Abstract
Pathological interplay between the heart and kidneys—also known as cardio-renal syndrome (CRS)—is frequently encountered in heart failure and is linked to worse prognosis and quality of life. Drug therapies for this complex situation may include nitroprusside or the recombinant B-type natriuretic peptide nesiritide for patients with acute CRS with normal or high blood pressure, and inotropes or inodilators for patients with acute CRS with low blood pressure. Clinical data for a renal-protective action of levosimendan are suggestive, and meta-analysis data obtained in a range of low-output states are consistent with a levosimendan-induced benefit. Evidence of favourable organ-specific effects of levosimendan, including pre-glomerular vasodilation and increased renal artery diameter and renal blood flow, were collected both in preclinical and clinical studies. Larger randomized controlled trials are however needed to confirm the renal effects of levosimendan in various clinical settings.
Keywords: Levosimendan, Kidney, Acute heart failure, Advanced heart failure, Cardio-renal syndrome
Introduction
Renal dysfunction is a frequent comorbidity in heart failure (HF) and is linked to worse prognosis and quality of life.1 Five types of cardio-renal syndrome (CRS) with distinct pathophysiologies and clinical presentations were identified by Ronco et al. in 2008.2 In their words, ‘CRS can be generally defined as a pathophysiologic disorder of the heart and kidneys whereby acute or chronic dysfunction of one organ may induce acute or chronic dysfunction of the other.’ This statement is recognition that the concept of CRS indicates a pathological relationship between cardiovascular system and renal function in which either the heart or kidney may be the prime mover of a pathological state. Nevertheless, the two forms of CRS most widely encountered in HF are Types 1 and 2, in which the heart may be regarded as the precipitant or initiating organ (Table 1). About 30% of patients with acute de novo HF show worsening of renal function when treated (Type 1 CRS) and some 60% with decompensated HF exhibit reduced (<60 mL/min/1.73 m2) glomerular filtration rate (GFR) indicative of Type 2 CRS. In both cases, the impairment of renal function is an independent predictor of worse prognosis, including hospitalization for HF,3–5 and, in the case of chronic HF (i.e. Type 2 CRS), that influence is seen in patients with both preserved and reduced left ventricular ejection fraction (LVEF).6 In Type 1 CRS, the extent of renal impairment is a function of the severity of LVEF reduction.7 Risk factors for Type 2 CRS include hypertension, diabetes, atherosclerosis, and older age.2
Table 1.
Classification of cardio-renal syndrome (CRS)
| Typology of CRS | Description |
|---|---|
| Type 1 | Rapid worsening of cardiac function influences renal function leading to an acute kidney injury |
| Type 2 | Chronically abnormal heart function exerts chronic deleterious effects on renal function |
| Type 3 | Sudden worsening of renal function that leads to acute cardiac injury |
| Type 4 | Chronic primary renal disease that may result in the course of time in chronic heart damage |
| Type 5 | Cardiac dysfunction in conjunction with renal dysfunction due to a chronic systemic disease |
From Ronco et al.2
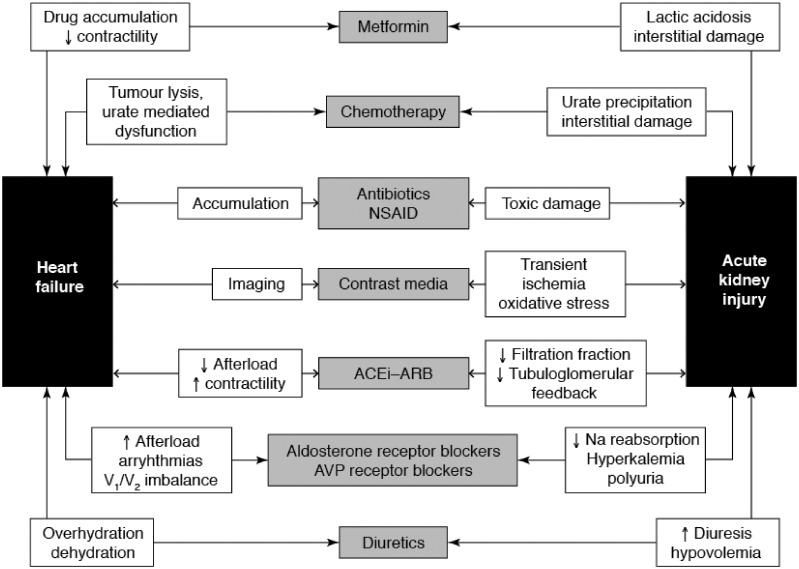
The mechanisms that lead to kidney damage in both these types of CRS are numerous and often complex. They include hypoperfusion, renal venous congestion, interstitial fibrosis, tubular damage and nephron loss caused by neurohormonal activation.8 The exact contribution of each process is likely to vary according to the particular circumstances of individual patients. Inter alia, it should be noted that a range of drugs and other substances (e.g. non-steroidal anti-inflammatory drugs, contrast media) may contribute to the development of renal impairment (Figure 1). Nevertheless, some general pathophysiological principles may be identified which, in turn, suggest a wide range of interventions to protect kidney function.
Figure 1.
A range of drugs may be implicated in the development of cardio-renal syndrome. See text for further discussion. NSAID, non-steroidal anti-inflammatory drug; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; AVP, arginine vasopressin.
Neurohormonal activity
Modulation of neuroendocrine activity is a major goal in the management of HF, with angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) among the primary agents for that purpose. However, those agents can also inhibit the autoregulation of glomerular blood flow leading to increased renal blood flow and, in some patients, to a reduction in GFR, a situation referred to as ‘pseudo-worsening renal function’.9 This effect is perhaps more pronounced at high doses and when intensive diuretic treatment is also being used10 but at least one large recent investigation in patients broadly approximating to a Type 2 CRS population found no evidence of a contribution of ACE inhibitor dosage to progression of chronic kidney disease.11 The scale of this potential hazard should be kept in proportion, particularly in Type 2 CRS, where the exceptional favourable effects of ACE inhibitors and ARBs on long-term survival and morbidity are a decisive consideration.12 In acute HF situations akin to Type 1 CRS, frequent measurement of renal function (blood urea, creatinine) and electrolytes is recommended12 and a case can be made for similar, although perhaps less intensive, monitoring in situations of chronic HF.11
One recent development in this area is the emergence of the dual ARB/neprilysin inhibitor LCZ696 (sacubitril–valsartan).13,14 Further research is needed to define the place of this agent.
Tolvaptan, a vasopressin inhibitor that reduces the reabsorption of free water, showed some beneficial short-term effects on weight, dyspnoea and oedema when given orally in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial but there was no evidence of significant benefits on cardiovascular death or hospitalization among 4133 patients with acute decompensated HF followed for 2 years.15 However, a subgroup analysis has confirmed that the benefits of improved symptoms, reduced body weight and increased serum sodium were attained in patients with hypotension and renal impairment.16 More recently, a series of investigations in patients with acute decompensated HF have reported that tolvaptan added to conventional therapy was more effective than conventional therapy alone in preventing deterioration of renal function in a population of high-risk patients with acute decompensated HF.17–19
Acute CRS with congestion
Despite their unquestioned utility for relief of congestion, loop diuretics may also precipitate acute kidney injury and Type 1 CRS in at-risk patients. Development of diuretic resistance via extra-renal mechanisms, such as reduction in levels of natriuretic peptides and activation of the sympathetic nervous system and renin–angiotensin system, and intra-renal mechanisms, such as increased tubular re-absorption of sodium and reductions in renal blood flow and GFR, is also a consideration.
Ultrafiltration has been advanced as a therapeutic approach for patients who already have, or are at risk of developing, diuretic resistance. In practice, however, the results of the Ultrafiltration Versus Intravenous (IV) Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) and Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trials offer little tangible support for this method, either in terms of its effects on renal function or its impact on larger prognostic criteria.20,21 It may be argued, however, that, at least in the short term, the benefits from relief of congestion outweigh longer-term considerations.
Diuretic resistance may arise from other physiological causes, which require specific therapeutic responses. It may be noted in passing that use of inotropes or vasodilators to increase the effective circulatory volume may be beneficial in cases where diuretic resistance is secondary to poor renal perfusion.
Acute CRS with normal or high blood pressure
High blood pressure is a frequent finding in acutely decompensated HF22 and calls for consideration of vasodilatation to unload the heart. The value of long-established agents such as nitroprusside and nitroglycerine should not be overlooked in this situation. In many cases, the improvement in cardiac output achieved through dilatation of the arterial circulation amply compensates for any fall in blood pressure, while venodilatation relieves congestion.23 The possibility of switching from intravenous vasodilator therapy to an oral regimen and maintaining the cardiac and haemodynamic effects while down-titrating dosage is a practical clinical consideration.24
Nesiritide is a recombinant B-type natriuretic peptide approved for the management of acute decompensated HF. Essentially a vasodilator in this context, nesiritide may be applicable to the treatment of patients in whom decompensated HF is characterized by neurohormonal activation and reduced EF and who have normal or relatively elevated systolic blood pressure (>110 mmHg).
Nesiritide produces modest improvements in dyspnoea and there is evidence for a renal-protective effect from small trials of a low-dose schedule (0.005 µg/kg/min without bolus).25,26 However, support for such an effect was not forthcoming from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial, which evaluated normal-dose nesiritide in addition to standard care including diuretics.27,28 The Renal Optimization Strategies Evaluation (ROSE) study revisited a low-dose nesiritide intervention (0.005 µg/kg/min over 72 h) in addition to standard therapy in patients with acute decompensated HF and kidney dysfunction and recorded no additional benefit on congestion or renal function.29 (There were trends towards lower serum cystatin C levels and improved urinary output in patients who had low EF or low systolic blood pressure.29) Meta-analyses of clinical experience with nesiritide performed in 2005 and again in 2016 identified either a significantly increased risk of worsening renal function30 or no obvious renal-protective benefit and some cardiovascular hazards (hypotension, bradycardia).31
Acute CRS with low blood pressure
When acute CRS presents in the context of low blood pressure, use of inotropes or inodilators may be appropriate to restore or preserve renal function by improving cardiac performance and hence renal perfusion. Options for this purpose include adrenergic (beta) agonists (e.g. dobutamine), phosphodiesterase inhibitors (e.g. milrinone) and calcium sensitizers (e.g. levosimendan). Reference must also be made to a recent retrospective analysis from the Digitalis Investigation Group (DIG) which reported that treatment with digoxin was associated with long-term improvement in kidney function, and that the overall effect of digoxin therapy in reducing death and hospitalizations was most pronounced among those patients exhibiting this favourable renal response.32
Use of low-dose dopamine with low-dose intravenous furosemide has been reported to be associated with lower rates of worsening renal function and electrolyte disturbances than high-dose intravenous furosemide alone in the Dopamine in Acute Decompensated Heart Failure (DAD-HF) trial.33 Worsening renal failure was more frequent with high-dose furosemide than with combination treatment (9 vs. 2 cases; P = 0.042) but substantial clinical endpoints (length of stay, 60-day mortality, re-hospitalization rates) were comparable in both groups.33 A similar disjunction between short-term renal outcomes and longer-term clinical effects has previously been reported from a meta-analysis of the effects of low-dose dopamine in >3300 patients at risk of acute renal failure.34 The ROSE trial, which involved 360 hospitalized patients with acute HF, could not demonstrate any improvements in decongestion or renal function when low-dose dopamine was added to standard diuretic therapy.29
Such data illustrate a general concern that use of conventional inotropes may worsen long-term prognosis, perhaps through adverse effects on the energy economy of at-risk tissues and organs subject to ischaemia.35,36 Levosimendan, an inodilator that enhances cardiac contractility and also has vasodilator and ischaemia-protective effects, exerted via opening of glibenclamide-sensitive cellular and mitochondrial calcium–adenosine triphosphate (ATP) channels, may represent an important alternative therapy in this setting. Evidence for a renal-protective action of levosimendan in preclinical experiments is persuasive but the clinical dataset supporting a renal-protective effect rests on a limited number of studies, many of them small and sometimes characterized by methodological limitations,37 and their results acquire significance only when pooled in meta-analyses.38 However, the findings of those meta-analyses and related investigations are suggestive of a renal-protective effect of levosimendan in a range of low-output states. Thus, Rafouli-Stergiou et al.39 have reported theoretically favourable effects on a range of markers of kidney function in patients with acutely decompensated HF and renal impairment, while meta-analyses in critical illness settings,40 in cardiac surgery41 and in heart transplantation42 have all produced findings indicative of a renal-protective effect of levosimendan.
Several lines of evidence suggest that any renal-protective effect of levosimendan is exerted at least in part via organ-specific effects, including pre-glomerular vasodilation and increased renal artery diameter and renal blood flow, without compromising renal oxygenation;43,44 signs of improved renal function are apparent before increases in cardiac index or left ventricular performance.43,45 This would be consistent with the observation in the Levosimendan Infusion versus Dobutamine (LIDO) trial,46 in which levosimendan was compared with dobutamine in severe low-output HF. In LIDO, dobutamine increased cardiac index and urine output but did not improve GFR, whereas levosimendan did. Also of note in this context is the observation of qualitative differences in the renal effects of levosimendan and dobutamine;47 the authors of that study suggested that the difference between the two drugs may be that the capacity of levosimendan to promote arterial and venous vasodilation through activation of ATP-sensitive potassium channels contributes to a lessening of central venous pressure, a property not shared by dobutamine and one that may be an important influence on GFR in some patients. A protective effect against kidney ischaemia/reperfusion injuries was also shown.48
These data are all suggestive of a renal-protective effect of levosimendan. Larger randomized controlled trials to evaluate possible renal effects of levosimendan in different clinical settings are, however, required. Pending the completion of such studies, levosimendan should be dosed according to published guidance and established principles, especially with regard to the presumption against use of initial bolus doses. Caution should always be exercised in patients with intrinsic kidney failure.
Drugs providing local renal protection
This category of interventions includes adenosine antagonists, exemplified by rolofylline. In theory, these inhibit the reductions in renal blood flow and GFR and the increased reabsorption of sodium and water that follow stimulation of renal adenosine A1 receptors by endogenous adenosine. Initial investigations were encouraging but the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial tested the effects of rolofylline as a renal-protective strategy in 2033 patients with acute HF and the results indicated no beneficial effect of rolofylline in this setting.49 Later subgroup analysis identified the possibility of a mortality benefit in subgroups of initially high-risk patients but that was offset by an increased mortality risk in lower-risk patients50 and will require further investigation.
Empagliflozin is a selective inhibitor of the sodium–glucose co-transporter in the proximal tubule and increases urinary excretion of sodium and glucose. That mechanism has been exploited to reduce rates of hyperglycaemia in patients with type 2 diabetes and has been shown in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) study to improve survival and reduce the likelihood of hospitalization for HF in patients with type 2 diabetes at high risk for cardiovascular events.51 The incidences of acute renal failure and acute kidney injury were also reduced significantly, although the absolute percentage reductions were small (0.6–1.4%). The applicability of this therapy to non-diabetic patients remains to be evaluated.
Also noteworthy in this category of candidate therapies is serelaxin (recombinant human relaxin-2 vasoactive peptide). In the Relaxin in Acute Heart Failure (RELAX-AHF) trial, which involved 1100 patients with acute HF plus dyspnoea, congestion, mild-to-moderate renal insufficiency and systolic blood pressure >125 mmHg, intravenous infusion of 30 µg/kg/day serelaxin for 48 h was associated with a significant early improvement in dyspnoea by visual analogue scale (but not by Likert scale) and, strikingly, by a significant reduction in 180-day mortality (although not with significant reductions in readmission to hospital for HF or renal failure).52
Conclusions
Renal impairment is very common in acute HF patients, who may also experience worsening of kidney function during hospitalization. The treatment of CRS in decompensated HF is a complex clinical challenge and there are currently only limited high-quality data to shape therapeutic choices. Moreover, the situation is one in which pathophysiology varies markedly between different patients. Identifying the underlying processes of kidney dysfunction is essential to successful management. We concur with Verbrugge et al.24 about volume status as a principal triaging indicator and with their identification of six factors proceeding from that initial staging, namely: accurate assessment of volume status; aggressive treatment of volume overload; avoidance of arterial hypotension and intravascular underfilling; removal of fluid accumulation in third spaces; increasing the effective circulatory volume; and optimization of renal perfusion.
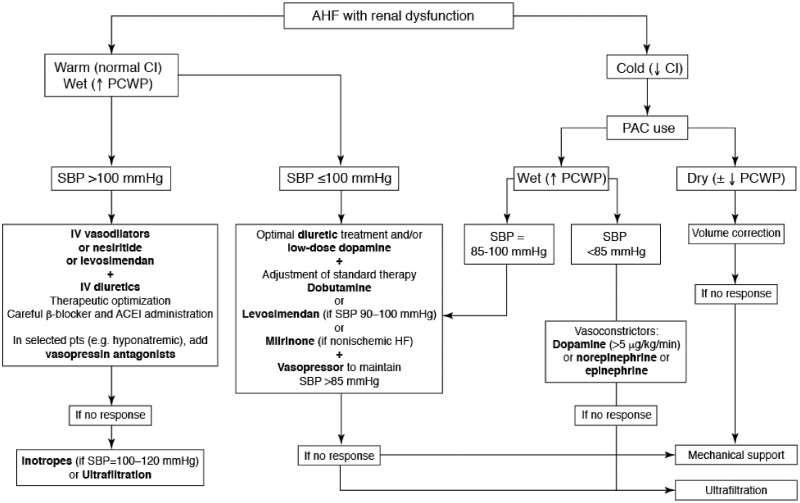
Inotropes may be indicated for short-term management of acute HF with renal dysfunction, mostly in cases of low-output HF that can provoke renal hypoperfusion. Parenterally administered inodilators are underutilized, especially in ‘wet’ patients (those with preserved or low blood pressure) who do not respond to diuretics (Figure 2).
Figure 2.
A treatment algorithm for the management of patients with acute heart failure plus renal dysfunction. From Rafouli-Stergiou et al.39 AHF, acute heart failure; CI, cardiac index; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; PAC, pulmonary artery catheter; IV, intravenous; ACEi, angiotensin-converting enzyme inhibitor; HF, heart failure.
Acknowledgements
We thank Hughes associates, Oxford, UK for editing the language of this manuscript.
Conflict of interest: none declared.
References
- 1. Maeder MT, Rickli H, Pfisterer ME, Muzzarelli S, Ammann P, Fehr T, Hack D, Weilenmann D, Dieterle T, Kiencke S, Estlinbaum W, Brunner-La Rocca HP.. TIME-CHF Investigators. Incidence, clinical predictors, and prognostic impact of worsening renal function in elderly patients with chronic heart failure on intensive medical therapy. Am Heart J 2012;163:407–414. [DOI] [PubMed] [Google Scholar]
- 2. Ronco C, Haapio M, House AH, Anavekar N, Bellomo R.. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D.. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J 2005;150:330–337. [DOI] [PubMed] [Google Scholar]
- 4. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J.. ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422–430. [DOI] [PubMed] [Google Scholar]
- 5. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL.. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 2007;13:599–608. [DOI] [PubMed] [Google Scholar]
- 6. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ.. Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006;113:671–678. [DOI] [PubMed] [Google Scholar]
- 7. Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD.. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol 2006;17:2886–2891. [DOI] [PubMed] [Google Scholar]
- 8. Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA.. The role of the kidney in heart failure. Eur Heart J 2012;33:2135–2142. [DOI] [PubMed] [Google Scholar]
- 9. Damman K, Tang WH, Testani JM, McMurray JJ.. Terminology and definition of changes renal function in heart failure. Eur Heart J 2014;35:3413–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Vecchis R, Di Biase G, Ariano C, Cioppa C, Giasi A, Ciccarelli A, Pucciarelli A, Cantatrione S.. ACE-inhibitor therapy at relatively high doses and risk of renal worsening in chronic heart failure. Arq Bras Cardiol 2011;97:507–516. [DOI] [PubMed] [Google Scholar]
- 11. Fröhlich H, Nelges C, Täger T, Schwenger V, Cebola R, Schnorbach J, Goode KM, Kazmi S, Katus HA, Cleland JG, Clark AL, Frankenstein L.. Long-term changes of renal function in relation to ACE inhibitor/angiotensin receptor blocker dosing in patients with heart failure and chronic kidney disease. Am Heart J 2016;178:28–36. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR.. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 14. Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, Solomon SD.. PARAMOUNT Investigators. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015;17:510–517. [DOI] [PubMed] [Google Scholar]
- 15. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C.. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 16. Vaduganathan M, Gheorghiade M, Pang PS, Konstam MA, Zannad F, Swedberg K, Grinfeld L, Burnett JC Jr, Krasa HB, Zimmer C, Blair J, Ouyang J, Maggioni AP.. EVEREST Investigators. Efficacy of oral tolvaptan in acute heart failure patients with hypotension and renal impairment. J Cardiovasc Med (Hagerstown) 2012;13:415–422. [DOI] [PubMed] [Google Scholar]
- 17. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR.. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016;22:423–432. [DOI] [PubMed] [Google Scholar]
- 18. Matsue Y, Suzuki M, Seya M, Iwatsuka R, Mizukami A, Nagahori W, Ohno M, Matsumura A, Hashimoto Y.. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure in high-risk population. J Cardiol 2013;61:169–174. [DOI] [PubMed] [Google Scholar]
- 19. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR.. Prognostic impact of early treatment with tolvaptan in patients with acute heart failure and renal dysfunction. Int J Cardiol 2016;221:188–193. [DOI] [PubMed] [Google Scholar]
- 20. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E.. Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA.. UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683. Erratum: J Am Coll Cardiol 2007; 49:1136. [DOI] [PubMed] [Google Scholar]
- 22. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP.. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 23. Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, Taylor DO, Tang WH.. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 2008;52:200–207. [DOI] [PubMed] [Google Scholar]
- 24. Verbrugge FH, Dupont M, Finucan M, Gabi A, Hawwa N, Mullens W, Taylor DO, Young JB, Starling RC, Tang WH.. Response and tolerance to oral vasodilator up-titration after intravenous vasodilator therapy in advanced decompensated heart failure. Eur J Heart Fail 2015;17:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC. Jr.. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation 2007;116:I134–I138. [DOI] [PubMed] [Google Scholar]
- 26. Riter HG, Redfield MM, Burnett JC, Chen HH.. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol 2006;47:2334–2335. [DOI] [PubMed] [Google Scholar]
- 27. O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM.. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 28. Gottlieb SS, Stebbins A, Voors AA, Hasselblad V, Ezekowitz JA, Califf RM, O'Connor CM, Starling RC, Hernandez AF.. Effects of nesiritide and predictors of urine output in acute decompensated heart failure: results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure). J Am Coll Cardiol 2013;62:1177–1183. [DOI] [PubMed] [Google Scholar]
- 29. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Dávila-Román VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM.. NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sackner-Bernstein JD, Skopicki HA, Aaronson KD.. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 2005;111:1487–1491. [DOI] [PubMed] [Google Scholar]
- 31. Gong B, Wu Z, Li Z.. Efficacy and safety of nesiritide in patients with decompensated heart failure: a meta-analysis of randomised trials. BMJ Open 2016;6:e008545.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Testani JM, Brisco MA, Tang WH, Kimmel SE, Tiku-Owens A, Forfia PR, Coca SG.. Potential effects of digoxin on long-term renal and clinical outcomes in chronic heart failure. J Card Fail 2013;19:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschöpe C, Triposkiadis F.. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail 2010;16:922–930. [DOI] [PubMed] [Google Scholar]
- 34. Friedrich JO, Adhikari N, Herridge MS, Beyene J.. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 2005;142:510–524. [DOI] [PubMed] [Google Scholar]
- 35. Thackray S, Easthaugh J, Freemantle N, Cleland JG.. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta-regression analysis. Eur J Heart Fail 2002;4:515–529. [DOI] [PubMed] [Google Scholar]
- 36. Morgan CJ, Gill PJ, Lam S, Joffe AR.. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med 2013;39:934–941. [DOI] [PubMed] [Google Scholar]
- 37. Yilmaz MB, Grossini E, Silva Cardoso JC, Édes I, Fedele F, Pollesello P, Kivikko M, Harjola VP, Hasslacher J, Mebazaa A, Morelli A, Le Noble J, Oldner A, Oulego Erroz I, Parissis JT, Parkhomenko A, Poelzl G, Rehberg S, Ricksten SE, Rodríguez Fernández LM, Salmenperä M, Singer M, Treskatsch S, Vrtovec B, Wikström G.. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther 2013;27:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pisano A, Monti G, Landoni G.. Levosimendan: new indications and evidence for reduction in perioperative mortality? Curr Opin Anaesthesiol 2016;29:454–461. [DOI] [PubMed] [Google Scholar]
- 39. Rafouli-Stergiou P, Parissis JT, Farmakis D, Bistola V, Frogoudaki A, Vasiliadis K, Ikonomidis I, Paraskevaidis I, Kremastinos D, Filippatos G, Lekakis J.. Effects of levosimendan on markers of kidney function in patients with acutely decompensated heart failure and renal impairment. J Cardiovasc Med (Hagerstown); doi:10.2459/JCM.0000000000000244. Published online ahead of print 31 January 2015. [DOI] [PubMed] [Google Scholar]
- 40. Bove T, Matteazzi A, Belletti A, Paternoster G, Saleh O, Taddeo D, Dossi R, Greco T, Bradic N, Husedzinovic I, Nigro Neto C, Lomivorotov VV, Calabrò MG.. Beneficial impact of levosimendan in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Heart Lung Vessel 2015;7:35–46. [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B.. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016;67:408–416. [DOI] [PubMed] [Google Scholar]
- 42. Knezevic I, Poglajen G, Hrovat E, Oman A, Pintar T, Wu JC, Vrtovec B, Haddad F.. The effects of levosimendan on renal function early after heart transplantation: results from a pilot randomized trial. Clin Transplant 2014;28:1105–1111. [DOI] [PubMed] [Google Scholar]
- 43. Fedele F, Bruno N, Brasolin B, Caira C, D’Ambrosi A, Mancone M.. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail 2014;16:281–288. [DOI] [PubMed] [Google Scholar]
- 44. Bragadottir G, Redfors B, Ricksten SE.. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med 2013;41:2328–2335. [DOI] [PubMed] [Google Scholar]
- 45. Zemljic G, Bunc M, Yazdanbakhsh AP, Vrtovec B.. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Card Fail 2007;13:417–421. [DOI] [PubMed] [Google Scholar]
- 46. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L.. Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002;360:196–202. [DOI] [PubMed] [Google Scholar]
- 47. Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I.. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther 2007;21:431–435. [DOI] [PubMed] [Google Scholar]
- 48. Grossini E, Molinari C, Pollesello P, Bellomo G, Valente G, Mary D, Vacca G, Caimmi P.. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther 2012;342:376–388. [DOI] [PubMed] [Google Scholar]
- 49. Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O’Connor CM, Givertz MM.. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function.). J Am Coll Cardiol 2011;57:1899–1907. [DOI] [PubMed] [Google Scholar]
- 50. Demissei BG, Postmus D, Liu LC, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Edwards C, Givertz MM, Bloomfield DM, Dittrich HC, Voors AA, Hillege HL.. Risk-based evaluation of efficacy of rolofylline in patients hospitalized with acute heart failure—post-hoc analysis of the PROTECT trial. Int J Cardiol 2016;223:967–975. [DOI] [PubMed] [Google Scholar]
- 51. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE.. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 52. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M.. RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]