Abstract
Background
We aimed to investigate the state of cardiovascular risk/protection factors in early psychosis patients.
Methods
A total 119 subjects were recruited during the first year after their first episode of psychosis. Eighty-five of these subjects were followed during the next 6 months. Cardiovascular risk/protection factors were measured in plasma and co-variated by sociodemographic/clinical characteristics. Multiple linear regression models detected the change of each biological marker from baseline to follow-up in relation to clinical scales, antipsychotic medication, and pro-/antiinflammatory mediators.
Results
Glycosylated hemoglobin is a state biomarker in first episode of psychosis follow-up patients and inversely correlated to the Global Assessment of Functioning scale. We found opposite alterations in the levels of VCAM-1 and E-selectin in first episode of psychosis baseline conditions compared with control that were absent in the first episode of psychosis follow-up group. Adiponectin levels decreased in a continuum in both pathological time points studied. E-Selectin plasma levels were inversely related to total antipsychotic equivalents and adiponectin levels inversely co-related to the Global Assessment of Functioning scale. Finally, adiponectin levels were directly related to antiinflammatory nuclear receptor PPARγ expression in first episode of psychosis baseline conditions and to proinflammatory nuclear factor nuclear factor κB activity in follow-up conditions, respectively.
Conclusions
Our results support the need for integrating cardiovascular healthcare very early after the first episode of psychosis.
Keywords: cardiovascular diseases, first episode, risk factors, neuroimmunology, antipsychotics
Significance Statement
The importance of early detection and intervention in psychosis has renewed interest in subtle psychopathology beyond positive and negative symptoms but also in the search of biological markers of the disease. In this vein, the determination of some initial changes in cardiovascular markers, including adhesion molecules (VCAM-1 and E-Selectin) and adiponectin, have interesting potential as biological markers and potential risk/protective factors. In this study, we have found evidence of subtle but concomitant alterations of classical cardiovascular and metabolic risk factors in a cohort of patients at the first onset of positive psychotic symptoms and 6 months after. In addition, alterations in adiponectin plasma levels could be a good clinical index, reflecting the grade of severity of the symptomatology, and could be affected by the balance between pro-/antiinflammatory mediators. Our results support the need of integrating CV health care very early in the first episode of psychosis.
Introduction
Increasing evidence supports the potential benefits of early intervention to enable disease prevention and to improve prognosis in psychiatric disorders, this being particularly important in schizophrenia (SZ) (Perala et al., 2007; van Os and Kapur, 2009). To consolidate this intervention, a deeper scientific effort is needed to properly identify and characterize the early phase of the disease (Cheng and Schepp, 2016). The First Episode of Psychosis (FEP) is classically considered the onset of the disease or, at least, the moment of the first presentation of psychotic symptoms, typically during late adolescent or early adult years. At this initial stage, the number of confounding variables produced by chronicity and medication is lower and consequently, the search for risk/protective biomarkers directly related to the etiopathogenesis of the disease is favored (Fond et al., 2015).
Chronic disease-induced lifestyle and the long-term use of antipsychotic medication are factors directly related to the appearance of comorbid metabolic disorders and cardiovascular (CV) alterations in the symptomatology of SZ, which are considered one of the main causes of premature death (Ringen et al., 2014). Specifically, chronic use of antipsychotics can induce metabolic alterations (weight gain, obesity, diabetes, hyperglycemia, dyslipidemia, and metabolic syndrome) directly related to an increased risk of suffering CV pathologies (Feinstein, 2002; Casey et al., 2004). However, even at the early stage of illness around FEP, metabolic abnormalities related to the first year of antipsychotic medication have been reported (Perez-Iglesias et al., 2014) as well as increased CV risk in patients with first-episode SZ spectrum disorders (Correll et al., 2014). In a more recent prospective and naturalistic study (Bioque et al., 2017), antipsychotic polypharmacy (and other factors) have been related to the extremely high risk for patients at early phases of SZ and other psychotic disorders of developing CV comorbidity, as well as the rapid worsening of the metabolic profile during the first 2 years.
The effects of antipsychotic drugs seem to be more pronounced at the onset of treatment (as soon as 8–12 weeks after initiation) in young antipsychotic-naive patients after FEP, including body weight gain and body mass index (BMI) increase (Pramyothin and Khaodhiar, 2010). However, there is growing evidence regarding the existence of subtle metabolic/CV alterations in drug-naïve psychotic populations or FEP subjects exposed to antipsychotics for a short, known period (2–4 weeks), suggesting the role of alternative susceptibility factors and premature etiopathogenic mechanisms not exclusively dependent on unhealthy lifestyle or antipsychotic medication. Thus, some authors have reported that drug-naïve, FEP patients are more insulin resistant (as assessed by the homeostatic model assessment index) compared with healthy controls (Petrikis et al., 2015). Lipid metabolism and abnormal QTc interval prolongation could also be affected in drug-naïve FEP patients (Zhai et al., 2017a, 2017b). The existence of concomitant individual risk factors in some FEP subjects such as subtle changes in hypertension, hypertriglyceridemia, abdominal obesity, and hyperglycemia without higher baseline metabolic syndrome prevalence compared with the general population of similar age needs to be further corroborated (Fleischhacker et al., 2013).
However, some discrepancy exists, and some studies did not find increased metabolic syndrome prevalence in naïve patients with a first episode of nonaffective psychosis compared with matched normal population (Garcia-Rizo et al., 2016). Other authors have found similar results reporting no signs of insulin resistance and dyslipidemia in drug-naïve FEP patients (Sengupta et al., 2008; Chen et al., 2013). Some meta-analysis concluded that there is no difference in CV risk assessed by weight gain or classical metabolic indices between individuals with an untreated FEP and matched controls (Foley and Morley, 2011).
These discrepant results suggest the need to search for novel metabolic alterations at a molecular level for the identification and early intervention of metabolic and CV risk in drug-naïve psychosis patients or FEP subjects exposed to antipsychotics for a short, known period of time. The search for systemic biomarkers for the early identification of individuals likely to suffer metabolic/CV alterations or to evaluate their future response to antipsychotics is continuously growing and crosslinks with other disciplines, as is the study of innate immunity/inflammation.
Inflammation is receiving special attention as an important component in the physiopathology of metabolic and CV disorders. Certain inflammatory mediators have been considered as potential biomarkers for these pathologies (Fortmann et al., 2004). In fact, there are consolidated (i.e., high-sensitivity C-reactive protein levels, cytokines) and emerging inflammatory markers for CV risk (Lubrano and Balzan, 2015) that need to be further characterized, considering the evidence for elevated systemic inflammation in patients with FEP and SZ (Garcia-Bueno et al., 2014a, 2014b; Leza et al., 2015).
Candidates to survey in FEP are cell adhesion molecules, including VCAM-1 and the selectins (P, E, and L), inflammatory markers implicated in the etiology and physiopathology of atherosclerosis, and therefore metabolic syndrome and CV disease (Brevetti et al., 2006). Recently abnormal VCAM-1 and selectin levels have been reported in SZ, being suggested as biomarkers for endothelial dysfunction (Nguyen et al., 2017). The capacity of atypical antipsychotics such as risperidone to upregulate V-CAM and selectin levels contributing to worsening endothelial function in diabetic rats has been also reported (Aboul-Fotouh and Elgayar, 2013). Another relevant mediator is the adipocytokine adiponectin, a master regulator of glucose metabolism and fatty acid oxidation and currently considered as a link between the inflammatory response and lipid metabolism. Reduced levels of adiponectin have been related to metabolic disorders such as obesity, insulin resistance, and type 2 diabetes (Shibata et al., 2017). Furthermore, several authors have reported decreased adiponectin levels also in mental disorders (Wedrychowicz et al., 2014).
Based on this background, we aimed to investigate the state of the inflammatory-related mediators VCAM-1, E-selectin, and adiponectin, considered to be putative CV risk/protective risk factors, in plasma samples of FEP patients from the Flamm-PEPs study. This is a multicenter, longitudinal, naturalistic, follow-up study designed to evaluate systemic inflammatory alterations inside the Spanish national network for mental health research (Garcia-Bueno et al., 2014a, 2014b). In this study, we applied previously validated robust multiple linear regression models to further analyze the change in every biological marker in relation to clinical characteristics classically related to increased metabolic and CV risk, confounding factors as diverse antipsychotic medication, and pro-/antiinflammatory mediators previously described in these same control, baseline, and 6-month follow-up FEP patients.
Materials and Methods
Subjects
We recruited a total of 119 FEP subjects during the first year after their FEP according to the DSM-IV criteria (American Psychiatric Association, 1994) in 5 tertiary hospitals in Spain as well as 108 gender-, ethnicity-, and age-matched controls between September 2010 and June 2011. From the initial sample, 85 FEP subjects were followed for the next 6 months (maximum 12 months after inclusion). In adults, the diagnosis was established by expert clinicians using the semistructured diagnostic interview designed to assess current and past psychopathology and personality disorders, according to DSM-IV criteria (SCID-I and II). For subjects <18 years old, diagnosis was made using the Spanish translation of the Kiddie-Schedule for Affective Disorders and SZ, Present and Lifetime Version (Ulloa et al., 2006). The duration of untreated psychosis (DUP) was defined as the number of days elapsed between the onset of positive psychotic symptoms (the first week with the PANSS items P1, P3, P5, P6, or G9 score ≥4) and the first appropriate treatment for psychosis. Being a naturalistic study, there were no specific guidelines for treatments, so patients received antipsychotic treatment based on the clinician’s choice. Dosing, co-medications, or treatment changes were based on clinical necessity. Following the inclusion/exclusion criteria, treatment with antipsychotics did not exceed 12 months at study entry (Bernardo et al., 2017).
Multiple sources, including medical records and interviews with relatives, were used to ascertain the length of this period. The initial number of recruited subjects, the final number of those to fulfill both clinical and the whole analytical study, and site recruitment details are shown in the supplementary information. This cohort was considered as the FEP baseline group. FEP subjects were followed up for 6 months (maximum 12 months after inclusion), and this cohort was considered as the FEP follow-up group.
Baseline and follow-up demographic details of patients and controls involved in the study are detailed in Table 1. In brief, mean age was 24.21±6.08 years, with a greater percentage of males (70.6%). To ensure diagnosis stability, clinical evaluations were repeated after 6 months of the patient’s inclusion. Tto not exclude early-onset psychotic patients, there was a broad age of inclusion allowed. The inclusion criteria for patients were: (1) age between 7 and 35 years at the time of first evaluation; (2) presence of psychotic symptoms of <12 months’ duration; (3) ability to speak Spanish correctly; and (4) signed the informed consent. The exclusion criteria for patients were: (1) mental retardation per the DSM-IV criteria, including not only an IQ <70 but also impaired functioning; (2) history of head trauma with loss of consciousness; and (3) organic disease with mental repercussions.
Table 1.
Demographic and Clinical Characteristics
| Patients Baseline (b) | Patients Follow- up (f) | p value (b-f) | Controls ( c) | P value (b-c) | P value (f-c) | |
|---|---|---|---|---|---|---|
| N | 85 | 85 | 106 | |||
| Age-years | 24,21(6,028) | 25,21 (6,028) | 25,43(6,428) | 0,21 | ||
| Sex- nº(%) | ||||||
| Male | 60(70,6) | - | 70(66,0) | 0,503 | ||
| Female | 25(29,4) | - | 36(34,0) | |||
| Age of psychosis onset | 24,37(5,93) | - | ||||
| Socioeconomic Status | ||||||
| High | 16(18,8) | - | 14(13,2) | 0,01 | ||
| Medium-High | 7(8,2) | - | 17(16,0) | |||
| Medium | 30(35,3)* | - | 54(50,9) | |||
| Medium-Low | 24(28,2) | - | 19(17,9) | |||
| Low | 8(9,4)* | - | 2(1,9) | |||
| Ethnic group | ||||||
| Caucasian | 79(92,9) | - | 96(90,6) | 0,344 | ||
| Gipsy | 1(1,2) | - | 0(0,0) | |||
| Maghrebian | 1(1,2) | - | 2(1,9) | |||
| Asian | 1(1,2) | - | 0(0,0) | |||
| Caribbean | 1(1,2) | - | 0(0,0) | |||
| Hispanic | 2(2,4) | - | 6(5,7) | |||
| Others | 0 (0,0) | - | 2(1,9) | |||
| Diagnosis nº(%) | ||||||
| Affective Psychosis* | 17(20) | 17(20) | 0,026 | - | ||
| Non-affective Psychosis | 68(80) | 62(72,9) | - | |||
| Drugs Psychosis | 0(0,0) | 0(0,0) | - | |||
| No psychosis | 0(0,0) | 6(7,1) | - | |||
| Psychopathology score | ||||||
| PANSS POSITIVE | 10,67(5,379) | 8,01(5,032) | <0,001 | - | ||
| PANSS NEGATIVE | 14,58(6,27) | 11,76(8,149) | 0,001 | - | ||
| PANSS GENERAL | 26,47(9,75) | 20,524(11,9) | <0,001 | - | ||
| PANSS TOTAL | 51,72(19,23) | 40,29(23,6) | <0,001 | - | ||
| YOUNG | 1,45(3,25) | 1,39(3,5) | 0,711 | - | ||
| Montgomery-Asberg | 6,51(6,52) | 5,75(6,71) | 0,096 | - | ||
| Global functioning score (GAF) | 68,60(13,88) | 72,08(17,22) | 0,005 | - | ||
| DUP | 68,58(77,29) | - | ||||
| Antipsycohotic medications - n(%) | ||||||
| None | 17(20,0) | 21(25,9) | - | |||
| Risperidone | 30(35,3) | 21(25,9) | - | |||
| Aripiprazole | 9(10,6) | 15(18,5) | - | |||
| Olanzapine | 9(10,6) | 6(7,4) | - | |||
| Quetiapine | 6(7,1) | 5(6,2) | - | |||
| Clozapine | 5(5,9) | 6(7,4) | - | |||
| Ziprasidone | 2(2,4) | 2(2,5) | - | |||
| Paliperidone | 7(8,2) | 5(6,2) | - | |||
| DDD | 282,19(253,04) | 298,06(303,16) | 0,903 | - | ||
| Lithium n(%) | 8(9,4) | 7(8,2) | 0,824 | - | ||
| Body mass index | 24,83(3,92)* | 24,65(5,73) | 0,06 | 23,14(3,16) | 0,002 | 0,003 |
| Tobacco use per month, n cigarettes | 238,9(259,08)* | 241,98(254,1) | 0,881 | 45,38(119,3) | <0,001 | <0,001 |
| Cotinine, ng/ml | 86,73(84,46) | 97,28(84,50) | 0,192 | 26,28(49,31) | <0,001 | <0,001 |
| Cotinine, ng/ml | ||||||
| minor PC n(%) | 31(39,7) | 29(34,9) | 0,529 | 60(70,6) | <0,001 | <0,001 |
| major PC n(%) | 47(60,3) | 54(65,1) | 25(29,4) | |||
| CANNABIS use n(%) | 18(21,2)* | 4(5,1) | 0,003 | 14(15,9) | 0,372 | 0,325 |
| CANNABIS use per month, n cigarettes | 4,71(19,36) | 1,09(6,37) | 0,092 | 1,15(6,36) | 0,657 | 0,345 |
DUP: duration of untreated psychosis.
DDD: Defined daily dose of chlorpromazine equivalents (mg).
* Affective psycho.sis includes DSM-IV diagnosis of unipolar depression or bipolar disorder with psychotic features and schizoaffective disorder.
Healthy controls were selected from the same geographic areas. The inclusion criteria for controls were: (1) same gender as patients; (2) similar age (±10%) as patients; (3) similar socioeconomic status as patients, measured by the Hollingshead-Redlich scale (±1 level); (4) no past or present psychiatric disorder per DSM-IV criteria; (5) ability to speak Spanish correctly; and (6) signed the informed consent. The exclusion criteria for controls were: (1) mental retardation according to DSM-IV criteria (American Psychiatric Association (Washington), 1994) including not only an IQ <70 but also impaired functioning; (2) history of head trauma with loss of consciousness; (3) organic disease with mental repercussions; and (4) history of psychotic disorder among first-degree relatives.
Clinical assessment of patients and controls included a complete medical history and physical examination, laboratory tests (thyroid function, liver function, renal function, electrolyte levels, complete blood cell count, and urinalysis), and electrocardiogram. Anthropometric measures were assessed: weight, height, and BMI(weight in kg/height2). A complete description of the clinical protocol has been published elsewhere (Bioque et al., 2017). The exclusion criteria were ongoing infections, fever, allergies, or the presence of other serious medical conditions (autoimmune, cardiac, pulmonary, endocrine, chronic infectious diseases, neoplasms). Having designed a real-life patient, naturalistic study, substance use was not an exclusion criterion. Neither the FEP patients nor the healthy control subjects received immunosuppressive drugs or vaccinations for at least 6 months prior to inclusion in the study or antiinflammatory analgesics the 2 days prior to the blood sample.
The study was approved by the ethics committees of the 5 participant hospitals. The subjects participated after receiving a full explanation of the study and providing written, informed consent in accordance with the Declaration of Helsinki II.
Specimen Collection and Preparation
Venous blood samples (10 mL) were collected by nursing personnel in polypropylene EDTA-containing tubes in the morning (between 8:00 and 10:00 am) after fasting overnight. All the sample collection and preparation protocols were approved by the technical committee of the Flamm-PEPs study (available on www.cibersam.es). The fresh blood samples were maintained at 4ºC until preparation after approximately 1 hour.
Blood tubes were centrifuged (641xg for 10 minutes, 4ºC). The resultant plasma samples were carefully collected and stored at -80ºC until further action was required.
Biochemical Determinations in Plasma
VCAM-1, E-Selectin, and adiponectin levels were measured by enzyme-linked immunosorbent assay following the manufacturer’s instructions. Plasma samples were diluted 1:200 for VCAM-1 and 1:100 for E-Selectin determinations with the assay diluent included in the commercial kits (RayBio). The sensitivity of the assay for VCAM-1 was 0.3 ng/mL and 30 pg/mL for E-Selectin; intra- and inter-assay CVs were <10% and <12%, respectively, in both kits. Plasma samples were diluted 1:5151 for determinations of total and high-molecular adiponectin fraction levels (Alpco). The detection limit was 0.019 ng/mL; intra- and inter-assay CVs were 5.4% and 5.0%, respectively. The absorbance measurements were determinate in the multi-mode plate reader Synergy 2 (BioTek).
Metabolic and CV risk parameter data of the subjects included in our study were already published as a part of the PEPs Project, which recruited 335 FEP subjects and 253 matched healthy controls (Bioque et al., 2017). Briefly, in all the participating sites, blood glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein cholesterol, and triglycerides were directly analyzed by enzymatic procedures with an Automatic Chemical Analyzer. Glycated hemoglobin was analyzed by high-performance liquid chromatography. The reference values at each site were recorded in a common database called GIDSAM, where individual values were homogenized and included (Bernardo et al., 2013).
Proinflammatory/antiinflammatory parameters data of the subjects included in our study were previously published (Garcia-Bueno et al., 2014a, 2014b).
Statistical Analysis
Differences between demographic, clinical, and biological markers for patients and controls were assessed using chi-square, t test, nonparametric Mann-Whitney U or Wilcoxon tests according to the distribution and scales of the variables. To calculate the association between the level of biological markers and FEP, multiple linear regression models were used in which we controlled for potential confounders (age, gender, CV history, cannabis, and tobacco use per month). Multiple linear regression analysis was used to analyze the change in each biological marker depending on the change in demographic (gender, age), clinical variables (CV history, lithium, DUP, GAF), antipsychotic medication (defined daily dose [DDD] and types), and cannabis and tobacco (cotinine). Correlations were analyzed to evaluate the association between biological markers and pro-/antiinflammatory parameters.
Results
Demographic and Clinical Features
The demographic and clinical characteristics of the FEP patients and the healthy control group are presented in Table 1 and can be also found in a previous study evaluating the status of systemic inflammation in the same groups of control and FEP subjects (Garcia-Bueno et al., 2014a, 2014b). Both baseline and follow-up FEP patients differed in BMI compared with matched controls (BMI, respectively; 24.83±3.92; 24.65±5.73 vs 23.14±3.16, P=.002; P=.003). Another important characteristic of our cohorts is the different type of antipsychotic medication used. In baseline FEP conditions, only 9 patients (10.6%) were treated with olanzapine and 6 (7.1%) with quetiapine. In the follow-up group, 6 (7.4%) received olanzapine and 5 (6.2%) quetiapine. PANSS and GAF clinical scales showed significantly less severe symptomatology in the follow-up group compared with baseline FEP condition.
Metabolic and CV Alterations in FEP Baseline and Follow-Up Groups
Firstly, we explored the general state of typical metabolic and CV parameters in the 3 groups of subjects studied. Bivariate analysis found a significantly higher basal triglyceride levels and abdominal perimeter in the FEP baseline group compared with controls, while HDL cholesterol values appeared lower in the pathological baseline group (Table 2). In the follow-up group, all these alterations remained present, and a significant increase in glycosylated hemoglobin was also found (Table 2). Baseline and follow-up groups only differed in glycosylated hemoglobin levels that were lower in baseline group (Table 2).
Table 2.
Classical cardiovacular and metabolic risk biomarkers in Control and FEP’s baseline and follow-up Patients.
| Baseline | Follow-up | Case Baseline - Case Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Patients Follow-up | Patients Baseline | Patients Follow-up | ||||||||||||
| Mean | SD | Mean | SD | Z | Sig. | Mean | SD | Z | Sig. | Mean | SD | Mean | SD | Z | Sig. | |
| BASAL_Glucose |
84,49
(83) |
7,97 |
84,49
(96) |
11,10 | -0,27 | 0,787** |
88,26
(70) |
15,51 | -0,89 | 0,373& |
84,40
(82) |
11,35 |
88,26
(70) |
15,51 | -1,68 | 0,093& |
| BASAL_glycosylated hemoglobin |
5,09
(79) |
0,32 |
5,08
(90) |
0,26 | -0,28 | 0,782** |
5,23
(71) |
0,32 | -2,76 | 0,006 & |
5,08
(78) |
0,26 |
5,23
(71) |
0,32 | -4,13 | <0,001 & |
| BASAL_Triglycerides |
75,81
(80) |
35,20 |
99,24
(95) |
57,10 | -3,37 | 0,001** |
104,21
(73) |
52,59 | -3,96 | <0,001 & |
99,28
(81) |
57,56 |
104,21
(73) |
52,59 | -1,23 | 0,220& |
| BASAL_Cholesterol |
170,73
(82) |
39,38 |
170,09
(96) |
36,55 | -0,36 | 0,722* |
167,26
(73) |
31,18 | -0,60 | 0,547* |
168,60
(82) |
37,35 |
167,26
(73) |
31,18 | -0,36 | 0,724* |
| BASAL_Cholesterol_HDL |
54,79
(82) |
12,49 |
47,09
(95) |
13,69 | -4,36 | <0,001** |
44,78
(73) |
13,36 | -5,02 | <0,001 & |
47,09
(81) |
13,16 |
44,78
(73) |
13,36 | -1,68 | 0,093& |
| BASAL_Cholesterol_LDL |
100,42
(83) |
32,74 |
102,71
(94) |
29,72 | 0,14 | 0,892* |
101,90
(73) |
26,66 | 0,31 | 0,759* |
101,10
(80) |
29,84 |
101,90
(73) |
26,66 | -0,63 | 0,529* |
| BASAL_SAP |
122,24
(82) |
15,53 |
120,13
(94) |
12,91 | -1,09 | 0,277** |
119,38
(72) |
13,01 | -1,15 | 0,252* |
120,68
(80) |
12,63 |
119,38
(72) |
13,01 | 1,10 | 0,277& |
| BASAL_DAP |
71,04
(82) |
8,20 |
71,41
(94) |
9,05 | -0,19 | 0,847** |
71,28
(72) |
9,79 | 0,17 | 0,868* |
71,51
(80) |
9,08 |
71,28
(72) |
9,79 | 0,30 | 0,766* |
| BASAL_ABDOMINAL perimeter |
84,32
(79) |
8,75 |
89,64
(92) |
10,57 | 3,45 | <0,001* |
90,42
(72) |
9,42 | 4,13 | <0,001* |
89,71
(78) |
10,71 |
90,42
(72) |
9,42 | -0,89 | 0,376* |
| VCAM-1 |
329,24
(85) |
194,53 |
278,29
(99) |
161,31 | -2,19 | 0,029** |
307,45
(82) |
212,85 | -1,42 | 0,157** |
283,19
(68) |
159,85 |
301,69
(81) |
207,89 | -0,25 | 0,801& |
| E-Selectin |
10.949,2
(84) |
7.580,2 |
13.723,3
(94) |
8.598,4 | -2,47 | 0,014** |
10.270,5
(82) |
6.857,06 | -0,31 | 0,759** |
13.750,0
(65) |
9.040,8 |
10.128,7
(81) |
6.777,7 | -3,76 | 0,000 & |
| Adiponectin HMW |
3,55
(76) |
2,40 |
3,13
(84) |
1,85 |
-1,20 | 0,229** |
2,68
(82) |
1,66 | -2,86 | 0,004** |
2,92
(68) |
1,67 |
2,70
(81) |
1,6623 | -2,17 | 0,030 & |
| Adiponectin total |
6,09
(37) |
2,51 |
5,78
(44) |
2,46 | -0,82 | 0,409** |
4,29
(47) |
1,57 | -4,13 | <0,001** |
5,66
(35) |
2,51 |
4,34
(81) |
1,54 | -4,35 | 0,000 & |
(N)= number of subjects for each determination
* parametric t-student test.
** non-parametric U-Mann Whitney test.
& non-parametric Wilcoxon test.
Novel CV Risk/Protective Factors in FEP Baseline and Follow-Up Groups
Next, we aimed to evaluate whether there were alterations in the plasma levels of the putative metabolic and CV risk (VCAM-1, E-Selectin) and protective (adiponectin, both in its high molecular weight [HMW] and total) markers between control, baseline, and follow-up groups.
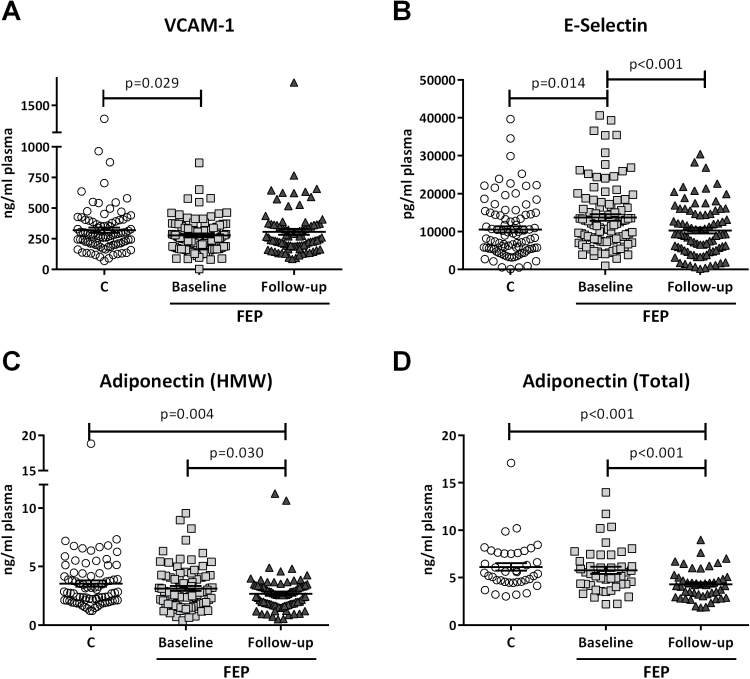
Bivariate analysis showed that VCAM-1 levels were significantly lower in the baseline group compared with the control group (Table 2; Figure 1A). This effect was partly due to an absence in the follow-up group, returning to values near control levels (Table 2; Figure 1A). In the case of the other adhesion molecule E-selectin, the pattern was the opposite. The baseline group’s E-selectin plasma levels were higher than control subjects (Table 2; Figure 1B). This effect was significantly reversed in the follow-up conditions, returning to control levels (Table 2; Figure 1B).
Figure 1.
Plasma levels of cardiovascular (CV) factors between first episode of psychosis (FEP) and controls. Mean differences (SD) on VCAM-1 (A), E-Selectin (B), adiponectin high (molecular weight [HMW]) (C), and total adiponectin (D) between controls (C). Baseline and follow-up FEP (for VCAM-1: controls n=89, baseline n=99, follow-up=82; for E-selectin and adiponectin [HMW]: controls n=80, baseline n=84, follow-up=82; for total adiponectin: controls n=40, baseline n=44, follow-up=47). Two-tailed nonparametric Mann-Whitney U test was used.
HMW and total adiponectin plasma levels were markedly lower in the follow-up group compared with both control and baseline groups, with no differences found between them (Table 2; Figure 1C-D).
In summary, we found opposite alterations to the levels of the CV risk factors VCAM-1 and E-selectin in FEP baseline conditions compared with control. These alterations were no longer present in the FEP follow-up group. In the case of the protective factor adiponectin, its HMW and total plasma levels were lower at both pathological time points studied.
Effects of Demographic and Clinical Features on Altered Metabolic and CV Parameters in FEP Baseline and Follow-Up Groups
Our next goal was to evaluate through ANCOVA the possible influence of selected demographic and clinical characteristics only on the significant alterations observed by bivariate analysis in general metabolic and CV risk factors and novel CV risk/protective parameters in control and FEP subjects.
When comparing control and FEP baseline groups, all the alterations previously found in the bivariate analysis remained significant (BMI, E-Selectin, triglyceride, and HDL cholesterol levels and abdominal perimeter) except for the effect observed in VCAM-1 levels (see supplemental Table 1A). In addition, BMI was directly affected by gender (higher in males) and the existence of familiar CV clinical record (supplemental Table 1A). Triglyceride levels were also directly related to age and cotinine levels (tobacco consumption) in plasma (supplemental Table 1A). Finally, abdominal perimeter was directly related to gender (higher in males), age, and familiar CV clinical record (supplemental Table 1A).
Next, when control and FEP follow-up groups were compared, all the effects remained significant (BMI, HMW and total adiponectin, glycosylated hemoglobin, triglyceride and HDL cholesterol levels, and abdominal perimeter) (supplemental Table 1B). Furthermore, BMI was again affected by gender (higher in males) and also by age (supplemental Table 1B). HMW adiponectin levels were also related to gender and age (supplemental Table 1B). Triglycerides and HDL cholesterol were affected by age and gender, respectively and, finally, abdominal perimeter positively corelated both with gender and age (supplemental Table 1B).
Finally, when FEP baseline and follow-up groups were compared, the analysis made was slightly different, including the effects of very relevant clinical variables such as the GAF score, DDD of antipsychotic medication, and DUP (see supplemental Table 1C). The effects observed on E-selectin levels remained unaffected for all demographic and clinical factors studied (supplemental Table 1C). Adiponectin alterations were affected by gender (HMW type) and age (total), and, finally, glycosylated hemoglobin alterations were directly related to the existence of familiar CV clinical record (supplemental Table 1C).
To further investigative the significant alterations observed between baseline and follow-up FEP groups, we carried out a complementary regression model that analyzed the change of each biological marker (from baseline to the follow-up point) depending on the other selected variables. In this approach, we also checked the putative effects of the use of the specific antipsychotic drugs clozapine and olanzapine, due to their remarkable capacity to produce metabolic/CV alterations from the time of their prescription, as occurred in our conditions.
Using this approach, the difference in total antipsychotic equivalents was inversely related to the difference in E-selectin plasma levels between both time points studied (for each DDD unit increased during this period, E-selectin decreased 7.575 units during follow-up, after controlling for the effect of the increase in the other explanatory variables; see Table 3). In addition, the difference in the GAF score was also inversely related to the difference in both types of adiponectin (for each GAF scale unit increased during this period, HMW and total adiponectin levels decreased 0.057 units and 0.069 units, respectively) (Table 3). The total adiponectin difference between baseline and follow-up was affected by gender (Table 3). The difference in glycosylated hemoglobin levels was also inversely related to the GAF score between both time-frames studied (for each GAF scale unit increased during this period, glycosylated hemoglobin levels decreased 0.011 units) (Table 3). No specific effects of clozapine and olanzapine use on the parameters tested were found (Table 3).
Table 3.
Change of each biological marker (from baseline to the follow-up point) depending on selected demographic and clinical variables. Multiple linear regression analysis. Abbreviations: ß, linear regression coefficient; SE, standard error; CI, Confidence interval; OL, over limit; LL, Lower limit; BMI, body mass index; DDD, defined daily dose of antipsychotic chlorpromazine equivalents; DUBM, number of days elapsed between the onset of the FEP and blood samplings; DUP, duration of untreated psychosis; GAF, Global Assessment of Functioning scale. The bold values in the table represents the values reaching statistical significance (P<.05).
| N=53 | E-SELECTIN | |||||
|---|---|---|---|---|---|---|
| B | SE | t | CI 95% (OL) | CI 95% (LL) | p | |
| Gender (ref Female) | 1111,603 | 1894,739 | ,587 | -2714,900 | 4938,107 | ,561 |
| Age | 306,990 | 162,144 | 1,893 | -20,466 | 634,446 | ,065 |
| Cardiovascular record | -887,788 | 7850,027 | -,113 | -16741,239 | 14965,663 | ,911 |
| Lithium | 439,704 | 3230,983 | ,136 | -6085,399 | 6964,806 | ,892 |
| Antipsychotic DDD | -7,575 | 3,347 | -2,263 | -14,334 | -,816 | ,029 |
| GAF | -51,375 | 79,835 | -0,644 | -212,604 | 109,854 | ,523 |
| Cannabis consume | 36,459 | 52,865 | ,690 | -70,305 | 143,223 | ,494 |
| Cotinin consume | -11,371 | 11,689 | -,973 | -34,978 | 12,236 | ,336 |
| DUBM | 7,477 | 2,708 | 2,761 | 2,008 | 12,945 | ,009 |
| DUP | 11,219 | 12,173 | 0,922 | -13,365 | 35,802 | ,362 |
| Olanzapine+Clozapine | -2649,334 | 2501,065 | -1,059 | -7700,337 | 2401,669 | ,296 |
| ADIPONECTIN HMW | ||||||
| N=56 | B | SE | t | CI 95% (OL) | CI 95% (LL) | p |
| Gender (ref Female) | -,036 | ,618 | -,059 | -1,281 | 1,209 | ,953 |
| Age | -,021 | ,050 | -,425 | -,122 | ,080 | ,673 |
| Cardiovascular record | -0,904 | 1,638 | -,552 | -4,204 | 2,396 | ,584 |
| Lithium | -,113 | 1,027 | -,110 | -2,183 | 1,957 | ,913 |
| Antipsychotic DDD | ,001 | ,001 | 1,018 | -,001 | ,003 | ,314 |
| GAF | -,057 | ,025 | -2,272 | -,108 | -,006 | ,028 |
| Cannabis consume | -,013 | ,016 | -,826 | -,044 | ,018 | ,413 |
| Cotinin consume | ,000 | ,004 | ,112 | -,007 | ,008 | ,911 |
| DUBM | ,000 | ,001 | -,238 | -,002 | ,002 | ,813 |
| DUP | -0,004 | 0,004 | -0,972 | -0,012 | 0,004 | ,336 |
| Olanzapine+Clozapine | 0,428 | 0,777 | 0,551 | -1,138 | 1,994 | ,584 |
| ADIPONECTIN TOTAL | ||||||
| N=24 | B | SE | t | CI 95% (OL) | CI 95% (LL) | p |
| Gender (ref Female) | 2,201 | ,557 | 3,949 | 0,997 | 3,404 | ,002 |
| Age | ,094 | ,048 | 1,967 | -,009 | ,196 | ,071 |
| Cardiovascular record | 1,967 | 1,325 | 1,485 | -0,895 | 4,829 | ,161 |
| Antipsychotic DDD | -,001 | ,002 | -,781 | -,005 | ,002 | ,449 |
| GAF | -,069 | ,025 | -2,800 | -,123 | -,016 | ,015 |
| Cannabis consume | ,021 | ,025 | ,842 | -,033 | ,076 | ,415 |
| Cotinin consume | -,003 | ,006 | -,507 | -,015 | ,009 | ,621 |
| DUBM | ,003 | ,001 | 2,504 | ,000 | ,005 | ,026 |
| DUP | 0,006 | 0,003 | 1,832 | -0,001 | 0,013 | ,090 |
| Olanzapine+Clozapine | 0,288 | 1,410 | 0,205 | -2,757 | 3,333 | ,841 |
| GLYCOSYLATED HEMOGLOBLIN | ||||||
| N=52 | B | SE | t | CI 95% (OL) | CI 95% (LL) | p |
| Gender (ref Female) | -,046 | ,100 | -,465 | -0,248 | 0,155 | ,644 |
| Age | ,003 | ,009 | ,346 | -,015 | ,021 | ,731 |
| Cardiovascular record | 0,072 | 0,286 | ,252 | -0,505 | 0,650 | ,802 |
| Lithium | -,205 | 0,173 | -1,187 | -0,555 | 0,144 | ,242 |
| Antipsychotic DDD | ,000 | ,000 | -1,228 | -,001 | ,000 | ,227 |
| GAF | -,011 | ,004 | -2,883 | -,019 | -,003 | ,006 |
| Cannabis consume | -,003 | ,003 | -1,053 | -,009 | ,003 | ,299 |
| Cotinin consume | ,001 | ,001 | ,793 | -,001 | ,002 | ,432 |
| DUBM | ,000 | ,000 | ,732 | ,000 | ,000 | ,469 |
| DUP | 0,000 | 0,001 | 0,124 | -0,002 | 0,002 | ,902 |
| Olanzapine+Clozapine | -0,105 | 0,124 | -0,849 | -0,356 | 0,145 | ,401 |
Relationship between Pro-/Antiinflammatory Mediators and Altered Metabolic and CV Parameters in FEP Baseline and Follow-Up Groups
The overactivation of the inflammatory response has been related to an increased risk of developing metabolic and CV alterations. We previously reported a systemic pro-/antiinflammatory dysregulation in our FEP baseline and follow-up groups (Garcia-Bueno et al., 2014a, 2014b). Considering this background, we applied the Pearson correlation analysis, trying to evaluate whether this inflammatory imbalance could be somehow affecting the metabolic and CV alterations found in FEP baseline and follow-up groups.
In this way, plasma levels of the proinflammatory mediator prostaglandin E2 directly correlated with E-selectin levels both in control and FEP baseline groups (Table 4). In addition, the activity of the proinflammatory nuclear factor κB (NFκB) in peripheral blood mononuclear cells directly correlated with both adiponectin HMW and total levels only in the FEP follow-up group (Table 4).
Table 4.
Relationship between cardiovascular and metabolic risk and protection factors and systemic pro/anti-inflammatory mediators. Multiple linear regression analysis. The bold values in the table represents the values reaching statistical significance (P<.05).
| Control | NFκB Act | COX2 WB | PGE2 | iNOS WB | TBARS | PPARy Act | PPARy WB | 15d-PGJ2 | IκBα WB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s Coefficient | -0,19 | -0,11 | 0,12 | -0,05 | -0,01 | 0,03 | 0,39 | -0,02 | -0,13 | |
| VCAM-1 | Sig (Bilat) | 0,118 | 0,204 | 0,131 | 0,564 | 0,866 | 0,742 | 0,041 | 0,766 | 0,126 |
| N | 67 | 130 | 150 | 131 | 149 | 124 | 28 | 150 | 131 | |
| Pearson’s Coefficient | -0,13 | -0,04 | 0,25 | -0,07 | 0,12 | -0,06 | -0,43 | -0,11 | 0,00 | |
| E-Selectin | Sig (Bilat) | 0,310 | 0,654 | 0,003 | 0,456 | 0,145 | 0,487 | 0,023 | 0,182 | 0,997 |
| N | 66 | 126 | 146 | 127 | 145 | 120 | 28 | 146 | 127 | |
| Pearson’s Coefficient | -0,15 | -0,07 | -0,09 | -0,04 | 0,04 | 0,11 | 0,20 | -0,06 | -0,05 | |
| Adiponectin HMW | Sig (Bilat) | 0,221 | 0,459 | 0,289 | 0,658 | 0,609 | 0,221 | 0,304 | 0,491 | 0,547 |
| N | 67 | 123 | 141 | 124 | 140 | 117 | 28 | 141 | 124 | |
| Pearson’s Coefficient | -0,38 | -0,11 | -0,02 | -0,14 | 0,11 | 0,11 | 0,13 | -0,08 | 0,05 | |
| Adiponectin total | Sig (Bilat) | 0,074 | 0,407 | 0,869 | 0,282 | 0,379 | 0,435 | 0,729 | 0,495 | 0,691 |
| N | 23 | 59 | 69 | 60 | 68 | 53 | 10 | 69 | 60 | |
| FEP’s Baseline | NFκB Act | COX2 WB | PGE2 | INOS WB | TBARS | PPARy Act | PPARy WB | 15d-PGJ2 | IκBα WB | |
| Pearson’s Coefficient | -0,23 | -0,11 | 0,01 | 0,14 | -0,06 | -0,07 | 0,12 | 0,06 | -0,22 | |
| VCAM-1 | Sig (Bilat) | 0,184 | 0,398 | 0,947 | 0,300 | 0,624 | 0,642 | 0,708 | 0,625 | 0,100 |
| N | 34 | 58 | 67 | 59 | 66 | 51 | 12 | 67 | 59 | |
| Pearson’s Coefficient | -0,21 | -0,06 | 0,27 | -0,07 | 0,05 | 0,17 | -0,64 | -0,15 | 0,01 | |
| E-Selectin | Sig (Bilat) | 0,247 | 0,673 | 0,030 | 0,593 | 0,671 | 0,242 | 0,026 | 0,233 | 0,957 |
| N | 33 | 55 | 64 | 56 | 63 | 48 | 12 | 64 | 56 | |
| Pearson’s Coefficient | -0,26 | -0,20 | -0,15 | 0,01 | 0,21 | 0,35 | 0,26 | -0,11 | -0,07 | |
| Adiponectin HMW | Sig (Bilat) | 0,143 | 0,131 | 0,234 | 0,955 | 0,089 | 0,011 | 0,406 | 0,380 | 0,597 |
| N | 34 | 58 | 67 | 59 | 66 | 51 | 12 | 67 | 59 | |
| Pearson’s Coefficient | -0,35 | -0,21 | -0,17 | -0,10 | 0,23 | 0,61 | 0,65 | -0,24 | -0,01 | |
| Adiponectin total | Sig (Bilat) | 0,197 | 0,284 | 0,343 | 0,593 | 0,205 | 0,003 | 0,351 | 0,175 | 0,959 |
| N | 15 | 29 | 34 | 30 | 33 | 22 | 4 | 34 | 30 | |
| FEP’s Follow-up | NFκB Act | COX2 WB | PGE2 | INOS WB | TBARS | PPARy Act | PPARy WB | 15d-PGJ2 | IκBα WB | |
| Pearson’s Coefficient | -0,01 | -0,07 | 0,06 | 0,08 | 0,03 | 0,16 | -0,24 | -0,10 | -0,15 | |
| VCAM-1 | Sig (Bilat) | 0,907 | 0,518 | 0,602 | 0,500 | 0,767 | 0,226 | 0,435 | 0,413 | 0,233 |
| N | 81 | 80 | 81 | 80 | 81 | 56 | 13 | 73 | 62 | |
| Pearson’s Coefficient | -0,21 | -0,08 | 0,20 | 0,07 | 0,18 | 0,13 | -0,42 | -0,14 | 0,05 | |
| E-Selectin | Sig (Bilat) | 0,063 | 0,459 | 0,072 | 0,564 | 0,101 | 0,328 | 0,152 | 0,242 | 0,690 |
| N | 81 | 80 | 81 | 80 | 81 | 56 | 13 | 73 | 62 | |
| Pearson’s Coefficient | 0,34 | -0,08 | -0,16 | -0,02 | 0,10 | -0,07 | -0,04 | 0,07 | -0,19 | |
| Adiponectin HMW | Sig (Bilat) | 0,002 | 0,493 | 0,161 | 0,875 | 0,363 | 0,603 | 0,896 | 0,579 | 0,140 |
| N | 81 | 80 | 81 | 80 | 81 | 56 | 13 | 73 | 62 | |
| Pearson’s Coefficient | 0,35 | -0,13 | -0,13 | 0,07 | 0,10 | 0,06 | 0,49 | -0,30 | 0,00 | |
| Adiponectin total | Sig (Bilat) | 0,018 | 0,389 | 0,375 | 0,662 | 0,527 | 0,793 | 0,404 | 0,065 | 0,991 |
| N | 46 | 45 | 46 | 45 | 46 | 25 | 5 | 38 | 31 |
The complementary analysis of the antiinflammatory mediators showed that the protein expression of the antiinflammatory nuclear peroxisome proliferator-activated receptor γ (PPARγ) in peripheral blood mononuclear cells negatively correlated with E-selectin levels and positively with VCAM-1 levels in control conditions (Table 4). Furthermore, in FEP baseline conditions, the protein levels PPARγ negatively correlated with E-selectin and PPARγ activity directly correlated with total and HMW adiponectin (Table 4).
Discussion
In this 6-month follow-up study with FEP subjects, we found specific alterations in the levels of classical and novel CV risk/protection biomarkers at the different time points analyzed. The complementary statistical approach made, considering the net difference between the values obtained at both temporal points studied for each parameter as dependent variables, allowed us to find an inverse relationship between the difference in total antipsychotic equivalents and the difference in E-Selectin plasma levels. In addition, the difference in GAF scores was also related to the difference in HMW and total adiponectin.
Finally, we also analyzed the possible relationship between CV risk/protective factor and pro-/antiinflammatory mediators. E-selectin levels were related to the levels of the proinflammatory mediator prostaglandin E2 both in control and FEP baseline groups. NFκB activity directly correlated with both HMW and total adiponectin levels only in the FEP follow-up group. In the case of antiinflammatory mediators, the protein levels PPARγ negatively correlated with E-selectin and PPARγ activity directly correlated with total adiponectin in the FEP baseline group.
Patients with chronic SZ present an increased susceptibility for suffering cardiac complications and even sudden cardiac death than the general population (Jindal et al., 2005); consequently, one of the fields of research is the study of CV risk and protection biomarkers, not only in full-blown SZ but also in earlier clinical manifestations and even in the prodromal phase. It is worth mentioning that the levels of all classical risk metabolic and CV factors studied in our cohort of FEP patients were in the normal range for subjects with a mean age of approximately 25 years, although we found some concomitant significant differences between groups that taken as a whole, could anticipate possible deleterious consequences in the physical condition of the FEP patients included in our study. The use of ratios between triglycerides, HDL and low density lipoprotein cholesterol to identify insulin resistance early in FEP patients of different gender is an interesting approach to explore in future studies (Quispe et al., 2016).
We have described a state in our cohort of FEP baseline subjects characterized by increased triglycerides and abdominal perimeter and decreased HDL cholesterol levels compared with control subjects. These alterations were also found in the follow-up group, and, in addition, FEP follow-up patients showed increased levels of glycosylated hemoglobin compared with both control and FEP basal groups. In this way, glycosylated hemoglobin could be considered a state biomarker, as the other trait biomarkers in our experimental conditions.
Previous studies identified the increased levels of glycosylated hemoglobin in plasma as a biomarker of poor control of blood glucose levels associated with CV alterations in FEP subjects with negative results (Phutane et al., 2011), although other glucose-metabolism related measures suggested, at least in one of the studies, that drug-naïve FEP subjects were more insulin resistant compared with matched controls (Petrikis et al., 2015). A recent meta-analysis corroborated that glycosylated hemoglobin levels were not altered in FEP patients compared with controls; however, other glucose metabolism-related parameters were clearly affected, suggesting an early impairment in this system (Pillinger et al., 2017). In chronic SZ, the increase in plasma glycosylated hemoglobin levels is well described (Balotsev et al., 2017). In addition to these results, the inverse relationship between the GAF score and glycosylated hemoglobin plasma levels found with our regression models supports the idea suggested by some authors that routine testing of glycosylated hemoglobin could be helpful for early detection of diabetes in psychotic inpatients and its relationship to symptomatology (Hinds et al., 2015; Steylen et al., 2015; Naidu et al., 2017).
Regarding the CV risk factors studied, the effects observed on E-selectin are the most relevant, because our multiple linear regression analysis suggested that antipsychotic medication from baseline to follow-up groups could be related to the restored levels of E-selectin observed in FEP follow-up subjects. In previous longitudinal studies, there is some controversy: FEP subjects treated for 24 weeks with antipsychotics showed increased levels of E-selectin compared with the onset of treatment (Graham et al., 2008), but in FEP subjects under antipsychotic medication for 3 months, patients treated with perphenazine, risperidone, and ziprasidone showed decreased levels of E-selectin in plasma compared with the baseline group (Meyer et al., 2009). It is worth mentioning that, in agreement with our report, patients treated with olanzapine or quetiapine did not present decreased levels of E-selectin in the 3-month follow-up, suggesting that each antipsychotic drug could be differentially affecting endothelial dysfunction and inflammation in pathological conditions.
The decreased levels of the CV protective factor adiponectin in the plasma of FEP follow-up patients compared with both control and FEP baseline groups also deserve further discussion. As in the case of E-selectin, some contradictory results have been reported. One study agreed with ours showing decreased levels of adiponectin in plasma samples of FEP subjects under antipsychotic medication for 24 weeks compared with the onset of treatment (Graham et al., 2008). However, high levels in drug-naive FEP patients (Song et al., 2013) or no changes on adiponectin levels after 1 year of treatment with antipsychotics in drug-naive FEP patients (Perez-Iglesias et al., 2008) have also been reported. A recent meta-analysis reported that subjects with SZ may not have lower levels of adiponectin than controls, but the specific treatment with olanzapine decreased adiponectin levels compared with other antipsychotics (Bartoli et al., 2015). However, the results derived from our statistical approach did not support a specific effect of olanzapine or clozapine on the observed reduced levels of adiponectin between FEP baseline and follow-up groups.
To our knowledge, there are no studies evaluating the association between adiponectin levels and GAF score in psychotic patients. Our results are contra-intuitive, taking into account that adiponectin is accepted as a protective mediator. It is worth mentioning that our patients presented improved symptomatology measured by several clinical scales (GAF included) in the follow-up group compared with the baseline group. Thus, our results could suggest a relationship between adiponectin and global functioning, at least in early FEP, but its nature as well as the putative effects of other confounding factors needs to be further corroborated in future studies.
Finally, we took advantage of previous studies evaluating the state of pro-/antiinflammatory mediators in the same cohorts of FEP patients and controls used here (Garcia-Bueno et al., 2014a, 2014b) to evaluate their putative relationship with VCAM-1, E-selectin, and adiponectin levels. We reported a direct correlation between NFκB activity and adiponectin only in the FEP follow-up group. The relationship between both factors is complex and the effects of adiponectin on NFκB depend on the isoform studied (Adya et al., 2015). Interestingly, the isoform HMW (that was specifically measured in our study) activated nuclear factor NFκB-mediated gene expression of the E-selectin promoter in adipocytes in vitro (Tsao et al., 2002), an effect that could be related to the decrease in E-selectin observed in the FEP follow-up group. The activation of NFκB by HMW-adiponectin has been also described in vascular endothelial cells and monocyte/macrophage U937 cells (Haugen and Drevon, 2007; Tomizawa et al., 2008). Indeed, further studies measuring the different adiponectin species are needed to elucidate the regulatory role of adiponectin on proinflammatory pathways, such as the one orchestrated by NFκB.
The correlation with antiinflammatory mediators only appeared with PPARγ and in the control and FEP baseline groups. The increased levels of E-selectin could be related to PPARγ expression downregulation as the inverse nature of their correlation suggests. PPARγ genetic or pharmacological positive modulation inhibited E-selectin expression in human endothelial cells both in vitro and in vivo (Wang et al., 2002; Genovese et al., 2013), although some negative results also exist (Pasceri et al., 2000).
In the FEP baseline group, total and HMW adiponectin were directly related to PPARγ activity. It is worth mentioning that adiponectin levels were not significantly different than in controls but were higher than in the FEP follow-up group. Whether a sustained level of adiponectin in the baseline group is related to PPARγ activity needs to be corroborated in future studies, but it has been recently described that adiponectin is induced by the pharmacological activation of PPARγ with the synthetic ligand rosiglitazone, affecting stress and negative emotion-related behaviors in rats submitted to a depression-like model (Guo et al., 2017). Future studies with larger cohorts are needed to evaluate the interactions between inflammation and metabolic syndrome considered as a whole clinical entity for cardiovascular disturbances in FEP, as they have been already studied in SZ (Leonard et al., 2012) and other neuropsychiatric disorders (Nousen et al., 2013).
The present study presents several limitations: (1) most patients were under antipsychotic treatment for several months before blood extraction for biochemical determinations; (2) as it was a naturalistic study, not a randomized controlled trial, patients could be changing treatments during the follow-up period according to the clinician’s choice; (3) all the participant sites are tertiary care centers linked to the Spanish network of translational research (CIBERSAM), so patient samples and therapeutic strategies may differ from those used in other areas; (4) a previous sample size calculation was not made. This calculation could have improved the design of the study and, in the case of a larger number of samples, we had probably found statistical differences in other variables, such as for example in E-selectin levels; (5) Control subjects were not followed 6 months after baseline as in case of FEP patients, and this limitation should be taken into account; and (6) there are statistically significant differences in BMI index between control and FEP patients. However, from a clinical point of view, all the subjects were in the range of normality following the BMI classical classification and thus, we consider that there are not significant clinical differences that could be decisively affecting the rest of results here reported.
In summary, we report here subtle but concomitant alterations of classical CV and metabolic risk factors in a cohort of FEP patients at the first onset of positive psychotic symptoms and at 6 months. Glycosylated hemoglobin emerged as a potentially relevant state biomarker for the diagnosis due to its relationship to the GAF score. In addition, among novel CV risk and protective factors analyzed, we concluded that alterations in plasma adiponectin levels could be a good clinical index, reflecting the degree of severity of the symptomatology. Our results support the need for integrating CV health care very early after the FEP.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Statement of interest
None.
Supplementary Material
Acknowledgments
Funded by CIBERSAM Intramural Projects 2010 (P02) Flamm-PEPs, Inflammatory alterations in schizophrenia: search of biological markers in first-psychotic episodes (J.C.L.). Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias. The results presented here formulate a supplementary hypothesis added to the initial hypothesis in the wide PEP study (ISCIII 2009–2011) (M.B.).
*FLAMM-PEPs is a multicentric, collaborative, and translational group inside CIBERSAM aimed to study inflammatory pathways in psychosis both as possible biomarkers and as possible new therapeutic targets incorporated in the PEPs study, a research project in first episodes of psychosis
References
- Aboul-Fotouh S, Elgayar N(2013)Atypical antipsychotics such as risperidone, but not paliperidone, worsen vascular endothelial function via upregulation of adhesion molecules VCAM-1, ICAM-1, and E-selectin in diabetic rats. Can J Physiol Pharmacol 91:1119–1126. [DOI] [PubMed] [Google Scholar]
- Adya R, Tan BK, Randeva HS(2015)Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res 2015:648239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (Washington) (1994)DSM-IV: diagnostic and statistical manual of mental disorders, 4th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- Balotsev R, Koido K, Vasar V, Janno S, Kriisa K, Mahlapuu R, Ljubajev U, Parksepp M, Veiksaar P, Volke V, Lang A, Haring L, Zilmer M, Vasar E(2017)Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur Psychiatry 39:1–10. [DOI] [PubMed] [Google Scholar]
- Bartoli F, Crocamo C, Clerici M, Carra G(2015)Second-generation antipsychotics and adiponectin levels in schizophrenia: a comparative meta-analysis. Eur Neuropsychopharmacol 25:1767–1774. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Bioque M, Parellada M, Saiz Ruiz J, Cuesta MJ, Llerena A, Sanjuan J, Castro-Fornieles J, Arango C, Cabrera B, Group PE(2013)Assessing clinical and functional outcomes in a gene-environment interaction study in first episode of psychosis (PEPs). Rev Psiquiatr Salud Ment 6:4–16. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Bioque M, Cabrera B, Lobo A, Gonzalez-Pinto A, Pina L, Corripio I, Sanjuan J, Mane A, Castro-Fornieles J, Vieta E, Arango C, Mezquida G, Gasso P, Parellada M, Saiz-Ruiz J, Cuesta MJ, Mas S, GROUP PE (2017)Modelling gene-environment interaction in first episodes of psychosis. Schizophr Res 189:181–189. [DOI] [PubMed] [Google Scholar]
- Bioque M, Garcia-Portilla MAP, Garcia-Rizo C, Cabrera B, Lobo A, Gonzalez-Pinto A, Diaz-Caneja CM, Corripio I, Vieta E, Castro-Fornieles J, Bobes J, Gutierrez-Fraile M, Rodriguez-Jimenez R, Mezquida G, Llerena A, Saiz-Ruiz J, Bernardo M, GROUP PE (2017)Evolution of metabolic risk factors over a two-year period in a cohort of first episodes of psychosis. Schizophr Res. doi: 10.1016/j.schres.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Schiano V, Chiariello M(2006)Cellular adhesion molecules and peripheral arterial disease. Vasc Med 11:39–47. [DOI] [PubMed] [Google Scholar]
- Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji MA, Lebovitz HE, Hennekens CH(2004)Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry 65:4–18; quiz 19–20. [PubMed] [Google Scholar]
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM(2014)Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 71:1350–1363. [DOI] [PubMed] [Google Scholar]
- Chen S, Broqueres-You D, Yang G, Wang Z, Li Y, Wang N, Zhang X, Yang F, Tan Y(2013)Relationship between insulin resistance, dyslipidaemia and positive symptom in Chinese antipsychotic-naive first-episode patients with schizophrenia. Psychiatry Res 210:825–829. [DOI] [PubMed] [Google Scholar]
- Cheng SC, Schepp KG(2016)Early intervention in schizophrenia: a literature review. Arch Psychiatr Nurs 30:774–781. [DOI] [PubMed] [Google Scholar]
- Feinstein RE.(2002)Cardiovascular effects of novel antipsychotic medications. Heart Dis 4:184–190. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Siu CO, Boden R, Pappadopulos E, Karayal ON, Kahn RS, group Es (2013)Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European first-episode schizophrenia trial. Int J Neuropsychopharmacol 16:987–995. [DOI] [PubMed] [Google Scholar]
- Foley DL, Morley KI(2011)Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 68:609–616. [DOI] [PubMed] [Google Scholar]
- Fond G, d’Albis MA, Jamain S, Tamouza R, Arango C, Fleischhacker WW, Glenthoj B, Leweke M, Lewis S, McGuire P, Meyer-Lindenberg A, Sommer IE, Winter-van Rossum I, Kapur S, Kahn RS, Rujescu D, Leboyer M(2015)The promise of biological markers for treatment response in first-episode psychosis: a systematic review. Schizophr Bull 41:559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortmann SP, Ford E, Criqui MH, Folsom AR, Harris TB, Hong Y, Pearson TA, Siscovick D, Vinicor F, Wilson PF, CDC, AHA (2004)CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: report from the population science discussion group. Circulation 110:e554–559. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Bioque M, MacDowell KS, Santabarbara J, Martinez-Cengotitabengoa M, Moreno C, Saiz PA, Berrocoso E, Gasso P, Fe Barcones M, Gonzalez-Pinto A, Parellada M, Bobes J, Mico JA, Bernardo M, Leza JC, Flamm-Peps Study Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) (2014a) Pro-/antiinflammatory dysregulation in early psychosis: results from a 1-year follow-up study. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martinez-Cengotitabengoa M, Pina-Camacho L, Rodriguez-Jimenez R, Saiz PA, Castro C, Lafuente A, Santabarbara J, Gonzalez-Pinto A, Parellada M, Rubio G, Garcia-Portilla MP, Mico JA, Bernardo M, Leza JC (2014b) Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Meseguer A, Cabrera B, Mezquida G, Bioque M, Penades R, Parellada E, Bernardo M, Kirkpatrick B(2016)Metabolic syndrome or glucose challenge in first episode of psychosis?Eur Psychiatry 41:42–46. [DOI] [PubMed] [Google Scholar]
- Genovese S, De Berardis G, Nicolucci A, Mannucci E, Evangelista V, Totani L, Pellegrini F, Ceriello A(2013)Effect of pioglitazone versus metformin on cardiovascular risk markers in type 2 diabetes. Adv Ther 30:190–202. [DOI] [PubMed] [Google Scholar]
- Graham KA, Cho H, Brownley KA, Harp JB(2008)Early treatment-related changes in diabetes and cardiovascular disease risk markers in first episode psychosis subjects. Schizophr Res 101:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY(2017)Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatry 22:1056–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen F, Drevon CA(2007)Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology 148:5478–5486. [DOI] [PubMed] [Google Scholar]
- Hinds A, Coulter L, Hudson J, Seaton V(2015)Screening for diabetes in patients receiving second-generation atypical antipsychotics. Am J Health Syst Pharm 72:70–73. [DOI] [PubMed] [Google Scholar]
- Jindal R, MacKenzie EM, Baker GB, Yeragani VK(2005)Cardiac risk and schizophrenia. J Psychiatry Neurosci 30:393–395. [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Schwarz M, Myint AM(2012)The metabolic syndrome in schizophrenia: is inflammation a contributing cause?J Psychopharmacol 26:33–41. [DOI] [PubMed] [Google Scholar]
- Leza JC, Garcia-Bueno B, Bioque M, Arango C, Parellada M, Do K, O’Donnell P, Bernardo M(2015)Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev 55:612–626. [DOI] [PubMed] [Google Scholar]
- Lubrano V, Balzan S(2015)Consolidated and emerging inflammatory markers in coronary artery disease. World J Exp Med 5:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, McEvoy JP, Davis VG, Goff DC, Nasrallah HA, Davis SM, Hsiao JK, Swartz MS, Stroup TS, Lieberman JA(2009)Inflammatory markers in schizophrenia: comparing antipsychotic effects in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Biol Psychiatry 66:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu P, Churilov L, Kong A, Kanaan R, Wong H, Van Mourik A, Yao A, Cornish E, Hachem M, Hart GK, Owen-Jones E, Robbins R, Lam Q, Samaras K, Zajac JD, Ekinci EI(2017)Using routine hemoglobin A1c testing to determine the glycemic status in psychiatric inpatients. Front Endocrinol (Lausanne) 8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Dev SI, Chen G, Liou SC, Martin AS, Irwin MR, Carroll JE, Tu X, Jeste DV, Eyler LT(2017)Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur Arch Psychiatry Clin Neurosci doi: 10.1007/s00406-017-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousen EK, Franco JG, Sullivan EL(2013)Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology 98:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Wu HD, Willerson JT, Yeh ET(2000)Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation 101:235–238. [DOI] [PubMed] [Google Scholar]
- Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J(2007)Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 64:19–28. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Vazquez-Barquero JL, Amado JA, Berja A, Garcia-Unzueta MT, Pelayo-Teran JM, Carrasco-Marin E, Mata I, Crespo-Facorro B(2008)Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J Clin Psychopharmacol 28:289–295. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Martinez-Garcia O, Pardo-Garcia G, Amado JA, Garcia-Unzueta MT, Tabares-Seisdedos R, Crespo-Facorro B(2014)Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol 17:41–51. [DOI] [PubMed] [Google Scholar]
- Petrikis P, Tigas S, Tzallas AT, Papadopoulos I, Skapinakis P, Mavreas V(2015)Parameters of glucose and lipid metabolism at the fasted state in drug-naive first-episode patients with psychosis: evidence for insulin resistance. Psychiatry Res 229:901–904. [DOI] [PubMed] [Google Scholar]
- Phutane VH, Tek C, Chwastiak L, Ratliff JC, Ozyuksel B, Woods SW, Srihari VH(2011)Cardiovascular risk in a first-episode psychosis sample: a ‘critical period’ for prevention?Schizophr Res 127:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD(2017)Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramyothin P, Khaodhiar L(2010)Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 17:460–466. [DOI] [PubMed] [Google Scholar]
- Quispe R, Martin SS, Jones SR(2016)Triglycerides to high-density lipoprotein-cholesterol ratio, glycemic control and cardiovascular risk in obese patients with type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 23:150–156. [DOI] [PubMed] [Google Scholar]
- Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA(2014)Increased mortality in schizophrenia due to cardiovascular disease: a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry 5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Parrilla-Escobar MA, Klink R, Fathalli F, Ying Kin N, Stip E, Baptista T, Malla A, Joober R(2008)Are metabolic indices different between drug-naive first-episode psychosis patients and healthy controls?Schizophr Res 102:329–336. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ohashi K, Murohara T(2017)The role of adipokines in cardiovascular disease. J Cardiol 70:329–334. [DOI] [PubMed] [Google Scholar]
- Song X, Fan X, Song X, Zhang J, Zhang W, Li X, Gao J, Harrington A, Ziedonis D, Lv L(2013)Elevated levels of adiponectin and other cytokines in drug naive, first episode schizophrenia patients with normal weight. Schizophr Res 150:269–273. [DOI] [PubMed] [Google Scholar]
- Steylen PM, van der Heijden FM, Hoogendijk WJ, Verhoeven WM(2015)Glycosylated hemoglobin as a screening test for hyperglycemia in antipsychotic-treated patients: a follow-up study. Diabetes Metab Syndr Obes 8:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa A, Hattori Y, Kasai K, Nakano Y(2008)Adiponectin induces NF-kappaB activation that leads to suppression of cytokine-induced NF-kappaB activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diab Vasc Dis Res 5:123–127. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF(2002)Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem 277:29359–29362. [DOI] [PubMed] [Google Scholar]
- Ulloa RE, Ortiz S, Higuera F, Nogales I, Fresan A, Apiquian R, Cortes J, Arechavaleta B, Foulliux C, Martinez P, Hernandez L, Dominguez E, de la Pena F(2006)[Interrater reliability of the Spanish version of schedule for affective disorders and schizophrenia for school-age children--present and lifetime version (K-SADS-PL)]. Actas Esp Psiquiatr 34:36–40. [PubMed] [Google Scholar]
- van Os J, Kapur S(2009)Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB(2002)Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem 277:34176–34181. [DOI] [PubMed] [Google Scholar]
- Wedrychowicz A, Zajac A, Pilecki M, Koscielniak B, Tomasik PJ(2014)Peptides from adipose tissue in mental disorders. World J Psychiatry 4:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai D, Lang Y, Dong G, Liu Y, Wang X, Zhou D, Cui T, Yang Y, Zhang W, Zhao Y, Zhang R (2017a) QTc interval lengthening in first-episode schizophrenia (FES) patients in the earliest stages of antipsychotic treatment. Schizophr Res 179:70–74. [DOI] [PubMed] [Google Scholar]
- Zhai D, Cui T, Xu Y, Feng Y, Wang X, Yang Y, Li S, Zhou D, Dong G, Zhao Y, Yang Y, Zhang R (2017b) Cardiometabolic risk in first-episode schizophrenia (FES) patients with the earliest stages of both illness and antipsychotic treatment. Schizophr Res 179:41–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.