Abstract
Levosimendan is an inodilator developed for treatment of acute heart failure. It was shown to enhance cardiac contractility, and to exert a vasodilatory effect in all vascular beds. In some trials, the use of levosimendan was associated with cardioprotective effects. These distinctive qualities may be relevant to its use in a range of acute heart failure settings and/or complications, including acute coronary syndromes and cardiogenic shock. It is conjectured that part of the benefit of levosimendan may arise from restoration of ventriculo-arterial coupling via optimization of the ratio of arterial to ventricular elastance and the transfer of mechanical energy. Full confirmation of the effectiveness of levosimendan is still awaited in many of these scenarios; however, the range of potential applications highlights both the versatility of levosimendan and the relative lack of proven interventions in many of these situations.
Keywords: Levosimendan, Inodilator, Cardiogenic shock, Acute coronary syndromes, Pulmonary hypertension, Left ventricular assist device
Levosimendan: context and some clinical applications
The latest iteration of comprehensive guidelines on all aspects of the diagnosis and treatment of acute and chronic heart failure (HF) was published by the European Society of Cardiology (ESC) in 2016.1 These guidelines form a framework from which to contextualize some clinical applications of levosimendan.
Full ESC recommendations for the use of inotropic drugs, including levosimendan, are shown in Table 1 and provide the basis for further discussion of the use of this agent. An essential point to make at the outset, however, is that levosimendan is set apart from other agents in the broad ‘inotropes’ class by the fact that it is the first such agent to promote cardiac contractility without increasing intracellular levels of ionic calcium in cardiomyocytes.2 This is an important distinguishing feature because elevated intracellular calcium levels have been correlated with increase in oxygen consumption, arrhythmia, remodelling, apoptosis, and general bad outcome when used in severely decompensated patients.3
Table 1.
2016 European Society of Cardiology recommendations for the use of inotropic drugs (dobutamine, dopamine, levosimendan, and PDE type-III inhibitors) in acute heart failure
| Level of evidence | ||
|---|---|---|
| Short-term, intravenous infusion of inotropic agents may be considered in patients with hypotension (SBP <90 mmHg) and/or signs/symptoms of hypoperfusion, despite adequate filling status, to increase cardiac output, increase blood pressure, improve peripheral perfusion and maintain end-organ function | IIb | C |
| An intravenous infusion of levosimendan or a PDE-III inhibitor may be considered to reverse the effect of beta-blockade if beta-blockade is thought to be contributing to hypotension with subsequent hypoperfusion | IIb | C |
| Inotropic agents are not recommended unless the patient is symptomatically hypotensive or hypoperfused because of safety concerns | III | A |
Reproduced with permission from Ponikowski et al.1
SBP, systolic blood pressure; PDE, phosphodiesterase.
Despite these theoretical attractions, the clinical development of levosimendan has not been linear: 4 Phase IIb–III regulatory studies (including LIDO and RUSSLAN) on a total of 1000 patients4–7 produced promising outcomes but a second round of Phase III regulatory trials on a total of 2000 patients produced more equivocal results.8,9 Nevertheless, a propensity score analysis based on a global registry of 5000 patients (ALARM-HF10) showed superiority of levosimendan compared with all conventional inotropes, inodilator, and inopressor as regards survival. This finding is supported by two recent meta-analyses that reported a favourable impact on mortality with levosimendan compared with either placebo or inotropes such as dobutamine.11,12
Levosimendan has in fact three distinct pharmacological actions:13
It exerts an inotropic effect by increasing the sensitivity of troponin C to ionic calcium in myocardial cells.
It causes vasodilatation by opening adenosine triphosphate-sensitive potassium channels (KATP channels) in smooth muscle cells.
It activates KATP channels in mitochondria, a property that seems to be central to its effects in protecting myocytes (and potentially other cell types) against ischaemia/reperfusion injury and similar insults.
Each and all of these qualities may be relevant to a range of HF settings and/or complications in which the drug may be used,14 including acute coronary syndromes (ACS), cardiogenic shock, as well as its use in conjunction with pulmonary hypertension, and left ventricular assist devices (LVADs). Those situations are briefly examined in this essay to illustrate the contributions of levosimendan as found in the clinical literature.
HF after ACS
The energy balance of the injured myocardium and adjacent tissue is often critically compromised in ACS. This is therefore a setting in which the ability of levosimendan to preserve and enhance myocardial function without increasing myocardial oxygen consumption or energy demand is a critical distinction between it and other drugs used to promote inotropy.
Levosimendan has been shown to improve systolic and diastolic function of stunned myocardium in the context of ACS and primary angioplasty15,16 and its use has been associated with improved outcomes in patients undergoing coronary artery bypass grafting surgery.17
There is extensive evidence that levosimendan may protect the myocardium against ischaemia and reperfusion injury. This effect is believed to be exerted principally via its action at mitochondrial KATP channels,18,19 but modulation of nitric oxide synthesis and the phosphoinositide-3-kinase pathway also seem to play a role.20,21
A group of European experts recently reviewed the role of levosimendan in acute HF complicating ACS and affirmed that: (i) levosimendan offers potential benefits in this setting due to a range of distinct effects, including positive inotropy, restoration of ventriculo-arterial coupling, increases in tissue perfusion, and anti-stunning and anti-inflammatory effects; (ii) in clinical trials, levosimendan improves symptoms, cardiac function, haemodynamics, and end-organ function; and (iii) adverse effects are generally less common than with other inotropic and vasoactive therapies, with the notable exception of hypotension.22
These favourable considerations must be qualified by the fact that only one large randomized trial has examined the use of levosimendan in this setting. The Randomised Study on Safety and Effectiveness of Levosimendan in Patients with Left Ventricular Failure after an Acute Myocardial Infarct (RUSSLAN) trial was a placebo-controlled, double-blind, parallel-group, randomized study in 504 patients enrolled within 5 days of an index infarction.7 Patients randomized to levosimendan were treated with a bolus dose of 6–24 μg/kg in 10 min, followed by a 6-h intravenous infusion at rates ranging from 0.1 to 0.4 μg/kg/min. Despite its positive results on mortality in both short- and long-term, RUSSLAN had some limitations: it was primarily an evaluation of the safety of levosimendan, not its efficacy; invasive haemodynamic data were not collected; and it compared four different dosing schedules (some of which were not adopted for the clinical practice recommendation) with a placebo arm.
Cardiogenic shock
The pathophysiological considerations identified for ACS are also pertinent in cardiogenic shock. Preservation or promotion of cardiac function is important and levosimendan has been demonstrated to achieve that goal.23 The use of levosimendan may be indirectly beneficial by reducing the requirement for catecholaminergic agents that exert less favourable effects on oxygen and energy consumption at the cellular level and demonstrate a propensity towards increased mortality.10,24
Of particular interest in this setting is the ability to use levosimendan successfully and without dose adjustment in patients who are on beta-blockers at presentation.9 This is not a quality shared by all inotropic drugs and assumes importance when it is considered that continuation of beta-blocker treatment is linked to significantly better prospects of survival.25
Table 2 summarizes the requirements for an ‘ideal’ inotrope and the extent to which available agents meet those requirements. No drug fully satisfies these criteria but, in the context of cardiogenic shock, levosimendan may offer some specific advantages, notably its ability to promote inotropy with little or no adverse effect on metabolic rate, energy demand or oxygen consumption in what is often a critically compromised myocardium.
Table 2.
The requirements for an ‘ideal’ inotrope and the extent to which available agents meet those requirements
| Calcium sensitizer | Beta-adrenergic agonist | Phosphodiesterase inhibitor | |
|---|---|---|---|
| Increased intracellular Ca2+ | No | Yes | Yes |
| Increased cAMP | No | Yes | Yes |
| Increased cardiac contractility | Yes | Yes | Yes |
| Increased oxygen demand | No | Yes | Yes |
| Tachyphylaxis | No | Yes | No |
| Antagonized by beta-blockers? | No | Yes | No |
| Adverse effects | Hypotension, headache | Tachycardia, arrhythmias | Hypotension, arrhythmias |
cAMP, cyclic adenosine monophosphate.
These energy-neutral inotropic effects of levosimendan are a striking contrast to the effects of other agents, whose haemodynamic improvements are often delivered at the cost of increased oxygen demand when the heart is failing as a contractile organ and the oxygen supply is already precarious. Other interventions of interest in this respect include omecamtiv medoxil, istaroxine, and sarcoplasmic reticulum Ca2+-ATPase modulation but data are lacking on these initiatives in cardiogenic shock.
These theoretically favourable considerations must be tempered by the consideration that formal experience with levosimendan in cardiogenic shock is limited26,27 and although the drug appeared to be generally well tolerated, improved multiple haemodynamic indices and was linked with substantially lower 30-day mortality than enoximone in one randomized (but open-label) trial,26 there is as yet no substantial indication of improved longer-term outcomes.22
Levosimendan has been reported to exert positive effects on ventriculo-arterial coupling in post-cardiotomy acute HF28,29 and in situations of ischaemic cardiomyopathy30 and those effects may be of interest in the context of cardiogenic shock.
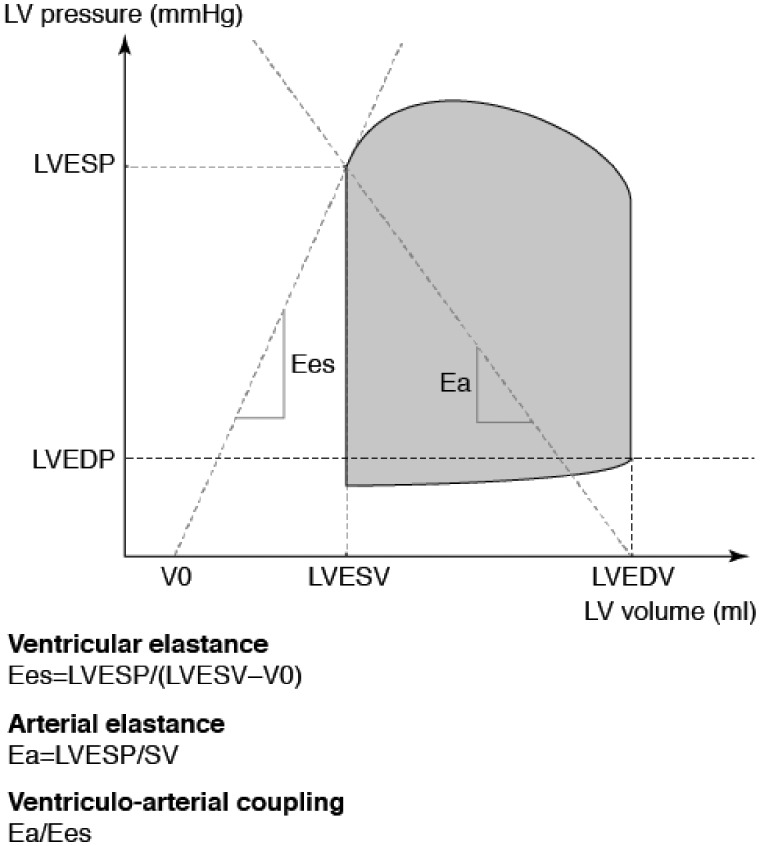
The area of the ventricular pressure–volume loop represents the total mechanical energy generated by the contraction of the left ventricle until the end of systole and is strongly and linearly correlated with myocardial oxygen consumption. The transfer of mechanical energy (i.e. stroke work from the ventricle to the major arteries) is optimal when the ratio of the arterial (Ea) and ventricular (Ees) elastances is in the range 0.8–1.1; higher values, as may be encountered in HF, cardiogenic or septic shock and a range of other conditions, are sub-optimal and identify a situation where the output of the heart is not providing maximal advantage for a quotient of energy consumption (In cardiogenic shock, the usual primary contributor to an elevated Ea/Ees ratio is the acute reduction in Ees31).
The physiological derivation of the two elastances is illustrated in Figure 1. One point to infer from this diagram is that any situation that displaces the pressure–volume curve to the right and upwards has potential to reduce Ees and increase Ea, thus producing an unfavourable Ea:Ees ratio. Such displacements may be the result of a decline in contractility (lower Ees) or increased afterload (raised Ea). In practice, all the necessary measurements and calculations may be made at the bedside, aided by echocardiography.32,33
Figure 1.
Derivation of arterial elastance (Ea) and ventricular elastance (Ees) for the assessment of ventriculo-arterial coupling efficiency. The pressure–volume loop area is shaded. The slopes of Ees and Ea are shown. See text for further discussion. LVESV, left ventricular end-systolic volume; LVESP, left ventricular end-systolic pressure; LVEDV, left ventricular end-diastolic volume; LVEDP, left ventricular end-diastolic pressure; LV, left ventricular; SV, stroke volume; V0, theoretical volume when no pressure is generated. Reproduced with permission from Guarracino et al.31
Whether or not rebalancing of the Ea:Ees ratio represents a new and productive therapeutic target is as yet undetermined but it is a concept deserving of investigation. Similarly, the impact of levosimendan on ventriculo-arterial coupling requires further and more detailed evaluation.
General advice for dosing of levosimendan in cardiogenic shock is to avoid bolus dosing in order to minimize the risk of hypotension and to administer a 24-h infusion at a rate of 0.05–0.1 µg/kg/min. The option exists to start with an infusion rate of 0.2 µg/kg/min for the first 60 min if a more rapid onset of effect is required.22 The circulating fluid volume should be assessed and optimized before initiating therapy. During levosimendan infusion, vascular tone should be manipulated to maintain mean arterial pressure at ≥70 mmHg.
Levosimendan use in conjunction with an LVAD
A major peri-operative hazard for the patient who receives an LVAD is RV failure secondary to raised pulmonary vascular resistance (PVR). This is characterized by LVAD dysfunction (due to under-filling of the left ventricle) and a low cardiac output (CO) state, hypotension, renal dysfunction, and bleeding (due to raised central venous pressure).
Estimates of the incidence of right-sided HF after placement of an LVAD vary but there is uniform agreement that the problem is widespread34,35 and that right-sided HF is associated with marked deterioration of survival prospects.36
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) has proposed criteria for the identification of post-LVAD right-sided HF37 and has identified three categories of severity:
Severe—the clinical response is installation of a right ventricular assist device (RVAD).
Moderate—inotropes or intravenous or inhaled pulmonary vasodilators are used.
Mild—a combination of ≥ 2 signs and symptoms is present but without the need for an RVAD or inotropic and/or vasodilator support.
In the INTERMACS category of moderate right-sided HF, the right ventricle can be supported with inotropes or inodilators such as milrinone, dobutamine or levosimendan, all of which facilitate pulmonary vasodilation. (Vasopressors such as dopamine and adrenaline should in general be avoided unless needed to overcome a vasodilatory situation and maintain systemic blood pressure.)
Current practice emphasizes the early weaning of inotropes, partly in response to a report that mortality is strongly correlated with duration of inotrope use.38 It should be noted, however, that those data were derived exclusively from patients who received milrinone or dobutamine. This may be an extension of the observation that conventional inotropes are associated with increased mortality in HF.11
In contrast, no similar deterioration in survival is apparent from meta-analyses of levosimendan in left-sided HF.12 It must be emphasized that there are at present no data from randomized controlled clinical trials for levosimendan in the setting of LVAD-associated right-sided HF. However, recently published data provide some indications that levosimendan may be useful as a predictive or prognostic instrument in this situation. In an uncontrolled study, observations on 21 patients who received pre-operative levosimendan (0.1–0.2 μg/kg/min for 48 h) in anticipation of LVAD implantation demonstrated significant (P < 0.05) improvements in cardiac index, pulmonary artery pressure, and central venous pressure, as might have been predicted, but also identified the lack of a significant reduction in the median level of N-terminal pro-brain natriuretic peptide after levosimendan infusion as strongly predictive of post-operative death due to right-sided HF.39
Levosimendan and pulmonary hypertension in advanced HF
Pulmonary hypertension/elevated PVR are recognized as relative contraindications to heart transplantation (HTx) because of the potential for causing right-sided HF.40 (For these purposes, ‘elevation’ is defined as PVR >5 Wood units, PVR index >6 or a transpulmonary pressure gradient in excess of 16–20 mmHg.)
Bringing PVR within acceptable limits may therefore be a crucial influence on a patient’s eligibility for a heart transplant. Where it is anticipated that the wait for a heart transplant will be lengthy, use of an LVAD to maintain PVR within acceptable limits is the appropriate course of action and has proven benefits.41 Where the waiting time is expected to be relatively short, or for patients for whom an LVAD is not an option, inodilator therapy may be regarded as a preferred option that avoids the surgical risk attendant on implantation of an LVAD. The objectives of therapy in this setting are to improve left ventricular contractility and reduce systemic vascular resistance. The resulting reduction in left atrial filling pressure is desirable as this has a substantial influence on PVR. It is well established that levosimendan reduces both PVR and pulmonary capillary wedge pressure4 and, as with several other aspects of its clinical profile, this effect is well sustained.42
As a limitation, we must emphasize that the effect of levosimendan in patients with right-side HF and pulmonary hypertension has not been studied in properly powered randomized studies.
Another important limitation is that levosimendan has not been tested in models or patients with clearly defined pre-capillary or combined pulmonary hypertension. It cannot therefore confidently be excluded that the effects of the drug might be detrimental in patients with pre-capillary pulmonary hypertension (with or without right-side HF). Caution is required if using levosimendan (or any other inodilator) in such patients.
These considerations notwithstanding, the capacity for occasional or intermittent use differentiates levosimendan from drugs such as dobutamine or milrinone and makes levosimendan preferable from the perspectives of patient convenience and the reduced risk of infection conferred by the avoidance of an indwelling catheter.
Levosimendan as a bridging therapy
In situations such as cardiogenic shock inodilators may be used, normally in conjunction with vasopressors, as part of life-sustaining medical care prior to emergency HTx or installation of a LVAD. In patients with advanced chronic HF who require (and are eligible for) either intervention, the time-frame is often substantially longer and the importance of inodilators such as levosimendan lies in their contribution to the twin goals of:
preserving renal function
lowering PVR and, with it, the risk of right ventricular (RV) failure.
Transplantation in the setting of significant renal dysfunction and unmanaged fluid overload often leads to post-transplantation right-sided HF and death. Renal function data from large registry sources are amply revealing of the adverse impact of pre-transplant renal dysfunction, as represented by serum creatinine levels43,44 or estimated glomerular filtration rate (eGFR),45 on the outcomes of HTx. Some of the roots of this unwelcome influence are located in what may be characterized as a ‘troublesome triad’ in which declining cardiac contractile performance undermines renal function and vice versa.
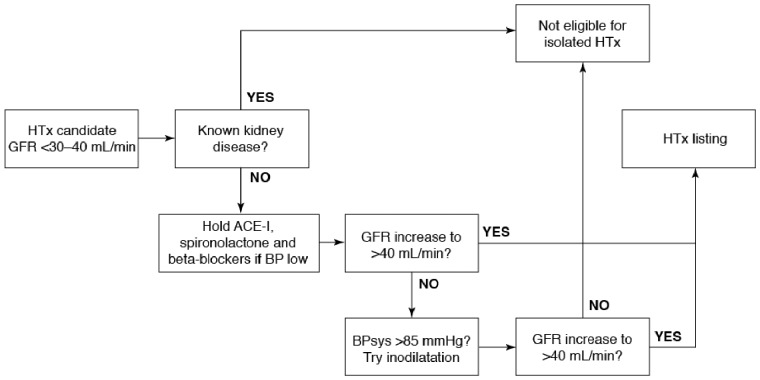
The value of inodilation in this setting is that it can break the cycle of debilitating organ dysfunction; at the same time, it may serve an important diagnostic role by differentiating primary renal disease (which is likely to rule out HTx) from impaired kidney function secondary to cardio-renal syndrome. The principles of this differentiation are illustrated in Figure 2, which shows how the GFR response to inodilation may be used to guide a patient’s eligibility for a place on the heart transplant register. (It should be noted that the box in the top right-hand corner of Figure 2 identifies patients who require combined heart and kidney transplantation; these are likely to be relatively young patients.)
Figure 2.
The GFR response to inodilatation may be used to guide a patient’s eligibility for a place on the heart transplant register. HTX, heart transplantation; ACE-I, angiotensin-converting enzyme inhibitor; GFR, glomerular filtration rate; BB, beta-blockers; BP, blood pressure; sys, systolic.
Conclusions
Levosimendan is approved for acutely decompensated HF characterized by low CO. Various lines of evidence, several of which have been reviewed in this essay, suggest that levosimendan may in addition be beneficial in a range of circumstances associated with acutely decompensated HF, including ACS and cardiogenic shock. However, the evidence currently available on many of these applications is preliminary: further clinical investigation and assessment in properly powered clinical trials will be needed to validate these possibilities.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Brixius K, Reicke S, Schwinger RH.. Beneficial effects of the Ca(2+) sensitizer levosimendan in human myocardium. Am J Physiol Heart Circ Physiol 2002;282:H131–H137. [DOI] [PubMed] [Google Scholar]
- 3. Pollesello P, Papp Z, Papp JG.. Calcium sensitizers: what have we learned over the last 25 years? Int J Cardiol 2016;203:543–548. [DOI] [PubMed] [Google Scholar]
- 4. Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W.. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study Investigators. Circulation 2000;102:2222–2227. [DOI] [PubMed] [Google Scholar]
- 5. Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ.. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 2000;36:1903–1912. [DOI] [PubMed] [Google Scholar]
- 6. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L.. Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002; 360:196–202. [DOI] [PubMed] [Google Scholar]
- 7. Moiseyev VS, Põder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, Kobalava ZD, Lehtonen LA, Laine T, Nieminen MS, Lie KI.. RUSSLAN Study Investigators. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 2002; 23:1422–1432. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T.. REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013;1:103–111. [DOI] [PubMed] [Google Scholar]
- 9. Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M.. SURVIVE Investigators Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 10. Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, Delgado JF, Follath F.. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med 2011;37:290–301. [DOI] [PubMed] [Google Scholar]
- 11. Belletti A, Castro ML, Silvetti S, Greco T, Biondi-Zoccai G, Pasin L, Zangrillo A, Landoni G.. The effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br J Anaesth 2015;115:656–675. [DOI] [PubMed] [Google Scholar]
- 12. Gong B, Li Z, Yat Wong PC.. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2015;29:1415–1425. [DOI] [PubMed] [Google Scholar]
- 13. Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikström BG, Jörgensen K, Filippatos G, Parissis JT, González MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F.. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012;159:82–87. [DOI] [PubMed] [Google Scholar]
- 14. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola VP, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten SE, Schwinger RH, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J.. Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol 2016;222:303–312. [DOI] [PubMed] [Google Scholar]
- 15. Harrison RW, Hasselblad V, Mehta RH, Levin R, Harrington RA, Alexander JH.. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth 2013;27:1224–1232. [DOI] [PubMed] [Google Scholar]
- 16. Järvelä K, Maaranen P, Sisto T, Ruokonen E.. Levosimendan in aortic valve surgery: cardiac performance and recovery. J Cardiothorac Vasc Anesth 2008;22:693–698. [DOI] [PubMed] [Google Scholar]
- 17. Tritapepe L, De Santis V, Vitale D, Guarracino F, Pellegrini F, Pietropaoli P, Singer M.. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth 2009;102:198–204. [DOI] [PubMed] [Google Scholar]
- 18. Pollesello P, Papp Z.. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol 2007;50:257–263. [DOI] [PubMed] [Google Scholar]
- 19. Du Toit EF, Genis A, Opie LH, Pollesello P, Lochner A.. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosimendan in the isolated guinea pig heart. Br J Pharmacol 2008;154:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Das B, Sarkar C.. Pharmacological preconditioning by levosimendan is mediated by inducible nitric oxide synthase and mitochondrial KATP channel activation in the in vivo anesthetized rabbit heart model. Vasc Pharmacol 2007;47:248–256. [DOI] [PubMed] [Google Scholar]
- 21. Leprán I, Pollesello P, Vajda S, Varró A, Papp JG.. Preconditioning effects of levosimendan in a rabbit cardiac ischemia-reperfusion model. J Cardiovasc Pharmacol 2006;48:148–152. [DOI] [PubMed] [Google Scholar]
- 22. Nieminen MS, Buerke M, Cohen-Solál A, Costa S, Édes I, Erlikh A, Franco F, Gibson C, Gorjup V, Guarracino F, Gustafsson F, Harjola VP, Husebye T, Karason K, Katsytadze I, Kaul S, Kivikko M, Marenzi G, Masip J, Matskeplishvili S, Mebazaa A, Møller JE, Nessler J, Nessler B, Ntalianis A, Oliva F, Pichler-Cetin E, Põder P, Recio-Mayoral A, Rex S, Rokyta R, Strasser RH, Zima E, Pollesello P.. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol 2016;218:150–157. [DOI] [PubMed] [Google Scholar]
- 23. Russ MA, Prondzinsky R, Christoph A, Schlitt A, Buerke U, Söffker G, Lemm H, Swyter M, Wegener N, Winkler M, Carter JM, Reith S, Werdan K, Buerke M.. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit Care Med 2007;35:2732–2739. [DOI] [PubMed] [Google Scholar]
- 24. Delaney A, Bradford C, McCaffrey J, Bagshaw SM, Lee R.. Levosimendan for the treatment of acute severe heart failure: a meta-analysis of randomised controlled trials. Int J Cardiol 2010;138:28128–28129. [DOI] [PubMed] [Google Scholar]
- 25. Böhm M, Link A, Cai D, Nieminen MS, Filippatos GS, Salem R, Cohen Solal A, Huang B, Padley RJ, Kivikko M, Mebazaa A.. Beneficial association of β-blocker therapy on recovery from severe acute heart failure treatment: data from the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support trial. Crit Care Med 2011;39:940–944. [DOI] [PubMed] [Google Scholar]
- 26. Fuhrmann JT, Schmeisser A, Schulze MR, Wunderlich C, Schoen SP, Rauwolf T, Weinbrenner C, Strasser RH.. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Crit Care Med 2008;36:2257–2266. [DOI] [PubMed] [Google Scholar]
- 27. García-Gonzáles MJ, Domínguez-Rodríguez A, Ferrer-Hita JJ, Abreu-González P, Muñoz MB.. Cardiogenic shock after primary percutaneous coronary intervention: effects of levosimendan compared with dobutamine on haemodynamics. Eur J Heart Fail 2006;8:723–728. [DOI] [PubMed] [Google Scholar]
- 28. De Santis V, Vitale D, Tritapepe L.. Levosimendan and cardiac surgery. J Cardiothorac Vasc Anesth 2010;24:210.. [DOI] [PubMed] [Google Scholar]
- 29. Toller W, Algotsson L, Guarracino F, Hörmann C, Knotzer J, Lehmann A, Rajek A, Salmenperä M, Schirmer U, Tritapepe L, Weis F, Landoni G.. Perioperative use of levosimendan: best practice in operative settings. J Cardiothorac Vasc Anesth 2013;27:361–366. [DOI] [PubMed] [Google Scholar]
- 30. Guarracino F, Cariello C, Danella A, Doroni L, Lapolla F, Stefani M, Baldassarri R, Vullo C.. Effect of levosimendan on ventriculo-arterial coupling in patients with ischemic cardiomyopathy. Acta Anaesthesiol Scand 2007;51:1217–1224. [DOI] [PubMed] [Google Scholar]
- 31. Guarracino F, Baldassarri R, Pinsky MR.. Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013;17:213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertini P, Baldassarri R, Simone V, Amitrano D, Cariello C, Guarracino F.. Perioperative non-invasive estimation of left ventricular elastance (Ees) is no longer a challenge; it is a reality. Br J Anaesth 2014;112:578.. [DOI] [PubMed] [Google Scholar]
- 33. Chirinos JA. Ventricular–arterial coupling: invasive and non-invasive assessment. Artery Res 2013;7:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fida N, Loebe M, Estep JD, Guha A.. Predictors and management of right heart failure after left ventricular assist device implantation. Methodist Debakey Cardiovasc J 2015;11:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, Katsikogiannis N, Kougioumtzi I, Machairiotis N, Tsiouda T, Tsakiridis K, Zarogoulidis K.. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:S52–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ: HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316–1324. [DOI] [PubMed] [Google Scholar]
- 37. Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB.. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant 2008;27:1065–1072. [DOI] [PubMed] [Google Scholar]
- 38. Schenk S, McCarthy PM, Blackstone EH, Feng J, Starling RC, Navia JL, Zhou L, Hoercher KJ, Smedira NG, Fukamachi K.. Duration of inotropic support after left ventricular assist device implantation: risk factors and impact on outcome. J Thorac Cardiovasc Surg 2006;131:447–454. [DOI] [PubMed] [Google Scholar]
- 39. Sponga S, Ivanitskaia E, Potapov E, Krabatsch T, Hetzer R, Lehmkuhl H.. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J 2012;58:6–11. [DOI] [PubMed] [Google Scholar]
- 40. Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M.. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant 2006; 25:1024–1042. [DOI] [PubMed] [Google Scholar]
- 41. Salzberg SP, Lachat ML, von Harbou K, Zünd G, Turina MI.. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg 2005;27:222–225. [DOI] [PubMed] [Google Scholar]
- 42. Kivikko M, Lehtonen L, Colucci WS.. Sustained hemodynamic effects of intravenous levosimendan. Circulation 2003;107:81–86. [DOI] [PubMed] [Google Scholar]
- 43. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J.. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report–2015; Focus theme: early graft failure. J Heart Lung Transplant 2015;34:1244–1254. [DOI] [PubMed] [Google Scholar]
- 44. Healy AH, Stehlik J, Edwards LB, McKellar SH, Drakos SG, Selzman CH.. Predictors of 30-day post-transplant mortality in patients bridged to transplantation with continuous-flow left ventricular assist devices—an analysis of the International Society for Heart and Lung Transplantation Transplant Registry. J Heart Lung Transplant 2016;35:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Habib PJ, Patel PC, Hodge D, Chimato N, Yip DS, Hosenpud JD, Wadei HM.. Pre-orthotopic heart transplant estimated glomerular filtration rate predicts post-transplant mortality and renal outcomes: an analysis of the UNOS database. J Heart Lung Transplant;doi:10.1016/j.healun.2016.05.028. Published online ahead of print 7 June 2016. [DOI] [PubMed] [Google Scholar]