Abstract
To assess the extent of water flow through channels in the membranes of intact higher plant cells, the effects of HgCl2 on hydraulic conductivity (LP) of wheat (Triticum aestivum L.) root cells were investigated using a pressure probe. The LP of root cells was reduced by 75% in the presence of 100 μm HgCl2. The K+-channel blocker tetraethylammonium had no effect on the LP at concentrations that normally block K+ channels. HgCl2 rapidly depolarized the membrane potential (Vm) of the root cells. The dose-response relationship of inhibition of LP and depolarization of Vm were not significantly different, with half-maximal inhibition occurring at 4.6 and 7.8 μm, respectively. The inhibition of LP and the depolarization of Vm caused by HgCl2 were partially reversed by β-mercaptoethanol. The inhibition of LP by HgCl2 was similar in magnitude to that caused by hypoxia, and the addition of HgCl2 to hypoxia-treated cells did not result in further inhibition. We compared the LP of intact cells with that predicted from a model of cortical cells incorporating water flow across both the plasma membrane and the tonoplast using measured values of water permeability from isolated membranes, and found that HgCl2 has other effects in addition to the direct inhibition of water channels.
The cloning and functional expression of aquaporins from higher plants (Maurel et al., 1993, 1997a; Daniels et al., 1994, 1996; Kammerloher et al., 1994; Yamada et al., 1997; Weig et al., 1997; Chaumont et al., 1998; Johansson et al., 1998) has indicated that water flow across intact higher plant membranes could be predominantly through aquaporins. The biophysical evidence for this in higher plants has lagged behind the molecular work, but recent studies have shown that biophysical characteristics of water transport are consistent with water flow occurring predominantly through channels in some membranes. This evidence includes the following: (a) the ratio of osmotic to diffusional water permeability is greater than unity (Niemietz and Tyerman, 1997); (b) the activation energy is low (Maurel et al., 1997b; Niemietz and Tyerman, 1997); and (c) water permeability is sensitive to sulfhydryl reagents, in particular HgCl2 (Maurel et al., 1997b; Niemietz and Tyerman, 1997).
In the membranes of intact giant charophyte cells, high diffusional water permeability, low activation energy, and inhibition by sulfhydryl reagents have been well established (Wayne and Tazawa, 1990; Henzler and Steudle, 1995; Steudle and Henzler, 1995; Tazawa et al., 1996; Schütz and Tyerman, 1997). The frictional interactions between the transport of water and highly permeant molecules (Tyerman and Steudle, 1982; Steudle and Henzler, 1995; Hertel and Steudle, 1997) are also indicative of water movement through aqueous pores in the membranes of characean cells.
Inhibition by mercurials of water flow through most (Maurel, 1997; Tyerman et al., 1999) but not all (Daniels et al., 1994) aquaporins has prompted experiments testing this effect in whole organs of plants (tomato roots, Maggio and Joly, 1995; wheat roots, Carvajal et al., 1996; barley roots, Tazawa et al., 1997). The strong inhibition that is often observed at high concentrations of HgCl2 has been interpreted as a direct block of water channels and has prompted the view that aquaporins could be involved in the regulation of water flow across roots. However, there is no direct evidence to show that the hydraulic conductivity (LP) of individual root cells is sensitive to HgCl2. There is also no direct evidence to exclude the possibility that HgCl2 inhibition may be indirect via metabolic inhibition, and recent studies have shown that HgCl2 rapidly depolarizes the membrane potential (Vm) of Chara corallina cells (Tazawa et al., 1996; Schütz and Tyerman, 1997).
The possibility of an indirect metabolic effect is especially relevant, since Niemietz and Tyerman (1997) found that the water permeability of isolated plasma membrane extracted from wheat roots was not inhibited by HgCl2. Previously, Zhang and Tyerman (1991) had shown that hypoxia and azide both substantially inhibit the LP of intact wheat root cells. Moreover, the evidence for water-channel-mediated water flow in isolated plasma membrane vesicles was overall not very strong (Niemietz and Tyerman, 1997). In contrast, the water permeability of tonoplast-enriched membrane vesicles was strongly inhibited by HgCl2 and other evidence pointed to water channels being active in the tonoplast (Niemietz and Tyerman, 1997). Qualitatively identical results were obtained by Maurel et al. (1997b) for plasma membrane and tonoplast from tobacco suspension-cultured cells, adding weight to the possibility that aquaporins are not significant in determining water flow in native plasma membranes.
At variance with these results, Kaldenhoff et al. (1998) recently demonstrated that expression of an antisense gene for PIP1b, a putative plasma membrane aquaporin in Arabidopsis, resulted in an increased root-to-shoot ratio and an apparently reduced water permeability of leaf mesophyll protoplasts. These results are consistent with PIP1b being involved in water flow. There is clearly a need to bridge the gap between measurements at the whole organ level and those at the isolated membrane level by investigating the effects of mercurials on single intact cells and the possibility of indirect effects of HgCl2 on water permeation.
There has also been a lack of attention to the possibility that K+ channels in the plasma membrane and tonoplast may also mediate water flow across the membranes, as suggested by work on C. corallina (Wayne and Tazawa, 1990; Homblé and Véry, 1992). The K+ outward and inward channels in the plasma membrane, which accommodate K+ efflux and influx, respectively, when activated, have been identified in various higher plant cells, including the protoplasts derived from wheat root cells (Schachtman et al., 1992; Findlay et al., 1994; Gassmann and Schroeder, 1994). However, whether the K+ channels could contribute to the LP of root cells remains unknown.
To address these issues, we investigated the effect of HgCl2 and a K+-channel blocker, TEA+, on the LP of cortical cells of wheat roots using a pressure probe. We also investigated the effect of HgCl2 on the Vm of cortical cells to compare it with the inhibition of LP. Finally, we compared measured water flow in intact cells with modeled water flow using measured water permeabilities of isolated membrane vesicles from wheat roots (Niemietz and Tyerman, 1997).
MATERIALS AND METHODS
Plant Material
Wheat (Triticum aestivum L. cv Machete) seeds were germinated in the dark for 48 h at 25°C on filter paper soaked with 0.5 mm CaSO4. The seedlings were then grown hydroponically in fully aerated one-half-strength Hoagland solution under controlled conditions, as described previously (Zhang and Tyerman, 1991).
Pressure Probe Measurements
The roots of 4- to 8-d-old plants were used in the pressure probe experiments. Measurements were made on the second to fourth layer of cortical cells 10 to 20 mm from the root apex. An excised root was held in a Perspex chamber positioned on the specimen stage of a light microscope. A glass capillary attached to the pressure probe was introduced into the root cortical cells through a small opening on one side of the chamber. The chamber was flushed with aerated one-half-strength Hoagland solution.
Details of the pressure probe measurements have been given previously (Zhang and Tyerman, 1991; Zhang et al., 1996). Once the pipette filled with silicone oil was introduced into a cortical cell, there was a sudden movement of cell sap into the micropipette, forming a meniscus between the oil and the sap. By moving the meniscus to a position adjacent to the root surface with the pressure probe, a stationary turgor pressure (P) output was recorded. The half-time for water flow equilibration (t1/2) induced by rapid changes in P was determined from the P relaxation curves recorded with a chart recorder and later digitized using an optical scanner and the program UnGraph (version 2.0, Biosoft, Cambridge, UK). For some experiments relaxation curves were fitted to both single- and double-exponential equations using the Prism program (GraphPad Software, San Diego, CA), which uses the Levenberg-Marquardt method to minimize the sum of squares. The equation that gave the best fit to the data was deduced by performing an F test within the Prism program that takes into account the difference in df from having different numbers of variables in the two equations.
The LP of the cells was calculated using the equation:
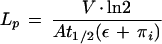 |
1 |
where V is the cell volume, A is the surface area, π is the intracellular osmotic pressure that is approximated to initial P because of the low π of the bathing solution, and ε is the volumetric elastic modulus determined independently by measuring changes in V (ΔV) and corresponding changes in P (ΔP):
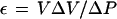 |
2 |
For each cell, measurements of ε and t1/2 were first performed in the absence of HgCl2 or TEA-Cl, and then the same measurements were repeated in the presence of HgCl2 or TEA-Cl. The cell was delineated by injecting silicon oil from the pressure-probe pipette at the end of the measurements, allowing for more accurate determinations of cell dimensions. The cells were approximated to cylinders.
Measurements of Vm
The Vm of the root cells was measured as described by Zhang and Tyerman (1997). The roots were bathed with aerated solution containing 1 mm KCl, 0.1 mm CaSO4, and 1 mm Hepes, pH 7.0. Microelectrodes were pulled from borosilicate glass capillaries with solid filaments (Clark Electromedical Instruments, Reading, UK). The micropipettes were filled with 1 m KCl. A reference electrode was filled with the same electrolyte solution as the micropipette plus 2% agar. The electrical potential difference was measured with an amplifier (model 1600 Neuroprobe, A-M Systems, Carlsborg, WA) with an input impedance of 1013 Ω.
RESULTS
Effect of HgCl2 on Water Relations of the Cells
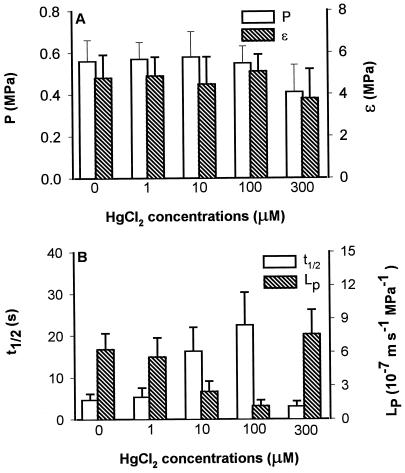
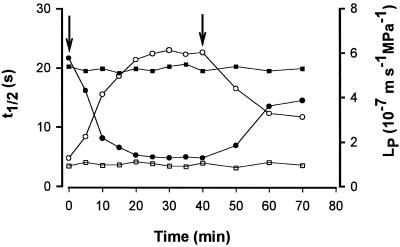
No significant changes in P or ε of wheat root cells were found when the roots were treated with HgCl2 concentrations up to 100 μm for 1 h (Fig. 1A). However, there was a significant increase in the t1/2 of the cells when 10 and 100 μm HgCl2 were added to the bathing medium (Fig. 1B). Because there was no significant change in ε, this increase in t1/2 indicates that there was a decrease in the LP (Fig. 1B). When the roots were treated with 300 μm HgCl2, an increase in LP (decrease in t1/2) was often observed (Fig. 1B). This increase in LP and a concurrent decrease in P suggest that the membranes become leaky. Figure 2 shows a time course of changes in LP (t1/2) of a cell upon adding 100 μm HgCl2 to the bathing medium. The reduction of LP by HgCl2 was not reversed when 1 mm β-mercaptoethanol was used to replace the HgCl2 (data not shown). However, when HgCl2 was washed out with 5 mm βmercaptoethanol, about 60% of the inhibition was recovered (Fig. 2).
Figure 1.
Effects of HgCl2 on cellular water relations in wheat roots. The values are means ± sd of 6 to 10 cells measured before HgCl2 addition and after 30 min.
Figure 2.
Time course of changes in t1/2 (□, ○) and LP (▪, •) of one root cell in response to 100 μm HgCl2 in the bathing medium (circles) or in control solution (squares). The first arrow indicates addition of 100 μm HgCl2 to the bathing medium, and the second arrow indicates removal of HgCl2 by 5 mm β-mercaptoethanol.
To rule out the possibility that the reduction of LP in the presence of HgCl2 could result from a coincidental time-dependent change in LP, measurements of LP (t1/2) of the root cells were repeatedly made on the root cells bathed in the control solution. No time-dependent change in the t1/2 (LP) of the cells was found (Fig. 2). The ratio of LP for endosmotic and exosmotic water flow, LenP/LexP, was 1.12 ± 0.11 (n = 21) for the cells in control solution and 1.02 ± 0.08 (n = 8) for the cells treated with 100 μm HgCl2. Therefore, HgCl2 reduced the LP of both endosmotic and exosmotic water flow.
A marked decrease in LP of wheat root cells was found when they were exposed to low-O2 treatments (Zhang and Tyerman, 1991); therefore, it would be interesting to determine whether the reduction of LP by HgCl2 and hypoxia is caused by a similar mechanism. The roots were pretreated with low O2 for 1 h, and the effect of HgCl2 on the LP of the cells was then examined under hypoxia. As shown in Table I, the hypoxia-treated cells had a low LP, and the addition of 100 μm HgCl2, a concentration that saturates the effect on LP, did not significantly change the LP of the cells (P = 0.12, t test).
Table I.
Effect of 100 μm HgCl2, TEA-Cl (1 and 10 mm) on LP of wheat root cells in aerated (control) and hypoxic solutions
| Treatment | LP (10−7 m s−1 MPa−1) | n |
|---|---|---|
| Control | 5.6 ± 1.6 | 21 |
| Hypoxia | 1.8 ± 0.7 | 6 |
| HgCl2 | 1.2 ± 0.5 | 8 |
| Hypoxia + HgCl2 | 1.6 ± 0.8 | 5 |
| TEA (1 mm) | 5.2 ± 2.1 | 8 |
| TEA (10 mm) | 5.5 ± 1.8 | 6 |
| Hypoxia + TEA (10 mm) | 2.1 ± 1.2 | 4 |
Values are means ± sd; n is the number of the cells measured for each treatment.
Effect of TEA+ on LP of Wheat Root Cells
In contrast to the HgCl2 treatments, when TEA+ at concentrations of 1 and 10 mm was added to the bathing medium for up to 1 h, no significant change in the LP of the cells was found (Table I), which could have been due to the K+ channels not being activated under our experimental conditions. In protoplasts of wheat root cells, a K+ outward channel is activated when the Vm becomes more positive than the equilibrium potential for K+ (EK) (Schachtman et al., 1992). The wheat root cells were rapidly depolarized by hypoxic treatments to a Vm more positive than EK (Zhang and Tyerman, 1997). The hypoxia-elicited membrane depolarization would be expected to activate the K+ outward channels. However, the LP of the cells was only slightly changed upon addition of 10 mm TEA+ to the hypoxic bathing medium (Table I).
Effect of HgCl2 on VP
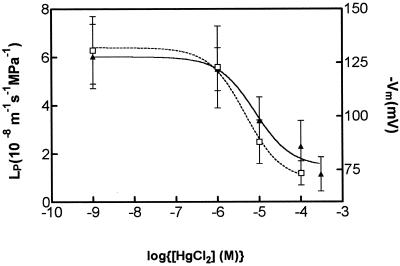
When HgCl2 was added to the bathing solution, the root cells showed a substantial membrane depolarization following an initial small hyperpolarization. The depolarization increased with increasing HgCl2 concentrations. These results have been plotted as a dose-response curve in Figure 3 so that they can be compared with the dose-response curve for HgCl2 inhibition of LP shown on the same graph. The control values without added HgCl2 where plotted against an arbitrarily small amount of HgCl2 to accommodate plotting the results on a logarithmic abscissa. The dose-response curves that were fitted gave half-maximal inhibitory constants of 4.6 μm HgCl2 for LP and 7.8 μm HgCl2 for Vm, which are not significantly different within 95% confidence limits. The membrane depolarization was not fully reversed when HgCl2 was washed out with mercaptoethanol. For the four cells examined, 100 μm HgCl2 depolarized Vm from −112 ± 15 to −81 ± 7 mV. Upon removal of HgCl2 and addition of 5 mm mercaptoethanol, Vm hyperpolarized to −92 ± 9 mV.
Figure 3.
Dose-response curves of Vm (▴) and LP (□) to HgCl2 concentrations in the bathing medium for root cortex cells. The values are means ± sd of 6 to 10 cells and have been fitted by sigmoidal dose-response curves of the form: y = ymax + (ymax − ymin)/(1 + 10(logEC50 − log[HgCl2 concentration]). The half-maximal inhibition constants (EC50) for LP and Vm were 4.6 and 7.8 μm, respectively.
Modeling the Effect of HgCl2 on Tonoplast and Plasma Membrane LP
To solve for the change in P as a function of time while taking into account the volume flows across the tonoplast and plasma membrane, the coupled differential equations for volume flow across the tonoplast and plasma membrane were solved using an iterative procedure (Eulers method). The procedure was performed in the program Mathcad (version 7.0, MathSoft, Cambridge, MA) based on the method outlined by Wendler and Zimmermann (1985). The finite difference equations used for the iteration were:
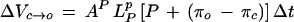 |
3 |
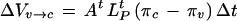 |
4 |
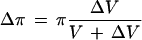 |
5 |
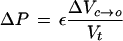 |
6 |
where ΔVc→o and ΔVv→c are the changes in V caused by water flow across the plasma membrane and tonoplast, respectively, over a small increment in time Δt; Ap and At are the surface areas of the plasma membrane and tonoplast, respectively; LPp and LPt are the LP of the plasma membrane and tonoplast, respectively; πc and πv are the π of the cytoplasm and vacuole, respectively. Equation 5 was used to adjust πc and πv with the relevant compartment volume and changes in V. It is assumed that ε and LP are constant with P over a small change in P and that the tonoplast and plasma membranes are effectively impermeable to the solutes that make up the total osmotic pressures in the compartments. The volume of the vacuole (Vv), cytoplasm (Vc), and cell (Vt) and the P were calculated as a function of time by iteration with small Δt (0.01 s) in the following order of calculations, ΔVc→o and ΔVv→c, then adjustment to P, πv, Vv, ΔVc, πc, and Vc. The calculations were essentially the same as that described by Wendler and Zimmermann (1985). The iteration was tested for variations in the size of the step size Δt and found to be stable for Δt less than 0.1 s.
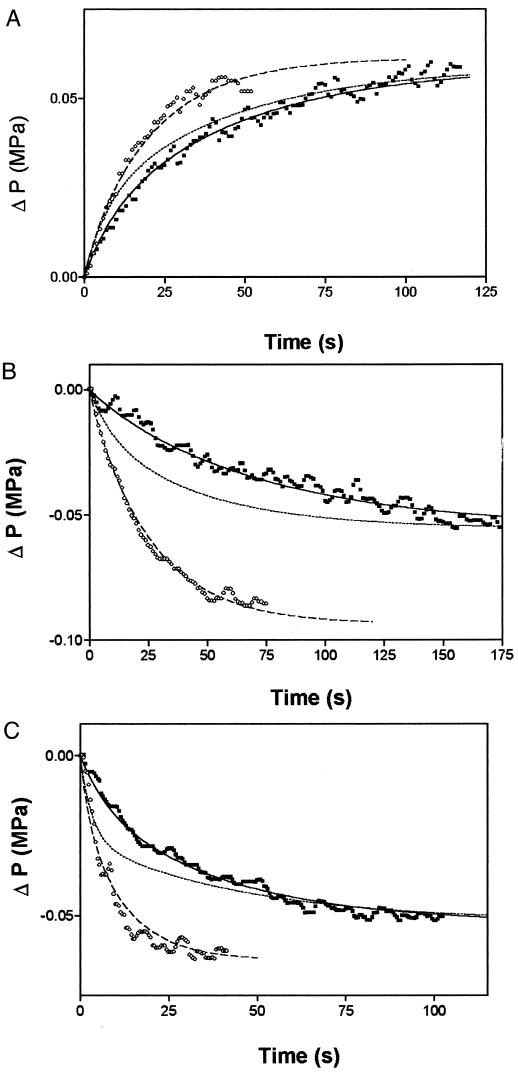
Using the Pos measured by Niemietz and Tyerman (1997) for the tonoplast and plasma membrane, and converting to units of LP, P as a function of time generated from the model was compared with P relaxation kinetics measured with the pressure probe (Fig. 4). The other parameters used in the model (π = πc = πv, ε, V, A, P [t = 0]) were taken from measurements made with the pressure probe on individual cells. The cytoplasm was assumed to be 2 μm in thickness.
Figure 4.
Examples of P relaxation curves for three cells before and after HgCl2 treatment. A, Cell no. 1412; B, cell no. 287; and C, cell no. 58. The fitted lines were generated from the three-compartment model of Wendler and Zimmermann (1985). The cell numbers correspond to those in Table II. Open symbols, Control; closed symbols, plus HgCl2; dashed lines, control fit; dotted lines, tonoplast inhibited; solid lines, plasma membrane and tonoplast inhibited.
For all six cells examined, the measured kinetics of the P relaxations were more rapid than predicted from the LP values obtained by Niemietz and Tyerman (1997). To obtain good fits for cells before HgCl2 treatment the plasma membrane LP had to be increased by between 1.2- and 10-fold (Fig. 4; Table II). The tonoplast LP did not have a significant effect on the kinetics except in one cell, in which it had to be increased by a factor of 3 before a reasonable fit could be obtained.
Table II.
Tonoplast and plasma membrane LP required to fit the pressure relaxations of individual cortical cells from wheat roots
| Cell No. | Control
|
HgCl2
|
||||
|---|---|---|---|---|---|---|
| ×LPt | ×LPp | Kinetics | ×LPt | ×LPp | Kinetics | |
| 237 | 1 | 1.18 | s | 0.25 | 0.65 | d |
| 58 | 1 | 5.50 | s | 0.20 | 1.80 | d |
| 287a | 1 | 1.35 | s | 0.30 | 0.50 | s |
| 287b | 1 | 5.00 | s | 0.20 | 1.40 | d |
| 1412 | 1 | 2.30 | s | 0.30 | 1.60 | d |
| 297 | 3 | 10.0 | s | 1.50 | 1.40 | s |
The three-compartment model of Wendler and Zimmerman (1985) was used, and the starting values of the tonoplast and plasma membrane LP were set at those obtained for isolated membrane vesicles from wheat roots of similar age obtained by Niemietz and Tyerman (1997). The values presented in the table are the multiplying factors used on the Niemietz and Tyerman (1997) LP values to obtain a good fit for each cell. LPt, 6.3 × 10−7 m s−1 MPa−1; LPP, 9.2 × 10−8 m s−1 MPa−1. Also given is whether the kinetics of the pressure relaxation were best fit by a single- (s) or double-exponential (d) equation. The other parameters for fitting to the model were set to the values measured with the pressure probe on the individual cells, assuming that the cytoplasm was 2 μm in thickness.
Niemietz and Tyerman (1997) found that the Pos of the plasma membrane was not inhibited by HgCl2, but that the tonoplast-enriched fraction was significantly inhibited. Incorporating the saturation inhibition by HgCl2 of the tonoplast LP (to 30% of control) but no inhibition of the plasma membrane LP into the model resulted in the half-time for equilibration being reduced (Fig. 4, dotted line), but not sufficiently to match the inhibition observed at 100 μm HgCl2 in pressure-probe experiments on intact cells. To fit the intact cell data both the plasma membrane and tonoplast LP had to be reduced from control values (Fig. 4; Table II). The relaxation of P was more often fitted by a double-exponential equation in the presence of 100 μm HgCl2 (Table II).
DISCUSSION
We demonstrated, using a pressure probe, that HgCl2 induced a rapid and significant decrease in the LP of wheat root cortical cells. This reduction in LP was comparable to that found in C. corallina internodal cells (Henzler and Steudle, 1995; Tazawa et al., 1996; Schütz and Tyerman, 1997), which was interpreted as an inhibition of the water channels. However, treatments of wheat root cells that cause general metabolic inhibition also reduce LP to a similar extent as that caused by HgCl2 treatment (Zhang and Tyerman, 1991). Furthermore, as shown in this study, there was no additional effect of HgCl2 treatment on the LP of cells already metabolically compromised by hypoxia treatment. This indicates that HgCl2 could reduce LP via general metabolic inhibition that may affect various water flow pathways, rather than by a direct block of water channels. This is further supported by the similarity between the dose response of cell Vm and LP to HgCl2.
The inhibition of LP by HgCl2 was only partly recovered when HgCl2 was replaced with the reducing agent mercaptoethanol. A similar effect was observed with Vm. In contrast, for C. corallina internodal cells, the effect of HgCl2 on LP could be fully reversed with mercaptoethanol (Henzler and Steudle, 1995; Schütz and Tyerman, 1997). This difference could arise if HgCl2 inhibition in wheat root cells were through a variety of different mechanisms, including direct blockage of water channels and metabolic inhibition. The LP of root cells treated with 0.3 mm HgCl2 increased rather than decreased, and this increase corresponded to a decrease in P, suggesting that the cell membranes become leaky in the presence of high concentrations of HgCl2. This finding highlights the potential nonspecific and detrimental effect of HgCl2 on the membranes of plant cells. Therefore, a low HgCl2 concentration is recommended for future studies, which should also take into account the nonspecificity of HgCl2 on Lp in intact plant cells.
It is possible that a substantial water flow occurs through plasmodesmata when pressure is altered in one cell within the symplast, as occurs with pressure-relaxation and pressure-clamp experiments (Murphy and Smith, 1998). The reduction of LP of cortical cells in wheat caused by metabolic inhibition has been suggested to be due to closure of plasmodesmata (Zhang and Tyerman, 1991). However, further investigations showed an increase in the solute size able to permeate plasmodesmata with anaerobic stress (Cleland et al., 1994) and no change in the cell-to-cell electrical resistance under hypoxia (Zhang and Tyerman, 1997). Therefore, to account for the reduced cell LP, either the water permeability of cell membranes is reduced under metabolic inhibition, or water and solutes take different pathways through plasmodesmata and metabolic inhibition reduces the LP of the water pathway.
The overall LP of cells measured in the pressure-probe experiments is most likely dominated by the LP of a composite membrane consisting of the plasma membrane and plasmodesmata in parallel, and the cytoplasm and tonoplast in series (Steudle, 1989; Maurel, 1997; Murphy and Smith, 1998). It is assumed that the tip of the pressure probe is located in the vacuole, because upon stabbing the cell, sap gushes into the capillary. Also, the osmotic volume of cells measured with the pressure-clamp technique was never significantly smaller than the geometric volume (Zhang and Tyerman, 1991), a result inconsistent with the tip of the microcapillary being situated in the cytoplasm (Murphy and Smith, 1998). A reduction of overall cell LP by HgCl2 could result from a decrease in the LP of the plasma membrane plus the plasmodesmata, the tonoplast, or both.
Recent studies using isolated membrane vesicles have shown that the LP of the tonoplast, measured as Pos, is much higher than that of the plasma membrane and is dominated by water flow through channels (Maurel et al., 1997b; Niemietz and Tyerman, 1997). The tonoplast LP, in contrast to that of the plasma membrane, is sensitive to HgCl2 (Maurel et al., 1997b; Niemietz and Tyerman, 1997). It has been suggested that the higher water permeability of the tonoplast allows the vacuole to effectively buffer the cytoplasm, thereby minimizing the magnitude of short-term volume transients in the cytoplasm that might have detrimental effects on the cytoskeleton and metabolism (for modeled cell, see Tyerman et al., 1999).
Using the LPs for the tonoplast and plasma membrane measured by Niemietz and Tyerman (1997), we could not reconstruct the pressure relaxations observed in the present study. First, the plasma membrane LP had to be increased significantly to fit the pressure relaxations of intact cells. Despite the tonoplast and plasma membrane LPs becoming more similar in magnitude, the model still indicated that water flow was dominated mostly by flow across the plasma membrane. This is indicated by the pressure relaxations being fit best by a single exponential equation, and is supported by the results of Oparka et al. (1991), who found that the t1/2 of turgor relaxation curves is not significantly different with the pressure probe located in either the cytoplasm or in the vacuole. Second, the inhibition of LP in the intact cells caused by HgCl2 could not be entirely accounted for by the inhibition of tonoplast LP. In all cases the plasma membrane LP had to be reduced to fit the pressure relaxations of inhibited cells. This is in contrast to the finding of Niemietz and Tyerman (1997) that the LP of isolated plasma membranes is not sensitive to HgCl2.
A possible explanation for these results is that the plasma membrane does contain functional water channels in intact cells that are inactivated in some way by treatments that disrupt the cells or inhibit metabolism. Perhaps during the plasma membrane isolation procedures used by Maurel et al. (1997b) and Niemietz and Tyerman (1997), the water channels also become inactivated by metabolic inhibition. This would reconcile the biophysical observations of lack of water channel activity in isolated plasma membrane (Maurel et al., 1997b; Niemietz and Tyerman, 1997) with the observations that aquaporins are located in the plasma membrane (Chrispeels and Maurel, 1994) at very high densities (Johansson et al., 1996). Phosphorylation of aquaporins seems to be a likely mechanism for the regulation of water permeation (Maurel, 1997). The plasma membrane aquaporin PM28A of spinach leaf is a major phosphoprotein (Johansson et al., 1996), and its water permeability is reduced upon dephosphorylation (Johansson et al., 1998). Therefore, reduced phosphorylation of the root cell aquaporins caused by metabolic inhibition provides one possible explanation for the reduction of the plasma membrane LP of wheat root cells under metabolic inhibition. It may also account for the observation that LP of isolated plasma membranes is less than the LP of plasma membranes of intact cells.
An alternative explanation is that plasmosdesmatal LP is reduced by metabolic inhibition. This would also explain the lack of agreement between the LP of isolated plasma membranes and the LP of the intact composite membrane of cells in tissues (plasma membranes plus plasmodesmata) required to fit the pressure relaxations. However, as outlined above, to fit the available evidence this explanation requires that solute and water take different pathways through plasmodesmata, and it begs the question of what the aquaporins are actually doing in the plasma membrane.
A reduction in cortical cell LP by HgCl2 may have a different effect on the overall root LP, depending upon the pathways of water flow across the root. Radial water flow within the root can in principle occur in three parallel pathways: apoplastic, symplastic via plasmodesmata, and transcellular pathways (Steudle, 1998). It is difficult to separate the symplastic from the transcellular (Murphy and Smith, 1998); therefore, the two pathways are generally considered as a cell-to-cell pathway (Steudle, 1998). If water flow is dominated by an apoplastic pathway, water flow across the root may not be controlled directly by water-channel activity and water channels may only facilitate local equilibrium of water with the apoplast in the pathway.
Since the exodermis could be a major hydraulic barrier for water flow due to the formation of suberin lamellae (Zimmermann and Steudle, 1998), it is expected that the aquaporins in the exodermal cells may be involved in regulating the root LP. However, if water flow through the root occurs via the cell-to-cell pathway, an inhibition of water channels in the cortical cells would have a marked effect on the LP of the whole root. In this context, an inhibition of LP of whole roots by HgCl2 has been shown in several plant species (wheat root, Carvajal et al., 1996; barley root, Tazawa et al., 1997; and tomato root, Maggio and Joly, 1995). For example, 50 μm HgCl2 reduced the wheat root LP by 66% (Carvajal et al., 1996), and the LP of barley root was reduced by 90% in the presence of 100 μm HgCl2 (Tazawa et al., 1997). It should be noted that higher concentrations of HgCl2 (0.5 mm) and mercaptoethanol (60 mm) were used in the study of HgCl2 effects on the LP of tomato roots (Maggio and Joly, 1995). It is conceivable that such high HgCl2 concentrations may have profound effects on root physiology in addition to the inhibition of water channels.
The inhibition of LP of individual wheat root cortical cells by HgCl2 is comparable to that found in whole wheat roots (Fig. 1; Carvajal et al., 1996) and provides an explanation for the reduction of the LP in wheat roots by HgCl2 (Carvajal et al., 1996). However, it cannot be assumed that this inhibition is caused exclusively by direct blockade of water channels; although it is likely that water channels are inhibited by HgCl2, this could be an indirect effect (especially for the plasma membrane). The average LP of the plasma membrane of root cells, which was deduced from fits to the three-compartment model of Wendler and Zimmermann (1985) in the absence of HgCl2, was 3.9 × 10−7 m s−1 MPa−1 (Table II). This value corresponds to a Pos of 5.8 × 10−5 m s−1, which is about 2 times higher than the Pd of wheat root protoplasts determined by NMR (Zhang and Jones, 1996). A Pos/Pd higher than unity is an indication of the involvement of water channels in water flow across the membranes (Finkelstein, 1987; Verkman, 1992).
The presence of functional water channels in root cells could be of importance in the regulation of water flow in response to environmental and developmental signals. A decrease in root LP seems to be a general phenomenon when plants are grown under unfavorable conditions such as salinity, hypoxia (Steudle, 1998), and nutrient deficiency (e.g. N and P) (Carvajal et al., 1996). Roots of N- and P-deficient wheat plants exhibited a whole-root LP similar to those treated with HgCl2, and the root LP of nutrient-deficient plants was no longer sensitive to HgCl2 (Carvajal et al., 1996). Since nutrient deficiency may not directly affect metabolism (Carvajal et al., 1996), mechanisms other than metabolic control are expected to be responsible for the regulation of water-channel activity.
The effect of HgCl2 on the LP of plant cells may not be a general phenomenon, as Rygol and Lüttge (1984) showed no effect of 0.1 mm HgCl2 on the LP of subepidermal cells of pepper fruits. This would indicate that the involvement of water channels in water flow through the cell membranes of plants is restricted to certain types of cells, and probably depends on physiological roles of the cells, as demonstrated in algae (Gutknecht, 1967; Wayne and Tazawa, 1990; Henzler and Steudle, 1995; Schütz and Tyerman, 1997; Tazawa et al., 1996) and animal cells (for review, see Verkman, 1992). This explanation may also account for the large variations in the LP of plant cells so far determined by the pressure probe (Steudle, 1989).
In contrast to HgCl2, the K+-channel blocker TEA+ showed no effect on the LP of wheat root cells (Table I), possibly due to K+ channels being closed during the TEA+ treatment. However, no significant effect of TEA+ on the LP of cells exposed to low-O2 treatments (Table I) seems to discount this possibility, as the Vm of the cells is depolarized to be more positive than the equilibrium potential of K+ under the hypoxic treatments (Zhang and Tyerman, 1997), and the K+ outward channels are likely to be activated at this depolarized Vm (Schachtman et al., 1992). The lack of effect of TEA on LP is unlikely to result from changes in Vm and consequently the voltage-gated K+ channels, as TEA+ had little effect on the Vm of wheat root cells (Zhang and Tyerman, 1997). Therefore, the extent of water flow through TEA-sensitive K+ channels, as far as can be determined from blocker studies, is probably minor.
In summary, the LP of intact wheat root cells is sensitive to HgCl2. The inhibition of LP by HgCl2 is comparable to that after hypoxia treatment and the inhibitions are not additive. HgCl2 rapidly depolarized the plasma membrane Vm at a similar half-maximal concentration to that causing inhibition of LP. These results suggest that cell metabolism may have a major effect on the activity of water channels in intact cells, which makes it difficult to attribute the effect of HgCl2 as direct blockage or blockage of water channels in intact cell or organ systems.
ACKNOWLEDGMENT
We wish to thank Dr. Christa Niemietz for comments concerning the manuscript.
Abbreviation:
- TEA+
tetraethylammonium ion
Footnotes
This study was supported in part by the Australian Research Council.
LITERATURE CITED
- Carvajal M, Cook DT, Clarkson DT. Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta. 1996;199:372–381. [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ. Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol. 1998;117:1143–1152. doi: 10.1104/pp.117.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. Aquaporins: the molecular basis of facilitated water movement through living plant cells. Plant Physiol. 1994;105:9–13. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE, Fujiwara T, Lucas WJ. Plasmodesmata-mediated cell-to-cell transport in wheat roots is modulated by anaerobic stress. Protoplasma. 1994;178:81–85. doi: 10.1007/BF01404123. [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ. Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell. 1996;8:587–599. doi: 10.1105/tpc.8.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ. The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GP, Tyerman SD, Garrill A, Skerrett M. Pump and K+ inward rectifiers in the plasmalemma of wheat protoplasts. J Membr Biol. 1994;139:103–116. doi: 10.1007/BF00232429. [DOI] [PubMed] [Google Scholar]
- Finkelstein A (1987) Water movement through lipid bilayers, pores and plasma membranes: theory and reality. In Distinguished Lecture Series of the Society of General Physiologists, Vol 4. John Wiley & Sons, New York
- Gassmann W, Schroeder JI. Inward-rectifying K+ channels in root hairs of wheat. Plant Physiol. 1994;103:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J. Membranes of Valonia ventricosa: apparent absence of water filled pores. Science. 1967;158:787–788. doi: 10.1126/science.158.3802.787. [DOI] [PubMed] [Google Scholar]
- Henzler T, Steudle E. Reversible closing of water channels in Chara internodes provides evidence for a composite transport model of the plasma membrane. J Exp Bot. 1995;46:199–209. [Google Scholar]
- Hertel A, Steudle E. The function of water channels in Chara: the temperature dependence of water and solute flows provides evidence for composite membrane transport and for a slippage of small organic solutes across water channels. Planta. 1997;202:324–335. [Google Scholar]
- Homblé F, Véry AA. Coupling of water and potassium ions in K+ channels of the tonoplasts of Chara. Biophys J. 1992;63:996–999. doi: 10.1016/S0006-3495(92)81666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell. 1998;10:451–459. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjellbom P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu J-J, Zimmermann U. Plant J. 1998;14:121–128. doi: 10.1046/j.1365-313x.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schäffner AR. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994;6:187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly JJ. Effects of mercuric chloride on the hydraulic conductivity of tomato root systems. Plant Physiol. 1995;109:331–335. doi: 10.1104/pp.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. Aquaproins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ, Lurin C, Tacnet F, Geelen D, Ripoche P, Guren J. Function and regulation of seed aquaporins. J Exp Bot. 1997a;48:421–430. doi: 10.1093/jxb/48.Special_Issue.421. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Tacnet F, Güclü J, Guern J, Ripoche P. Purified vesicles of tobacco cell vacuolar and plasma membranes exhibited dramatically different water permeability and water channel activity. Proc Natl Acad Sci USA. 1997b;94:7103–7108. doi: 10.1073/pnas.94.13.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Smith JAC. Determination of cell water-relation parameters using the pressure probe: extended theory and practice of the pressure-clamp technique. Plant Cell Environ. 1998;21:637–657. [Google Scholar]
- Niemietz C, Tyerman SD. Characterization of water channels in wheat root membrane vesicles. Plant Physiol. 1997;115:561–567. doi: 10.1104/pp.115.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Murphy R, Derrick PM, Prior DAM, Smith JAC. Modification of the pressure-probe technique permits controlled intracellular microinjection of fluorescent probes. J Cell Sci. 1991;98:539–544. [Google Scholar]
- Rygol J, Lüttge U. Effects of various benzene derivatives, dodecylbenzensulfonate and HgCl2 on water relations parameters at the cellular level. Physiol Veg. 1984;22:783–792. [Google Scholar]
- Schachtman DP, Tyerman SD, Terry BR. The K/Na selectivity of a cation channel in the plasma membrane of root cells does not differ in salt-tolerant and salt-sensitive wheat species. Plant Physiol. 1992;97:598–605. doi: 10.1104/pp.97.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz K, Tyerman SD. Water channels in Chara corallina. J Exp Bot. 1997;48:1511–1518. [Google Scholar]
- Steudle E. Water flow in plants and its coupling to other processes: an overview. Methods Enzymol. 1989;174:183–225. [Google Scholar]
- Steudle E. How does water get through roots? J Exp Bot. 1998;49:775–788. [Google Scholar]
- Steudle E, Henzler H. Water channels in plants: do basic concepts of water transport change? J Exp Bot. 1995;46:1067–1076. [Google Scholar]
- Tazawa M, Asai K, Iwasaki N. Characteristics of Hg- and Zn-sensitive water channels in the plasma membrane of Chara cells. Bot Acta. 1996;105:388–396. [Google Scholar]
- Tazawa M, Ohkuma E, Shibasaka M, Nakashima S. Mercurial-sensitive water transport in barley roots. J Plant Res. 1997;110:435–442. [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot. 1999;50:1055–1071. [Google Scholar]
- Tyerman SD, Steudle E. Comparison between osmotic and hydrostatic water flows in higher plant cells: determination of hydraulic conductivities and reflection coefficients in isolated epidermis of Tradescantia virginoana. Aust J Plant Physiol. 1982;9:461–479. [Google Scholar]
- Verkman VS. Water channels in cell membranes. Annu Rev Physiol. 1992;54:97–108. doi: 10.1146/annurev.ph.54.030192.000525. [DOI] [PubMed] [Google Scholar]
- Wayne R, Tazawa M (1990) Nature of water channels in the internodal cells of Nitellopsis. J Membr Biol 116, 31–39 [DOI] [PubMed]
- Weig A, Deswarte C, Chrispeels MJ. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997;114:1347–1357. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler S, Zimmermann U. J Membr Biol. 1985;85:133–142. [Google Scholar]
- Yamada S, Nelson DE, Ley E, Marquez S, Bohnert HJ. The expression of an aquaporin promoter from Mesembryanthemum crystallinum in tobacco. Plant Cell Physiol. 1997;38:1326–1332. doi: 10.1093/oxfordjournals.pcp.a029125. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Atwell BJ, Patrick JW, Walker NA. Turgor-dependent efflux of assimilates from coats of developing seed of Phaseolus vulgaris L: water relations of the cells involved in efflux. Planta. 1996;199:25–33. [Google Scholar]
- Zhang WH, Jones GP. Water permeability in wheat root protoplasts determined from nuclear magnetic resonance relaxation times. Plant Sci. 1996;118:97–106. [Google Scholar]
- Zhang WH, Tyerman SD. Effect of low O2 concentration and azide on hydraulic conductivity and osmotic volume of the cortical cells of wheat roots. Aust J Plant Physiol. 1991;18:603–613. [Google Scholar]
- Zhang WH, Tyerman SD. Effect of hypoxia on the electrical properties of wheat root cells. J Plant Physiol. 1997;150:567–572. doi: 10.1016/s0176-1617(97)80320-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann HM, Steudle E. Apoplastic transport across young maize roots: effect of the exodermis. Planta. 1998;206:7–19. [Google Scholar]