Abstract
Background
We evaluated vortioxetine’s effects on functional capacity in demographic and clinical subgroups of patients with major depressive disorder.
Methods
This was an exploratory analysis of the CONNECT study (NCT01564862) that evaluated changes in functional capacity using University of California San Diego Performance-based Skills Assessment data, categorized by sex, age, education, employment status, and baseline disease severity (Montgomery-Åsberg Depression Rating Scale, Clinical Global Impressions–Severity of Illness).
Results
Greater changes in University of California San Diego Performance-based Skills Assessment composite scores were observed with vortioxetine vs placebo in specific subgroups: males (∆+3.2), females (∆+2.9), 45–54 or ≥55 years (∆+5.6, ∆+3.4), working (∆+2.8), high school or greater education (∆+2.7, ∆+2.8), disease severity (Montgomery-Åsberg Depression Rating Scale, <30, ∆+3.5; ≥30, ∆+2.5; Clinical Global Impressions–Severity of Illness ≤4, ∆+2.8; >4, ∆+3.0), major depressive episodes (≤2, >2 [∆+2.7,+3.3]), and episode duration (≤22, >22 weeks [∆+3.7,+2.4]).
Conclusions
Our findings support the need for additional studies to assess whether vortioxetine improves functional capacity within specific patient subgroups.
Clinical Trial Registry
clinicaltrials.gov: NCT01564862
Keywords: major depressive disorder, vortioxetine, functional capacity, UPSA
Introduction
Major depressive disorder (MDD) is a common psychiatric disorder and is the second leading cause of disability worldwide, affecting individuals across all socio-demographic strata (Ferrari et al.,2013; Vos et al.,2015). In the United States, the onset of MDD typically occurs between ages 12 and 40 y, and the duration of an individual’s longest episode on average lasts approximately 24 weeks (Hasin et al.,2005). Many patients with MDD experience multiple depressive episodes throughout their lifetime, and the risk for recurrence increases with each successive episode (Hasin et al.,2005).
Cognitive dysfunction can be a hallmark feature of MDD that correlates with impairments in functional capacity, or the ability to perform skills relevant for everyday functioning, even after successful treatment of an acute episode (Reppermund et al., 2009; Bortolato et al., 2014). Therefore, restoration of patient functional capacity is an important goal of MDD treatment (Lam et al., 2011). The University of California, San Diego Performance-Based Skills Assessment (UPSA) is an objective measure of everyday living skills, initially developed to assess functional capacity in older, community-dwelling patients diagnosed with schizophrenia (Patterson et al., 2001). Since then, the UPSA has been used to assess functional capacity in clinical studies across a wide range of populations, including patients with other psychiatric disorders and patients with medical conditions not related to psychiatric disorders (Mausbach et al., 2010; Kaye et al., 2014; Allen et al., 2015). Because of the UPSA’s reliability in subsequent testing, its practicality, and shared variance with cognitive performance, the UPSA is widely used as an index of treatment response (Mausbach et al., 2010; Green et al., 2011).
Vortioxetine is an antidepressant with multimodal activity approved for the treatment of adults with MDD. With a distinct pharmacological profile compared with other conventional antidepressants, vortioxetine modulates several neurotransmitter systems involved in cognitive processes that are dysregulated in MDD (i.e., serotonergic, noradrenergic, dopaminergic, cholinergic, histaminergic, and glutamatergic) (Mørk et al., 2013; Pehrson et al., 2014; Sanchez et al., 2015). In preclinical and clinical studies, vortioxetine exhibited improvements in depression and cognitive functioning, suggesting the potential for vortioxetine to improve functional capacity in patients with MDD (Katona et al., 2012; Fava et al., 2014; McIntyre et al., 2014; Pehrson et al., 2014; Mahableshwarkar et al., 2015).
The CONNECT study (NCT01564862) evaluated the cognitive effects of vortioxetine (10/20 mg QD for up to 8 weeks) in adults with MDD and self-reported symptoms of cognitive dysfunction (Mahableshwarkar et al., 2015). Using the UPSA as a prespecified endpoint in the study protocol, Mahableshwarkar et al. (2015) reported that vortioxetine significantly improved functional capacity relative to placebo and duloxetine. However, whether improvements with vortioxetine were observed across patient subgroups had not been explored.
In this posthoc analysis of the CONNECT study, we evaluated the effects of vortioxetine on functional capacity in patient subgroups based on socio-demographic factors and clinical characteristics at baseline.
Methods
Study Population
CONNECT was a randomized, 8-week, multicenter, double-blind, parallel-group, placebo-controlled, duloxetine-referenced study conducted in outpatients with acute recurrent MDD (baseline Montgomery-Åsberg Depression Rating Scale [MADRS] ≥26) who reported cognitive dysfunction at baseline. Depression symptoms had to be rated as moderate to severe at screening and baseline. The full analysis set (FAS) comprised all patients who received ≥1 dose of study drug and had at least 1 valid post-baseline assessment of the primary efficacy variable (Digit Symbol Substitution Test [DSST]) and was used for posthoc analyses. Patients treated with vortioxetine received a dose of 10 or 20 mg/d (as determined for each individual by the treating clinician), and patients treated with duloxetine were administered the approved dose of 60 mg/d. Since the post-baseline UPSA value for one patient in the FAS was missing, all UPSA analyses excluded this subject from the analysis dataset. Full details of the CONNECT study design, patient population, treatments, major results, and outcome assessments have been published (Mahableshwarkar et al., 2015).
Assessments
The changes from baseline in MADRS total score and UPSA composite score were predefined endpoints in the CONNECT study that were evaluated in this posthoc analysis (Mahableshwarkar et al., 2015). Two forms of the UPSA were used: the UPSA–Validation of Intermediate Measures (UPSA-VIM) in English-speaking countries and the UPSA Brief form (UPSA-B) in non-English-speaking countries. The results on the UPSA-VIM and UPSA-B were standardized to a 0 to 100 scale and analyzed together as the UPSA composite score, since the UPSA-B has been shown to be highly correlated with the UPSA-VIM (r=0.91), where higher scores indicate greater functional capacity (Lam et al., 2011).
In this posthoc analysis, change from baseline to week 8 in UPSA composite scores was evaluated based on the following baseline characteristics: sex (male, female), age (<35, 35–44, 45–54, ≥55 years), education level (less than high school, high school, post-high school), employment status (employed/student, unemployed), the number of previous major depressive episodes (MDEs ≤2, >2), duration of current MDE (≤22 weeks, >22 weeks), baseline MADRS score (moderate <30, severe ≥30), and Clinical Global Impressions–Severity of Illness (CGI-S) score (≤4, >4). The median value for all quantitative variables assessed (i.e., age, number/duration of MDEs, MADRS score, and CGI-S score) was used to determine the cutoff value that defined patient subgroups.
Statistical Analyses
The change from baseline in the UPSA composite score after 8 weeks of treatment was analyzed using ANCOVA in observed cases (OC), with treatment and pooled center as fixed factors and baseline UPSA as the covariate. Two-sided statistical tests comparing vortioxetine with placebo were assessed at the 5% significance level. The term “statistical trend” was used to describe changes that did not achieve statistical significance at the 5% level, but whose P value was <.10.
Results
Patients
In the total randomized population of the CONNECT study (N=602; vortioxetine, n=198; placebo, n=194; duloxetine, n=210), baseline demographics and clinical characteristics were similar across all treatment groups (Mahableshwarkar et al., 2015). This analysis evaluated UPSA efficacy data from 528 patients included in the FAS (vortioxetine, n=175; placebo, n=166; duloxetine, n=187).
Analysis of Functional Capacity across Baseline Demographics Subgroups
The overall effect of vortioxetine treatment on functional capacity was reported in the CONNECT study (Mahableshwarkar et al., 2015). Briefly, the change in UPSA composite score from baseline to week 8 was ∆+2.94 for vortioxetine vs placebo (P<.001) and ∆+0.38 for duloxetine vs placebo (P=.637) (Mahableshwarkar et al., 2015).
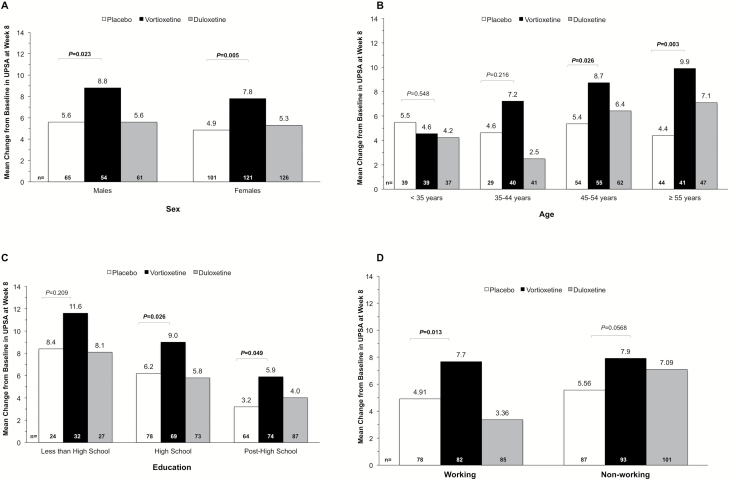
This analysis evaluated the potential relationship between vortioxetine’s effects on functional capacity (UPSA composite scores) and baseline demographic or clinical characteristics. The outcome of this analysis showed that the change in UPSA composite scores for vortioxetine vs placebo was ∆+3.2 in males (n=54, P=.023), ∆+2.9 in females (n=121, P=.005) (Figure 1A), ∆+3.3 in patients aged 45 to 54 years (n=55, P=.026), and ∆+5.5 in those aged ≥55 years (n=41, P=.003) (Figure 1B). Across education levels, a change of ∆+2.8 was observed in the high school education subgroup (n=69, P=.026), ∆+2.7 in the post-high school education subgroup (n=74, P=.049) and ∆+3.2 in the lower education subgroup (n=32, P=.209), although the change for the latter did not reach statistical significance (Figure 1C). In patients who were working (e.g., full- or part-time employee or student) at the time of their baseline visit, the change in UPSA composite score with vortioxetine was ∆+2.8 (n=82, P=.013) (Figure 1D). In patients who were not working (eg, unemployed, retired, or nonworking spouse) at baseline, the treatment difference between vortioxetine and placebo groups was ∆+2.4 (n=93, P=.06) and was significant only at the level of a statistical “trend” (i.e., 0.05 > P≤.10) (Figure 1D).
Figure 1.
Change from baseline to week 8 in UPSA composite score in subgroups based on (A) sex, (B) age, (C) education level, and (D) working status (FAS, ANCOVA, OC). The number of patients in each of the subgroups is indicated at the bottom of each figure. Abbreviations: ANCOVA, analysis of covariance; FAS, full analysis set; OC, observed case; UPSA, University of California San Diego Performance-based Skills Assessment.
Analysis of Functional Capacity across Baseline Clinical Characteristics
The change from baseline in UPSA composite scores (i.e., vortioxetine vs placebo) was analyzed in patient subgroups categorized by baseline levels of disease severity, number of previous MDEs, and duration of current MDE. Patients with moderate (MADRS <30) or severe (MADRS ≥30) disease severity levels at baseline saw changes of ∆+3.5 (n=63, P=.014) and ∆+2.5 (n=112, P=.015), respectively, in UPSA composite scores with vortioxetine compared with placebo. Similar effects of vortioxetine in functional capacity were observed in patient subgroups categorized by baseline CGI-S scores (CGI-S ≤4: ∆+2.8, n=84, P=.01; CGI-S >4: ∆+3.0, n=91, P=.02), number of previous MDEs (≤2: ∆+2.7, n=108, P=.011; >2: ∆+3.3, n=67, P=.02), or duration of current MDE (≤22 weeks: ∆+3.7, n=89, P=.003; >22 weeks: ∆+2.4, n=86, P=.031) (Table 1). The change from baseline in UPSA composite scores was not significantly different between duloxetine and placebo treatment allocations in any of the patient subgroups investigated.
Table 1.
Change from Baseline to Week 8 in UPSA Composite Score in Subgroups Based on Baseline Clinical Characteristics (FAS, ANCOVA, OC)
| Placebo | Vortioxetine | Duloxetine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | CFB | N | CFB | ∆ | P | N | CFB | ∆ | P | |
| MADRS total score | ||||||||||
| <30 | 50 | 5.2 | 63 | 8.7 | 3.5 | .014 | 58 | 3.7 | −1.5 | .307 |
| ≥30 | 116 | 4.9 | 112 | 7.4 | 2.5 | .015 | 129 | 6.0 | 1.1 | .274 |
| CGI-S score | ||||||||||
| ≤4 | 80 | 4.7 | 84 | 7.5 | 2.8 | .010 | 85 | 4.5 | −0.2 | .833 |
| >4 | 86 | 5.0 | 91 | 8.0 | 3.0 | .020 | 102 | 6.2 | 1.2 | .342 |
| Number of previous MDEs | ||||||||||
| ≤2 | 100 | 6.0 | 108 | 8.6 | 2.7 | .011 | 114 | 5.9 | −0.1 | .941 |
| >2 | 66 | 4.1 | 67 | 7.4 | 3.3 | .020 | 73 | 4.4 | 0.3 | .823 |
| Duration of current MDE | ||||||||||
| ≤22 weeks | 98 | 5.9 | 89 | 9.6 | 3.7 | .003 | 89 | 5.8 | −0.1 | .952 |
| >22 weeks | 68 | 3.6 | 86 | 6.0 | 2.4 | .031 | 98 | 4.7 | 1.1 | .300 |
Abbreviations: ∆, difference from placebo in CFB; ANCOVA, analysis of covariance; CFB, (least squares mean) change from baseline; CGI-S, Clinical Global Impression-Severity; FAS, full analysis set; MADRS, Montgomery-Åsberg Depression Rating Scale; MDE, major depressive episode; OC, observed case; UPSA, University of California San Diego Performance-based Skills Assessment.
All P values are vs placebo. Bold indicates significant P value.
Discussion
The findings from these posthoc analyses demonstrate vortioxetine’s effects on functional capacity in patient subgroups categorized by demographic and clinical characteristics. Overall, the change from baseline to week 8 in UPSA composite score was greater for vortioxetine than placebo for most demographic and clinical patient subgroups.
Emerging evidence from several clinical studies has revealed a potential effect of vortioxetine treatment on cognition, functioning, and symptoms of depression. In a short-term switch study conducted in MDD patients with inadequate response to selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors (REVIVE, NCT01488071), outcome results showed improvements in overall functional capacity and productivity (based on Sheehan Disability Score and the Work Limitations Questionnaire) with vortioxetine compared with agomelatine, a potent melatonin agonist and 5-HT (2C) receptor antagonist (Papakostas G, Nielsen R, Dragheim M, Tonnoir B, unpublished data). Further, a recently published exploratory analysis of the FOCUS clinical study (NCT01422213) reported a treatment benefit with vortioxetine in working adult patients with MDD on depression (MADRS) and cognitive functioning (DSST, Trail Making Test A/B) (McIntyre et al., 2014, 2017).
In the current study, statistically significant improvements were observed with vortioxetine vs placebo in patients who were employed; however, the improvements with vortioxetine observed in the nonworking subgroup were observed only at the level of a statistical “trend” (i.e., 0.05 ≤ P < .10). Across education levels, the effect size vs placebo was comparable, although limited to those with a high school or greater level of education. It may be that the changes from baseline are smaller on the UPSA scores in patients with less education; however, the small sample size of this subgroup had substantially less statistical power than the comparisons in the other subgroups. Because the UPSA was included as a predefined endpoint in the primary study, this exploratory analysis was not powered to directly compare and detect statistical differences between treatment cohorts (e.g., vortioxetine and duloxetine) or patient subgroups.
While this study was not powered to investigate potential dose-dependent effects, our current analysis raises the question of whether more pronounced changes from baseline in UPSA composite score would be observed at the higher vortioxetine dose (e.g., 20 mg/d) and whether this effect would differ between patient subgroups. Published clinical studies demonstrating vortioxetine’s benefits on improving depressive symptoms have not evaluated a potential dose-response relationship at the approved 5- to 20-mg dose range; however, the observed linear and dose-proportional PK profile of vortioxetine, together with the reported dose escalations from 10 to 20 mg to achieve therapeutic benefit in clinical studies, suggest the potential for dose-dependent effects on psychiatry outcome measures (D’Agostino et al., 2015; Thase et al., 2016). Further studies will be required to directly assess whether between vortioxetine dose level directly correlates with improvements in functional capacity.
In the CONNECT study, functional capacity was evaluated using 2 different versions of the UPSA (UPSA-B and UPSA-VIM) to accommodate the regional diversity of the study population (Mahableshwarkar et al., 2015). The UPSA-B has been shown to be highly correlated with the UPSA-VIM (r=0.91) (Mausbach et al., 2007), therefore enabling the two to be analyzed as a composite score. Whereas it remains unclear whether there are subtle differences in the ability of these forms to detect changes in these patients, findings from a recent posthoc analysis of CONNECT study data suggest that these differences may not have a significant impact on the outcome (Merikle et al., 2017). In that study, a posthoc scoring adjustment was made to the UPSA-VIM Financial and Communication Skills domains to be consistent with the UPSA-B scoring, yielding the UPSA-B-Aligned score (Merikle et al., 2017). The UPSA-B-Aligned demonstrated similar distributional characteristics to the UPSA-B and UPSA-VIM at baseline and good psychometric properties across the outcomes assessed (Merikle et al., 2017).
The mechanism by which vortioxetine positively influences functional capacity is not completely understood. Effects on cognitive functioning are thought to arise from the enhancement of cholinergic and histaminergic neurotransmission as well as modulation of monoaminergic and glutamatergic neurotransmission (Sanchez et al., 2015). The baseline scores for functional capacity (UPSA) and cognitive functioning (DSST) appear to be moderately related (r=0.36, P<.001) and perhaps reflect common underlying mechanisms (Harvey et al., 2017). Data from long-term studies designed to evaluate treatment effects on functional capacity and cognitive functioning may provide insight into common underlying mechanisms.
In conclusion, this analysis of UPSA data from the CONNECT study provides further evidence to support a consistent effect for vortioxetine on functional capacity in patients with MDD and self-reported cognitive symptoms, even when analyzed across most baseline demographic and clinical patient subgroups. While the interpretation of these findings is limited by the design of the primary clinical study and the statistical power to detect between-cohort differences, this analysis may lay the groundwork for future dedicated prospective studies evaluating vortioxetine’s effects on functional capacity in specific patient subpopulations.
Funding
Funding This work was supported by Takeda Pharmaceutical Company, Ltd., and H. Lundbeck A/S.
Statement of Interest
Dr. Keefe currently or in the past 3 years has received investigator-initiated research funding support from Brain Plasticity, Inc., Department of Veteran’s Affairs, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, PsychoGenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past 3 years has received honoraria from or has served as a consultant or advisory board member for AbbVie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biogen Idec, BioMarin, Boehringer Ingelheim, Eli Lilly, EnVivo, GW Pharmaceuticals, H. Lundbeck, Helicon, Merck, Minerva Neurosciences, Inc., Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, and Targacept. Dr. Keefe receives royalties from the BAC testing batteries and the MATRICS Battery (BACS Symbol Coding). He is also a shareholder in NeuroCog Trials, Inc., and SenGenix. Dr. Nomikos and Dr. Jacobson were employees of Takeda Pharmaceuticals at the time this study was undertaken. Dr. Jacobson holds stock in Pfizer and UnitedHealth Care. Dr. Zhong is a current employee of Takeda Pharmaceuticals. Dr. Christensen is an employee of H. Lundbeck A/S.
Acknowledgments
Medical writing assistance was provided by Gina M. DeStefano, PhD, and Martina Schwarzkopf, PhD, from inVentiv Medical Communications. The authors acknowledge Elizabeth Merikle, PhD, a former employee of Takeda Pharmaceuticals U.S.A., Inc., for her contributions to this study and early work on this manuscript.
References
- Allen DN, Bello DT, Thaler NS(2015)Neurocognitive predictors of performance-based functional capacity in bipolar disorder. J Neuropsychol 9:159–171. [DOI] [PubMed] [Google Scholar]
- Bortolato B, Carvalho AF, McIntyre RS(2014)Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNS Neurol Disord Drug Targets 13:1804–1818. [DOI] [PubMed] [Google Scholar]
- D’Agostino A, English CD, Rey JA(2015)Vortioxetine (brintellix): a new serotonergic antidepressant. P T 40:36–40. [PMC free article] [PubMed] [Google Scholar]
- Fava M, Lophaven S, Olsen CK(2014)Effects of vortioxetine on cognitive symptoms of major depressive disorder (MDD) [abstract P-04-026]. Int J Neuropsychopharmacol 17:61. [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA(2013)Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. Plos Med 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Schooler NR, Kern RS, Frese FJ, Granberry W, Harvey PD, Karson CN, Peters N, Stewart M, Seidman LJ, Sonnenberg J, Stone WS, Walling D, Stover E, Marder SR(2011)Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry 168:400–407. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Jacobson W, Zhong W, Nomikos GG, Cronquist Christensen M, Kurre Olsen C, Merikle E(2017)Determination of a clinically important difference and definition of a responder threshold for the UCSD performance-based skills assessment (UPSA) in patients with major depressive disorder. J Affect Disord 213:105–111. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF(2005)Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 62:1097–1106. [DOI] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK(2012)A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27:215–223. [DOI] [PubMed] [Google Scholar]
- Kaye JL, Dunlop BW, Iosifescu DV, Mathew SJ, Kelley ME, Harvey PD(2014)Cognition, functional capacity, and self-reported disability in women with posttraumatic stress disorder: examining the convergence of performance-based measures and self-reports. J Psychiatr Res 57:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Filteau MJ, Milev R(2011)Clinical effectiveness: the importance of psychosocial functioning outcomes. J Affect Disord 132:S9–S13. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS(2015)A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 40:2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL(2007)Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 33:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Pulver AE, Depp CA, Wolyniec PS, Thornquist MH, Luke JR, McGrath JA, Bowie CR, Patterson TL(2010)Relationship of the brief UCSD performance-based skills assessment (UPSA-B) to multiple indicators of functioning in people with schizophrenia and bipolar disorder. Bipolar Disord 12:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK(2014)A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 17:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Florea I, Tonnoir B, Loft H, Lam RW, Christensen MC(2017)Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry 78:115–121. [DOI] [PubMed] [Google Scholar]
- Merikle E, Zhong W, Olsen CK, Perez V, Jacobson W(2017)UCSD performance-based skills assessment (UPSA): psychometric evaluation of the communication and financial skill domains. In: American Society of Clinical Psychopharmacology (ASCP) Annual meeting Miami, FL. [Google Scholar]
- Mørk A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y, Gulinello M, Sanchez C(2013)Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav 105:41–50. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV(2001)UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 27:235–245. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C(2014)Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr 19:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund S, Ising M, Lucae S, Zihl J(2009)Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med 39:603–614. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F(2015)Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. [DOI] [PubMed] [Google Scholar]
- Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E(2016)A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol 26:979–993. [DOI] [PubMed] [Google Scholar]
- Vos T, Barber R, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson FJ, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin V, Ferrari AJ(2015)Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]