Abstract
Inotropes may be an appropriate response for some patients with advanced heart failure who remain highly symptomatic despite optimization of evidence-based therapy. These patients need to be supported waiting for a heart transplant or ventricular assist device, or may be candidates for inotropy as an intervention in its own right to maintain a patient in the best achievable circumstances. Objectives in such a situation include relieving symptoms, improving quality of life and reducing unplanned hospitalizations and the costs associated with such admissions. Levosimendan, a calcium sensitizer and potassium channel opener with inotrope and vasodilator actions, has emerged as a potentially valuable addition to the armamentarium in this context, used in repeated or intermittent cycles of therapy. Detailed proposals and guidance are offered for the identification of candidate patients with good prospects of a beneficial response to levosimendan, and for the safe and effective implementation of a course of therapy.
Keywords: Advanced heart failure, Levosimendan, Repetitive, Intermittent, Rehospitalization
Introduction
Some answers to the question ‘Why use inodilators in patients with advanced heart failure (AHF)?’ can be found in Table 1 of this essay, which summarizes the European Society of Cardiology criteria for a diagnosis of ‘advanced heart failure’.1 The salient point in the context of the question ‘Why use inodilators?’ is shown in the bottom row of the table: these are patients for whom even the full repertoire of established medical therapies is no longer sufficient to avoid severe symptoms and impairment of exercise capacity, often with frequent and repeated hospitalizations. The two periods of highest risk for readmission are immediately following discharge and just before death, with risk being very much higher in the second of these periods.2–4
Table 1.
European Society of Cardiology criteria for a diagnosis of ‘advanced heart failure’
| NYHA functional class III or IV |
| Episodes of fluid retention and/or reduced cardiac output |
| Severe cardiac dysfunction (at least one of): LVEF <30%, pseudonormal or restrictive mitral inflow pattern, high ventricular filling pressures or high BNP/NTproBNP |
| Severe impairment of functional capacity: inability to exercise, 6-min walk distance <300 m or peak oxygen uptake 12–14 ml/kg/min |
| At least one hospitalization in the past 6 months |
| Despite optimal medical therapy |
Reproduced with permission from Metra et al.1
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide; NTproBNP, N-terminal pro-brain natriuretic peptide.
The second of these peaks in risk, which coincides with, and signifies, a patient’s progression into AHF, has been described, pertinently, as the ‘phase of palliation and priorities’.3 Many patients and their families are drawn into the recurring crises of readmissions in ways that preclude calm consideration of the implications or alternatives. Early introduction of palliative care measures in oncology has been shown to improve quality of life for patients and their families without shortening survival5 and might reasonably be expected to provide similar benefits in patients with AHF, a condition that resembles late-stage cancer in terms of its symptom burden and prognosis. In this context, a recourse to inodilator therapy to provide symptomatic relief, preserve functional capacity and maintain quality of life can help to create time and opportunity for patients and their relatives to establish realistic priorities of care.
Hence, the first justification for intermittent inodilator therapy in patients with AHF is the most fundamental of all those encountered in medicine: to provide the best possible humane care for a patient in extremis and, by doing so, to enable them and their relatives to make the best arrangements they can for the end of life.
A secondary consideration is the management of costs associated with HF-related readmissions. Recent data from the Health Department of Catalonia indicate that during 2013, a total of €536.2 million was spent on the care of 88 195 HF patients, of which substantially the largest single locus of expense was hospitalizations; two-thirds of the total recorded expenditure was assigned to use of hospital facilities (including renal services) or to outpatient services.6 (It should be noted that the proportion of costs accruing to hospital services may be even larger in AHF, where unplanned or urgent admissions are frequent.)
What options are there?
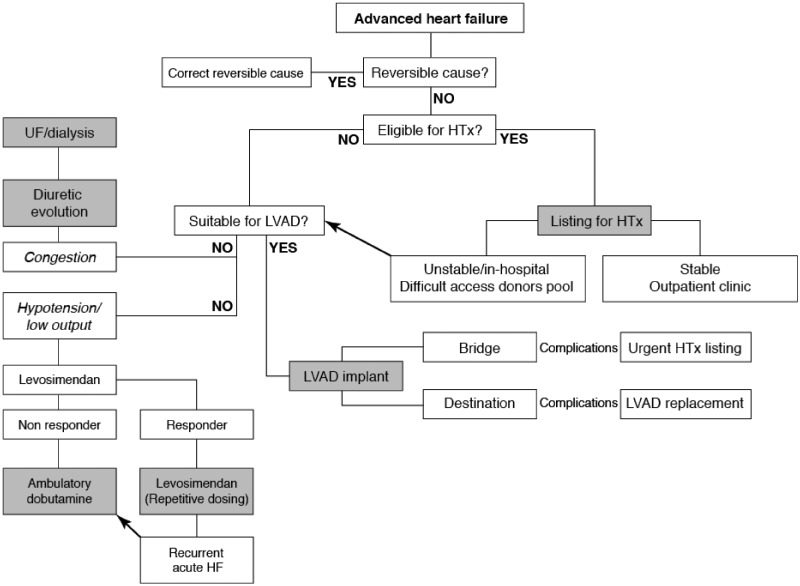
These positive possibilities notwithstanding, it is essential to register that in the wider context of AHF management, intermittent or episodic inodilator therapy is not the first or preferred option. Rather, as illustrated in Figure 1, it may be seen as the medical option of first (or last) resort in AHF patients with low cardiac output and hypotension who are not candidates for heart transplantation or the installation of a left ventricular assist device (LVAD). The significance and value of intermittent inodilator therapy rest on the fact that, for a variety of reasons, those other interventions are not available (or suitable) in every instance.
Figure 1.
How intermittent therapy with levosimendan fits into a larger strategy of treatment for patients with advanced heart failure. HTx, heart transplant; LVAD, left ventricular assist device; UF, ultrafiltration.
For properly selected patients, heart transplantation is the definitive, curative intervention for HF and is now associated with very good 1- and 5-year survival rates.7–9 Unfortunately the number of candidate recipients usually exceeds the number of donors: data from the 8-country Eurotransplant initiative indicate that, between 2007 and 2015, the total number of heart transplants in participating countries varied from ≈580 to 604 (all but one from cadaveric donors); during the same period, the number of patients waiting for a transplant consistently exceeded 1000. The subsequent decline to 1170 patients in 2015 was almost exclusively the result of a shorter waiting list in Germany.10 It is beyond the scope of this essay to explore whether that reduction was due to reforms in a German transplant prioritization scheme described in 2012 as being ‘on a straight line to a default’11 but the persisting disparity between the number of potential recipients and the number of hearts available to be transplanted is indisputable; the population of candidates for transplant grows more rapidly than the donor pool.7 Some of the consequences of time spent on the transplant waiting list, misallocation of resources and ‘gaming’ the prioritization schemes adopted in different countries have been described elsewhere and are not considered further in this essay.11–13
One positive aspect of the demand–supply mismatch for heart transplantation is that waiting list mortality rates appear to be stable.14 The reasons for this are likely to be many and various, and mostly outside the remit of this essay, but it may be conjectured that increased availability of LVADs may have contributed to the ability to maintain potential transplant recipients in a stable condition and with acceptable quality of life for extended periods of time.15 Use of these devices has undoubtedly expanded in the past decade7, aided by technical innovations and improvements such as conversion from external to internal placement of the device and from pneumatic to electrical power, and the transition from pulsatile to continuous-flow devices. Present-day LVADs are much smaller in size and weight, and quieter in operation than their predecessors and these characteristics improve patient satisfaction and extend the range of patients who may be candidates for these devices.
Survival rates with the latest continuous-flow devices are ≈70% at 2 years16 and ≈50% at 4 years, comparing reasonably well with heart transplantation and raising the possibility of LVADs as a permanent alternative to transplantation for some categories of patients who are not candidates for a transplant or for individuals who, for whatever reason, never reach the top of the transplant list. Examples in the first of these categories might include very elderly or obese patients, or those with diabetes, HIV-positive status, pulmonary hypertension or a history of recent malignancy.17–23 It should be noted, however, that in none of these situations should LVAD installation be regarded as a panacea.21,24 There are, in addition, acknowledged contraindications to LVADs, just as for every other medical resource (Table 2).
Table 2.
Contraindications to the use of left ventricular assist devices
| Systemic illness with a life expectancy of <2 years |
| Active malignancy with poor prognosis |
| Severe aortic disease |
| Severe chronic obstructive pulmonary disease |
| Irreversible renal or hepatic dysfunction |
| Severe right ventricle dysfunction |
Does intermittent inodilator therapy work?
At this juncture it is necessary to address a question that does not feature in the title of this essay: what evidence is there to support intermittent inodilator use in AHF? The answer, framed specifically for levosimendan, is that there is a growing database of evidence for a survival benefit from intermittent therapy and one that is certainly sufficient to make the case for a properly scaled randomized trial with mortality and quality of life metrics as endpoints.
A series of meta-analyses on this subject have all produced strong indications of a survival benefit vs. placebo; not only have all these exercises produced similar estimates of the odds ratio in favour of levosimendan (≈0.5) but, within each meta-analysis, there has been strong consistency in the trends for effect from each contributing study.25–27
These data are in marked contrast to experience with dobutamine, which has been associated with worse survival in AHF, notwithstanding that it improves New York Heart Association (NYHA) functional status.28,29
Whether this difference relates in any way to differences in the haemodynamic responses to levosimendan and dobutamine in HF patients remains an open question, but the lack of an (adverse) haemodynamic interaction between levosimendan and beta-blockers is a recorded fact30 and is relevant in an era when use of beta-blockers in HF is widespread.
Effects on quality of life that are not directly attributable to inotropy are also relevant. In the Randomized EValuation of Intravenous Levosimendan Efficacy (REVIVE)-II trial31, patients’ self-reporting of dyspnoea exhibited a clear and sustained trend for benefit from levosimendan across the entire period of observation (P = 0.018 vs. placebo), despite both study groups receiving optimal conventional therapy throughout the trial. Levosimendan has also been shown to exert some positive effects on renal function.32,33
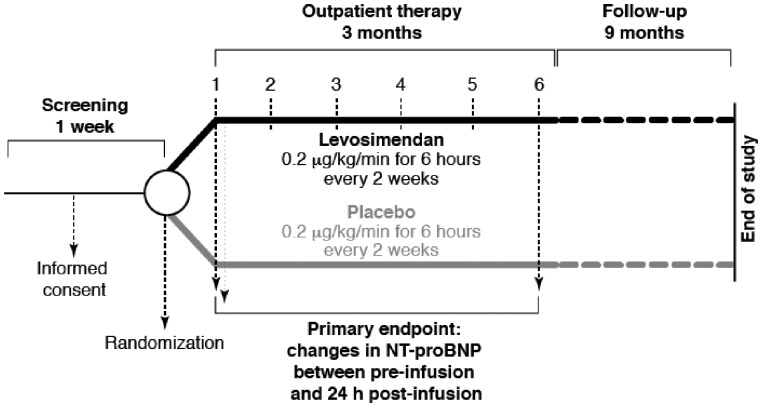
Additional data relating to effects of intermittent levosimendan on quality of life in AHF are now emerging from the Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients (LION-HEART) study (NCT01536132), the protocol for which is shown in Figure 2.
Figure 2.
Protocol of the LION-HEART study.
The purpose of this multicentre, double-blind, placebo-controlled, randomized trial was to evaluate the efficacy and safety of intravenous administration of intermittent doses of levosimendan in ambulatory patients with advanced congestive HF. The primary endpoint of LION-HEART was change in natriuretic peptide levels between baseline and 3 months; secondary endpoints included 12-month hospitalization and 12-month mortality.
Very highly significant (P < 0.001) reductions in N-terminal pro-brain natriuretic peptide (NTproBNP) were documented in the patients randomized to levosimendan; these were accompanied by profound reductions in the risk of HF-related hospitalization (12-month cumulative hospitalization rate ≈20% vs. ≈65% in the placebo group; P < 0.001) and in the composite endpoint of all-cause mortality or HF-related hospitalization (12-month cumulative hospitalization rate ≈25% vs. ≈65% in the placebo group; P < 0.001).
The percentage of patients requiring cessation of therapy was higher with levosimendan than with placebo (15% vs. 9.5%; P > 0.7) but this was offset by a very large reduction in the percentage of patients registering a clinically significant decline in quality of life at 6 months (≈20% vs. 63%; P = 0.022). Levosimendan-treated patients were significantly more likely to record an improvement of at least one NYHA functional class after 6 months of therapy than placebo-treated peers (odds ratio 4.3, 95% confidence interval 1.1–18.3; P = 0.042).
It must be highlighted, however, that LION-HEART was not powered for mortality, and the fact that levosimendan significantly reduced NT-proBNP level is per se not sufficient for providing a strong recommendation for its use. In the past, in the SURVIVE trial on acute heart failure patients,34 a significant short-term reduction of BNP was not correlated to a significant long-term reduction of mortality. Even so, the trend identified in the LION-HEART trial seems to indicate some value from repetitive doses of levosimendan.
This trend complements the experience in the LevoRep study. The principal findings of that study have been published.35 Addition of levosimendan (0.2 µg/kg/min for 6 h at 2-week intervals over 6 weeks) to standard therapy was associated with non-significant trends in the 6-min walk test and the Kansas City Cardiomyopathy Questionnaire score over 24 weeks of follow-up, but with significant effects on secondary endpoints such as survival.
There is therefore a strong rationale for a properly powered study to be conducted.
How to deliver intermittent inodilator therapy
The ‘how?’ of intermittent levosimendan therapy in AHF starts with the identification of possible candidate patients and the goals of therapy.
Patients are characterized by AHF, defined for this purpose as:
left ventricular ejection fraction (LVEF) <35%
NYHA class IV or IIIb, or NYHA class IIIa with frequent decompensation
mean systolic blood pressure (BP) >90 mmHg (or 80–90 mmHg, if the patient has previously tolerated levosimendan).
The objective of therapy, which should be clearly explained to the patient, is to prevent disease progression, reduce hospitalizations and improve quality of life. Reference to improved survival may not be appropriate, depending on the patient’s mood and outlook, and the decision to mention any effect on survival should be taken on a case-by-case basis.
Patients should be admitted as day cases. Blood tests should be performed prior to commencement of infusion. Patients should not be treated if at this stage there are signs of:
severe hypotension (BP < 90 or < 80 mmHg if previous infusion well tolerated) or tachycardia
severe renal or hepatic impairment
hypokalaemia
significant mechanical obstructions affecting ventricular filling or outflow.
A history of torsades de pointes is also a contraindication.
Weight-specific infusion rates for levosimendan are summarized in Table 3. An initial bolus dose should not be used. As patient characteristics and needs vary considerably, as does their response to treatment, we recommend a flexible dosing schedule of 0.05 to 0.2 μg/kg/min, for 6 to 24 h, every 2 to 4 weeks. The first-time infusion should be initiated at a rate of 0.1 µg/kg/min. If that dose is well tolerated during the first 1–2 h it may be increased to 0.2 µg/kg/min; if the initial dose is not well tolerated (as evidenced by hypotension) it should be halved to 0.05 µg/kg/min and re-evaluated. If that lower dose is also not tolerated, treatment should be stopped.
Table 3.
Weight-specific infusion rates (ml/h) for use of intermittent or repeated levosimendan infusions in AHF
| Weight (kg) | Levosimendan dose (µg/kg/min) |
||
|---|---|---|---|
| 0.05 | 0.1 | 0.2 | |
| 50 | 6 | 12 | 24 |
| 60 | 7 | 14 | 29 |
| 70 | 8 | 17 | 34 |
| 80 | 10 | 19 | 38 |
| 90 | 11 | 22 | 43 |
| 100 | 12 | 24 | 48 |
| 110 | 13 | 26 | 53 |
Some real-world experience
Levosimendan became available in Kiel (Germany) in 2002, initially as an off-label therapy restricted to use in the intensive care unit (ICU). Use was extended to the intermediate medical care unit (IMC) in 2014. It should be noted at the outset that the assignment of any given patient to either the ICU or the IMC is partly determined by structural rather than medical considerations: facilities such as mechanical or non-invasive ventilation, extracorporeal membrane oxygenation and haemofiltration are available only in the ICU and so any patient in need of those interventions is directed to the ICU even if they might otherwise be candidates for treatment in the IMC.
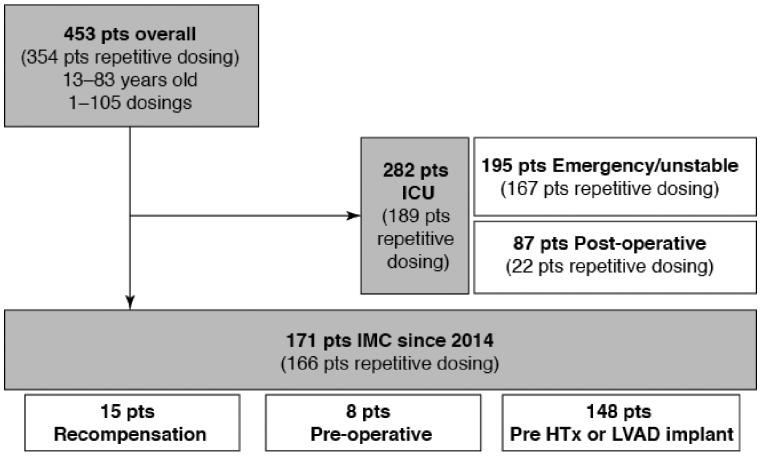
Figure 3 summarizes a preliminary inspection of data on levosimendan usage between 2009 and 2015 in both the ICU and IMCU. In total, 453 patients were treated with levosimendan, most of whom (n = 354) received repeated dosing. The longest sequence of repeat dosing was 105 cycles in a patient who was eventually successfully transplanted for a failing Fontan heart.
Figure 3.
Distribution of patients in Kiel (Germany) treated with levosimendan between 2009 and 2015. IMC, intermediate care unit; ICU, intensive care unit; HTx, heart transplant; LVAD, left ventricular assist device.
Among the 171 patients treated in the IMCU, 15 were recompensated and discharged, eight were stabilized prior to valve or bypass surgery and 148 were stabilized as a prelude to heart transplantation or installation of an LVAD.
The critical first stage of inodilator/inotrope therapy in Kiel is clinical assessment. This should always be undertaken by an experienced HF consultant and should aim, so far as the urgencies of the situation allow, to take a broad view of the patient’s circumstances: clinical status should be ascertained, with special attention paid to any indications of frailty.36 Medical history should also be examined, including comorbidities, medications and, in particular, the time course of the presenting decompensation: rapid decline over a few weeks may signify a loss of control that has its origins in lapses from patient self-care and good outpatient follow-up and may respond to the performance of those activities with renewed vigour, whereas a crisis in a patient who has been following a downward trajectory for an extended period of time may signify a new and adverse phase of late-stage HF. Echocardiography at this stage should emphasize the right ventricle and the possibility of pulmonary hypertension. Electrocardiography, determination of BP and oxygen saturation, and a blood-gas analysis are also required, along with the standard chest X-ray and a blood assay for electrolytes, kidney function, liver congestion, infection and natriuretic peptides (NTproBNP in Kiel). Tests additional to this core set are commissioned as required by individual cases.
Medical factors that might then lead a patient to be referred for treatment in the ICU include the presence of acute coronary syndrome, supraventricular tachycardia (heart rate >10 beats/min), low systemic BP or evidence of low tissue perfusion. Levosimendan might then be used as part of an inopressor regimen that may also include milrinone and intravenous adrenaline or noradrenaline.
In the centres where levosimendan is the first-choice agent for inotropic support, a common operating practice is to deliver a single ampoule of 12.5 mg per 24 h: this approach does not have the exactitude of the schedule proposed in Table 3 but is a robust and practical method that simplifies dose calculations and, given the weights and body mass index values of patients encountered in northern Germany, generally delivers a low initial dose, one in the region of 0.1 µg/kg/min.
With therapy initiated, close clinical observation is paramount. The standard schedule at Kiel specifies nurse observations at 30-min intervals, physician visits every 3 h and daily (usually evening) discussions with an experienced HF consultant. The patient is maintained in an upright (‘pilot seat’) position in bed, and heart rate and BP are monitored continuously.
Patients not responding to this intervention, or who require higher doses of levosimendan or other vasoactive agents, are transferred to the ICU.
Where the response to levosimendan is satisfactory, heavy patients (>110 kg) in whom BP remains >120/70 mmHg may have their dosage increased to 12.5 mg/12 h: this approximates to 0.15 µg/kg/min, and further evaluations are performed at 3-h intervals.
Hypotension (BP <85/55 mmHg plus increased heart rate) or dizziness or headache are indicators for a dose reduction to 0.025–0.05 µg/kg/min. Here also, responses should be re-evaluated every 3 h.
Practice in Kiel is to maintain beta-blockers at the dose in use at presentation, drawing on observations in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial that showed no attenuation of levosimendan effect by beta-blockade.37 Antihypertensive therapies are adjusted according to BP or afterload status (suspend dosing if afterload low, increase dosing if afterload high). Diuretics are paused if there is evidence of volume depletion but, in patients with indications of fluid overload, there is no prohibition against using levosimendan with increased oral doses of diuretic or intravenous treatment where required. (These adjustments are part of a wider scheme of fluid status adjustment based on clinical monitoring tailored to the circumstances of individual patients.)
The tolerability profile of levosimendan in this context has been highly satisfactory. Between 2013 and 2015, 171 patients were treated with levosimendan in the IMCU, with 166 receiving repetitive doses. No serious adverse events were recorded in these patients; there were also no episodes of ventricular tachycardia and the only withdrawal of therapy (following development of hypotension) was effected by the attending resident without reference to senior colleagues. There were five episodes of mild hypotension (BP ≈80/55 mmHg) and one case of headache, none of which led to withdrawal of levosimendan.
Examination of data from 111 patients treated with levosimendan in 2015 in either the IMCU or the ICU at Kiel identified no serious adverse events and three cases of mild-to-moderate hypotension (defined as above), none of which resulted in withdrawal of levosimendan.
Other real-world experiences have been reported previously in detail by several authors, e.g. in Sweden38, Australia39, and Greece.40 In addition, a Swedish registry collected data on planned repetitive use of levosimendan for heart failure in cardiology and internal medicine in Sweden, where this therapy is the overwhelming inotrope of choice.41 An Italian registry (RELEVANT) is currently collecting data from 130 patients treated with levosimendan in the past 2 years.
Conclusions
Intermittent inodilator therapy may be used as a medical option for AHF patients with low cardiac output and hypotension who are not candidates for heart transplantation or the installation of a LVAD. It may be a good therapeutic option for patients on transplant waiting lists in order to avert readmissions.
Data supporting the use of inodilators in this way are suggestive rather than conclusive but warrant further investigation of this strategy in eligible patients. Importantly, meta-analyses indicate that, in contrast to conventional (mostly adrenergic) inotropes, use of the inodilator levosimendan is not associated with worse longer-term survival. In addition, levosimendan may be used in conjunction with beta-blockers, which are widely used in HF.
Practical guidance is available for the safe implementation of intermittent levosimendan therapy and real-world experience indicates that this option is feasible and generally well tolerated.
Acknowledgements
We thank Hughes associates, Oxford, UK, for editing the language of this manuscript.
References
- 1. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M.. Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007;9:684–694. [DOI] [PubMed] [Google Scholar]
- 2. Desai AS. The three-phase terrain of heart failure readmissions. Circ Heart Fail 2012;5:398–400. [DOI] [PubMed] [Google Scholar]
- 3. Desai AS, Stevenson LW.. Rehospitalization for heart failure: predict or prevent? Circulation 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 4. Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, Lee DS.. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 2012;5:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ.. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 6. Farré N, Vela E, Clèries M, Bustins M, Cainzos-Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdú-Rotellar JM, Comín-Colet J.. Medical resource use and expenditure in patients with chronic heart failure: a population-based analysis of 88 195 patients. Eur J Heart Fail 2016;18:1132–1140. [DOI] [PubMed] [Google Scholar]
- 7.OPTN/SRTR 2012 Annual Data Report: Heart. http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/05_heart_13.pdf(10 September 2016).
- 8. Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, Stehlik J.. International Society for Heart and Lung Transplantation. The registry of the international society for heart and lung transplantation: Thirtieth Official Adult Heart Transplant Report—2013; focus theme: age. J Heart Lung Transplant 2013;32:951–964. [DOI] [PubMed] [Google Scholar]
- 9. Starling RC. Improved quantity and quality of life: a winning combination to treat advanced heart failure. J Am Coll Cardiol 2010;55:1835–1836. [DOI] [PubMed] [Google Scholar]
- 10. Eurotransplant Statistics Report Library. http://statistics.eurotransplant.org(10 September 2016).
- 11. Smits JM. Actual situation in Eurotransplant regarding high urgent heart transplantation. Eur J Cardiothorac Surg 2012;42:609–611. [DOI] [PubMed] [Google Scholar]
- 12. Stevenson LW. Crisis awaiting heart transplantation: sinking the lifeboat. JAMA Intern Med 2015;175:1406–1409. [DOI] [PubMed] [Google Scholar]
- 13. Givens RC, Dardas T, Clerkin KJ, Restaino S, Schulze PC, Mancini DM.. Outcomes of multiple listing for adult heart transplantation in the United States: analysis of OPTN data from 2000 to 2013. JACC Heart Fail 2015;3:933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller CD, Callahan ER, Snyder JJ, Israni AK, Kasiske BL.. OPTN/SRTR 2013 annual data report: heart. Am J Transplant 2015;2:1–28. [DOI] [PubMed] [Google Scholar]
- 15. Colvin M, Miranda-Herrera D, Gustafson SK, Heubner B, Skeans M, Wang X, Snyder JJ, Kasiske BL, Israni AK.. Impact of increased time at the highest urgency category on heart transplant outcomes for candidates with ventricular assist devices. J Heart Lung Transplant 2016;35:326–334. [DOI] [PubMed] [Google Scholar]
- 16. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB.. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495–1504. [DOI] [PubMed] [Google Scholar]
- 17. Go PH, Nemeh HW, Borgi J, Paone G, Morgan JA.. Effect of body mass index on outcomes in left ventricular assist device recipients. J Card Surg 2016;31:242–247. [DOI] [PubMed] [Google Scholar]
- 18. Mohamedali B, Yost G, Bhat G.. Obesity as a risk factor for consideration for left ventricular assist devices. J Card Fail 2015;21:800–805. [DOI] [PubMed] [Google Scholar]
- 19. Vest AR, Mistak SM, Hachamovitch R, Mountis MM, Moazami N, Young JB.. Outcomes for patients with diabetes after continuous-flow left ventricular assist device implantation. J Card Fail; doi:10.1016/j.cardfail.2016.02.010. Published online ahead of print 26 February 2016. [DOI] [PubMed] [Google Scholar]
- 20. Sims DB, Uriel N, González-Costello J, Deng MC, Restaino SW, Farr MA, Takayama H, Mancini DM, Naka Y, Jorde UP.. Human immunodeficiency virus infection and left ventricular assist devices: a case series. J Heart Lung Transplant 2011;30:1060–1064. [DOI] [PubMed] [Google Scholar]
- 21. Al-Kindi SG, Farhoud M, Zacharias M, Ginwalla MB, ElAmm CA, Benatti RD, Oliveira GH.. Left ventricular assist devices or inotropes for decreasing pulmonary vascular resistance in patients with pulmonary hypertension listed for heart transplantation. J Card Fail; doi:10.1016/j.cardfail.2016.06.421. Published online ahead of print 30 June 2016. [DOI] [PubMed] [Google Scholar]
- 22. Ozturk P, Engin AY, Nalbantgil S, Oguz E, Ayik F, Engin C, Yagdi T, Erkul S, Balcioglu O, Ozbaran M.. Comparison of continuous-flow and pulsatile-flow blood pumps on reducing pulmonary artery pressure in patients with fixed pulmonary hypertension. Artif Organs 2013; 37:763–767. [DOI] [PubMed] [Google Scholar]
- 23. Atluri P, Fairman AS, MacArthur JW, Goldstone AB, Cohen JE, Howard JL, Zalewski CM, Shudo Y, Woo YJ.. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg 2013;28:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnold SV, Jones PG, Allen LA, Cohen DJ, Fendler TJ, Holtz JE, Aggarwal S, Spertus JA.. Frequency of poor outcome (death or poor quality of life) after left ventricular assist device for destination therapy: Results from the INTERMACS Registry. Circ Heart Fail; doi:10.1161/CIRCHEARTFAILURE.115.002800. Published online ahead of print August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silvetti S, Greco T, Di Prima AL, Mucchetti M, de Lurdes CM, Pasin L, Scandroglio M, Landoni G, Zangrillo A.. Intermittent levosimendan improves mid-term survival in chronic heart failure patients: meta-analysis of randomised trials. Clin Res Cardiol 2014;103:505–513. [DOI] [PubMed] [Google Scholar]
- 26. Nieminen MS, Altenberger J, Ben-Gal T, Böhmer A, Comin-Colet J, Dickstein K, Edes I, Fedele F, Fonseca C, García-González MJ, Giannakoulas G, Iakobishvili Z, Jääskeläinen P, Karavidas A, Kettner J, Kivikko M, Lund LH, Matskeplishvili ST, Metra M, Morandi F, Oliva F, Parkhomenko A, Parissis J, Pollesello P, Pölzl G, Schwinger RH, Segovia J, Seidel M, Vrtovec B, Wikström G.. Repetitive use of levosimendan for treatment of chronic advanced heart failure: clinical evidence, practical considerations, and perspectives: an expert panel consensus. Int J Cardiol 2014;174:360–367. [DOI] [PubMed] [Google Scholar]
- 27. Silvetti S, Nieminen M.. Repeated or intermittent levosimendan treatment in advanced heart failure: an updated meta-analysis. Int J Cardiol 2016;202:138–143. [DOI] [PubMed] [Google Scholar]
- 28. Thackray S, Easthaugh J, Freemantle N, Cleland JG.. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta-regression analysis. Eur J Heart Fail 2002;4:515–529. [DOI] [PubMed] [Google Scholar]
- 29. Oliva F, Latini R, Politi A, Staszewsky L, Maggioni AP, Nicolis E, Mauri F.. Intermittent 6-month low-dose dobutamine infusion in severe heart failure: DICE multicenter trial. Am Heart J 1999;138:247–253. [DOI] [PubMed] [Google Scholar]
- 30. Bergh CH, Andersson B, Dahlström U, Forfang K, Kivikko M, Sarapohja T, Ullman B, Wikström G.. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail 2010;12:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T.. REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013;1:103–111. [DOI] [PubMed] [Google Scholar]
- 32. Yilmaz MB, Grossini E, Silva Cardoso JC, Édes I, Fedele F, Pollesello P, Kivikko M, Harjola VP, Hasslacher J, Mebazaa A, Morelli A, Le Noble J, Oldner A, Oulego Erroz I, Parissis JT, Parkhomenko A, Poelzl G, Rehberg S, Ricksten SE, Rodríguez Fernández LM, Salmenperä M, Singer M, Treskatsch S, Vrtovec B, Wikström G.. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther 2013; 27:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grossini E, Molinari C, Pollesello P, Bellomo G, Valente G, Mary D, Vacca G, Caimmi P.. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther 2012;342:376–388. [DOI] [PubMed] [Google Scholar]
- 34. Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M.. SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 35. Altenberger J, Parissis JT, Costard-Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G.. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014;16:898–906. [DOI] [PubMed] [Google Scholar]
- 36. Jha SR, Hannu MK, Chang S, Montgomery E, Harkess M, Wilhelm K, Hayward CS, Jabbour A, Spratt PM, Newton P, Davidson PM, Macdonald PS.. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation 2016;100:429–436. [DOI] [PubMed] [Google Scholar]
- 37. Mebazaa A, Nieminen MS, Filippatos GS, Cleland JG, Salon JE, Thakkar R, Padley RJ, Huang B, Cohen-Solal A.. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail 2009;11:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barbici I, Hedman A, Ewaldsson CA.. Use of levosimendan in patients with heart failure in different settings: case reports and treatment guidance. Heart Lung Vessel 2015;7:143–150. [PMC free article] [PubMed] [Google Scholar]
- 39. Parle NM, Thomas MD, Dembo L, Best M, Driscoll GO.. Repeated infusions of levosimendan: well tolerated and improves functional capacity in decompensated heart failure—a single-centre experience. Heart Lung Circ 2008;17:206–210. [DOI] [PubMed] [Google Scholar]
- 40. Spargias KS, Anifantakis A, Papadakis M, Iakovis P, Chatzigeorgiou G, Koutsogiannis N, Karatasakis G, Athanassopoulos G, Manginas A, Maounis T, Cokkinos DV.. Preliminary clinical experience with the repetitive administration of levosimendan in patients with end-stage heart failure. Ital Heart J 2003;(4 Suppl 2):45S–49S. [PubMed] [Google Scholar]
- 41. Thorvaldsen T, Benson L, Hagerman I, Dahlström U, Edner M, Lund LH.. Planned repetitive use of levosimendan for heart failure in cardiology and internal medicine in Sweden. Int J Cardiol 2014;175:55–61. [DOI] [PubMed] [Google Scholar]