Abstract
Objective
We aimed to clarify the onset of diabetes.
Design
Data from 27,392 nondiabetic health examinees were retrospectively analyzed for a mean of 5.3 years. Trajectories of fasting plasma glucose (FPG), body mass index (BMI), and the single point insulin sensitivity (Si) estimator (SPISE), an index of Si, 10 years before diagnosis of prediabetes (PDM; n = 4781) or diabetes (n = 1061) were separately assessed by a mixed effects model. Diabetes and PDM were diagnosed by the American Diabetes Association definition on the basis of FPG and glycosylated hemoglobin A1c values.
Results
In individuals who developed diabetes, mean FPG and BMI were significantly higher (P < 0.01 each) and SPISE lower than those who did not at −10 years: FPG 101.5 mg/dL vs 94.5 mg/dL, BMI 24.0 kg/m2 vs 22.7 kg/m2, and SPISE 7.32 vs 8.34, P < 0.01 each. These measurements, in subjects who developed prediabetes, were slightly but definitely different from those who did not, already at −10 years: FPG 91.8 mg/dL vs 89.6 mg/dL, BMI 22.6 kg/m2 vs 22.1 kg/m2, and SPISE 8.44 vs 8.82, P < 0.01 each. In both cases, the differences were progressively greater toward year 0, the time of diabetes, or PDM diagnosis.
Conclusions
FPG was significantly elevated in those who developed diabetes at least 10 years before diagnosis of diabetes, and this was also the case in those who developed PDM. Glucose dysregulation precedes diagnosis of diabetes at least for 20 years.
Keywords: diabetes mellitus, Japanese, prediabetes, starting point of diabetes, trajectory
FPG already increased at 10 years before diagnosis of diabetes and also at 10 years before diagnosis of prediabetes. Diabetes may begin at least >20 years before its diagnosis.
It has been well established that a long-lasting, prodromal stage exists before clinical diagnosis of type 2 diabetes mellitus, which consists of progressive elevation of plasma glucose (PG) within a nondiabetic range, weight gain, and attenuation of insulin sensitivity (Si) [1–5]. In general, such abnormalities may be detected already 10 years before diagnosis of diabetes [1–8]. An increase in glucose-stimulated insulin secretion, which may well be a compensation for attenuated Si during this period, was demonstrated in some [2, 8] but not in other studies [1, 9]. Variability in glucose-stimulated insulin secretion is likely a result of ethnic diversity of Si [10] and in part, a result of a variable degree of obesity [8].
Japanese people are relatively lean, and the average body mass index (BMI) in the general population is ~22 kg/m2 [11], whereas that of patients with diabetes in a representative cohort was slightly >23 kg/m2 [12]. Increase in insulin resistance before clinical diagnosis of diabetes was reported to be modest, irrespective of participants’ body weight in the Japanese population [8]. Despite the low degree of obesity, accompanied with increased Si in the Japanese population, the trajectory of glucose before diabetes [6, 8] and the prevalence of diabetes are not substantially different between Japanese [13] and whites [2, 14]. With the assumption that attenuated Si and β-cell dysfunction are the two primary drivers for the development of diabetes, these data are compatible with a significant role of β-cell dysfunction in diabetes causation [15, 16] in Japanese individuals.
Despite accumulation of data, the timeline of diabetes has not been fully understood. Particularly, the onset of glucose dysregulation leading to diabetes has not been clarified, i.e., the trajectories before diagnosis of diabetes have been assessed for 10 to 15 years, and the patients destined to develop diabetes exhibited significant elevation of PG already at the earliest time points in all studies [1–8]. In this regard, the time point when progressors to diabetes and nonprogressors first become significantly different from each other is currently unknown. It was hypothesized that β-cell dysfunction starts 12 years before the clinical diagnosis of diabetes [17]. However, such a hypothesis is not necessarily consistent with a more recent epidemiological study [2]. Therefore, we assessed the trajectories of fasting PG (FPG), BMI, and Si before development of diabetes or prediabetes (PDM) separately. Si was quantified by a new index—the single point Si estimator (SPISE) [18].
1. Materials and Methods
A. Study Participants and Study Design
The main dataset was obtained at the Health Center of the Aizawa Hospital, Matsumoto, Japan, which was designated as the Aizawa Cohort. Another data set was obtained at Juntendo University, Tokyo, Japan. Written, informed consent was obtained from all participants, and the Review Board of Aizawa Hospital and Juntendo University approved the study protocol. This study was performed in accordance with the principles outlined in the Declaration of Helsinki.
A-1. Aizawa Cohort
A total of 49,781 participants received a health examination, including FPG and glycosylated hemoglobin A1c (HbA1c), between July 2005 and May 2016. Of those, 22,389 were excluded, as they were newly diagnosed with diabetes at baseline, or they did not receive follow-up examinations during the study period. The difference between the characteristics of the nondiabetic subjects who were followed up and not followed up was minimal (data not shown). Thus, the data from 27,392 eligible individuals were analyzed. The endpoint of the follow up was the development of PDM or diabetes.
For the analysis of subjects who developed PDM, data of 15,778 participants with normal glucose regulation (NGR) were collected at baseline and until participants developed PDM or until the last examination (whichever occurred first).
For the analysis of subjects who developed diabetes, data were collected from the entire nondiabetic participant cohort (n = 27,392, NGR and PDM combined) until participants developed diabetes or until the last examination (whichever occurred first). Participants who developed diabetes during the observation period were defined as DM-Progressors, and those who did not develop diabetes until the last examination were defined as Non-DM (NDM)-Nonprogressors.
Trajectories were assessed for FPG, BMI, and a new index of Si, SPISE [18]. The following formula was used to calculate SPISE, where HDL-c is high-density lipoprotein cholesterol (milligrams per deciliters), TG is triglyceride (milligrams per deciliters), and BMI is unit of kilograms per square meters:
SPISE is a quantitative index of Si, suited for the health examination, because of its low cost and derivation from the ordinary data. We used it after validation of it in the Japanese population, as it is originally developed and validated in whites [18].
A-2. Juntendo Cohort
This cohort was used for verification of SPISE [18] in the Japanese adults. The characteristics of this cohort were reported elsewhere [19]. Nondiabetic Japanese men, aged between 30 and 50 years, were recruited through posters at major companies and internet advertisements. Subjects being treated for hypertension, lipid disorders, diabetes, cardiovascular disease, chronic lung disease, cancer, renal failure, serious hepatic dysfunction, and hepatitis B and C were excluded. Blood samples, after overnight fasting, were withdrawn for measurement of TG and HDL-c, and BMI was determined. Subjects (n = 236) volunteered to participate in the study, and after exclusion or refusal, a euglycemic hyperinsulinemic glucose clamp test with PG, clamped at 95 mg/dL, was performed in 111. We used rate of glucose disappearance (Rd) during 180 to 360 minutes as an index of muscle sensitivity. Further detail of the clamp study is provided elsewhere [19].
B. Diagnosis of Glucose Metabolism
Diabetes was diagnosed when FPG ≥ 126 mg/dL or HbA1c ≥ 6.5% [20]. Diagnosis of diabetes was also made based on information by the participants who had been diagnosed with diabetes at a medical facility. PDM was diagnosed when FPG was between 100 and 125 mg/dL and/or HbA1c, 5.7% to 6.4%. Participants with FPG < 100 mg/dL and HbA1c < 5.7% were diagnosed as NGR.
C. Variables
Sex, age, BMI, systolic blood pressure, FPG, HbA1c, HDL-c, low-density lipoprotein cholesterol, TG, and alanine aminotransferase were recorded. SPISE [18] was also recorded as an index of Si. For analysis of trajectories before diabetes, the date of examination was rounded to the nearest year by setting the date of diagnosis of diabetes as day 0. For assessment of trajectories before PDM, the date of diagnosis of PDM was taken as day 0. The date of the last examination was taken as day 0 for those who did not develop PDM or diabetes. It should be noted that the data from individuals with NGR at baseline were used for the trajectory assessment before PDM, and the data from those with NGR and/or PDM at baseline were used for the trajectory analysis before diabetes.
D. Statistics
The trajectories were assessed using the mixed effects model with adjustment for sex and age, and the data shown in the figures were the estimated marginal means and 95% confidence interval at each year. In DM-Progressors, the number of subjects who received a health examination at −9, −10, and −11 years were 94, 41, and 3, respectively. It was rather small, and therefore, the subjects in the three year ranges were combined, and this group, containing the data from −9, −10, and −11 years, was designated simply as −10 years in the figures. The correlation between the clamp-based Rd and SPISE was examined using Spearman’s rank correlation. Mann-Whitney U test and χ2 test were used as required. Comparison of the estimated marginal means was performed by Medcalc online calculator (https://www.medcalc.org/calc/comparison_of_means.php). For the linear and cubic weighted minimum square regression, Bayesian information criterion (BIC) and r2 values were determined to compare fitness of the regression.
JMP 12.2.0 (SAS Institute) or Statistical Package for Social Sciences 21.0 (IBM) statistical software was used, and P < 0.05 (two tailed) was considered significant.
2. Results
A. Baseline Characteristics of the Aizawa Cohort
Baseline anthropometric and laboratory data in individuals who developed PDM (PDM-Progressors) showed slightly but significantly atherogenic or metabolic characteristics compared with individuals who remained NGR (NGR)-Nonprogressors, except for plasma HDL-c, which was not significantly different between the two groups (Table 1). Such trend was unequivocal in DM-Progressors compared with NDM-Nonprogressors (Table 1).
Table 1.
Characteristics of the Participants Used for Trajectory Assessment Before PDM and Diabetes
| Variable | Cohorts Used for Trajectory Assessment Before PDM | Cohorts Used for Trajectory Assessment Before Diabetes | ||
|---|---|---|---|---|
| PDM-Progressors (n = 4781) | NGR-Nonprogressors (n = 10,994) | DM-Progressors (n = 1061) | NDM-Nonprogressors (n = 26,331) | |
| Males, n (%) | 2,554 (53.4%) | 6,094 (55.4%) | 743 (70.0%) | 15,154 (57.6) |
| Age (y) | 49 (42–55) | 44 (38–52) | 53 (46–60) | 48 (41–56) |
| BMI (kg/m2) | 22.5 (20.6–24.6) | 21.8 (20.1–23.8) | 24.8 (22.5–27.3) | 22.5 (20.6–24.6) |
| SBP (mmHg) | 119 (109–130) | 116 (106–127) | 127 (117–138) | 119 (109–131) |
| FPG (mg/dL) | 92 (89–96) | 90 (86–94) | 106 (99–114) | 93 (89–99) |
| HbA1c (%) | 5.5 (5.2–5.6) | 5.2 (5.1–5.5) | 6.0 (5.7–6.2) | 5.5 (5.2–5.7) |
| HDL-c (mg/dL) | 60 (50–71) | 60 (51–71) | 53 (44–63) | 59 (49–69) |
| LDL-c (mg/dL) | 118 (100–139) | 111 (92–131) | 127 (108–151) | 117 (98–139) |
| TG (mg/dL) | 84 (60–121) | 76 (55–110) | 121 (84–169) | 85 (61–126) |
| ALT (U/L) | 19 (14–27) | 18 (14–25) | 26 (18–38) | 19 (15–27) |
| SPISE | 8.15 (6.73–9.81) | 8.71 (7.20–10.38) | 6.43 (5.40–7.82) | 8.10 (6.67–9.82) |
| Follow up (examination no./y) | 5.5/6.2 | 4.4/5.1 | 4.2/4.2 | 4.8/5.2 |
All variables for progressors and nonprogressors were significantly different (P < 0.01) except for HDL-c in PDM-Progressors and NGR-Nonprogressors. Values are median (25% to 75%), except for categorical data, which are shown as number and percent. Values for follow up represent the mean.
Abbreviations: LDL-c, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
B. Correlation Between SPISE and Clamp-Based Rd
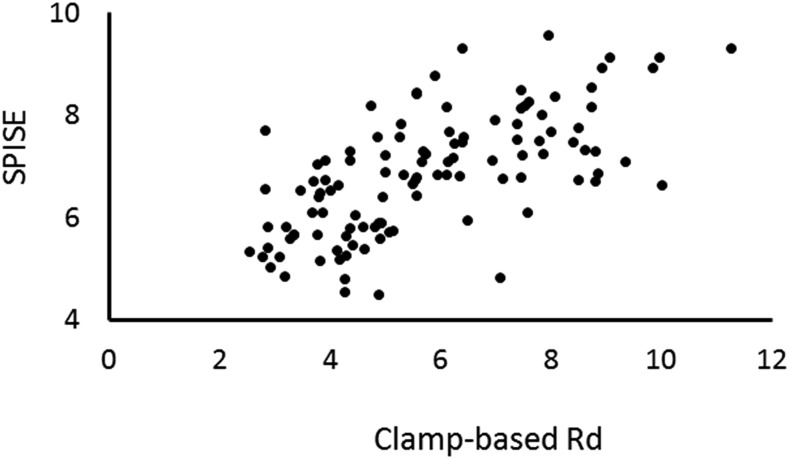
The correlation between the clamp-based index of Si and SPISE appears to be better in the Juntendo Cohort than in the original adult cohort: Spearman rank correlation coefficient 0.688 in the former and Pearson correlation coefficient 0.474 in the latter.
SPISE was positively and robustly correlated with clamp-based Rd adjusted for body weight (Spearman ρ = 0.688, P < 0.01; Fig. 1).
Figure 1.
Validation of the SPISE. The clamp-based Rd values (adjusted for body weight) strongly correlated with SPISE: Spearman ρ = 0.668, P < 0.01.
C. Trajectories of FPG
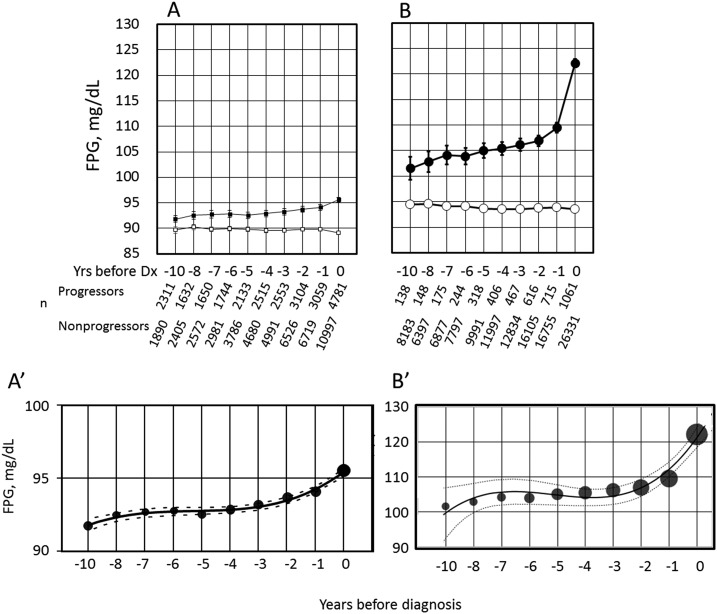
FPG was significantly higher in PDM-Progressors than NGR-Nonprogressors, already at 10 years before PDM (Fig. 2A). FPG gradually rose toward year 0 (the time of PDM diagnosis) in PDM-Progressors, whereas FPG exhibited no significant rise in NGR-nonprogressors.
Figure 2.
Trajectories of FPG before (A) PDM and (B) diabetes and weighted cubic regression of the estimated marginal means of FPG trajectory before (A′) PDM and (B′) diabetes. (A and B) Values in the progressors and nonprogressors at each time point were all significantly different (P < 0.01). (A) PDM-Progressors (▪) and NGR-Nonprogressors (□); (B) DM-Progressors (●) and NDM-Nonprogressors (○). The axis scale was intentionally maintained the same to facilitate visual comparison. (A′ and B′) The sizes of the circles are proportional to the number of individuals. The lines are the best-fit cubic regression, and broken lines indicate 95% confidence intervals. Dx, diagnosis; n, number of participants examined each year; Yrs, years.
FPG was significantly higher in DM-Progressors than NDM-Nonprogressors at year −10 (Fig. 2B). A gradual elevation of FPG occurred in DM-Progressors thereafter until year −2, which was followed by an accelerated increase toward year 0 (the time of diabetes diagnosis). There was no significant increase in FPG in NDM-Nonprogressors during the observation period (Fig. 2B).
The FPG trajectory in PDM-Progressors fitted to weighted cubic regression clearly better than linear regression (Fig. 2A′). BIC was 97.11 and 113.36 for cubic and linear regressions, respectively, and r2 was 0.944 and 0.548, respectively. Similar results were obtained for the FPG trajectory in DM-Progressors (Fig. 2B′): BIC for cubic and linear regression was 108.57 and 125.42, respectively, and r2 was 0.956 and 0.623, respectively.
D. Trajectories of BMI
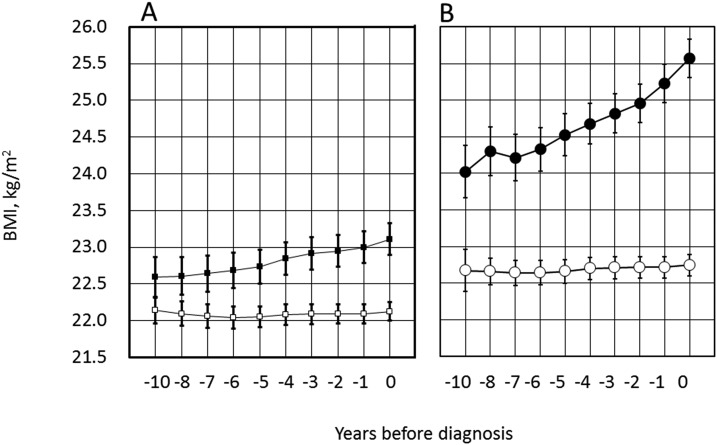
BMI was significantly greater in PDM-Progressors than NGR-Nonprogressors, already at year −10 (P < 0.01; Fig. 3A), and in the former, BMI progressively increased toward year 0. BMI was also significantly greater in DM-Progressors than NDM-Nonprogressors at year −10 (Fig. 3B). It gradually increased thereafter in DM-Progressors. At the time of diabetes diagnosis, BMI was 25.6 kg/m2 and 22.7 kg/m2 in DM-Progressors and NDM-Nonprogressors, respectively (P < 0.01). BMI did not significantly change during the observation period in the two nonprogressor groups (Fig. 3A and 3B, open symbols).
Figure 3.
Trajectories of BMI before diagnosis of (A) PDM and (B) diabetes. Symbols are the same as in Fig. 2. (A and B) Values in the progressors and nonprogressors at each time point were significantly different both in A and B (P < 0.01 for each). The axis scale was intentionally maintained the same to facilitate visual comparison. See Fig. 2A and 2B for the number of individuals examined each year.
E. Trajectories of SPISE
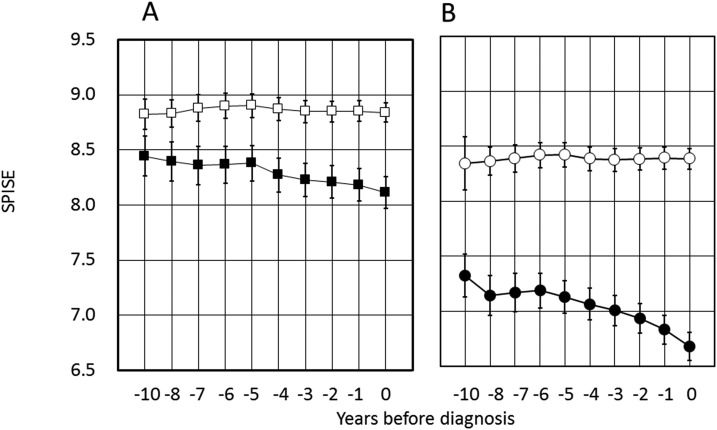
SPISE was significantly lowered in PDM-Progressors compared with NDM-Nonprogressors, already at year −10 (Fig. 4A). SPISE values in PDM-Progressors, but not in NGR-Nonprogressors, were progressively small thereafter (Fig. 4A). Likewise, SPISE in DM-Progressors showed an unequivocal lowering compared with NDM-Nonprogressors at 10 years before diagnosis of diabetes. There was no significant time-dependent change of SPISE in NDM-Nonprogressors (Fig. 4B).
Figure 4.
Trajectories of SPISE before (A) PDM and (B) diabetes. (A and B) The axis scale was intentionally maintained the same to facilitate visual comparison. See Fig. 2A and 2B for the number of individuals examined each year.
3. Discussion
Glucose trajectory before development of diabetes has been studied by many groups [1–8]. Consistent with these previous studies, we showed that FPG in individuals destined to develop diabetes is already elevated at 10 years before diagnosis compared with those who did not develop diabetes. In individuals who developed diabetes, a relatively slow elevation of FPG occurred until −3 to −5 years, which was followed by an accelerated escalation of FPG toward the time of diagnosis of diabetes. Thus, the onset of diabetes has not been identified. In Japanese people, the data were obtained in the cohorts with exclusively [6, 7] or mostly [8] males in the previous studies. We analyzed the data of the cohort with an approximately equal sex distribution. Nonetheless, our data on the trajectories in subjects who developed diabetes mostly confirmed previous results. In other words, the onset of glucose dysregulation leading to diabetes could not be identified in the Aizawa Cohort with this length of observation.
SPISE is a surrogate measure of Si, developed and validated in whites [18], and therefore, we needed to confirm its validity in the Japanese people using the data from the Juntendo Cohort [19]. With the knowledge that SPISE is a reliable index of Si in the Japanese people, we applied it to the Aizawa Cohort.
In an attempt to uncover the onset of dysglycemia preceding diabetes, we assessed the trajectories of FPG, as well as BMI and SPISE, before PDM diagnosis, at the earliest stage of diabetes evolution [21]. We hypothesized that until a certain time point, FPG may be indistinguishable between individuals who developed PDM and those who did not. In this study, we investigated the glucose trajectory before diagnosis of PDM. The results were clear. Contrary to our hypothesis, the individuals destined to develop PDM (PDM-Progressors) had significantly higher FPG value already at −10 years compared with those who did not develop PDM (NGR-Nonprogressors). In addition, BMI and Si were greater and attenuated decreased, respectively, already at −10 years. Therefore, the primary abnormalities of diabetes, dysglycemia, increased body weight, and attenuated Si had started >10 years before diagnosis of PDM. The deviation from nonprogressors was highly important, although the degree of abnormality was smaller compared with that seen in those destined to develop DM. Taken together, we consider that dysglycemia leading to diabetes begins even >10 years before diagnosis of PDM so that it would be >20 years before diagnosis in the majority of patients with diabetes, if not all.
We noticed that the trajectories of FPG, before both PDM and diabetes, fitted nicely to the cubic regression (Fig. 2A′ and 2B′), which suggested a time-dependent pathophysiology. The most likely interpretation would be as follows. At the initial stage of development of PDM or diabetes, a minute elevation of PG may occur as a result of modest weight gain with a minimum attenuation of Si. This may cause increased insulin secretion and stabilization of PG at slightly elevated levels. Eventually, as Si attenuates further, β-cells cannot meet the increased demand of insulin secretion, and a relatively sharp rise of PG ensues. Of note, the well-fit cubic regression was not a product of setting a threshold value for the diagnosis, as the fitness of cubic regression was present even after omission of the value at the diagnosis of diabetes or PDM (year 0; data not shown).
There were limitations in this study. First, our study population was a group of potentially health-conscious individuals. Therefore, the timeline of diabetes in the general population may be steeper than the Aizawa Cohort. Second, reliability of SPISE in the Japanese subjects was confirmed. Nonetheless slight over or under estimation of Si by SPISE cannot be completely ruled out. Third, the duration between diagnosis of PDM and that of diabetes is currently unknown so that an entire timeline of diabetes evolution still remains to be clarified. Finally, the insulin level was not determined in the Aizawa Cohort; therefore, the trajectory of the β-cell function was not ascertained.
In conclusion, we assessed trajectories of FPG, BMI, and Si before diagnosis of diabetes and PDM separately. These measurements were apparently abnormal at least −10 years of diagnosis of not only diabetes but also PDM. Diabetes may start as early as 10 years before development of PDM, which means >20 years before diagnosis of diabetes in the majority of patients.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BIC
Bayesian information criterion
- BMI
body mass index
- DM
diabetes mellitus
- FPG
fasting plasma glucose
- HbA1c
glycosylated hemoglobin A1c
- HDL-c
high-density lipoprotein cholesterol
- NDM
nondiabetes mellitus
- NGR
normal glucose regulation
- PDM
prediabetes
- PG
plasma glucose
- Rd
rate of glucose disappearance
- Si
insulin sensitivity
- SPISE
single point insulin sensitivity estimator
- TG
triglyceride
References and Notes
- 1. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II Study. Lancet. 2009;373(9682):2215–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Færch K, Witte DR, Tabák AG, Perreault L, Herder C, Brunner EJ, Kivimäki M, Vistisen D. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: a post-hoc analysis of the longitudinal Whitehall II Cohort Study. Lancet Diabetes Endocrinol. 2013;1(1):43–51. [DOI] [PubMed] [Google Scholar]
- 4. Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes. 2004;53(1):160–165. [DOI] [PubMed] [Google Scholar]
- 5. Looker HC, Knowler WC, Hanson RL. Changes in BMI and weight before and after the development of type 2 diabetes. Diabetes Care. 2001;24(11):1917–1922. [DOI] [PubMed] [Google Scholar]
- 6. Heianza Y, Arase Y, Fujihara K, Hsieh SD, Saito K, Tsuji H, Kodama S, Yahagi N, Shimano H, Yamada N, Hara S, Sone H. Longitudinal trajectories of HbA1c and fasting plasma glucose levels during the development of type 2 diabetes: the Toranomon Hospital Health Management Center Study 7 (TOPICS 7). Diabetes Care. 2012;35(5):1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heianza Y, Arase Y, Kodama S, Tsuji H, Tanaka S, Saito K, Hara S, Sone H. Trajectory of body mass index before the development of type 2 diabetes in Japanese men: Toranomon Hospital Health Management Center Study 15. J Diabetes Investig. 2015;6(3):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuwahara K, Honda T, Nakagawa T, Yamamoto S, Hayashi T, Mizoue T. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Rep. 2017;7:43521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aizawa T, Yamauchi K, Yamada M. Longitudinal changes in insulin sensitivity, insulin secretion, beta cell function and glucose effectiveness during development of non-diabetic hyperglycemia in a Japanese population. Springerplus. 2014;3(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arakaki RF. Ethnic differences and β-cell changes. J Clin Endocrinol Metab. 2013;98(9):3595–3597. [DOI] [PubMed] [Google Scholar]
- 11. Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, Arima H, Tanaka K, Ibayashi S, Fujishima M. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama Study. Stroke. 2000;31(11):2616–2622. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka S, Tanaka S, Iimuro S, Yamashita H, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Sone H; Japan Diabetes Complications Study Group . Cohort profile: the Japan Diabetes Complications Study: a long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int J Epidemiol. 2014;43(4):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukai N, Doi Y, Ninomiya T, Hirakawa Y, Nagata M, Yoshida D, Hata J, Fukuhara M, Nakamura U, Kitazono T, Kiyohara Y. Trends in the prevalence of type 2 diabetes and prediabetes in community-dwelling Japanese subjects: the Hisayama Study. J Diabetes Investig. 2014;5(2):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 15. Seike M, Saitou T, Kouchi Y, Ohara T, Matsuhisa M, Sakaguchi K, Tomita K, Kosugi K, Kashiwagi A, Kasuga M, Tomita M, Naito Y, Nakajima H. Computational assessment of insulin secretion and insulin sensitivity from 2-h oral glucose tolerance tests for clinical use for type 2 diabetes. J Physiol Sci. 2011;61(4):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katakura M, Komatsu M, Sato Y, Hashizume K, Aizawa T. Primacy of beta-cell dysfunction in the development of hyperglycemia: a study in the Japanese general population. Metabolism. 2004;53(7):949–953. [DOI] [PubMed] [Google Scholar]
- 17. Lebovitz HE. Management of hyperglycemia with oral antihyperglycemic agents in type 2 diabetes In: Kahn RC, Weir GC, King GL, Jacobson AM, and Moses AC, eds. Joslin Diabetes Mellitus. 14th ed.Boston: Lippincott Williams & Wilkins; 2005:687–710. [Google Scholar]
- 18. Paulmichl K, Hatunic M, Højlund K, Jotic A, Krebs M, Mitrakou A, Porcellati F, Tura A, Bergsten P, Forslund A, Manell H, Widhalm K, Weghuber D, Anderwald CH; Beta-JUDO Investigators; RISC Investigators . Modification and validation of the triglyceride-to-HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the single point insulin sensitivity estimator (SPISE). Clin Chem. 2016;62(9):1211–1219. [DOI] [PubMed] [Google Scholar]
- 19. Takeno K, Tamura Y, Kawaguchi M, Kakehi S, Watanabe T, Funayama T, Furukawa Y, Kaga H, Yamamoto R, Kim M, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Taka H, Fujimura T, Sawada SS, Giacca A, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Relation between insulin sensitivity and metabolic abnormalities in Japanese men with BMI of 23–25 kg/m2. J Clin Endocrinol Metab. 2016;101(10):3676–3684. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. [DOI] [PubMed] [Google Scholar]
- 21. Cefalu WT. “Prediabetes”: are there problems with this label? No, we need heightened awareness of this condition! Diabetes Care. 2016;39(8):1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]