Abstract
A computational screen for novel small nucleolar RNAs in Drosophila melanogaster uncovered 15 novel snoRNAs and snoRNA-like long non-coding RNAs. In contrast to earlier surverys, the novel sequences are mostly poorly conserved and originate from unusual genomic locations. The majority derive from precurors antisense to well-known protein-coding genes, and four of the candidates are produced from exon-coding regions. Only a minority of the new sequences appears to have canonical target sites in ribosomal or small nuclear RNAs. Taken together, these evolutionary young, poorly conserved, and genomically atypical sequences point at a class of snoRNA-like transcripts with predominantly regulatory functions in the fruit fly genome.
Keywords: snoRNA, long non-coding RNA, sno-lncRNAs, Drosophila
1. Introduction
Small nucleolar RNAs (snoRNAs) are among the few ancient non-coding RNA (ncRNA) classes that predate the radiation of Eukaryotes. Their biology has been well studied and often reviewed [1,2,3,4]. Their primary function is to act as “address labels” for small nucleolar ribonucleotide protein (snoRNP) complexes, defining the specific locations at which chemical modifications are introduced into their RNA targets by means of complementary base pairing. There are two main types of snoRNAs, distinguished by characteristic sequence boxes as well as characteristic secondary structures: Box C/D snoRNAs, with two short motifs C (RUGAUGA) and D (CUGA), and a short helix connecting the 3′- with the 5′-end, direct 2′-O-methylation of nucleotides. Box H/ACA have a more elaborate secondary structure, usually comprising two hairpin structures separated by motif H (ANANNA) and featuring the sequence ACA three positions from their 3′-end. H/ACA snoRNPs catalyze the specific conversion of uridines into pseudouridines. Both classes of snoRNAs primarily target ribosomal RNAs (rRNAs) and spliceosomal small nuclear RNAs (snRNAs), thereby influencing stability, folding, and interactions of their targets. An increasing number of non-canonical functions have been discovered in recent years. These include the regulation of mRNA editing, alternative splicing, and gene silencing through microRNA-like mechanisms. While some snoRNAs are “orphans” in the sense that they have no known targets, others seem perform both traditional and non-canonical functions, see [4,5,6] for recent reviews. Recent evidence has implicated snoRNAs as important regulators of cellular functions—dysfunction of snoRNAs in particular appears to have a key role in oncogenesis [7,8].
Almost all animal snoRNAs are encoded in introns. Nevertheless, the expression patterns of snoRNAs are highly variable, as has been observed particularly in cancer samples [9]. In a recent study [10], we analyzed in some detail the genomic organization of annotated snoRNAs in the fruit fly Drosophila melanogaster and found that genes involved in cell division and cytoskeleton organization are preferred snoRNA hosts. SnoRNAs exhibit dynamic developmental expression patterns that are often distinct from the temporal profiles of other snoRNAs encoded in introns of the same host gene. This decoupling of expression levels may be achieved by alternative splicing and alternative processing of different isoforms [11]. The many potential regulatory roles of snoRNAs also suggest that—as in the case of microRNAs—novel snoRNA families may arise de novo and take on lineage-specific roles.
The snoRNA complement of fruit flies has been studied extensively in the past [12,13]. Earlier studies, in particular, used computationally predicted candidates derived from known targets [14,15,16,17]. Many of the fruit fly snoRNAs appear in clusters, more precisely in different introns of a single host gene [10,18], albeit in sometimes unusual arrangements. Several snoRNAs, for example, are encoded in introns in alternative splicing and polyadenylation variants of the pseudouridine synthase gene [19]. In some cases, however, the transcript structure deviates drastically from the vertebrate standard. MeU1b-A234, for example, derives from a longer, unspliced precursor transcribed from the reverse strand of the egr gene, a key regulator of cell differentiation, apoptosis and immune response in flies [20].
Motivated by such aberrant examples, we report here on a computation-based search for snoRNAs and snoRNA-like RNAs in the D. melanogaster genome, which includes snoRNA candidates in unusual genomic contexts as well as candidates that exhibit only a very limited phylogenetic distribution. Such snoRNAs are of particular interest because they plausibly constitute lineage-specific regulators rather than canonical guide RNAs.
2. Materials and Methods
2.1. Computational Analysis
We combined bioinformatic approaches based on different screening parameters to consecutively scan the fruit fly genomic sequence, with the aim to enhance the sensitivity and specificity of our survey.
We used SnoScan-0.99b with default parameters [21] to scan the complete D. melanogaster genome. This tool recognizes the terminal stem, the characteristic box C and D sequence motives, and the sequence complementary to putative target sites. It is therefore limited to box C/D snoRNAs with known target sites. SnoRNAs with a SnoScan-0.99b higher than 15 were kept. To alleviate this limitation, we used every position in the D. melanogaster rRNAs as a putative methylation site. Genomic regions surrounding these initial candidates were re-evaluated with snoReport-1.0 [22] using default parameters, to increase the sensitivity of the search. This program is designed to improve the prediction of snoRNAs with non-canonical antisense elements, and thus with no obvious RNA target, and can thus also recognize orphans of either C/D and H/ACA family. This feature is particularly relevant since, due to frequent degeneration of consensus boxes observed for Drosophila snoRNAs, experimentally validated candidates are often not included in the list of those displaying top scores with the SnoScan-0.99b program [16]. Considering the frequent clustering of snoRNAs, screening with snoReport also allows the discovery of H/ACA snoRNAs flanking C/D putative genes, a type of organization not common in Drosophila.
Candidate sequences were investigated for homologs in other drosophilid genomes using the available genome-wide multiple sequence alignments and a blast search. Consensus secondary structures of snoRNA candidates were analyzed with RNAalifold program [23] to check for conserved typical secondary structure.
We used RNAsnoop [24] and PLEXY [25] to predict possible targets for the novel candidate sequences. Both programs were run with default parameters.
A diagram of the work flow is represented in Figure S3.
2.2. RNA Extraction and Analysis
Canton S was used as wild-type strain in all experiments. Total RNA from 0–24 h mixed-stage embryos, a mixed population of first-second-third instar larvae, mixed-stage pupae and adults of both sexes at 4 days after eclosion, was extracted using TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions.
RNA was treated with TurboDNase (Life Technologies, Carlsbad, CA, USA) and phenol:chloroform extraction, 1 μg RNA was reverse transcribed using SuperSript III RT (Life Technologies) using the manufacturer’s recommended conditions and diluted 1:10. To check for gDNA elimination, 1:10 dilutions of RT plus and minus reactions were used as a template for amplification of 7SL-RNA by qualitative PCR using DreamTaq (Life Technologies) and applying the manufacturer’s recommended conditions; negative amplification of RT minus reaction was used to verify the complete digestion of gDNA.
For Northern blot analysis, 6 μg of total RNA was electrophoresed and transferred onto Hybond-NX (GE Healtcare, Fairfield, CT, USA) membranes. Probes were produced by amplifying PCR gDNA fragments (0.3–0.5 kb in length) spanning the detected sequences. PCR fragments were then 32P-labeled using the Nick Translation Kit (Hoffmann-La Roche, Basel, Switzerland). DNA extraction, manipulation and labelling, RNA electrophoresis, and blotting were carried out according to [26]. The size of RNAs was determined by using high range and low range RNA molecular weight markers (Life Technologies)
Quantitative real-time RT-PCR (qRT-PCR) experiments were performed in triplicate using iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) as previously described [20]. All PCR reactions were carried out in a final volume of 15 μL using 1 μL of diluted cDNA, 7.5 μL of 2X SYBR-Green (Bio-Rad) and 5 pmol of each primer. All primer sequences were designed using Primer 3 software (bioinfo.ut.ee/primer3-0.4.0/) [27] and are available under request. RpL32 was used as endogenous control for sample normalization. qRT-PCR experiments were restricted to snoRNAs in the canonical size range that do not overlap exons.
Quantitative PCR analysis was performed using the -method [28]. Sequencing was performed by the external firm PRIMM (Milano, Italy).
All newly identified sequences were named according to the FlyBase Nomenclature rules that are found at http://flybase.org/wiki/FlyBase:Nomenclature [33]. These rules were extended to scaRNAs.
3. Results
We employed different computational approaches to extend the current snoRNA annotation of drosophilids. We identified and experimentally validated (by Northern blot and RT-PCR) fifteen novel genes, whose main features are summarized in Table 1. All identified sequences were named following the FlyBase nomenclature rules. Sequences of all identified specimens were well conserved within the melanogaster subgroup, but none of them has a recognizable homolog outside the Drosophila genus, see Figure 1 and Figure S1. Only snoRNA Or-ACA8 and possibly Or-CD15 appears to be present in the entire Drosophila genus, while the other genes are later innovations (For clarity, we omit the prefixes snoRNA: and scaRNA: from gene names throughout the text.). Even more surprisingly, only a minority of the sequences are under stabilizing selection, as measured by phastcons [29] scores displayed in the UCSC genome browser. Among these, only Or-ACA8 and, to a lesser extent, Or-ACA6, Or-CD13, Or-CD14, Or-CD15, and Me28S-C2789, show appreciable levels of sequence conservation, even though the conserved parts do not cover the entire length of the ncRNAs.
Table 1.
Summary of newly identified snoRNAs. Subscripts to the host gene identify the intron, superscripts indicate an exonic position, and * indicates that the snoRNA overlaps a splice junction.
| Name | Class | Length | Genomic Location | Accession | Host Gene | Opposite to | ||
|---|---|---|---|---|---|---|---|---|
| snoRNA:Or-ACA6 | ACA | 137 | chr2R | 5,968,693–5,968,830 | - | KJ808674 | Uhg46E3 | egr1 |
| snoRNA:Or-ACA7 | ACA | 81 | chr2R | 5,968,118–5,968,199 | - | KJ808675 | Uhg46E3 | egr1 |
| snoRNA:Or-CD13 | CD | 69 | chr2R | 5,969,758–5,969,827 | - | KJ808676 | Uhg46E3 | egr2 |
| snoRNA:Or-CD15 | CD | 96 | chr3L | 1,667,151–1,667,247 | - | KJ808681 | RpL23A3* 4 | |
| snoRNA:Me28S-A2629 | CD | 140 | chr3L | 10,530,718–10,530,858 | + | KJ808678 | A2bp12 | |
| snoRNA:Or-ACA8 | ACA | 93 | chr3L | 11,882,942–11,883,035 | + | KJ808677 | CG58971 | |
| snoRNA:Or-CD14 | CD | 126 | chr2L | 2,014,449–2,014,575 | - | KJ808682 | CG42381 | |
| snoRNA:Me28S-C2789 | CD | 148 | chr2L | 21,034,775–21,034,923 | + | KJ808683 | CG422381 | |
| snoRNA:Me18S-G1506 | CD | 147 | chr2L | 16,561,050–16,561,197 | - | KJ808679 | CG423891 | |
| scaRNA:MeU1:95C-A24 | CD | 106 | chr2L | 901,976–902,082 | - | KJ808680 | ||
| snoRNA:Or-ACA9 | ACA | 600 | chr3R | 11,297,723–11,298,370 †‡ | - | KJ808684 | AdamTS-A1 | |
| snoRNA:Or-CD16 | CD | 400 | chr2L | 3,504,032–3,504,182 ‡ | - | Suppl. data | tim2 | |
| scaRNA:MeU4:25F-C137 | CD | 600 | chr3R | 1,410,224–1,410,366 ‡ | - | Suppl. data | CG29263 | |
| snoRNA:Me28S-C993 | CD | 400,1200 | chr3L | 10,189,262–10,189,354 ‡ | - | Suppl. data | ||
| snoRNA:Or-CD17 | CD | 500 | chr2R | 12,958,091–12,958,193 ‡ | + | Suppl. data | ||
† The start of the transcript is only approximate, due to a discrepancy between the deposited and verified (KJ808686) genomic sequences. ‡ The snoRNA coordinates were determined with in silico methods, while the transcript length is deduced from Northern Blots.
Figure 1.
Two examples of novel snoRNAs. (A) Two box H/ACA and one box C/D snoRNAs were detected antisense to the eiger (egr) gene. Presumably, they derive from a common precursor with the scaRNA MeU1b-A234 (scaRNA:46E3); (B) Or-ACA8 is the best conserved among the novel snoRNAs, dating back at least to the ancestor of the Drosophila genus.
Ten of the transcripts conform to the typical length range of snoRNAs, while five are much longer and may constitute snoRNA-like long non-coding RNAs. Such sno-lncRNAs that feature snoRNA-like ends have been found as predominantly species-specific transcripts in mammals [30,31], making their presence in flies a credible proposition. The sequences of 11 well-characterized transcripts were deposited in GenBank. For the remaining four snoRNA-like lncRNAs the true extent of the RNAs remains unknown. These sequences can be found in the supplementary material together with Northern blots.
The majority of newly identified snoRNAs had no obvious target on rRNAs or snRNAs and were thus classified as orphans. Plausible target sites in rRNAs are predicted only for the three methylation guides Me28S-A2629, Me28S-C2789, Me18S-G1506 as well as for the long transcript Me28S-C993. The two methylation guides MeU1:95C-A24 and MeU4:25F-C137 might target snRNAs and thus are tentatively classified as scaRNAs. In particular, MeU1:95C-A24 is predicted to recognize all D. melanogaster U1 snRNA isoforms, see also Table S1.
Targets for the orphan snoRNAs were then sought genome-wide using RNAsnoop and PLEXY with default parameters. No plausible target site was found for any of the box H/ACA snoRNAs. This may be explained by their reduced sequence length, which impedes the formation of the stabilizing upper stem upon binding. We hypothesize, therefore, that these box H/ACA may have non-canonical functions, such as the production of small RNAs. In contrast, we identified putative targets for all five orphan box C/D snoRNAs. The best interactions are compiled in Table S2.
Surprisingly, except for two specimens (Me28S-A2629 in A2bp1 and the long Or-ACA9 in AdamTS-A) none of the new transcripts is located in the expected intronic position. Instead, they exhibit peculiar genomic arrangements that may have precluded their detection in previous screens. Eight snoRNAs were found antisense to protein-coding genes.
A particularly interesting case are the snoRNAs Or-ACA6, Or-ACA7 and Or-CD13 that map close to each other in the vicinity of the previously identified MeU1b-A234 (formerly known as scaRNA:46E3) [20]. The tight clustering of these snoRNAs is suggestive of the polycistronic transcription common to Uhgs. At least MeU1b-A234 derives from a longer precursor antisense to egr [20]. We therefore name this new snoRNA cluster and its putative precursor Uhg-46E3. It is atypical in several respects. First, it is heterogeneously composed of an equal number of H/ACA and C/D specimens. All of its components show very little sequence conservation and are very different, so they cannot have originated through a series of local gene duplications. Third, the whole cluster overlaps, with opposite polarity, the eiger gene (egr), a member of the TNF family involved in programmed cell death and immune response acting through the JNK pathway [32]. Although unique among previously annotated Uhgs, this antisense arrangement is highly conserved across the melanogaster subgroup (see Figure S1), suggesting that it might have functional significance, possibly directly affecting egr. This hypothesis is supported by the earlier observation that MeU1b-A234 is significantly up-regulated in mutants in which egr transcription was reduced [20].
Another four snoRNAs (Or-CD15, Or-CD16, MeU4:25F-C137, and Me28S-C993) are released from an exon of protein-coding genes transcribed with the same polarity. The existence of these putative snoRNAs has been validated by Northern blots (Figure S2). Similar exonic arrangements were previously reported for a few Drosophila snoRNAs (snoRNA:660, snoRNA:83E4-5, snoRNA:Me28S-G2596, snoRNA:Or-CD11a, snoRNA:Or-CD11b, snoRNA:Or-ACA2), see http://Flybase.org [33]. Three of them derive from coding regions, posing intriguing questions about their expression strategy and the possibility of an influence on host mRNA processing in a way similar to yeast U18 snoRNA [34].
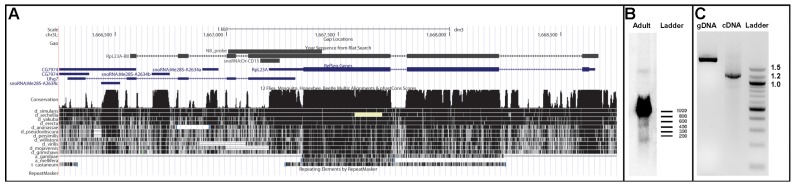
The biogenesis of the snoRNA Or-CD15 is closely related to other members of the Uhg7 cluster. It originates from an exon shared by RpL23A and Uhg7 (Uhg7-RA FlyBase ID: FBtr0300232) genes and appears to be particularly puzzling. Indeed, Uhg7 itself exhibits a complex genomic arrangement, since sequence of a long annotated EST (GenBank: BP542227) indicates that it partially overlaps with both RpL23A and CG7974 flanking genes, Figure 2. To better explore the expression strategy of snoRNA Or-CD15 we performed RT-PCR experiments by using primers annealing on the RpL23A exon 1 and Uhg7 exon 4, respectively. This approach lead to productive amplification of a cDNA fragment (Figure 2), whose sequencing defined a previously undescribed long transcript of the RpL23A locus (RpL23A-RB, accession number KJ808685) that ends at an alternative 3′-distal site and extends over Uhg7 sequences. Structure of this cDNA thus revealed that RpL23A and Uhg7 can be included together in a common, long, spliced RNA precursor. However, we found that this precursor still retained the unspliced Or-CD15 sequence, suggesting that release of this snoRNA might depend on a rare alternative splicing event or, alternatively, by a splicing-independent mechanism [11,34,35].
Figure 2.
Genomic organization and expression strategy of Or-CD15. (A) Screen shot of the RpL23A/Uhg7 region taken from the UCSC genome browser. The RpL23A-RA and Uhg7 transcripts are depicted in blue, the location of Or-CD15 and the newly identified transcript RpL23A-RB are shown in black. Below, sequence conservation as measured by phastcons and the coverage of a multiple genome alignment is indicated; (B) Northern blot analysis of adult fruit fly RNA. The genomic position of the utilised probe is outlined in (A). The band at about 100 nt corresponds to snoRNA Or-CD15; sizes are indicated by an RNA molecular weight ladder (RiboRuler Low Range, Life Technologies); (C) PCR experiments using primers annealing to RpL23A exon 1 and Uhg7 exon 4; amplification of the genomic DNA (gDNA) produces the expected fragment of about 2 kb; the same primers used in RT-PCR experiments successfully amplified a fragment of 1.2 kb, representative of the new RpL23A-RB transcript. In this transcript, RpL23A and Uhg7 sequences are fused to each other. On the right, a DNA molecular weight ladder (100bp DNA ladder, New England BioLabs, Ipswich, MA, USA) is given.
An unusual arrangement was noticed also for MeU1:95C-A24, which maps, as an independent singlet, only about 300 bp upstream of the divergently transcribed snRNA U1:21D that represents one of its potential targets (Figure S1). This type of genomic organization raises the possibility that this gene pair might share the same upstream regulatory region and be coordinately regulated.
In the light of recent suggestions that C/D snoRNA abundance can oscillate according to circadian rhythm [36], it is also interesting to note that a new specimen, Or-CD16, originates from timeless (tim), a regulatory gene involved in the response to light stimulus.
Northern blot analysis often identified transcripts whose size was longer than that of canonical snoRNAs (see Figure S2), in line with the finding that regardless of their genomic arrangement, snoRNAs are often part of longer stable transcripts, possibly related to sno-lncRNAs [30,31].
We evaluated the developmental expression profile of those eight newly identified snoRNAs by qRT-PCR that are within the expected size range for canonical snoRNAs and that do not overlap exons of other transcripts. To account for experimental errors, sex-related bias and stochastic fluctuation in expression profiles, we define a snoRNA as developmentally regulated if it shows a fold change of at least 2× relative to the embryo reference stage. According to this rule, and in agreement with previous data [10], only two of the assayed snoRNAs (Me28S-A2629 and Me28S-C2789) show a time course of expression that is not dependent on the developmental stage. All snoRNAs hosted in Uhg-46E3 (Or-ACA6, Or-ACA7, and Or-CD13) show a reduction of expression along the development. The remaining snoRNAs (Me18S-G1506, Or-ACA8 and Or-CD14) are all constantly up-regulated along development, reaching maximum expression at adult stage (see Figure 3). These data confirm the fine modulation of snoRNA expression.
Figure 3.
Developmental expression profiles of the eight novel snoRNAs that are within the typical size range for snoRNAs and that do not overlap known exons.
4. Discussion and Conclusions
We have conducted a computational survey for novel snoRNAs in D. melanogaster using a combination of established methods. In contrast to earlier work, we have focused on recent innovations and poorly conserved snoRNAs, i.e., deep sequence conservation did not play a major role in our approach. We discovered 15 novel snoRNAs and snoRNA-like long non-coding RNAs. Surprisingly, the majority of these transcripts are located in a genomic context very atypical for snoRNAs, namely antisense to protein-coding genes or overlapping coding sequences in the sense direction. Three of the novel snoRNAs originated from the novel polycistronic untranslated host gene Uhg-46E3, antisense to the regulatory gene eiger. In another case, the expression of a non-coding host gene can be coupled to that of the upstream RpL23A gene. This arrangement is highly reminiscent of the gene structure previously observed for the Drosophila pseudouridinylase mfl and the associated Uhg6 snoRNA host genes [19]. The complexity of these genomic loci highlights the complexity underlying snoRNA regulation and indicates that the expression strategy of some previously annotated Uhgs needs to be revised.
Taken together, these evolutionary young, poorly conserved, and genomically atypical sequences point at a class of snoRNA-like transcripts with predominantly regulatory functions in the fruit fly genome. It is quite plausible that many, or even most, of the novel snoRNAs do not have canonical functions in rRNA or snRNA processing. Instead, effects on splicing or a role as precursor of microRNA-like small RNAs might well be their primary biological function. This view is supported by the disparate developmental expression patterns in Figure 3. Out of eight assayed snoRNAs, three are strongly upregulated from embryo to adult, the three members of Uhg46E3 are significantly downregulated, and only two snoRNAs show a nearly constant expression pattern.
Finally, the findings reported here indicate that despite the plethora of available NGS data we have by no means a complete or comprehensive overview of the repertoires of structured regulatory RNAs—even in well-studied model organisms. Several effects may have conspired to hide the transcripts reported here: low sequence conservation, low or moderate expression levels, and rapid processing might have removed the loci from NGS analysis pipelines. At the same time a failure to exhibit characteristic features of Dicer-cleavage may have precluded the recoding of potential small processing products—the latter are nearly ubiquitous for snoRNAs [37,38]. The RNA deriving from coding sequences, of course, cannot be detected as independent entities by any of the usual NGS analysis work flows. We suspect that a large number of small and medium size RNAs with potentially important biological function still await discovery.
Acknowledgments
This work was supported by Assessorato alla Ricerca Scientifica, Regione Campania (Legge 5), by University Federico II of Naples and by P.O.R. Campania FSE 2007-2013 Project CREMe—CUP B25B09000050007 which funded Alberto Angrisani’s postdoctoral fellowship.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/2311-553X/1/2/139/s1.
Author Contributions
A.A. and H.T. conducted the computational analysis, A.A. performed the wet lab experiments, P.F.S. and M.F. designed the study. All authors contributed to the interpretation of the data and the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bachellerie J.P., Cavaillé J., Hüttenhofer A. The expanding snoRNA world. Biochimie. 2002;84:775–790. doi: 10.1016/S0300-9084(02)01402-5. [DOI] [PubMed] [Google Scholar]
- 2.Henras A.K., Dez C., Henry Y. RNA structure and function in C/D and H/ACA s(no)RNPs. Curr. Opin. Struct. Biol. 2004;14:335–343. doi: 10.1016/j.sbi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Dieci G., Preti M., Montanini B. Eukaryotic snoRNAs: a paradigm for gene expression flexibility. Genomics. 2009;94:83–88. doi: 10.1016/j.ygeno.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Bratkovič T., Rogelj B. The many faces of small nucleolar RNAs. Biochim. Biophys. Acta. 2014;1839:438–443. doi: 10.1016/j.bbagrm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Makarova J.A., Ivanova S.M., Tonevitsky A.G., Grigoriev A.I. New functions of small nucleolar RNAs. Biochemistry (Mosc.) 2013;78:638–650. doi: 10.1134/S0006297913060096. [DOI] [PubMed] [Google Scholar]
- 6.Falaleeva M., Stamm S. Processing of snoRNAs as a new source of regulatory non-coding RNAs: snoRNA fragments form a new class of functional RNAs. Bioessays. 2013;35:46–54. doi: 10.1002/bies.201200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams G.T., Farzaneh F. Are snoRNAs and snoRNA host genes new players in cancer? Nat. Rev. Cancer. 2012;12:84–88. doi: 10.1038/nrc3195. [DOI] [PubMed] [Google Scholar]
- 8.Herter E.K., Stauch M., Gallant M., Wolf E., Raabe T., Gallant P. snoRNAs are a novel class of biologically relevant Myc targets. BMC Biol. 2015;13:25. doi: 10.1186/s12915-015-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronchetti D., Todoerti K., Tuana G., Agnelli L., Mosca L., Lionetti M., Fabris S., Colapietro P., Miozzo M., Ferrarini M., et al. The expression pattern of small nucleolar and small Cajal body-specific RNAs characterizes distinct molecular subtypes of multiple myeloma. Blood Cancer J. 2012;2:e96. doi: 10.1038/bcj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angrisani A., Tafer H., Stadler P.F., Furia M. Developmentally regulated expression and expression strategies of Drosophila snoRNAs. Insect Biochem. Mol. Biol. 2015;61:69–78. doi: 10.1016/j.ibmb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Lykke-Andersen S., Chen Y., Ardal B.R., Lilje B., Waage J., Sandelin A., Jensen T.H. Human nonsense-mediated RNA decay initiates widely by endonucleolysis and targets snoRNA host genes. Genes Dev. 2014;28:2498–2517. doi: 10.1101/gad.246538.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tycowski K.T., Steitz J.A. Non-coding snoRNA host genes in Drosophila: expression strategies for modification guide snoRNAs. Eur. J. Cell Biol. 2001;80:119–125. doi: 10.1078/0171-9335-00150. [DOI] [PubMed] [Google Scholar]
- 13.Jung C.H., Hansen M.A., Makunin I.V., Korbie D.J., Mattick J.S. Identification of novel non-coding RNAs using profiles of short sequence reads from next generation sequencing data. BMC Genomics. 2010;11:77. doi: 10.1186/1471-2164-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renalier M.H., Nicoloso M., Qu L.H., Bachellerie J.P. SnoRNA U21 is also intron-encoded in Drosophila melanogaster but in a different host-gene as compared to warm-blooded vertebrates. FEBS Lett. 1996;379:212–216. doi: 10.1016/0014-5793(95)01511-6. [DOI] [PubMed] [Google Scholar]
- 15.Enerly E., Mikkelsen O.L., Lyamouri M., Lambertsson A. Evolutionary profiling of the U49 snoRNA gene. Hereditas. 2003;138:73–79. doi: 10.1034/j.1601-5223.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- 16.Accardo M.C., Giordano E., Riccardo S., Digilio F.A., Iazzetti G., Calogero R.A., Furia M. A computational search for box C/D snoRNA genes in the Drosophila melanogaster genome. Bioinformatics. 2004;20:3293–3301. doi: 10.1093/bioinformatics/bth394. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z.P., Zhou H., Liang D., Qu L.H. Different expression strategy: multiple intronic gene clusters of box H/ACA snoRNA in Drosophila melanogaster. J. Mol. Biol. 2004;341:669–683. doi: 10.1016/j.jmb.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z.P., Zhou H., He H.L., Chen C.L., Liang D., Qu L.H. Genome-wide analyses of two families of snoRNA genes from Drosophila melanogaster, demonstrating the extensive utilization of introns for coding of snoRNAs. RNA. 2005;11:1303–1316. doi: 10.1261/rna.2380905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riccardo S., Tortoriello G., Giordano E., Turano M., Furia M. The coding/non-coding overlapping architecture of the gene encoding the Drosophila pseudouridine synthase. BMC Mol. Biol. 2007;8:15. doi: 10.1186/1471-2199-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortoriello G., Accardo M.C., Scialò F., Angrisani A., Turano M., Furia M. A novel Drosophila antisense scaRNA with a predicted guide function. Gene. 2009;436:56–65. doi: 10.1016/j.gene.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Lowe T.M., Eddy S.R. A computational screen for methylation guide snoRNAs in yeast. Science. 1999;283:1168–1171. doi: 10.1126/science.283.5405.1168. [DOI] [PubMed] [Google Scholar]
- 22.Hertel J., Hofacker I.L., Stadler P.F. snoReport: Computational identification of snoRNAs with unknown targets. Bioinformatics. 2008;24:158–164. doi: 10.1093/bioinformatics/btm464. [DOI] [PubMed] [Google Scholar]
- 23.Bernhart S.H., Hofacker I.L., Will S., Gruber A.R., Stadler P.F. RNAalifold: improved consensus structure prediction for RNA alignments. BMC Bioinformatics. 2008;9:474. doi: 10.1186/1471-2105-9-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tafer H., Kehr S., Hertel J., Stadler P.F. RNAsnoop: Efficient target prediction for box H/ACA snoRNAs. Bioinformatics. 2010;26:610–616. doi: 10.1093/bioinformatics/btp680. [DOI] [PubMed] [Google Scholar]
- 25.Kehr S., Bartschat S., Stadler P.F., Tafer H. PLEXY: Efficient Target Prediction for Box C/D snoRNAs. Bioinformatics. 2011;27:279–280. doi: 10.1093/bioinformatics/btq642. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J., Russell D.W. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2001. [Google Scholar]
- 27.Untergrasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3=–new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Siepel A., Bejerano G., Pedersen J.S., Hinrichs A., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L.W., Richards S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15 doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Q.F., Yang L., Zhang Y., Xiang J.F., Wu Y.W., Carmichael G.G., Chen L.L. Long noncoding RNAs with snoRNA ends. Mol. Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X.O., Yin Q.F., Wang H.B., Zhang Y., Chen T., Zheng P., Lu X., Chen L.L., Yang L. Species-specific alternative splicing leads to unique expression of sno-lncRNAs. BMC Genomics. 2014;15:28. doi: 10.1186/1471-2164-15-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igaki T., Miura M. The Drosophila TNF ortholog Eiger: emerging physiological roles and evolution of the TNF system. Semin. Immunol. 2014;26:267–274. doi: 10.1016/j.smim.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 33.McQuilton P., St Pierre S.E., Thurmond J., FlyBase Consortium FlyBase 101—The basics of navigating FlyBase. Nucleic Acids Res. 2012;40:D706–D714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitali P., Basyuk E., le Meur E., Bertrand E., Muscatelli F., Cavaillé J., Hüttenhofer A. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J. Cell Biol. 2005;169:745–753. doi: 10.1083/jcb.200411129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo D., Raabe C.A., Reinhardt R., Brosius J., Rozhdestvensky T.S. Alternative processing as evolutionary mechanism for the origin of novel nonprotein coding RNAs. Genome Biol. Evol. 2013;5:2061–2071. doi: 10.1093/gbe/evt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes M.E., Grant G.R., Paquin C., Qian J., Nitabach M.N. Deep sequencing the circadian and diurnal transcriptome of Drosophila brain. Genome Res. 2012;22:1266–1281. doi: 10.1101/gr.128876.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taft R.J., Glazov E.A., Lassmann T., Hayashizaki Y., Carninci P., Mattick J.S. Small RNAs derived from snoRNAs. RNA. 2009;15:1233–1240. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langenberger D., Çakir M.V., Hoffmann S., Stadler P.F. Dicer-Processed Small RNAs: Rules and Exceptions. J. Exp. Zool. Mol. Dev. Evol. 2012;320:35–46. doi: 10.1002/jez.b.22481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.