6.7) |
| n | 11 | 14 | 17 | 30 | 15 | 15 | |
Cultures were acclimated to three target CO2 concentrations (Low CO2 = 180 μatm, Mid CO2 = 380 μatm and High CO2 = 720 μatm), saturating light intensity (400 μmol photons m−2 s−1), optimal temperature (26°C), across a range of Fe′ concentrations (~20–1,010 pM). Iron treatments are grouped together to present the growth conditions for each CO2 and light treatment. The pH was measured prior to every dilution, while the bicarbonate (), carbonate (), CO2, and pCO2 concentrations were calculated via CO2SYS using the post-culturing measurements of pH and the initial alkalinity (AT) concentration. Note that the individual pH-values were converted to a H+ concentration, allowing a mean pH value to be calculated.
Iron limited response of growth rate and photophysiology
Growth rates decreased significantly with a decrease in Fe′ (Figures 1A,B). Acclimation to high CO2 enabled growth to occur at comparatively lower Fe′ concentrations at both low and high light (Figures 1A,B). Maximum (iron-replete) growth rates (μm) exhibited a CO2 response; where at low light, μm increased significantly by 30% from low to mid CO2 [F(2, 8) = 14.00, p < 0.05], and 29% from low to high CO2 [F(2, 8) = 6.00, p < 0.05]. At high light, μm increased significantly by 74% from low to mid CO2 [F(2, 10) = 26.25, p < 0.05], and 90% from low to high CO2 [F(2, 10) = 51.17, p < 0.05; Table 2]. There were no significant differences in μm between mid and high CO2 treatments at low [F(2, 8) = 0.01, p > 0.05] or high light [F(2, 10) = 1.25, p > 0.05; Supplementary Table 2].
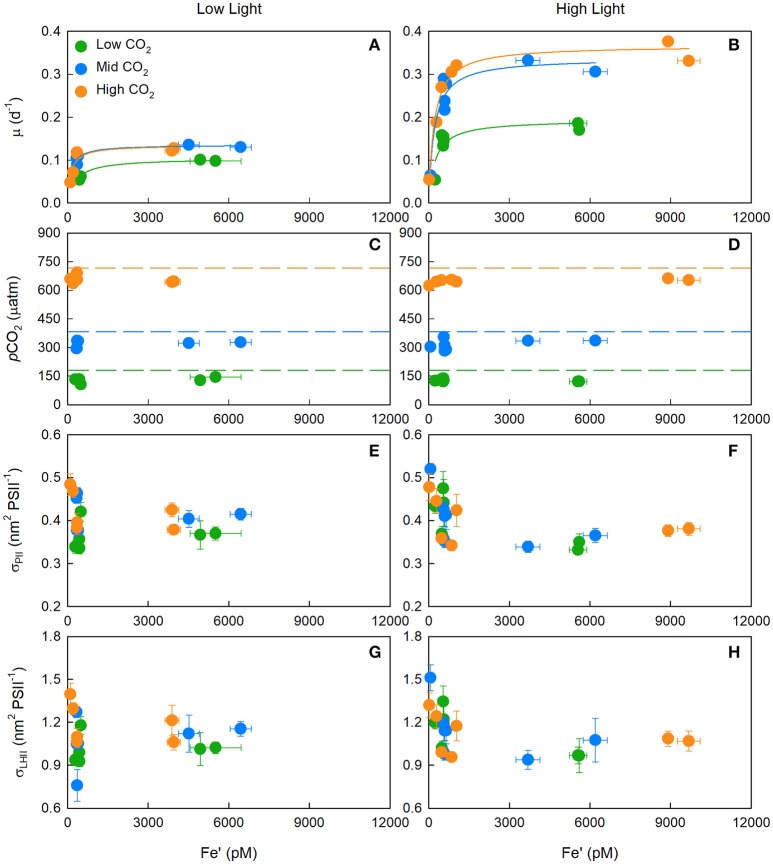
Figure 1.
The median (± SE) growth rate (μ) (A,B), and mean (± SE) pCO2 (C,D), absorption cross section of PSII photochemistry (σPII) (E,F), and absorption cross section of PSII light harvesting (σLHII = σPII/Fv/Fm) (G,H) for T. erythraeum IMS101. Prior to calculating σLHII, the photochemical efficiency of PSII in the dark-acclimated state (Fv/Fm) was corrected for baseline fluorescence (Fb) by assuming Fv/Fm* = 0.362. Left-hand panels (A,C,E,G) are low light treatments while right-hand panels (B,D,F,H) are high light treatments. Cultures were acclimated to three targeted CO2 concentrations [Low = 180 μatm (green circles), Mid = 380 μatm (blue circles) and High = 720 μatm (orange circles)], two light intensities (LL = 40 μmol photons m−2 s−1, HL = 400 μmol photons m−2 s−1), across a range of Fe′ concentrations (~20–9,600 pM) and at optimal temperature (26°C).
Table 2.
The iron dependence of T. erythraeum IMS101 growth.
| Parameters | Units | Low CO2 | Mid CO2 | High CO2 |
|---|---|---|---|---|
| LOW LIGHT | ||||
| μm | d−1 | 0.104 (0.005)[A]* | 0.136 (0.006)[B]* | 0.134 (0.012)[B]* |
| Km | pM | 307 (56)[A] | 116 (29)[B] | 124 (48)[B] |
| Affinity | d−1 (nM)−1 | 0.339 (0.028)[A]* | 1.165 (0.125)[B]* | 1.083 (0.259)[B]* |
| HIGH LIGHT | ||||
| μm | d−1 | 0.193 (0.021)[A]* | 0.337 (0.025)[B]* | 0.367 (0.016)[B]* |
| Km | pM | 236 (104) | 206 (72) | 201 (46) |
| Affinity | d−1 (nM)−1 | 0.819 (0.248)* | 1.639 (0.321)* | 1.823 (0.175)* |
T. erythraeum IMS101 was acclimated to three target CO2 concentrations (Low CO2 = 180 μatm, Mid CO2 = 380 μatm, and High CO2 = 720 μatm), saturating light intensity (400 μmol photons m−2 s−1), optimal temperature (26°C), across a range of Fe′ concentrations (~ 20–9,600 pM). Median Fe-dependent growth rate curves were fitted using a Michaelis-Menten function, defining parameter values (± SE) for μm (d−1), the maximum growth rate; Km (pM Fe′), the half saturation concentration and affinity [d−1 (nM Fe′)−1], the initial slope of the growth- Fe′ curve. At low light, there were 6 growth rate data points per CO2 treatment with two estimated parameters (df = 4); and at high light, there were 7 growth rate data points per CO2 treatment with two estimated parameters (df = 5). Letters in parenthesis indicate significant differences between CO2 treatments (F-test, P < 0.05); where [B] is significantly greater than [A]. An asterisk indicates a significant difference between light treatments.
At low light, increasing from low to mid CO2 caused a significant three-fold decrease in the half saturation concentration (Km) for growth [F(2, 8) = 14.979, p < 0.05], as well as a three-fold increase in the affinity (initial slope) of the growth-Fe′ curve [F(2, 8) = 11.222, p < 0.05]. Increasing from mid to high CO2 did not cause significant differences to either Km [F(2, 8) = 0.144, p > 0.05] or affinity [F(2, 8) = 1.031, p > 0.05]. At high light, the variability in Km [F(4, 15) = 0.083, p > 0.05] and affinity [F(2, 8) = 0.056, p > 0.05] between CO2 treatments was not significant. Due to the associated standard errors, there were no significance differences in Km between low and high light treatments at the low [F(4, 9) = 0.441, p > 0.05], mid [F(4, 9) = 2.049, p > 0.05] or high [F(4, 9) = 1.158, p > 0.05] CO2 treatments (Supplementary Tables 3–5).
For both low [F(4, 12) = 0.333, p > 0.05] and high light [F(4, 15) = 1.429, p > 0.05] treatments, combining the mid and high CO2 growth rate data did not cause a significant difference in curve fit parametrisations. However, incorporating the low CO2 data into a combined fit (i.e., low + mid + high CO2 growth rate data) caused the combined curve fit parameterisations to be significantly different to the separate CO2 growth rate-Fe′ curves for both low [F(4, 12) = 14.143, p < 0.05] and high treatments [F(4, 12) = 27.743, p < 0.05; Supplementary Table 2].
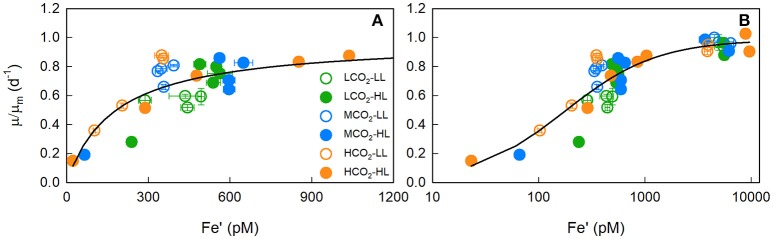
Growth-Fe′ curves were normalised to the modelled maximum growth rate (μm) at each CO2 and light treatment to generate a single μ/μm-Fe′ curve. Low CO2 data deviated either side of the modelled curve fit (Figure 2A), highlighting that the significant differences in the growth rate-Fe′ curve fits arise from differences in the growth response to iron-limitation rather than the maximal rate achieved under iron-replete conditions.
Figure 2.
The iron dependency of maximum-normalised growth rates (μ/μm). Panel (A) shows the iron-limitation range (0–1,200 pM) plot on a linear scale while panel (B) shows the full range (0–12 nM) of data plot on a log scale, and includes the data points from Boatman et al. (2017). T. erythraeum IMS101 was cultured at three targeted CO2 concentrations [Low = 180 μatm (green circles), Mid = 380 μatm (blue circles) and High = 720 μatm (orange circles)], two light intensities [LL = 40 μmol photons m−2 s−1 (open circles), HL = 400 μmol photons m−2 s−1 (closed circles)], across a range of Fe′ concentrations (~20–1,010 pM), at optimal temperature (26°C). The solid line is μ/μm modelled by a Michaelis-Menten function (Km of 177 pM with an r2 of 0.803).
Dark-acclimated absorption cross-sections of PSII photochemistry (σPII) showed little variation between CO2 or light treatments, but did significantly increase from ~0.4 nm2 PSII−1 under iron-replete concentrations to ~0.45 nm2 PSII−1 under iron-limited concentrations (One-Way ANOVA on Ranks, Dunn's post-hoc; p < 0.05) (Figures 1E,F). The non-baseline (Fb) corrected photochemical efficiency of PSII (Fv/Fm) exhibited a strong negative correlation to the non-Fb corrected absorption cross section of PSII light harvesting (i.e., σLHII = σPII/Fv/Fm) (r2 = −0.871, p < 0.05) (Supplementary Figure 1).
The highest Fv/Fm achieved from iron-replete cultures (~0.362) was used in the calculation of Fb (i.e., Fv/Fm*). The σLHII, calculated from Fb corrected Fv/Fm values, showed no significant difference between CO2 or light treatments, but did significantly increase from ~1.0 nm2 PSII−1 under iron-replete conditions to >1.4 nm2 PSII−1 under iron-limiting concentrations (One-Way ANOVA on Ranks, Dunn's post-hoc; p < 0.05) (Figures 1G,H).
Iron limited response of relative PSII electron transport rates and PSII photochemical efficiency
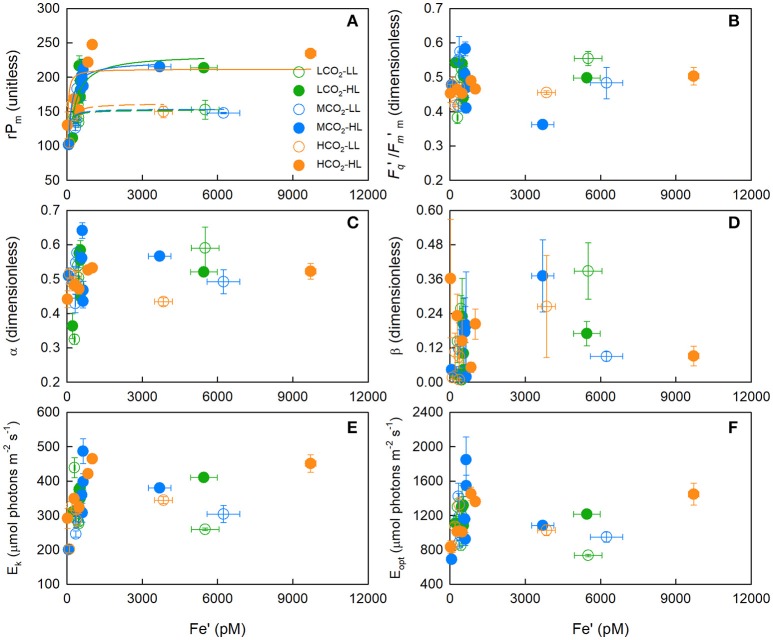
The highest maximum relative PSII electron transport rates (rPm) remained relatively constant down to an Fe′ concentration of ~1,000 pM (Figure 3A), and then decreased linearly with decreasing Fe′ [t(2, 25) = 8.535, p < 0.0001 [rPm = Fe′ · 0.1455 + 100.53, r2 = 0.732]]; where rates declined by ~ 55% as concentrations decreased from ~1,000 to 20 pM Fe′ (Supplementary Figure 2). Whilst Fe′ concentrations were dissimilar between treatments, a CO2 response at low and high light was evident under iron-limited conditions; where acclimation to higher CO2 concentrations (Fe′ < 300 pM) resulted in a comparatively higher rPm, α and Ek (Figures 3A–C). For example, at the lowest Fe′ treatments, rPm was only 8% lower and 18% higher at mid and high CO2 relative to low CO2, despite Fe′ being 70 and 90% lower, respectively.
Figure 3.
The mean (± SE) maximum relative PSII electron transport rate (rPm) (A), photochemical efficiency of PSII in the dark-acclimated state (Fv/Fm) (B), initial slope of the rP-Fe′ curve (α) (C), slope of photoinhibition (β) (D), light-saturated parameter (Ek) (E), and light intensity at which rP was maximal (Eopt) (F) for T. erythraeum IMS101. Cultures were acclimated to three targeted CO2 concentrations [Low = 180 μatm (green circles), Mid = 380 μatm (blue circles) and High = 720 μatm (orange circles)], two light intensities [LL = 40 μmol photons m−2 s−1 (open circles), HL = 400 μmol photons m−2 s−1 (closed circles)], across a range of Fe′ concentrations (~20–9,600 pM), at optimal temperature (26°C). In (A), the dashed and solid lines are the Michaelis-Menten function curve fits for the low light and high light treatments, respectively; where green, blue and orange lines are for low, mid and high CO2 treatments, respectively. Data in the iron-limited region only (20–1,010 pM Fe′) is presented in Supplementary Figure 2.
Under iron-replete conditions, CO2 did not affect the rPm (One-Way ANOVA, Tukey post-hoc; p > 0.05) or half saturation concentration for rPm () at low [F(3, 13) = 0.063, p > 0.05] or high light [F(3, 13) = 2.860, p > 0.05; Supplementary Tables 6, 7]. Although there was a significant difference between light treatments, where low light iron-replete cultures exhibited a rPm ~ 150 and high light cultures ~220 (unitless) (One-Way ANOVA, Tukey post-hoc; p < 0.05).
There were significant positive correlations between rPm and the light-saturation parameter (Ek) (r2 = 0.797, p < 0.05), rPm and the light intensity at which rP was maximal (Eopt) (r2 = 0.501, p < 0.05), and rPm and the initial slopes of rP-Fe′ curves (α) (r2 = 0.435, p < 0.05) (Figures 3E,F). In contrast, the photoinhibition slopes (β) were not correlated to CO2, light or Fe′ concentration (r2 < 0.5, p > 0.05) (Figure 3D).
The highest light-acclimated, baseline-corrected operating efficiency of PSII (Fq′/Fm′m) was not correlated to Fe′ (r2 = 0.036, p > 0.05) (Figure 3B). Although there was a significant CO2 response on the operating efficiency of PSII (Fq′/Fm′) at the highest actinic light intensity (i.e., 1400 μmol photons m−2 s−1) for low and high light cultures, where acclimation to higher CO2 concentrations under iron-limited conditions resulted in a comparatively higher Fq′/Fm′ value (Figures 3A–C). This reflects the trends reported for rPm, which is to be expected given rPm is a product of Fq′/Fm′ and actinic light intensity.
Discussion
Iron, CO2, and light dependencies on balanced growth rates
Accounting for differences in growth conditions (i.e., light intensity, CO2 etc.), iron-replete growth rates were similar to the majority of previous research, exhibiting similar responses under comparable Fe′ concentrations (Berman-Frank et al., 2001, 2007). Our findings show a significant CO2 response, affirming the observation that elevated CO2 produces higher growth rates, with the magnitude of the CO2 response increasing more under iron-replete conditions. In contrast, Shi et al. (2012) reported a decrease in growth rate at elevated CO2 under iron-replete conditions, which they attribute to a direct pH-mediated change in Fe′, that in turn lowers the iron uptake rate. While we cannot offer a definitive explanation for this discrepancy, it may arise from differences in culturing techniques. Specific differences could include culture medium (YBCII vs. Gulf Stream seawater), the method used to manipulate the inorganic carbon chemistry (bubbling vs. HCl/NaOH additions) and the acclimated state of the cultures; where balanced growth is only achieved after many generations (Boatman et al., 2017).
A recent study by Hong et al. (2017) reported that the positive effect of ocean acidification was due to a pH induced shift of the NH3/ equilibrium, where NH3 concentrations in the medium declines at lower pH (i.e., higher CO2). Dissolved inorganic N concentrations were measured in parallel cultures (Supplementary Table 8), and for all CO2 and light treatments were undetectable at the start of culturing and approximately 0.8 μM post-culturing. Similar concentrations have been reported in previous studies using standard YBCII media (Mulholland and Capone, 2001; Mulholland et al., 2004; Mulholland and Bernhardt, 2005), and likely indicates some cellular leakage of from cells rather than contamination of the growth medium. In any case, the concentrations we measured are sufficiently low (<10 μM) to have a negligible effect on nitrogenase activity and cellular metabolism (Mulholland et al., 2001), and will not be toxic to Trichodesmium cells (Hong et al., 2017).
Growth rates from our highly-buffered 20 μM EDTA cultures started saturating at ~1 nM Fe′, and were comparable to the iron-replete maximum growth rates reported by Boatman et al. (2017), which used standard YBCII EDTA concentrations (2 μM). In addition, Fv/Fm of the 2 μM and 20 μM EDTA, iron-replete cultures were comparable (Supplementary Figure 1). This suggests that cultures from both experiments were not affected by trace metal toxicity, as one would expect a decrease in growth and/or Fv/Fm during exposure to toxic Cu2+ concentrations, as the primary reactions of photosynthetic will become inhibited (Cid et al., 1995; Yruela et al., 1996; Nielsen et al., 2003).
Under iron-limitation there were no differences in the dark-acclimated absorption cross-sections of PSII photochemistry (σPII) or maximum photochemical efficiency of PSII (Fv/Fm) between low and high light treatments. Therefore, the lower initial slopes (Affinity) of the low light growth-Fe′ curves are likely due to a decrease in cellular chlorophyll a. Decreasing the chlorophyll a concentration conserves energy, alters the stoichiometry of iron containing components in the PET chain (Falkowski and LaRoche, 1991), and may be of importance during iron-limited, low light conditions; where a ten-fold decrease in light intensity (500 to 50 μmol photons m−2 s−1) can cause a four-fold increase in the cellular iron requirement of marine phytoplankton (Sunda and Huntsman, 1997). The combination of iron-limitation and low light conditions can significantly decrease iron uptake rates (Shi et al., 2012), creating a situation where Trichodesmium's cellular requirements for growth cannot be supported. Thus, when the light intensity is less than the Ek for Trichodesmium growth [~80 μmol photons m−2 s−1 at low CO2 and ~130 μmol photons m−2 s−1 at mid and high CO2 (Boatman et al., 2017)], productivity may well be constrained by a co-limitation of light and iron.
This low light mediated response also exhibited a CO2 dependency where (i), elevated CO2 enabled growth rates to occur at significantly lower Fe′ concentrations (ii), increased CO2 yielded higher growth rates (Figure 2) and (iii), acclimation to low CO2 yielded the lowest initial slope of the growth-Fe′ response (Affinity) as well as the highest iron saturation parameter (Km). We attribute these CO2 responses directly to the operation of the CCM and the ATP spent/saved for CO2 uptake and transport at low and high CO2, respectively. At high light, Trichodesmium likely has a lowered iron quota; therefore, whilst under iron-limitation, a low CO2 concentration will limit growth rates less than low light. Based on the relative growth rate curve fits (μ/μm) and the F-test results (Supplementary Tables 2–5), we suggest that at limiting and saturating light intensities, Trichodesmium's present (i.e., mid CO2) or future (i.e., high CO2) μ-Fe′ response can be defined using shared model parameterisations.
Resource allocation under iron limitation
T. erythraeum IMS101 exhibited an array of responses to CO2, light and iron-limiting conditions, indicating a high degree of phenotypic plasticity. Whilst not measured here, it is well-documented that under iron-limitation, T. erythraeum IMS101 downregulates the genes that encode for major iron-binding proteins. For example, Shi et al. (2007) reported a decrease in psbA and psbE (PSII), psaA and psaC (PSI), petB and petC (Cyt b6f complex) and nifH (nitrogenase); where expression of nifH decreased significantly more than PSI or PSII genes. By contrast, Küpper et al. (2008) found that both the abundance of iron requiring PSI and the total phycobiliproteins measured from single-cell in vivo spectra remained constant under iron-limitation. The selective decrease in nifH over genes encoding for components of the photosynthetic machinery of the electron transport chain may aid in reducing the risk of photodamage and conserve energy which can be used to increase the carbon concentrating mechanism (CCM), CO2 fixation or up-regulate photoprotective (i.e., IdiA and IsiA) or iron-scavenging (i.e., TonB, ExbB, and ExbD) proteins.
Proteins associated with N2 fixation are more affected under iron-limitation than those associated with photosynthesis (Paerl et al., 1994; Shi et al., 2007; Brown et al., 2008; Fu et al., 2008; Küpper et al., 2008). Decreased nitrogenase activity induced by iron-limitation (Berman-Frank et al., 2001, 2007) decreases a major sink for reductant and energy that is otherwise supplied by respiratory electron flow through the Cyt b6f complex. This complex couples PSII to PSI by transferring electrons from hydroplastoquinone (PQH2) to plastocyanin (PC) (Supplementary Figure 3). Thus, under iron-limitation, electrons originating from photosynthesis (oxidation of water) and respiration (oxidation of organic carbon) could be bottlenecked at the Cyt b6f complex, resulting in a highly reduced plastoquinone (PQ) pool and consequent decrease in non-Fb corrected Fv/Fm (Supplementary Figure 1).
Alternatively, under iron-limited conditions, it may be that photoinactivated PSII reaction centres accumulate within the thylakoid membrane, which could account for the lower values for Fv/Fm. In addition, connectivity between active and photoinactivated PSIIs within a dimer or less efficient connectivity among monomeric PSIIs could account for the increase in σPII observed at very low iron concentrations (Figure 1).
Additionally, non-Fb corrected Fv/Fm may also decrease in part to an increased expression of IsiA, which forms an antenna around PSI, increasing the absorption cross-sections of PSI light harvesting (σLHI) (Bibby et al., 2001a,b; Melkozernov and Blankenship, 2005; Wang et al., 2008). This mechanism may also play a critical role in non-photochemical quenching as (i), blue light converts IsiA from one form (efficient in harvesting photons) to another (converts excess energy to heat) (Cadoret et al., 2004) and (ii), high light increases the affinity of IsiA for phycobilisomes thus reducing the high fluorescence of free phycobilisomes (Joshua et al., 2005). In addition, IsiA can aggregate to form empty multimeric rings (without PSI) which exhibits a strong quenched state (Yeremenko et al., 2004), and are responsible for the dissipation of thermal energy (Ihalainen et al., 2005).
Iron, CO2, and light dependencies on photophysiology
Under iron-limitation, the initial slopes of the rP-light curves declined due to a decrease in Fq′/Fm′ (Supplementary Figure 4). Due to light-dependent state transitions, Fo′ can't be calculated using the initial, pre-FLC dark step measure of Fo and Fv/Fm only; and as such Fq′/Fm′ was not separated into its two contributing processes; the PSII photochemical efficiency factor (Fq′/Fv′) and the maximum efficiency of PSII photochemistry (Fv′/Fm′). The lower operating efficiency of PSII (Fq′/Fm′), and subsequent lower rP at low CO2 is likely attributed to the an up-regulated CCM and/or to a down-regulation of the IdiA protein, which is thought to maintain optimal PSII activity (Michel and Pistorius, 2004).
Trichodesmium has high PSI:PSII ratios ranging between 1.3 and 4 under iron-replete diazotrophic conditions (Berman-Frank et al., 2001, 2007; Levitan et al., 2007, 2010; Brown et al., 2008). Previous studies have shown PSII to be less sensitive to iron-limitation than PSI at both an mRNA and protein level (Richier et al., 2012). It has been proposed that decreasing the PSI:PSII ratio is a physiological response to conserve iron (Berman-Frank et al., 2001), and one which could be compensated by increasing the cross-section of PSI light harvesting via IsiA-PSI super-complexes (Bibby et al., 2001a; Ryan-Keogh et al., 2012).
Pseudocyclic electron transport describes the movement of electrons around PSI, and the Mehler reaction (via flavodoxin) which is a photo-catalysed reaction consuming O2 (helping prevent nitrogenase inhibition) whilst simultaneously supplying ATP to nitrogenase (Supplementary Figure 3). Mehler activity can consume up to 75% of the O2 evolved from PSII (Milligan et al., 2007). Prolonged exposure to iron-limiting concentrations could lead to the occurrence of reactive oxygen species as several key proteins (e.g., catalase, peroxidise, superoxidase dismutase) associated to the Mehler reaction are dependent on iron as a co-factor. However, as the nitrogenase pool is significantly reduced under iron-limitation, maintaining the same degree of intracellular anoxia may be less critical.
As reported here, the PSII operating efficiency decreased significantly under iron-limitation, yielding lower rPm. Given that IsiA proteins can be 4 and 6 times more abundant than PSI and PSII proteins in iron-starved cultures and natural populations, respectively (Richier et al., 2012), we suggest that under iron-limitation, Trichodesmium increases pseudocyclic electron transport, with energy being re-directed from nitrogenase to enhance production of key proteins associated with iron stress (i.e., IsiA and IsiD).
Conclusion
Our findings highlight iron as a major influencing factor on Trichodesmium growth, productivity and biogeographical distribution. Whilst iron-limitation constrains maximal Trichodesmium growth and productivity in the open ocean (Rueter, 1988; Rueter et al., 1990), integrated effects of elevated CO2 and/or high light intensities may act as negative feedbacks to climate change. Under iron-limitation the positive effects of elevated CO2 likely arise from a down-regulation of the CCM, whilst the positive effects of saturating light are likely due to a decrease in the requirement for key metalloenzymes, and lead to an increase in iron scavenging mechanisms and high light induced proteins (HLIP). Our findings are important given predictions of an increase in water stratification, a decrease in upwelling and wind-driven mixing, a shoaling of the mixed layer depth, increases in CO2 and sea surface temperatures (SSTs), as well as higher daily light exposures within the water column over the coming decades (Doney, 2006).
A major source of both iron and phosphorus to the Atlantic Ocean is Aeolian dust, which provides up to 16 Tg Fe yr−1 (Jickells et al., 2005) and 1.15 Tg P yr−1 (Mahowald et al., 2008), or 82% and 83% of total input, respectively. Although toxic to picoeukaryotes and Synechococcus (Paytan et al., 2009), Aeolian dust benefits Trichodesmium by providing a source of bioavailable iron (Carpenter and Romans, 1991) coupled with a low N:P ratio. This combination of features stimulates diazotrophic growth without providing a competitive advantage to other phytoplankton groups (Krishnamurthy et al., 2010).
One might expect changes in light and temperature to have a minimal direct effect on iron concentrations in the ocean. However, increased light intensity will increase Fe′ through the photolysis of ferric chelates and the resulting iron redox cycle. Temperature also influences the light requirement for algal growth, as light absorption by photosynthetic pigments and associated photochemistry within the photosynthetic reaction centres are insensitive to temperature, whereas downstream “dark” metabolic reactions which support growth are highly temperature dependent (Geider, 1987; Raven and Geider, 1988). Consequently, higher light is needed to support growth at a higher temperature (Davison, 1991). As such, increased CO2 and higher light intensities in the surface waters could help enhance Trichodesmium's productivity and growth in the future, potentially expanding its distribution into more iron-limited regions. However, it is worth considering whether the direct and indirect benefits of elevated CO2 and higher light intensity outweigh the disadvantages of supra-optimal SSTs.
Author contributions
TB and RG: Conceptualisation. TB, MG, and RG: Methodology. TB and MG: Software. TB and RG: Validation. TB and KO: Formal analysis. TB: Investigation. TB and RG: Resources. TB: Data curation. TB: Writing (original draft preparation). TB, KO, MG, and RG: Writing (review and editing). TB: Visualisation. RG and TL: Supervision. TB: Project administration. RG and TL: Funding acquisition.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
TB was supported by a UK Natural Environment Research Council Ph.D. studentship (NE/J500379/1 DTB).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00624/full#supplementary-material
References
- Anderson M. A., Morel F. M. M. (1982). The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol. Oceanogr. 27, 789–813. 10.4319/lo.1982.27.5.0789 [DOI] [Google Scholar]
- Berman-Frank I., Cullen J. T., Shaked Y., Sherrell R. M. F. P. G. (2001). Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 46, 1249–1260. 10.4319/lo.2001.46.6.1249 [DOI] [Google Scholar]
- Berman-Frank I., Quigg A., Finkel Z. V., Irwin A. J., Haramaty L. (2007). Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol. Oceanogr. 52, 2260–2269. 10.4319/lo.2007.52.5.2260 [DOI] [Google Scholar]
- Bibby T. S., Nield J., Barber J. (2001a). Iron deficiency induces the formation of an antenna ring around trimeric, photosystem I in cyanobacteria. Nature 412, 743–745. 10.1038/35089098 [DOI] [PubMed] [Google Scholar]
- Bibby T. S., Nield J., Partensky F., Barber J. (2001b). Antenna ring around photosystem I. Nature 413:590. 10.1038/35098153 [DOI] [PubMed] [Google Scholar]
- Boatman T. G., Lawson T., Geider R. J. (2017). A key marine diazotroph in a changing ocean: the interacting effects of temperature, CO2 and light on the growth of Trichodesmium erythraeum IMS101. PLoS ONE 12:e0168796. 10.1371/journal.pone.0168796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Killmann H. (1999). Bacterial solutions to the iron-supply problem. Trends Biochem. Sci. 24, 104–109. 10.1016/S0968-0004(99)01359-6 [DOI] [PubMed] [Google Scholar]
- Brown C. M., MacKinnon J. D., Cockshutt A. M., Villareal T. A., Campbell D. A. (2008). Flux capacities and acclimation costs in Trichodesmium from the Gulf of Mexico. Mar. Biol. 154, 413–422. 10.1007/s00227-008-0933-z [DOI] [Google Scholar]
- Cadoret J. C., Demoulière R., Lavaud J., van Gorkom H. J., Houmard J., Etienne A. L. (2004). Dissipation of excess energy triggered by blue light in cyanobacteria with CP43 (isiA). Biochim. Biophys. Acta Bioener. 1659, 100–104. 10.1016/j.bbabio.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Campbell L., Carpenter E., Montoya J., Kustka A., Capone D. (2005). Picoplankton community structure within and outside a Trichodesmium bloom in the southwestern Pacific Ocean. Vie et milieu 55, 185–195. [Google Scholar]
- Capone D. G., Burns J. A., Montoya J. P., Subramaniam A., Mahaffey C., Gunderson T., et al. (2005). Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem. Cycles 19:GB2024 10.1029/2004GB002331 [DOI] [Google Scholar]
- Capone D. G., Zehr J. P., Paerl H. W., Bergman B., Carpenter E. J. (1997). Trichodesmium, a globally significant marine cyanobacterium. Science 276, 1221–1229. 10.1126/science.276.5316.1221 [DOI] [Google Scholar]
- Carpenter E. J., Capone D. G. (eds.). (1992). Nitrogen fixation in Trichodesmium blooms, in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs, NATO ASI Series (Series C: Mathematical and Physical Sciences), Vol. 362 (Dordrecht: Springer; ), 211–217. [Google Scholar]
- Carpenter E. J., Romans K. (1991). Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254, 1356–1358. 10.1126/science.254.5036.1356 [DOI] [PubMed] [Google Scholar]
- Chappell P. D., Webb E. A. (2010). A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium. Environ. Microbiol. 12, 13–27. 10.1111/j.1462-2920.2009.02026.x [DOI] [PubMed] [Google Scholar]
- Chen Y. B., Zehr J. P., Mellon M. (1996). Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. J. Phycol. 32, 916–923. 10.1111/j.0022-3646.1996.00916.x [DOI] [Google Scholar]
- Cid A., Herrero C., Torres E., Abalde J. (1995). Copper toxicity on the marine microalga Phaeodactylum tricornutum: effects on photosynthesis and related parameters. Aquat. Toxicol. 31, 165–174. 10.1016/0166-445X(94)00071-W [DOI] [Google Scholar]
- Croot P. L., Heller M. I. (2012). The importance of kinetics and redox in the biogeochemical cycling of iron in the surface ocean. Front. Microbiol. 3:219. 10.3389/fmicb.2012.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison I. R. (1991). Environmental effects on algal photosynthesis: temperature. J. Phycol. 27, 2–8. 10.1111/j.0022-3646.1991.00002.x [DOI] [Google Scholar]
- Dickson A. G. (1990). Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. A Oceanogr. Res. Pap. 37, 755–766. 10.1016/0198-0149(90)90004-F [DOI] [Google Scholar]
- Dickson A. G. (1993). pH buffers for sea water media based on the total hydrogen ion concentration scale. Deep Sea Res. I Oceanogr. Res. Pap. 40, 107–118. 10.1016/0967-0637(93)90055-8 [DOI] [Google Scholar]
- Doney S. C. (2006). Oceanography: plankton in a warmer world. Nature 444, 695–696. 10.1038/444695a [DOI] [PubMed] [Google Scholar]
- Eichner M., Kranz S. A., Rost B. (2014). Combined effects of different CO2 levels and N sources on the diazotrophic cyanobacterium Trichodesmium. Physiol. Plant. 152, 316–330. 10.1111/ppl.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski P. G., LaRoche J. (1991). Acclimation to spectral irradiance in algae. J. Phycol. 27, 8–14. 10.1111/j.0022-3646.1991.00008.x [DOI] [Google Scholar]
- Falkowski P. G., Raven J. A. (1997). Aquatic Photosynthesis. Malden, MA: Blackwell Science. [Google Scholar]
- Fu F. X., Mulholland M. R., Garcia N. S., Beck A., Bernhardt P. W., Warner M. E., et al. (2008). Interactions between changing pCO2, N2 fixation, and Fe limitation in the marine unicellular cyanobacterium Crocosphaera. Limnol. Oceanogr. 53, 2472–2484. 10.4319/lo.2008.53.6.2472 [DOI] [Google Scholar]
- Gao Y., Kaufman Y., Tanre D., Kolber D., Falkowski P. (2001). Seasonal distributions of aeolian iron fluxes to the global ocean. Geophys. Res. Lett. 28, 29–32. 10.1029/2000GL011926 [DOI] [Google Scholar]
- Geider R. J. (1987). Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton. N. Phytol. 106, 1–34. 10.1111/j.1469-8137.1987.tb04788.x [DOI] [Google Scholar]
- Geider R. J., La Roche J. (1994). The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosyn. Res. 39, 275–301. 10.1007/BF00014588 [DOI] [PubMed] [Google Scholar]
- Gledhill M., Achterberg E. P., Li K., Mohamed K. N., Rijkenberg M. J. (2015). Influence of ocean acidification on the complexation of iron and copper by organic ligands in estuarine waters. Mar. Chem. 177, 421–433. 10.1016/j.marchem.2015.03.016 [DOI] [Google Scholar]
- Gustafsson J. (2012). Visual MINTEQ, Version 3.0, Compiled in Visual Basic NET2005, KTH. Department of Land and Water Resources Engineering, Stockholm.
- Hong H., Shen R., Zhang F., Wen Z., Chang S., Lin W., et al. (2017). The complex effects of ocean acidification on the prominent N2-fixing cyanobacterium Trichodesmium. Science 356, 527–531. 10.1126/science.aal2981 [DOI] [PubMed] [Google Scholar]
- Ihalainen J. A., van Stokkum I. H. M., Gibasiewicz K., Germano M., van Grondelle R., Dekker J. P. (2005). Kinetics of excitation trapping in intact Photosystem I of Chlamydomonas reinhardtii and Arabidopsis thaliana. Biochim. Biophys. Acta Bioener. 1706, 267–275. 10.1016/j.bbabio.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Ivanov A., Park Y.-I., Miskiewicz E., Raven J., Huner N., Öquist G. (2000). Iron stress restricts photosynthetic intersystem electron transport in Synechococcus sp. PCC 7942. FEBS Lett. 485, 173–177. 10.1016/S0014-5793(00)02211-0 [DOI] [PubMed] [Google Scholar]
- Jickells T., An Z., Andersen K. K., Baker A., Bergametti G., Brooks N., et al. (2005). Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308, 67–71. 10.1126/science.1105959 [DOI] [PubMed] [Google Scholar]
- Joshua S., Bailey S., Mann N. H., Mullineaux C. W. (2005). Involvement of phycobilisome diffusion in energy quenching in cyanobacteria. Plant Physiol. 138, 1577–1585. 10.1104/pp.105.061168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D., Letelier R., Tupas L., Dore J., Christian J., Hebel D. (1997). The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533–538. 10.1038/41474 [DOI] [Google Scholar]
- Kranz S. A., Levitan O., Richter K. U., Prášil O., Berman-Frank I., Rost B. (2010a). Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiol. 154, 334–345. 10.1104/pp.110.159145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz S. A., Wolf-Gladrow D., Nehrke G., Langer G., Rost B. (2010b). Calcium carbonate precipitation induced by the growth of the marine cyanobacteria Trichodesmium. Limnol. Oceanogr. 55, 2563–2569. 10.4319/lo.2010.55.6.2563 [DOI] [Google Scholar]
- Krishnamurthy A., Moore J. K., Mahowald N., Luo C., Zender C. S. (2010). Impacts of atmospheric nutrient inputs on marine biogeochemistry. J. Geophys. Res. Biogeosci. 115, 1–13. 10.1029/2009JG001115 [DOI] [Google Scholar]
- Küpper H., Šetlík I., Seibert S., Prášil O., Šetlikova E., Strittmatter M., Levitan O., et al. (2008). Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. N. Phytol. 179, 784–798. 10.1111/j.1469-8137.2008.02497.x [DOI] [PubMed] [Google Scholar]
- Lee K., Kim T.-W., Byrne R. H., Millero F. J., Feely R. A., Liu Y-M. (2010). The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Acta 74, 1801–1811. 10.1016/j.gca.2009.12.027 [DOI] [Google Scholar]
- Levitan O., Rosenberg G., Setlik I., Setlikova E., Grigel J., Klepetar J., et al. (2007). Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 13, 531–538. 10.1111/j.1365-2486.2006.01314.x [DOI] [Google Scholar]
- Levitan O., Sudhaus S., LaRoche J., Berman-Frank I. (2010). The influence of pCO2 and temperature on gene expression of carbon and nitrogen pathways in Trichodesmium IMS101. PLoS ONE 5:e15104. 10.1371/journal.pone.0015104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E., Wallace D. (1998). CO2SYS Program. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory Environmental Sciences Division, Oak Ridge, TN.
- Liu X., Millero F. J. (1999). The solubility of iron hydroxide in sodium chloride solutions. Geochim. Cosmochim. Acta 63, 3487–3497. 10.1016/S0016-7037(99)00270-7 [DOI] [Google Scholar]
- Mahowald N., Jickells T. D., Baker A. R., Artaxo P., Benitez-Nelson C. R., Bergametti G., et al. (2008). Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem. Cycles 22, 1–19. 10.1029/2008GB003240 [DOI] [Google Scholar]
- Martínez-Pérez C., Mohr W., Löscher C. R., Dekaezemacker J., Littmann S., Yilmaz P., et al. (2016). The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat. Microbiol. 1:16163. 10.1038/nmicrobiol.2016.163 [DOI] [PubMed] [Google Scholar]
- Melkozernov A. N., Blankenship R. E. (2005). Structural and functional organization of the peripheral light-harvesting system in Photosystem, I. Photosyn. Res. 85, 33–50. 10.1007/s11120-004-6474-5 [DOI] [PubMed] [Google Scholar]
- Michaelis L., Menten M. L. (1913). Die kinetik der invertinwirkung. Biochem. Z. 49:352. [Google Scholar]
- Michel K. P., Pistorius E. K. (2004). Adaptation of the photosynthetic electron transport chain in cyanobacteria to iron deficiency: the function of IdiA and IsiA. Physiol. Plant. 120, 36–50. 10.1111/j.0031-9317.2004.0229.x [DOI] [PubMed] [Google Scholar]
- Millero F. J. (2010). Carbonate constants for estuarine waters. Mar. Freshw. Res. 61, 139–142. 10.1071/MF09254 [DOI] [Google Scholar]
- Milligan A. J., Berman-Frank I., Gerchman Y., Dismukes G. C., Falkowski P. G. (2007). Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J. Phycol. 43, 845–852. 10.1111/j.1529-8817.2007.00395.x [DOI] [Google Scholar]
- Mulholland M. R., Bernhardt P. W. (2005). The effect of growth rate, phosphorus concentration, and temperature on N2 fixation, carbon fixation, and nitrogen release in continuous cultures of Trichodesmium IMS101. Limnol. Oceanogr. 50, 839–849. 10.4319/lo.2005.50.3.0839 [DOI] [Google Scholar]
- Mulholland M. R., Capone D. G. (2001). Stoichiometry of nitrogen and carbon utilization in cultured populations of Trichodesmium IMS101: implications for growth. Limnol. Oceanogr. 46, 436–443. 10.4319/lo.2001.46.2.0436 [DOI] [Google Scholar]
- Mulholland M. R., Bronk D. A., Capone D. G. (2004). Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat. Microb. Ecol. 37, 85–94. 10.3354/ame037085 [DOI] [Google Scholar]
- Mulholland M. R., Ohki K., Capone D. G. (2001). Nutrient controls on nitrogen uptake and metabolism by natural populations and cultures of Trichodesmium (Cyanobacteria). J. Phycol. 37, 1001–1009. 10.1046/j.1529-8817.2001.00080.x [DOI] [Google Scholar]
- Nielsen H. D., Brownlee C., Coelho S. M., Brown M. T. (2003). Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. N. Phytol. 160, 157–165. 10.1046/j.1469-8137.2003.00864.x [DOI] [PubMed] [Google Scholar]
- Oxborough K. (2012). FastPro8 GUI and FRRf3 Systems Documentation. West Molesey: Chelsea Technologies Group Ltd. [Google Scholar]
- Paerl H. W., Prufert-Bebout L. E., Guo C. (1994). Iron-stimulated N2 fixation and growth in natural and cultured populations of the planktonic marine cyanobacteria Trichodesmium spp. Appl. Environ. Microbiol. 60, 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paytan A., Mackey K. R., Chen Y., Lima I. D., Doney S. C., Mahowald N., et al. (2009). Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. U.S.A. 106, 4601–4605. 10.1073/pnas.0811486106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Jassby A. D. (1976). The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. J. Phycol. 12, 421–430. 10.1111/j.1529-8817.1976.tb02866.x [DOI] [Google Scholar]
- Raven J. A., Geider R. J. (1988). Temperature and algal growth. N. Phytol. 110, 441–461. 10.1111/j.1469-8137.1988.tb00282.x [DOI] [Google Scholar]
- Richier S., Macey A. I., Pratt N. J., Honey D. J., Moore C. M., Bibby T. S. (2012). Abundances of iron-binding photosynthetic and nitrogen-fixing proteins of Trichodesmium both in culture and in situ from the North Atlantic. PLoS ONE 7:e35571. 10.1371/journal.pone.0035571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M., Berman-Frank I., Shaked Y. (2011). Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat. Geosci. 4, 529–534. 10.1038/ngeo1181 [DOI] [Google Scholar]
- Rueter J. G. (1988). Iron stimulation of photosynthesis and nitrogen fixation in Anabaena 7120 and Trichodesmium (Cyanophyceae). J. Phycol. 24, 249–254. 10.1111/j.1529-8817.1988.tb00084.x [DOI] [Google Scholar]
- Rueter J. G., Hutchins D. A., Smith R. W., Unsworth N. L. (1992). Iron nutrition of Trichodesmium, in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs, eds Carpenter E. J., Capone D. G. (Dordrecht: Springer; ), 289–306. [Google Scholar]
- Rueter J. G., Ohki K., Fujita Y. (1990). The effect of iron nutrition on photosynthesis and nitrogen fixation in cultures of Trichodesmium (Cyanophyceae). J. Phycol. 26, 30–35. 10.1111/j.0022-3646.1990.00030.x [DOI] [Google Scholar]
- Ryan-Keogh T. J., Macey A. I., Cockshutt A. M., Moore C. M., Bibby T. S. (2012). The cyanobacterial chlorophyll-binding-protein IsiA acts to increase the in vivo effective absorption cross-section of photosystem I under iron limitation. J. Phycol. 48, 145–154. 10.1111/j.1529-8817.2011.01092.x [DOI] [PubMed] [Google Scholar]
- Saito M. A., Bertrand E. M., Dutkiewicz S., Bulygin V. V., Moran D. M., Monteiro F. M., et al. (2011). Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc. Natl. Acad. Sci. U.S.A. 108, 2184–2189. 10.1073/pnas.1006943108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Kranz S. A., Kim J. M., Morel F. M. M. (2012). Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proc. Natl. Acad. Sci. U.S.A. 109, 3094–3100. 10.1073/pnas.1216012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Xu Y., Hopkinson B. M., Morel F. M. M. (2010). Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679. 10.1126/science.1183517 [DOI] [PubMed] [Google Scholar]
- Shi T., Sun Y., Falkowski P. G. (2007). Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS101. Environ. Microbiol. 9, 2945–2956. 10.1111/j.1462-2920.2007.01406.x [DOI] [PubMed] [Google Scholar]
- Sunda W. G., Huntsman S. A. (1997). Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature (London, UK: Elsevier; ), 390:389 10.1038/37093 [DOI] [Google Scholar]
- Sunda W. G., Price N. M., Morel F. M. M. (2005). Trace metal ion buffers and their use in culture studies, in Algal Culturing Techniques, ed Anderson R. A. (Elsevier; Academic Press; ), 35–64. [Google Scholar]
- Wang Q., Jantaro S., Lu B., Majeed W., Bailey M., He Q. (2008). The high light-inducible polypeptides stabilize trimeric photosystem I complex under high light conditions in Synechocystis PCC 6803. Plant Physiol. 147, 1239–1250. 10.1104/pp.108.121087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E. A., Moffett J. W., Waterbury J. B. (2001). Iron stress in open-ocean cyanobacteria (Synechococcus, Trichodesmium, and Crocosphaera spp.): identification of the IdiA protein. Appl. Environ. Microbiol. 67, 5444–5452. 10.1128/AEM.67.12.5444-5452.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sunda W., Boyle E. A., Karl D. M. (2000). Phosphate depletion in the western North Atlantic Ocean. Science 289, 759–762. 10.1126/science.289.5480.759 [DOI] [PubMed] [Google Scholar]
- Yeremenko N., Kouril R., Ihalainen J. A., D'Haene S., van Oosterwijk N., Andrizhiyevskaya E. G., et al. (2004). Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 43, 10308–10313. 10.1021/bi048772l [DOI] [PubMed] [Google Scholar]
- Yruela I., Pueyo J. J., Alonso P. J., Picorel R. (1996). Photoinhibition of photosystem II from higher plants effect of copper inhibition. J. Biol. Chem. 271, 27408–27415. 10.1074/jbc.271.44.27408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.