Heavily glycosylated secreted mucin MUC5AC, which serve as a protective barrier under normal physiologic conditions, gets aberrantly expressed, distributed and glycosylated during malignant transformation. Delineation of MUC5AC function, structure and glycosylation has created new avenues for early diagnosis and therapeutic targeting for multiple malignancies.
Abstract
Heavily glycosylated secreted mucin MUC5AC, by the virtue of its cysteine-rich repeats, can form inter- and intramolecular disulfide linkages resulting in complex polymers, which in turn craft the framework of the polymeric mucus gel on epithelial cell surfaces. MUC5AC is a molecule with versatile functional implications including barrier functions to epithelial cells, host–pathogen interaction, immune cell attraction to sites of premalignant or malignant lesions and tumor progression in a context-dependent manner. Differential expression, glycosylation and localization of MUC5AC have been associated with a plethora of benign and malignant pathologies. In this era of robust technologies, overexpression strategies and genetically engineered mouse models, MUC5AC is emerging as a potential diagnostic, prognostic and therapeutic target for various malignancies. Considering the clinical relevance of MUC5AC, this review holistically encompasses its genomic organization, domain structure, glycosylation patterns, regulation, functional and molecular connotation from benign to malignant pathologies. Furthermore, we have here explored the incipient and significant experimental tools that are being developed to study this structurally complex and evolutionary conserved gel-forming mucin.
Introduction
Mucins are a family of gigantic glycoproteins expressed on the epithelial cell surfaces including ducts of lacrimal glands in the eye, salivary glands, the lining of the respiratory, gastrointestinal, urothelial and reproductive tracts (1). They play a central role in the mechanochemical protection of the epithelial cell surfaces, configure and maintain the local microenvironment and promote cell survival by impacting overall homeostasis in adverse physiological and pathological conditions (2–5). All members of the mucin family are characterized by the presence of tandem repeat regions (TRR), with a high proportion of proline, threonine and serine residues (called PTS sequences). The serine and threonine residues in the TRR undergo O-linked glycosylation post-translationally, as per the distinct requirements of the epithelia (6). The variability in their tandem repeats renders a high degree of polymorphism to the members of mucin family (1). Owing to the large molecular weight, glycosylations and formation of polymer-based high-ordered structures, mucins have complex biophysical properties. In response to external stimuli, mucins transduce signals through multiple domains present in their structure, resulting in altered proliferation, migration, differentiation, apoptosis, cell adhesion and downstream signaling in cellular pathology (7). Deregulated mucin expression and function act as an important link between inflammation and cancer (8). Mucin-associated glycans may act as ligands for receptors, confer hygroscopic properties, sequester various cytokines and growth factors and provide stoichiometric amplification owing to their high degree of multivalency for oligosaccharide structures (4). The deregulated expression of mucins, their differential glycosylation and altered localization has been implicated in multiple pathologies including properties of tumor and its microenvironment (8).
To date, 21 different mucin family members have been identified. These have both unique and shared structural features and are classified into two broad subfamilies, namely the transmembrane mucins and secreted mucins (1). Transmembrane mucins (MUC1, MUC3A/B, MUC4, MUC11–13, MUC15–17, MUC20 and MUC21) have hydrophobic plasma membrane-spanning domain and short cytoplasmic tails that facilitate mucin-mediated intracellular signaling (9). Secreted mucins include gel-forming (MUC2, MUC5AC, MUC5B, MUC6 and MUC19) and non-gel forming (MUC7) mucins. The gel-forming mucins form a physical barrier as a mucous gel, providing protection to epithelial surfaces such as respiratory (MUC5B, MUC5AC) and gastrointestinal tracts (MUC2, MUC6) (5). As a part of defense mechanism to maintain the integrity of epithelial surfaces, secretory mucins seems to appear early in metazoan evolution followed by transmembrane mucins (8).
Among the gel-forming secretory mucins, the MUC6, MUC2, MUC5AC and MUC5B genes are thought to have evolved from the common gene ancestor, human von Willebrand factor on chromosome 11p15 by gene multiplications and subsequent domain duplications (10). For the first time, MUC5AC was identified as a tracheobronchial mucin gene MUC5 localized on chromosome 11p15 (11). Numerous genetic clones were identified and thought to encode unique mucins MUC5A, MUC5B and MUC5C. Subsequently, MUC5A and MUC5C were found identical, and henceforth, the gene was designated as MUC5AC (12). Multiple histological studies highlighted that polymeric gel-forming secretory mucin MUC5AC is expressed in conjunctiva, middle ear, nasopharynx, lungs, gallbladder and stomach under normal conditions providing protection to corresponding epithelial surfaces from different factors under physiological conditions (Table 1) (13,14). However, aberrant expression of MUC5AC is observed in various benign pathologies (Table 2) and malignant conditions (Table 1). Pathological significance of MUC5AC expression and the associated complications have led to extensive studies discerning its regulation and therapeutic inhibition of its expression (15). However, to date, molecular implications of MUC5AC and modes of its functioning remain obscure, thus hindering the progress of successful therapeutic interventions against these pathologies.
Table 1.
Expression of MUC5AC in physiological and malignant conditions
| Organ | Expressiona | Cancer | Expression/association and functional implications to cancer |
|---|---|---|---|
| Salivary glands | + (16) | Salivary gland carcinoma | ++/Higher grade mucoepidermoid carcinoma with tumor- associated lymphoid infiltrates (P < 0.05) (17); ++/signet-ring cells in mucin-rich variant of salivary duct carcinoma (18) |
| Breast | − (19) | Breast cancer | ++/Shorter disease-free survival (20); coexpression of MUC1 and MUC5AC in invasive carcinomas (21); hypermethylated promoter of MUC5AC gene in non-expressing cells (MDA- MB-453) and low methylation levels in MUC5AC-expressing cells (MCF-7) expression (22) |
| Lung | ++ (13) | Lung cancer | ++++/Postoperative relapse (23); postoperative distant metastasis, shorter survival (24) |
| Stomach | +++ (25) | Gastric cancer (GC) | Shorter survival associated with reduced MUC5AC expression (26); higher tumor stage, increased invasion (27–29) |
| Gallbladder/intrahepatic bile ducts | ++ (30,31) | Gallbladder cancer | +/Adenoma, dysplasia and carcinomas of gallbladder (32); poor survival with decreased expression (33); MUC5AC expression in 86% of mucinous carcinomas of the gallbladder cases from 606 carcinomas (34) |
| Liver | − | Cholangiocarcinoma | +++/More advanced tumors (35); neural invasion (36); lymph node metastasis, poor prognosis (37,38); serum to bile ratio of MUC5AC as an effective marker to discriminate cholangiocarcinoma from benign biliary disorders (39); involvement of Lewis antigen CA-S27-associated modification of MUC5AC in promoting cholangiocarcinoma cell growth, adhesion, migration and invasion (40) |
| Pancreas | − (41) | Pancreatic cancer | +++/Better survival (42–44); shorter survival (45); lymph node metastasis (46) |
| Ileum | − (47) | CRC | +++/Less aggressive (48); increased tumor grade (49); increased invasion (50) |
| Colon | − (51) | ||
| Ovary | − | Ovarian cancer | ++/97.2% ovarian mucinous tumors (52); MUC5AC over-expression leads to ovarian cancer-peritoneal cell interaction resulting in invasion to distant sites (53) |
| Endocervix | ++ (54) | Cervical cancer | +/Neoplastic glandular lesions; −/tubal metaplasia and endometriosis (55); poor survival with loss of MUC5AC (56) |
| Urothelial cells | − (57) | Urothelial bladder cancer (UBC) | ++/Various forms of UBCs (58,59); simultaneous expression of MUC1 and MUC5AC in 83% of UBC (57); no correlation with survival (57) |
Table 2.
Expression and implications of MUC5AC in benign pathologies
| Benign conditions | Pathologies | MUC5AC status |
|---|---|---|
| Allergic rhinitis | Disorders of nasopharyngeal mucosa with goblet cell hyperplasia, mast cell infiltration and lymphocytes (66) | MUC5AC is differentially overexpressed during allergic rhinitis, causing airway obstruction and infection (67) |
| Chronic rhinosinusitis | Goblet cell and submucosal gland cell hyperplasia resulting in mucus production and hypersecretion with defective mucociliary clearance of nasopharyngeal mucosa (68) | MUC5AC mRNA and protein levels are observed to be significantly increased in goblet cells of the epithelium in chronic rhinosinusitis compared with those in normal sinus mucosa (68–70) |
| Otitis media | Uncontrolled, excessive mucus production in the eustachian tube and middle ear contributing significantly to conductive hearing loss (71) | Post-transcriptionally modified longer length of MUC5AC mucin results in the higher viscosity of mucin, leading to abnormal mucociliary clearance, middle ear fluid stasis and ultimately chronic disease condition (72) |
| Chronic airway diseases (CADs) | CADs (asthma, cystic fibrosis and chronic obstructive pulmonary disease) are associated with mucous cell metaplasia, resulting in mucus hypersecretory phenotype (15) that involves increased expression, production and release of mucins | A marked increase in MUC5AC mRNA and protein expression is observed in CADs (73). Expression of MUC5AC is also found to be elevated in the cystic fibrosis sputum (74). A 6.4 kb VNTR in the MUC5AC gene has been significantly associated with the severity of cystic fibrosis lung disease (75) |
| Barrett’s esophagus | Premalignant condition of the esophagus consisting of mucosa with a metaplastic columnar epithelium (76) | MUC5AC expression and secretion during Barrett’s esophagus can be attributed to the conjugated bile acids present in the refluxate (77) |
| Helicobacter pylori infections | Helicobacter pylori (H.pylori) colonizes the gastric mucosa to induce chronic inflammation and intestinal metaplasia through genetic and epigenetic changes (78) | H.pylori colonize by its adhesion via BabA and SabA adhesins to the blood group antigens LeB and Sialyl LeX present on gastric mucin MUC5AC (79). The reduced MUC5AC expression might be associated with H.pylori mediated gastric carcinogenesis and the progression to GC (80). Among H.pylori virulence factors, urease virulence factor downregulate MUC5AC mucin expression in cell-specific manner and independent of NF-kB (81) |
| Pancreatitis | Inflammation of the pancreas that may progress to necrosis of the pancreas or surrounding fatty tissue. The two main types include acute and chronic pancreatitis (82) | In a case of autoimmune pancreatitis, the atypical cells were tested positive for the MUC5AC overexpression (83). In case of mouse models, chronic pancreatic injury results in hyperplastic PDGs along with de novo expression of mouse Muc5ac (84) |
| Inflammatory bowel disease | Dysfunctional mucus barrier caused by host immune, microbial, genetic or environmental | MUC5AC expression was detected in the mucus of 70% ulcerative colitis cases and associated with gastric metaplasia during long-standing inflammation to exhibit foveolar differentiation in intestinal mucosa. MUC5AC expression was observed predominantly in columnar cells of the significantly distorted crypts and positively correlated with disease activity and goblet cell depletion (85,86) |
This review, for the first time, provides a comprehensive and updated overview of the genomic organization, structure, expression, regulation and functional significance of secretory mucin MUC5AC in physiological conditions, as well as during development and progression of benign and malignant pathologies. Furthermore, it highlights the diagnostic utility and possible implications associated with direct/indirect therapeutic targeting of MUC5AC expression in various pathologies including cancers.
Biosynthesis, genomic organization, domain structure and domain-specific antibodies of MUC5AC
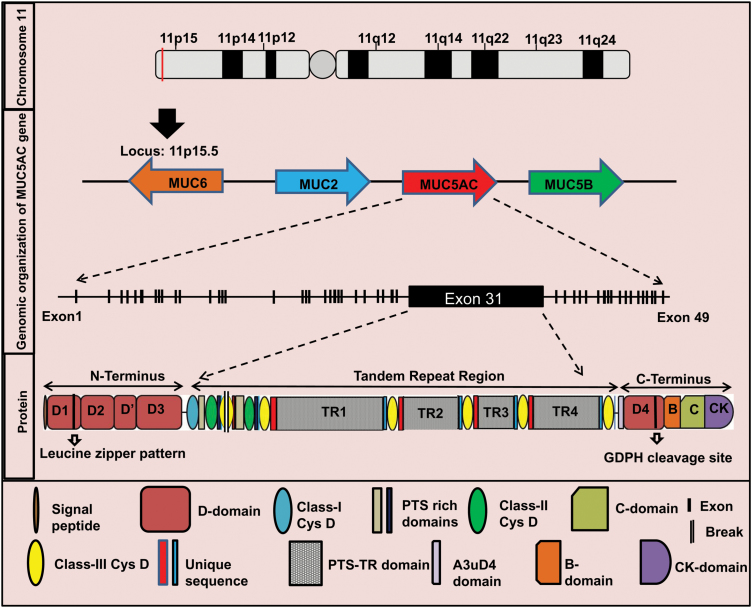
The high degree of sequence similarity among different mucin genes and their guanine-cytosine rich extended repeats make them difficult targets for molecular cloning, thereby rendering their structure and sequence hard to characterize (5,10). Polymeric gel forming secretory mucins share a similar gene structure composed of a large central exon representing TRR and flanking 5′ and 3′ regions. MUC5AC gene is located within a single 400 kb genomic deoxyribonucleic acid (DNA) segment in the subtelomeric, recombination-rich region on chromosome 11p15.5, clustered with four other mucin genes in a sequence MUC6-MUC2-MUC5AC-MUC5B from telomere to centromere (Figure 1) (87).
Figure 1.
Schematic representation of genomic and structural organization of MUC5AC. MUC5AC gene is located within a single 400 kb genomic DNA segment on chromosome 11 in the subtelomeric locus 11p15.5. On this locus, MUC5AC is clustered with four other mucin genes in a sequence MUC6-MUC2-MUC5AC-MUC5B from telomere to centromere. The array indicates direction of transcription (not to scale). MUC6 transcribes in opposite direction to MUC2, MUC5AC and MUC5B. The genomic DNA of MUC5AC transcribes to ~17.5 kb of mRNA comprised of 49 exons. The exon 31 is the largest domain. MUC5AC mRNA translates to a multidomain protein of ~5654 amino acids. Protein structure of MUC5AC is broadly divided into N-terminus, TRR and C-terminus. The N-terminus is made up of a 19 amino acid leader peptide and four von Willebrand factor type D domains-D1, D2, D′ and D3. The D1 domain also contains a leucine zipper pattern. The TRR consists of nine CysD domains and four PTS-rich tandem repeats (TR1–TR4). The C-terminus of MUC5AC is made up of D4, B, C (von Willebrand factor type C domain) and CK (cysteine knot domain) domains. D4 domain contains a putative GDPH cleavage site.
MUC5AC is a huge multidomain oligomeric secretory molecule with a heavily O-glycosylated apoprotein core and extensive intramolecular disulfide bonds between the cysteine-rich amino and carboxy-terminal domains (15). The completely annotated MUC5AC cDNA sequence (~17.5 kb) (http://www.ncbi.nlm.nih.gov/nuccore/748983075) consists of total 49 exons that encode a protein of ~5654 residues. The location and sequences of the intron–exon boundaries, intron–exon sizes and splice junction sequences have previously been elucidated and were recently updated (87). The 5′ region of MUC5AC is composed of 30 exons that encode cysteine-rich domains D1, D2, D′ and D3 [similar to structural domains within von Willebrand factor (vWF)] and a short domain corresponding to the MUC11p15-type (88). The 5′ region also contains a potential 19 amino acid signal sequence and 7 potential N-glycosylation sites (89). It has been proposed that Cys-Gly-Leu-Cys motifs in 5′-region D-domains of MUC5AC drive disulfide-linked multimer formation (88). The D1 domains of two consecutive MUC5AC molecules are involved in non-covalent interactions via their leucine zipper motif (between residues 273 and 300) resulting in homo-oligomerization and further multimerization via the N-termini of the homo oligomers (89). Overall, N-terminal domains are predicted to form inter- and intrachain disulfide bonds resulting in the assembly of MUC5AC as multimers.
The large central exon (exon 31, ~10.5 kb) in MUC5AC encompass PTS-rich tandem repeats (TR) and cysteine-rich CysD domains. The 4 TRRs (TR1–TR4) in MUC5AC consists of 24 nucleotides repeated units that translate into 8 amino acid PTS repeats. Although the consensus repeat sequence TTSTTSAP is observed more frequently, other motifs such as GTTPSPVP are also frequently observed (88). The four TR regions are interspersed by a total of nine CysD domains (87). Each of these CysD domains contains 10 cysteine residues per 110 amino acids (88). The amino-terminal region of Cys subdomain is thought to possess a C-mannosylation consensus sequence (W-x-x-W) (10). Interestingly, different individuals may have varying number of CysD domains due to segmental duplications resulting in MUC5AC structural variants (87). The TR domains of MUC5AC are heavily O-glycosylated, coupled with unique sialylation and sulfation patterns. Glycosylation not only impacts the packaging of MUC5AC in the secretory granules and passage through the secretory pathway but also leads to conformational alteration impacting overall gel volume and formation of the MUC5AC networks within the mucus layer. The differential glycosylation patterns of MUC5AC in different pathologies may act as a mode of host defense against the ever-evolving pathogens and other diverse classes of environmental insults.
The 3′ region of MUC5AC has 18 exons, the last one containing the 3′-untranslated region. These C-terminus exons encode MUC11p15-type, A3uD4 and cysteine-rich vWF-like (D4, B, C and CK) subdomains (90). The C-terminal cysteine-rich part of MUC5AC also contains a GDPH (Gly-Asp-Pro-His) cleavage sequence (91). MUC5AC is cleaved at GDPH site in the endoplasmic reticulum at a neutral pH, and cleavage is accelerated by a lower pH (91). However, the exact biological implications of this cleavage are still obscure necessitating further investigations. Studies suggest that the MUC5AC polypeptide form approximately within 20 min followed by folding, dimerization via disulfide linkage between the C-termini in the endoplasmic reticulum within 1 h. The dimeric polypeptide is then O-glycosylated by addition of N-acetyl galactosamine (GalNAc) residues (1–2 h) in Golgi complex. Further, oligo- and multimerization of MUC5AC happens in the Golgi network via disulfide linkage between N-termini (92).
It may be possible that the different domains and subdomains of MUC5AC bear interaction sites for extracellular matrix components and other members of mucus layer, thereby indicating toward yet to be elucidated functional roles of MUC5AC apart from being an indispensable arm of innate barrier function. Recently, Ermund et al. illustrated that MUC5AC secreted from the airway surface goblet cells transiently coat the MUC5B bundles secreted from submucosal glands of trachea, probably through their homologous CysD domains. This interaction seems to facilitate slow movement of mucus bundles and its attachment to the surface epithelium preventing their fall off into the lumen (93). Several monoclonal and polyclonal antibodies (Table 3) having reactivity towards unique domains of MUC5AC are available. Interestingly, multiple monoclonal antibodies reactive against MUC5AC were generated by animal immunization with mucins purified from ovarian cyst, pancreatic cancer cell line SW1990 xenografts or gastric mucin (Table 3). Depending upon their affinity toward mature mucin or native polypeptide, differential reactivity pattern has been observed for anti-MUC5AC antibodies. Ho et al. observed that 21M1, 45M1 and Nd2 antibodies reacted with mature MUCAC having high degree of glycosylation. In contrast, the CLH2 antibody reacted principally with immature, less glycosylated mucin. Furthermore, on immunostaining of SW1990 cultured cells, the CLH2 antibody showed intense staining in the perinuclear region, while Nd2 stained perinuclear and cytoplasmic region. On stimulation by forskolin, the immunostaining by CLH2 remained perinuclear, while extracellular staining pattern was observed for 45M1, Nd2 and 21M1. Hence, CLH2 has preferential reactivity toward immature form, whereas 21M1, 45M1 and Nd2 show strong reactivity toward mature secreted form (94). Furthermore, NPC-1C reacts with specific epitopes expressed by colorectal, pancreatic tumor-associated MUC5AC, while it does not appear from MUC5AC from normal tissues and other tumor cell types (95). Furthermore, the preclinical activity of NPC-1C showed promise for targeting colorectal and pancreatic tumors. Of note, in certain scenario, differential pattern in expression of MUC5AC levels (increased versus decreased) has been observed by different groups for same pathological conditions (96,97). The information regarding antibodies reactive epitope characteristic could be of immense significance in delineating the observed differences. Considering their unique characteristic, MUC5AC antibodies can serve as useful tools for studying its biosynthesis, polymerization, degradation and interactome.
Table 3.
List of domain-specific antibodies for MUC5AC
| Antibody | Antibody type | Antigen/peptide | Domain | Reference |
|---|---|---|---|---|
| SO-MU1 | Mouse mAb | Deglycosylated gastric mucin | Cys domains (3, 5, 6, 7, 8 and 9) | (98) |
| PDM5 | Mouse pAb | Synthetic peptide | VNTR of MUC5AC | (99) |
| LUM5-1, MAN5AC-1 | Rabbit pAb | RNQDQQGPFKMC | TRR & C-terminal peptide | (99,100) |
| 1-13M1 | Mouse mAb | Mucinous ovarian cyst | Cys2, Cys4 | (101–103) |
| 2-11M1 | Mouse mAb | Mucinous ovarian cyst | D1/D2 | |
| 9-13M1 | Mouse mAb | Mucinous ovarian cyst | D3 | |
| 19M1/21M1 | Mouse mAb | Mucinous ovarian cyst | MUC11p15/A3uD4 | |
| 62M | Mouse mAb | Mucinous ovarian cyst/native gastric mucin | C and CK domain | |
| 463M | Mouse mAb | Mucinous ovarian cyst/native gastric mucin | D4-vWF-like domain | |
| 589M | Mouse mAb | Mucinous ovarian cyst/native gastric mucin | D4-vWF-like domain | |
| CLH2 | Mouse mAb | CTTSTTSAPTTSTTSAPTTS | Tandem repeat | (101,104) |
| 45M1 | Mouse mAb | Mucinous ovarian cyst | Last CysD domain | (105) |
| 2-12M1 | Mouse mAb | Mucinous ovarian cyst | C-terminal | |
| 166-M1 | Mouse mAb | Native gastric mucin | C-terminal to GDPH cleavage site | |
| 660 | Monoclonal | Rat gastric mucin | (106) | |
| L56/C | Rabbit pAb | Normal gastric mucin | C-terminal | (103,107) |
| Anti-M1 antiserum | Rabbit pAb | Ovarian mucinous cysts and gastric mucosa | (108) | |
| HO8 | Chicken pAb | Mouse gastric mucin/QTSSPNTGKTSTISTT | Tandem repeat | (109) |
| Nd2 | Monoclonal | Pancreatic cancer cell line SW1990 xenografts purified mucins | C-terminal (competes with 45M1) | (94,110) |
| RGM23 | Monoclonal | Rat gastric mucin | NA | (111) |
| M5P-c1; M5P-b1 | Chicken and rabbit pAb | TTSAPTTSTTSAPTTS | Tandem repeat | (112) |
| NPC-1C (ensituximab) | Monoclonal | NPC-1C antigen (epitopes of tumor-associated MUC5AC) | NA | (95,113) |
mAb, mouse monoclonal antibody; NA, not applicable; pAb, rabbit polyclonal antibody.
MUC5AC glycosylation
Study conducted to understand the biosynthetic process of MUC5AC concluded that MUC5AC mucin forms a dimer during initial biosynthetic step. MUC5AC forms dimers in N-glycosylation-dependent manner in the rough endoplasmic reticulum within 15 min of the initiation of biosynthesis (114). De Bolos C et al. observed a distinct distribution pattern of MUC6 and MUC5AC along with different patterns of Lewis antigen expression within normal gastric tissues. A correlation was observed for simultaneous expression of MUC6 and Lewis (y) in antral mucous cells. The superficial epithelium and neck glands expressed MUC5AC along with Lewis type 1 antigens [Lewis (b) and sialyl-Lewis (a)] simultaneously (115). Looking at these observations, it could be proposed that the carbohydrate heterogeneity represented by these mucins might be associated with their cell and tissue-specific functions and might have different pathological implications. It is still unclear whether cell type-specific glycosylation is guided by a cell-specific set of glycosyltransferases or apomucin itself instruct its own glycosylation leading to multiple carbohydrate chains to bind each mucin type. In this context, it was observed that fucosyltransferase 2 (FUT2) is expressed in normal stomach only when MUC5AC is present. The authors concluded that in normal gastric epithelium, MUC5AC glycosylation pattern might be dictated by a specific set of fucosyltransferase instead of the apomucin sequence (116). Furthermore, in colon cancer cell lines like HT-29/M3, it has been shown that overexpression of enzyme FUT1 catalyzes the addition of α-1,2-fucose to MUC5AC, thus underscoring cell-specific glycosyltransferase-mediated apomucin glycosylation (117). Furthermore, it has been shown that MUC5AC-associated Lewis type-1 antigens play a crucial role in binding of Helicobacter Pylori (H. Pylori) to gastric mucosa that may cause gastritis, peptic ulcer disease and gastric cancer (GC) (118). Magalhaes et al. observed that in FUT2 knockout mice, the loss of MUC5AC fucosylation impaired gastric mucosal binding of H.Pylori Bab A adhesin decreasing the risk of disease (119). Furthermore, Raklawska et al. observed that FUT2-mediated fucosylation of MUC5AC (α1,2-fucosylation) increases mucus viscoelasticity that is associated with severe asthma exacerbation risk (120). Furthermore, Hennebicq-Reig S et al. studied the impact of the growing state and differentiation stage of the cells on O-glycosylation of MUC5AC. In HT-29 MTX cells, expression of MUC5AC was not observed up to day 5, while the activity of GalNAc transferase and 3-β-galactosyltransferase were at high rates. Interestingly, postconfluent cultures of HT-29 MTX cells expressed MUC5AC with high sialic acid content in α-2,3 linkage to galactose residues due to the differential activity of ST3Gal I enzyme (121). This study highlights that different growth state and differentiation state of cancer cells implicate in differential glycosylation leading to differential oncogenic phenotypes. Furthermore, it has been known that inflammatory microenvironment of pancreatic cancer exposes pancreatic cancer cells to multiple cytokines and growth factors. In order to reason the origin of different mucin glycosylation seen in pancreatic cancer, Wu et al. utilized antibody-based lectin arrays for summarizing the changes in different glycan structures on multiple proteins. Interestingly, the pro-inflammatory stimuli altered the expression and glycosylation of secretory mucin MUC5AC in a cell line-dependent manner (122). It is now established that the mucin-associated altered glycosylation may impact the mitogenic and metastatic properties of pancreatic cancer cells and can be clinically utilized for generation of tumor-specific markers. Hence, the identification of combined mucins and specific glycoforms has been attempted to increase the specificity of these cancer biomarkers. Pan et al. investigated altered glycoproteome associated with pancreatic tumorigenesis and observed aberrant N-glycosylation of MUC5AC during pancreatic cancer compared with normal pancreas (123). Furthermore, owing to glycan alterations in pancreatic cancer, the novel antibody-lectin sandwich array technology utilized the combination of antibody-based capture along with lectin detection of glycan levels. It was observed that lectin Vicia villosa (bind to core the GalNAc), Jacalin lectin [bind Tn antigen, related T antigen (Galb1, 3GalNAc)], WGA [binds N-acetylglucosamine (GlcNAc), other saccharides] and Erythrina cristagalli lectin (binds lactosamine) increasingly bind to MUC5AC from mucin-producing cystic tumors. Use of lectin wheat germ agglutinin assay led to the detection of a MUC5AC glycan variant, which could ultimately differentiate between mucin-producing cystic tumors from benign cystic lesions with 78% sensitivity and 80% specificity (124). Results from the study by Cao et al. (125) show that detection of oligosaccharides terminating in GlcNAc or N-acetyl-lactosamine with sporadic α1,2-linked fucose on MUC5AC distinguished mucinous pancreatic cysts from non-mucinous cysts with 93% accuracy. Furthermore, utilizing proximity ligation assay and the glycoform-specific antibodies, the expression of Tn-MUC5AC, STn-MUC5AC and SLea-MUC5AC glycoforms has been observed in carcinomas from stomach, ampulla of Vater, lung and ovary. Furthermore, extensive studies are necessitated to establish these MUC5AC-specific glycoforms as potential biomarker candidates in these malignancies (126).
Dysregulation of MUC5AC in benign pathologies
By forming a polymeric gel, MUC5AC acts as a physicochemical barrier against environmental, chemical and enzymatic insults, allergens and infectious pathogens, thus forming the first line of defense (13). The secretory mucin MUC5AC is synthesized under physiological conditions by various epithelial cell surfaces like conjunctiva (60), middle ear epithelium (63), lungs, stomach, gallbladder and endocervix (14). At the ocular surface, the conjunctival goblet cell-derived MUC5AC acts as a component of the tear fluid providing lubrication as well as maintaining hydration of the epithelial surface (61). MUC5AC is a major secretory mucin in the middle ear epithelium and plays a major role in protecting the surface from pathogenic invasion, pathogen-mediated damage and pathogen clearance (127). MUC5AC is one of the major airway mucins where it is expressed mainly by the goblet cells (13) and is thought to play a prime role in mucosal defense system. However, to date, the exact mechanisms behind these functions are poorly understood (13). MUC5AC messenger RNA (mRNA) has been detected in the stomach at 23 weeks of gestation, and it is the major gel-forming mucin consistently transcribed and expressed in the adult stomach (54,128,129). As a component of the mucus layer covering the gastric mucosa, MUC5AC functions to protect gastric tissues, from the hazardous gastric environment and multiple infectious agents (130,131). MUC5AC is also expressed in endocervix; however, the definitive role played by it is still unclear (54). Frequent expression of MUC5AC has also been observed in the gallbladder (30). Interestingly, MUC5AC is secreted into bile fluid (132). Besides all these, a moderate signal of MUC5AC mRNA has been detected in primitive intestine both on villi and in crypts at 8 weeks of gestation with its inconsistent levels after that. Furthermore, at 12 weeks, MUC5AC mRNAs were observed in the ileum but not in the colon with no expression of MUC5AC detected in any region of the intestine after 12 weeks. Interestingly, MUC5AC expression is never detected in adult bowel unless early stages of neoplasia develop (51). Also, expression of MUC5AC protein had been observed early in embryonic epidermis from day 13 of gestation until 7 days after birth, when the surface epidermis became negative, and the expression restricts to secreting sebum cells (65). Expression of secretory mucin MUC5AC is deregulated in different disease conditions as reviewed in Table 2.
Molecular alterations of MUC5AC in various malignancies
MUC5AC expression is significantly altered in various malignant conditions. Few studies conducted to functionally characterize this mucin in GC (133), pancreatic cancer (134) and lung cancer (135) indicate both tumor suppressive as well as oncogenic roles of this mucin, suggesting its context-dependent functions. The mechanistic basis of these functions is still obscure. Detailed information regarding expression and molecular implications of MUC5AC in various malignancies is discussed in this section:
Lung cancer
Variable expression pattern of MUC5AC has been observed in bronchial epithelium from normal individuals. MUC5AC is expressed in goblet cells in the normal respiratory mucosa. In initial studies, a reduced expression of MUC5AC was observed in non-small cell carcinomas, regardless of their histologic subtype (136). In contrast to these, expression of MUC5AC was shown to be the most intense in mucinous-type bronchoalveolar carcinomas (137). In a study conducted to evaluate the expression of the secretory mucins (MUC2, MUC5AC and MUC6) in 79 surgical specimens of small adenocarcinoma of the lung cases, 7.6% cases expressed MUC5AC. No significant correlation was observed between MUC5AC expression and nodal involvement (138). While assessing progression from adenomatous hyperplasia to adenocarcinoma with mixed subtypes (MX), an increase in the MUC5AC expression correlated significantly with p53 gene abnormalities, suggesting that the expression of MUC5AC might be associated with the progression of lung adenocarcinoma (139). Furthermore, overexpression of MUC5AC gene was significantly associated with postoperative relapse and lung cancer metastases (23). In non-small cell lung carcinoma samples, expression of MUC5AC was detected in 27% samples with epidermal growth factor receptor EGFR) mutations and 30% samples with wild-type EGFR. However, regardless of EGFR mutation status, expression of MUC5AC in non-small cell lung carcinoma was not associated with overall and relapse-free survival (140). As non-small cell lung carcinoma cases bearing sialomucin expression tended to relapse earlier than those without sialomucin (141), hence, Yu et al. investigated whether the expression of sialomucin in lung cancer is due to an altered glycosylation event or by differential expression of mucin genes. Interestingly, the significantly higher MUC5AC gene expression in adenocarcinomas positively correlated with sialomucin expression (P = 0.012) (142). Furthermore, studies conducted to test the hypothesis that sialomucin on MUC5AC may contribute to increased metastasis of lung cancer cells revealed that the patients bearing coexpression of MUC5AC and sLe(x) in tumors had a higher probability of postoperative distant metastasis and shorter overall survival (24). Also, overexpression of secretory mucin MUC5AC in lung tumors of smokers has been associated with increased probability of postoperative recurrence (23). Interestingly, investigation of the reversible and irreversible expression changes in lung epithelium upon smoking cessation indicated that only partial reversal of MUC5AC on cessation of smoking might account for the persistent lung cancer risk and/or progression to lung cancer (143).
Gastric cancer
The normal adult stomach is characterized by strong expression of MUC5AC in the foveolar epithelium (144,145). However, the expression rate of MUC5AC is decreased with the loss of tumor differentiation, increase in tumor progression, invasion depth and with a heightened occurrence of lymph node metastasis (144). MUC5AC expression is reduced in GC with 38–42% of gastric carcinomas expressing MUC5AC (146–148). Reduction of MUC5AC expression is associated with the significant reduction of gastric differentiation, poor prognosis and a high-risk subgroup of pTNM stage-I patients (26). Similarly, MUC5AC expression has been inversely associated with tumor stage, depth of invasion, lymphovascular invasion, lymph node and liver metastasis (27,133,147). The patients with MUC1+/MUC5AC− staining in gastric carcinoma tissues showed the lowest survival rate in contrast to the patients with MUC1−/MUC5AC+ staining pattern (147). Furthermore, MUC5AC expression was negative in mucinous gastric carcinomas having a deeper invasion, more frequent lymph node metastasis, more advanced pathologic state, lower survival rates than patients with non-mucinous gastric carcinoma (28). In 11 retrospective cohort studies comprising 2135 patients conducted to assess the association between MUC5AC expression and overall survival and clinicopathological characteristics revealed that decreased MUC5AC expression was significantly correlated with poor overall survival of GC patients. Furthermore, in these analyses, decreased MUC5AC expression was also significantly associated with tumor invasion depth and lymph node metastasis in GC (29).
Pancreatic cancer
It has been observed that the expression of MUC5AC transcripts was undetectable in all the cell types of normal pancreatic tissue and chronic pancreatitis adjacent to pancreatic cancer. However, MUC5AC transcript expression was observed in the areas of papillary hyperplasia and pancreatic cancer. MUC5AC mRNA detected by in situ hybridization was expressed in 83% of the 24 intraductal papillary mucinous tumors versus in only 13% of the 38 invasive ductal carcinomas (42). All the three subtypes of intraductal papillary mucinous tumors (villous dark cell type, papillary clear cell type and compact cell type) expressed MUC5AC (42). Immunohistochemically, expression of MUC5AC was observed in 100% of intraductal papillary mucinous adenomas compared with only 33.33% of benign mucinous cystadenomas of the pancreas (149). Besides this, MUC5AC was expressed in all the cases of gastric and intestinal type of intraductal papillary mucinous neoplasm (IPMN) (150). Meta-analysis of 39 studies having 1235 IPMN samples to determine the relationship between specific genetic alterations and malignant transformation in IPMN indicated differential expression of MUC5AC as a common feature across IPMN cases. Interestingly, among eight markers, a weak positive correlation of MUC5AC and Kirsten rat sarcoma viral oncogene (Kras) and a strong positive correlation of hTERT (human telomerase reverse transcriptase) and Shh (Sonic hedgehog) were observed for histologic progression of IPMN to malignancy (151). Also, pancreatic mucinous cysts without histologic characteristics of neoplasia and non-invasive mucinous cystic neoplasms were observed to be expressing MUC5AC (152,153).
MUC5AC is maximally expressed among all the mucins in all the histologically well-defined precursor ductal lesions of pancreatic cancer called pancreatic intraepithelial neoplasia (PanIN-1A, -1B, -2, -3) and pancreatic cancer compared with its rare expression in normal pancreas (154). Matsuyama et al. examined the differences in mucin expression between normal PanIN lesions and PanINs in pancreatic ductal adenocarcinoma (PDAC-PanINs). MUC5AC expression was observed in 41, 65.7 and 36.4% of normal PanIN-1A, PanIN-1B and PanIN-2 specimens, respectively. Notably, expression positivity was increased to 80.9, 75.8 and 78.3% for PDAC-associated PanIN-1A, PanIN-1B and PanIN-2 specimens, respectively. Particularly, significant differences were observed in the frequency of MUC5AC expression between normal and PDAC-PanIN-1A (P < 0.0001) and PanIN-2 (P < 0.05) lesions (155).
Among pancreatic tumors, utilizing 21M1 Mab that reacts with C-terminal region of MUC5AC, the expression of MUC5AC was observed in all 100% cases of well-differentiated pancreatic tumors, 96% moderately differentiated and 59% of poorly differentiated tumors (154). In another study, the expression of MUC5AC was observed in 73.9% of cancerous regions, 48.7% of the dysplastic regions and 72% of the hyperplastic regions but not in the normal pancreatic duct. Notably, stromal expression of MUC5AC was observed in 60.9% of the cancerous regions with no such observation in stromal regions associated with hyperplasia (156). Furthermore, immunohistochemistry utilizing CLH2 Mab on invasive ductal carcinomas cases of the pancreas in the Japanese population (total cases = 33) revealed that MUC5AC was expressed in 63.6% cases (N = 21), and the investigator observed strong association MUC5AC absence with lymphatic invasion, venous invasion, lymph node metastasis with MUC5AC positive patients (N = 12) showing significantly better survival than MUC5AC-negative patients (43). Contrary to this, in an important study done on 161 pancreatic tumors taking samples from Surveillance, Epidemiology and End Results registries, the authors observed and presented a statistically significant association between expression of MUC5AC and shorter survival time of patients after adjusting for various covariate including race, sex, stage, time period of diagnosis and treatment. This association was further strengthened in well-to-moderately differentiated ductal adenocarcinomas. Authors highlighted that tumor histologies differed in this study compared with Japanese cohorts and concluded that pancreatic tumor types differ across populations and that interpretation of biomarkers may also vary by pancreatic tumors lineages (45). Takano et al. classified PDAC samples (59 specimens) by histopathological changes and the propensity of heterogeneous gastric and intestinal elements. Tissues expressing MUC5AC (using Mab CLH2) were classified as gastric type PDAC accounted for approximately 25.4% of cases. Interestingly, PDAC with gastric elements were well-differentiated types with the significantly higher rate of lymph node metastasis (46). In endoscopic ultrasound-guided fine needle aspiration specimens from PDAC patients (N = 114) in the Japanese cohort, expression of MUC5AC detected using CLH2 Mab was observed in 78.9% cases. Interestingly, advanced-stage PDAC patients with MUC5AC expression had a significantly better outcome than those who were MUC5AC negative (P = 0.002) (44). Overall, observations regarding MUC5AC expression and pancreatic cancer pathogenesis vary across Japanese cohort versus other regions. These differences could be due to differential pathological classification criterion, sample type, antibodies used, population cohort and stage at which samples were collected.
One of the biggest challenges associated with pancreatic cancer is lack of early-stage diagnostic markers. By the time patient is diagnosed, the disease is in its advanced stages or cancer has metastasized to distant locales. In this context, analysis of upregulated MUC1 and MUC5AC mRNA in pancreatic juice is a better diagnostic modality than that of MUC4 and MUC6 mRNA during the development of high-grade PanIN lesions to invasive ductal carcinomas (157). In the non-neoplastic endoscopic ultrasound-guided fine needle aspirations (EUS-FNA) of the pancreas, MUC5AC was observed to be absent. Interestingly, the MUC1/MUC2/MUC5AC panel was considered to be the most optimum for the diagnosis of ductal adenocarcinoma (158). Furthermore, in EUS-FNA specimens, higher specificity of the panel MUC1−/MUC2−/MUC5AC+ and MUC1−/MUC2+/MUC5AC+ were highlighted to diagnose pancreatic cancer and pancreatic mucinous neoplasms, respectively (159). In a study conducted on sera of pancreatic cancer patients, MUC5AC was found to be elevated at the protein level (in 35% of the patients) and had the most glycan alterations. The most frequent elevations involved fucose, the CA 19-9 (Cancer antigen 19-9) and terminal mannose. The N-glycosylation, as well as O-glycosylation, may play important roles in modifying the behaviors of MUC5AC in disease (160). Hence, identification and functional characterization of MUC5AC-specific glycan alterations during pancreatic cancer are necessitated. In fact, detection of glycan variants on MUC5AC utilizing antibody-lectin sandwich microarray method could discriminate benign cystic lesions (serous cystadenomas + pseudocysts) from mucin-producing cystic tumors (mucinous cystic neoplasms and IPMN) with sensitivity/specificity of 78%/80%. Interestingly, the combination with cyst fluid CA 19-9 improved sensitivity/specificity to 87%/86% (124). Furthermore, measurement of glycans on specific proteins may improve a biomarker performance (161). Interestingly, combined detection of the standard CA 19-9 along with CA 19-9 on MUC5AC and MUC16 improved the sensitivity of pancreatic cancer detection over CA 19-9 alone in each sample set (161). Moreover, a serum enzyme-linked immunosorbent assay has been developed using the NPC-1C antibody that detects specific epitopes expressed by tumor-associated MUC5AC in pancreatic cancer but not by normal tissues. This serum test may be a new tool to aid in the improved diagnosis of pancreatic cancer (113). In a major leap forward toward the early stage diagnosis of pancreatic cancer, a recent multicenter study investigated circulating levels of MUC5AC using sandwich enzyme-linked immunosorbent assay and established it as a valuable diagnostic biomarker. It was observed that MUC5AC efficiently differentiated resectable early-stage PC cases from healthy controls, benign controls and chronic pancreatitis cases with 83%/80% sensitivity/specificity, 67%/87% sensitivity/specificity and 83%/77% sensitivity/specificity, respectively. The diagnostic accuracy for differentiating resectable cases from controls was significantly improved (P-value < 0.001) on a combination of CA 19-9 and MUC5AC (162).
Colorectal cancer
Expression of MUC5AC has been extensively explored during colon adenoma–carcinoma sequence. The absence of MUC5AC expression in the normal colon has been observed by multiple groups. However, MUC5AC is aberrantly expressed in adenomas that are known to be the precursor lesions of colorectal cancer (CRC). Intense overexpression of MUC5AC was a feature of rectosigmoid villous adenomas with low-grade dysplasia than cases with high-grade dysplasia. The study suggested that by acting as a specific marker for rectosigmoid villous adenomas, MUC5AC may be useful for the early detection of rectosigmoid villous adenoma recurrences (163). In another study, MUC5AC was rarely detected in normal colon, while its low positivity was observed in hyperplastic polyps. MUC5AC immunoreactivity was greatest in larger adenomas of moderate villous histology and dysplasia, while it tended to decrease in highly villous polyps and with severe dysplasia (164). Interestingly, in a larger cohort of colorectal carcinoma samples, expression of MUC5AC was strongly associated with carcinogenic features via the serrated neoplasia pathway, including CpG island methylator phenotype (CIMP) positivity, somatic BRAF p.V600E mutation, mismatch repair deficiency, proximal location, poor differentiation, lymphocytic response and increased T-stage (P < 0.001) (165,166). Interestingly, on the basis of significantly increased expression of secretory mucin MUC5AC and MUC2 in sporadic microsatellite instability-high cancers, it was concluded that serrated polyps of the colorectum may represent precursors of microsatellite instability-high cancers (167). Furthermore, MUC5AC hypomethylation status could be a useful marker to identify serrated neoplasia pathway-related precursor lesions (168). Krishn et al. (169) observed that MUC2, MUC5AC and MUC17 could provide a critical aid in effectively discriminating adenoma–adenocarcinoma from benign hyperplastic polyps. Furthermore, MUC5AC was found to be associated with both goblet cells as well as crypt for sessile serrated precursor lesions of colon compared with only goblet cells in hyperplastic lesion (170). Besides precursor lesions of CRC, increased expression of MUC5AC has been observed in 90% of high-grade dysplasia associated with ulcerative colitis (UC; N = 10), 89% of low grade dysplasia in UC (N = 9), 73% of colitic cancer (N = 22) (171), 33% of signet-ring carcinoma of the colorectum (N = 12) (172), 34.1% of colon adenocarcinoma samples (N = 41), 60% of mucinous carcinoma (N = 5) and 19% of lymph node metastatic cases (N = 21) (48,50,173). On the basis of observed higher incidence of MUC5AC in UC-associated neoplasms than sporadic tumors, its utility in the differential diagnosis of UC-associated neoplasms and sporadic ones was also highlighted (171). Furthermore, MUC5AC autoantibodies have been detected in the sera of 27.3% healthy subjects, 45% patients with polyps and 60% CRC patients. Interestingly, MUC5AC antibody positive patients had shorter disease-free survival and overall survival (174). Presence of MUC5AC has been significantly correlated with the lack of mismatch repair protein MutL homolog 1 (MLH1) expression (P < 0.005) in colorectal carcinomas (175). Besides this, expression of MUC5AC in colorectal adenocarcinoma was significantly correlated with tumor differentiation, invasion (P < 0.01) along with positive correlation to E-cadherin expression (50). Another study on T1-stage colorectal carcinoma patients revealed that MUC5AC-positive patients had significantly higher incidences of right-sided carcinoma (P = 0.005), polypoid growth carcinoma (P = 0.009) and mucinous adenocarcinoma (P = 0.035) than in MUC5AC-negative patients (176). MUC5AC expression in CRC has a significant positive correlation with tumor grade (P = 0.006), and MUC5AC expression increased as the stage of disease progressed from 1 to 4 (49). Not only in humans but also in rat colon cancer model (MNNG treatment based), increased Muc5ac expression was observed in typical aberrant crypt foci in the colon mucosae underscoring utility of Muc5ac as an early marker of colon carcinogenesis (177,178).
In addition to these malignancies, expression status and functional association of MUC5AC in other cancers have been elaborated in Table 1.
Functional and mechanistic implications of MUC5AC in cancer
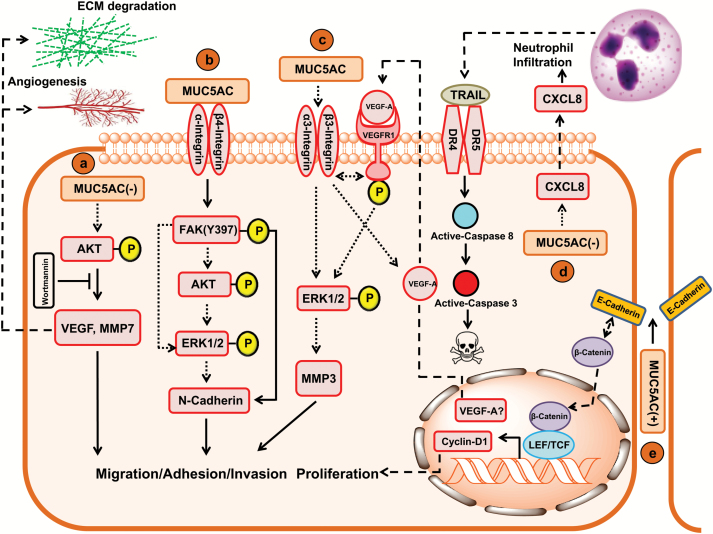
Despite information regarding differential expression of MUC5AC in multiple malignancies, fewer studies have focused to investigate context-dependent role of MUC5AC in various pathologies. Study conducted to comprehend the functional implication of MUC5AC in pancreatic cancer concluded that MUC5AC mediates accelerated pancreatic cancer progression by inducing the vascular endothelial growth factor receptor-1 (VEGFR-1) signaling pathway in an autocrine manner. Suppression of MUC5AC resulted in significant decrease in the propensity of cells to adhere or invade the extracellular matrix components as compared with controls along with downregulation of genes encoding for the adhesion and invasion factors like matrix metalloproteinase-3 (MMP-3), integrins and vascular endothelial growth factor (VEGF) and reduced activation of ERK1/2. Inhibition of MUC5AC led to the reduced production of VEGF and subsequent phosphorylation of VEGFR-1 (Figure 2). Subcutaneous tumor formation was significantly reduced on knockdown of MUC5AC in nude mice. However, the mechanisms associated with MUC5AC-mediated in vivo tumor suppression remain obscure (179). Furthermore, in another study, Inaguma et al. (134) observed that GLI-mediated expression of MUC5AC modulated pancreatic cancer cell properties by regulation through the E-cadherin/β-catenin axis. Observations from the study indicated that homophilic interaction and membrane stabilization of E-cadherin might be hindered by MUC5AC expression at the junctions of pancreatic cancer cells. Therefore, it was inferred that targeted collapse of the MUC5AC polymeric gel would allow E-cadherin of adjacent cells to undergo their conventional ligation pattern and thereby restrain β-catenin-mediated expression of genes encoding for migration and invasion factors of pancreatic cancer cells (Figure 2) (134). Another study showed that presence of MUC5AC does not impact the in vitro growth of pancreatic cancer cells; however, its expression was directly correlated with tumor growth in vivo. Mechanistically, infiltration of CD45R/B220+ and Gr-1+ cells was observed in si-MUC5AC cells derived tumor tissues of mice, suggesting that infiltrated neutrophils might have antitumor effects mediated by antibody-dependent cell cytotoxicity via phagocytosis, elastase or superoxide generation. Another possible reason for the attenuation of tumorigenicity in knockout mice was a possible association of MUC5AC in immune suppression and avoidance (180). Furthermore, Hoshi et al. (181) observed that suppression of MUC5AC inhibits in vivo tumor growth due to increased infiltration of neutrophils resulting in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated increased apoptosis (Figure 2). However, extensive studies should be directed to explore the immunosuppressive role of MUC5AC in human pancreatic cancer.
Figure 2.
Molecular and signaling impact of MUC5AC in various malignancies. (a) GC: The absence of MUC5AC results in increased migratory and invasive capability of GC cells through increased expression of VEGF-A and MMP-7 via increased phosphorylation of AKT. The inhibition of AKT phosphorylation by Wortmannin (PI3K/AKT pathway inhibitor) resulted in decreased expression of VEGF-A and MMP-7 that are implicated in decreased migration and invasion of GC cells by angiogenesis and degradation of extracellular matrix. The mechanisms by which MUC5AC mediates AKT phosphorylation are still obscure (depicted by dotted arrow). (b) Lung cancer: The increased migratory potential of lung cancer cells is mediated by interaction of secretory mucin MUC5AC with β4 integrin. This interaction results in increased phosphorylation of focal adhesion kinase at Y397 residue, resulting in increased N-cadherin expression and epithelial to mesenchymal transition. High MUC5AC expression in lung cancer cells is also associated with increased phosphorylation of AKT and ERK1/2 by still unknown mechanisms (probable pathways depicted by dotted arrows). (c) Pancreatic cancer: In pancreatic cancer cells, high MUC5AC expression is associated with increased migratory potential. Attempts to decipher underlying mechanisms revealed increased MUC5AC-associated high expression levels of α3 integrin, β3 integrin, VEGF-A and MMP-3 along with increased phosphorylation of VEGFR1 and ERK1/2. However, the exact mechanisms by which above mentioned factors implicate pancreatic cancer cell migration remained obscure (probable pathways depicted by dotted arrows). (d) The absence of MUC5AC in pancreatic cancer cells results in increased expression of chemokine CXCL8 by unknown mechanisms. Under in vivo conditions, increased secretion of chemokine CXCL8 from pancreatic cancer cells act as a chemoattractant for neutrophils. The tumor-associated neutrophils then secrete apoptotic factor TRAIL, which binds and activates DR4/DR5 on plasma membrane of pancreatic cancer cells, resulting in increased cell death via extrinsic apoptotic pathway. (e) In PDAC cells, overexpressed MUC5AC may be basolaterally sorted to the intercellular junctions by unknown mechanisms. MUC5AC in intercellular junctions may interfere with the E-cadherin (E-Cadh) homotypic interaction, thereby increasing nuclear localization of β-catenin (β-Cat), which results in increased expression of cyclin-D1 (plays an important role in cell cycle regulation and proliferation).
Elevated levels of MUC5AC are associated with poor prognosis in lung cancer; wherein, it was associated with epithelial to mesenchymal transition of the cancer cells. MUC5AC-mediated increased lung cancer cell migration was also attributed to its interaction with integrin β4 and increased focal adhesion kinase (FAK) phosphorylation at Y397 residue (Figure 2) (135). On the contrary, MUC5AC knockdown increased tumorigenicity and metastasis of GC cells (SNU216 and AGS). Increased Akt (v-akt murine thymoma viral oncogene homolog 1) phosphorylation, elevated VEGF and matrix metalloproteinase-7 (MMP-7) expression were indicated as the underlying mechanism behind the increased oncogenic properties in the knockdown cells because the presence of MUC5AC can lead to inhibition of the phosphatidylinositol 3-kinase/Akt pathway (Figure 2) (133). A recent study highlighted MUC5AC as a potential therapeutic candidate for CRC. The genetic inhibition of MUC5AC in colon cancer cells impaired the colony-forming ability of these cells by reducing cell viability, increased apoptosis and G1 cell cycle arrest. Interestingly, loss of MUC5AC dramatically repressed the cell migration and invasion of colon cancer cells (182).
Overall, MUC5AC is an emerging conspicuous glycoprotein differently regulated in multiple malignancies with variable prognostic marks in a context-dependent manner. However, to date, limited studies have explored its mechanistic significance, and further emphases are necessary to delineate its functional contributions during neoplastic pathogenesis.
Muc5ac mice models
Shekels et al. for the first time established that the MGM gene identified from murine gastric cDNA, was a murine homolog of human MUC5AC (109). Similar to human homolog, mouse Muc5ac is organized with other mucins as Muc6-Muc2-Muc5ac-Muc5b indicating the organizational conservation during evolution (183). The genomic region encoding for Muc5ac was found to be of 29 kb size and was expected to produce a 9 kb mRNA transcript bearing 49 exons (184). The size of Muc5ac central exon was estimated to be approximately 6.5 kb (183). With respect to orientation, the mucins Muc2, Muc5ac and Muc5b were arranged in the same direction as in humans, while Muc6 gene has reverse orientation. Further investigations indicated that the peptide sequences from different secretory mucins had striking similarities in their N- and C-terminal regions and also to pre-pro-von Willebrand factor-like domains [D, B, C and C-terminal cystine knot (CK) domains]. However, the PTS-rich central region of different secretory mucins was specific for mucin-type and species. The murine MUC5AC gene encompasses a stretch of 48 nucleotides that encodes a serine and threonine-rich consensus sequence of 16 amino acid (QTSSPNTGKTSTISTT) residues, comparable with the human 8 amino acids long TR regions (109,185). Expression studies also showed that alike human MUC5AC, murine Muc5ac was observed most prominently in airways, stomach and conjunctiva of mice (109). Overall, this information laid the foundation for the investigation of Muc5ac gene control and functional implications utilizing the animal models (183). Multiple studies have utilized the genetically modified mice models of Muc5ac, to understand the mechanisms associated with its regulation and functionally characterize this secretory mucin in pathologies related to different organs.
Hasnain et al. highlighted the role of Muc5ac as a crucial mediator during infections of the intestine, besides being a major component of the immune barrier. Cytokines IL-13- and IL-4-induced Muc5ac protects from Trichuris muris (T.muris) infection, whereas its deficiency (Muc5ac−/−) renders mice more susceptible to chronic T.muris infection along with a significant delay in the removal of other infectious agents such as Trichinella spiralis and Nippostrongylus brasiliensis from the intestines of infected mice (186). Interestingly, Muc5ac-deficient mice infected with T.muris revealed significantly elevated IFN-γ, increased goblet cell numbers, Muc2 levels, significantly higher levels of adenosine triphosphate in worms compared with the worms isolated from the infected WT mice. There was also a slight increase in IFN-γ in uninfected Muc5ac-deficient mice (186). In this study, authors also concluded that Muc5ac plays an important role in alleviation of inflammatory response, thus playing a protective role. Ehre et al. investigated relationship between inflammation, Muc5ac hypersecretion and airways obstruction using Muc5ac transgenic animal and highlighted the protective role of Muc5ac during airway pathologies. Muc5ac transgenic mouse was developed by cloning full-length Muc5ac cDNA in murine small and large airways. The resultant Muc5ac transgenic mice showed a 17.7-fold increased Muc5ac production in bronchoalveolar lavage fluid and approximately 2-fold increase over the ovalbumin-challenged model compared with WT mice (184). There was no compensatory impact on other mucins suggesting independent regulation of these mucins in the absence of disease. Furthermore, there was no evidence of small airways, large airways obstruction suggesting its efficient clearance from airway surfaces (184). Interestingly, the increased mucus was recovered by increasing the height of the mucus layer, without any alterations in its concentration. Moreover, the protective antiviral impact of Muc5ac was uncovered by the resistance of Muc5ac-transgenic mice to PR8/H1N1 influenza in vivo (184). It was shown that α-2,3-linked sialic acid residues on the Muc5ac act as a decoy to bind virus, limiting its access to the airways epithelial surfaces, thus preventing infection (184). Interestingly, reduced neutrophilic response thus limiting the lung tissue damage was observed in Muc5ac-transgenic mice exposed to infection (184). However, the exact mechanisms addressing the impact of Muc5ac on reduced neutrophil infiltration remained elusive, needing further investigations. This study cautions for careful titration of any future therapeutics designed to reduce Muc5ac production in order to amend airway obstruction, as this may lead to compromised immune functions (184).
Contrary to above mentioned protective roles of Muc5ac, another study provided the evidence of the detrimental role of Muc5ac during acute lung injury. For this, the Muc5ac−/− mice were subjected to ventilator-induced lung injury (VILI) (187). WT mice exposed to VILI revealed increased Muc5ac transcript and protein levels mediated by transcription factor NF-κB (187). Intriguingly, Muc5ac−/− mice exposed to VILI had significantly improved pulmonary gas exchange, significantly reduced pulmonary edema, complete abolishment of myeloperoxidase levels, attenuated regulation of cytokine, chemokine and chemokine receptor levels (Cxcl1, Cxcl2 and IL-6 transcripts and proteins) and significantly increased survival than WT littermate controls. The presence of Muc5ac also increased in trafficking of Ly6Ghigh/CD11bhigh polymorphonuclear cells in the pulmonary tissue of WT mice exposed to VILI (187). Mechanistically, it is possible that by acting as a cytokine ‘sponge,’ Muc5ac may prolong the half-life of these inflammatory cytokines and other inflammatory mediators, thereby resulting in enhanced lung inflammation and increased neutrophil trafficking. Evans et al. identified the novel role of mucin plug formed by Muc5ac overproduction in exacerbating the pathologies of airway hyperreactivity to methacholine (MCh) in mice challenged with pulmonary allergens Ovalbumin and Aspergillus oryzae (AOE) extract. Since no significant variation in the eosinophil infiltration was observed among WT and Muc5ac−/− mice upon OVA and AOE challenge, airway hyperreactivity was reasoned to be caused by Muc5ac-mediated physical constriction of the airways. Furthermore, airway diameters and airway smooth muscle contraction thickness were significantly reduced in Muc5ac−/− mice after allergen exposure. The authors commented that both the airway smooth muscle and Muc5ac hypersecretion act synergistically to induce airway hyperreactivity (188).
Besides studies delineating the role of Muc5ac in airway pathophysiology, its potential role in the maintenance of ocular homeostasis has also been investigated. In Muc5ac−/− mice model, there was a compensatory increase of another secretory mucin Muc5b at mRNA and protein level along with significant enlargement of mucous cells on the ocular surface (189). Lack of Muc5ac markedly destabilized the tear film resulting in qualitative tear film insufficiency with the tear volume remaining intact (189). Besides this, a few Muc5ac−/− developed opacification on their ocular surface (mainly on the cornea) warranting further investigations on the molecular and mechanistic basis of these observations. This study highlighted the therapeutic potential of artificial tear fluid supplemented with Muc5ac to have a beneficial effect on dry eye patient (189). Another study contradicted a dry eye phenotype in mice on loss of Muc5ac. They did not observe any significant alterations in the overall appearance of the cornea or eyelids and concluded that Muc5ac−/− mice are inappropriate animal model for studying human dry eye disease (60). The authors proposed that the corneal opacification observed in the previous study might be a secondary phenotype caused by infection due to debris or pathogens and do not directly relate to the deletion of the Muc5ac gene. Besides this, they did not observe any qualitative or quantitative changes in goblet cells of conjunctival epithelium in Muc5ac deficient mice, thus disproving the observations from the previous study (60).
Our group evaluated the expression of Muc5ac in the genetically engineered KrasG12D, Pdx1-Cre (KC) murine models of pancreatic cancer and observed an elevated expression of secretory mucin Muc5ac in the pancreas of KC mice both at mRNA and protein level. De novo expression of Muc5ac was observed as early as at 10 weeks compared with no expression in LSL-KrasG12D mice (190). Interestingly, MUC5AC expression progressively increased from 10th week to the 50th week of progression compared with none in the pancreas of age-matched unfloxed LSL-KrasG12D mice (190). Interestingly, Muc5ac was strongly expressed in the metastatic lesions involving liver, small intestines and lungs at 50 weeks of age (190).
Unfortunately, majority of mouse models used for Muc5ac studies involves its germline deletion. Considering its context-dependent role in different organ, the obtained information might not represent true picture in terms of both functionality as well as modulating the innate and adaptive immunity. In this direction, Ehre et al. observed that overexpression of Muc5ac in lung epithelial reduced pulmonary tissue damage by limiting the neutrophil infiltration upon infection, while Koeppen et al. observed reduced infiltration of neutrophil in conjunction with lung inflammation in Muc5ac knockout animals compared with WT mice upon antigen challenge. Taking into consideration the above example, mouse models bearing organ-specific knockout of this mucin may enhance the robustness and reproducibility of the functional annotations of this molecule. In order to delineate organ-specific functioning of MUC5AC for pancreatic cancer, our group is putting concerted efforts to develop a pancreas-specific knockout model for Muc5ac.
Regulation of MUC5AC expression
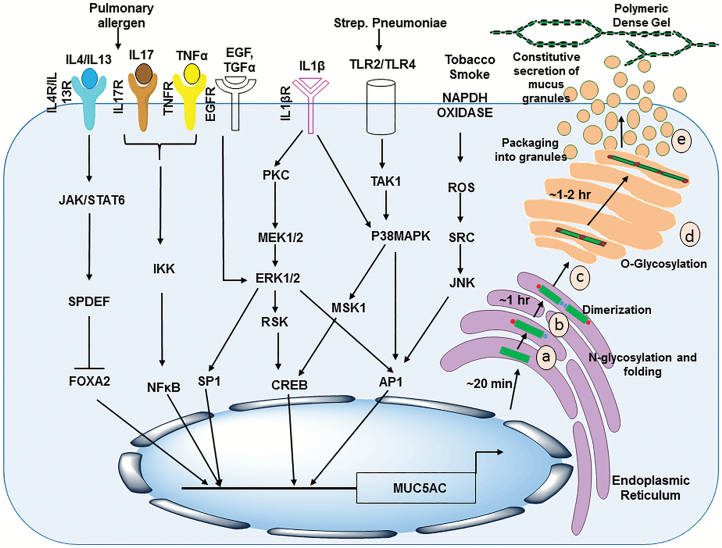
Secretory mucin MUC5AC is aberrantly expressed under inflammatory conditions and multiple malignancies such as pancreatic cancer, lung cancer, and CRC. A plethora of studies in last decade has investigated the regulation of MUC5AC expression, in different physiological and pathological contexts. Briefly, MUC5AC has been shown to be regulated by environmental pollutants [cigarette smoke (CS) extracts, acrolein, ozone], viral mediators (respiratory syncytial virus, rhinovirus), bacterial products [lipopolysaccharides (LPS), lipoteichoic acid (LTA), peptidoglycans, flagellin], cytokines (TNF-α, IL-1β, IL-4, IL-6, IL-9, IL-13, IL-17), growth factors (EGF, TGF-α, RA, thyroid hormones) and proteases (neutrophil elastase) (73,191,192) (Figure 3). A recent study by Qin et al. showed that TMEM16A, a calcium-activated chloride channel-mediated MUC5AC hypersecretion in the human airway epithelia, can be induced by IL13 and was mechanistically modulated by STAT6 and ERK1/2 signaling pathways (193). Furthermore, MUC5AC is also regulated by microRNAs (miRNAs) underscoring the utility of their therapeutic targeting for modulation of MUC5AC expression (194). It has been shown that mucus overproduction during inflammatory airway diseases can be managed by manipulation of miRNAs. MUC5AC production by airway epithelial cells stimulated by neutrophil elastase was downregulated by miR-146a-mediated inactivation of c-Jun N-terminal kinase (JNK) and NF-κB signaling (195). Notably, overexpression of hemeoxygenase-1 (HMOX1) decreased the expression of miR-378 (Oncomir/Angiomir) and further reversed miR-378-mediated increased MUC5AC, VEGF, interleukin-8 (IL-8) and Ang-1 production leading to decreased proliferation, migration and angiogenic potential of NCI-H292 lung cancer cells both in vitro and in vivo. Thus, this study underscores a prospective therapeutic strategy, to inhibit miR-378-mediated impact on lung cancer cell growth and metastasis (196). Another study showed that in IL-13-stimulated nasal epithelial cells, mRNA and protein expression levels of MUC5AC could be significantly decreased by the forced expression of miR-143 (197).
Figure 3.
Secreted mucin MUC5AC regulation and secretion. Regulation of MUC5AC: Multiple mediators affect transcriptional regulation of MUC5AC gene expression in goblet and epithelial cells. Various ligands (EGF, TGFα, TNFα and ILs) induced downstream signaling leads to induction in MUC5AC expression (denoted by arrows). These events are also associated with goblet cell metaplasia and transdifferentiation. Biosynthesis of secretory mucin MUC5AC: After transcription, the translated MUC5AC polypeptide undergoes N-glycosylation and intramolecular disulfide bond formation and folding in endoplasmic reticulum (a), intermolecular dimerization takes place within 1 h by disulfide linkage between C-terminal CK domains (b) followed by MUC5AC dimerization and translocation to cis Golgi (c). In cis Golgi, O-glycosylation takes place at the serine and threonine residues present in tandem repeat domains of MUC5AC. During transit through Golgi, the glycan chains are further extended (d) and MUC5AC dimers then polymerize in trans Golgi or secretory granules via the intermolecular disulfide linkage between N-terminal D3 domains (e). During the constitutive secretion process, the polymeric mucins are released outside the cell where they form a dense polymeric gel-like structure.
Little is known about the mechanisms associated with atypical expression of MUC5AC in pancreatic tumors. It has been shown that forskolin and vasoactive intestinal peptide stimulate adenylyl cyclase and protein kinase A pathway to increase MUC5AC antigen expression and release from SW1990 pancreatic cancer cells (94). Furthermore, transcription factor specificity protein-1 (SP-1) is involved in basal transcription of the MUC5AC gene (198). The AP-1 (Activator protein-1) transcription factor is also involved in both basal and PMA-induced MUC5AC promoter activation via PKC/ERK and PKC/JNK pathways (198). As detailed earlier, MUC5AC is a direct transcriptional target of GLI1 in pancreatic cancer cells. GLI1 and GLI2 could activate the promoter of MUC5AC through its conserved CACCC-box-like cis-regulatory elements (134). Furthermore, Kageyama-Yahara et al. identified a highly conserved 15 bp sequence between −111 and −125 bp in the 5′ promoter upstream region of MUC5AC gene in gastrointestinal cells as the Gli-binding site, and it was observed that Gli regulates MUC5AC transcription via direct protein–DNA interaction (199). Promoter characterization of mucin genes in lung carcinoma cell line NCI H292 indicated that the EGF and TGFα responsive regulatory element is localized at -202/-1 region of MUC5AC gene. Simultaneous activation of the EGFR/Ras/Raf/Extracellular signal-regulated kinase-signaling axis led to overexpression of MUC2 and MUC5AC upon Sp1 binding to the promoter (200).
Furthermore, utilizing human pancreatic ductal epithelial cells (HPDE and PK-8), it was concluded that mutation of GNAS induces MUC5AC expression in IPMNs through interaction with MAPK (mitogen-activated protein kinase) and phosphoinositide 3 kinase (PI3K) pathways (201).
In order to understand the epigenetic regulation of mucin expression, Vincent et al. examined DNA methylation and histone modification associated with the gel-forming mucin gene promoters by designing luciferase reporter promoter deletion constructs in different epithelial cancer cell lines. This study indicated that MUC5AC gene expression is rarely regulated by direct histone deacetylation or DNA methylation and probably involve indirect mechanisms (202). Interestingly in a later study, CpG methylation and Histone H3 Lysine 9 modification at the distal region of the MUC5AC promoter were associated with its expression profile (22).
Differential expression of MUC5AC is observed in hypoxic environment. A consensus-binding motif for hypoxia-inducible factor 1-α (HIF1-α) has been observed in the core promoters of all the mammalian MUC5AC orthologs, inferring direct impact of hypoxia on the expression of MUC5AC. Zhou et al. observed that −64 bp hypoxia response element (HRE) on MUC5AC promoter is responsible for hypoxia-mediated MUC5AC induction, and site-specific mutation in this element abolished the activity of MUC5AC promoter (203). Under inflammatory conditions, IL-13 and EGF-mediated induction of HIF1-α binding to Muc5ac promoter resulted in increased transcription of Muc5ac, while a mutation in the binding motif dramatically reduced the Muc5ac promoter activity (204,205). Interestingly, human neutrophil elastase induced generation and activation of HIF-1α-activated protein kinase C (PKC), subsequently sensitized transient receptor potential vanilloid subtype 1 (TRPV1), resulting in increased production of MUC5AC and pro-inflammatory cytokines (206). Kim et al. (207) observed that increased MUC5AC expression and production during sinusitis can be reduced either by increasing the oxygen tension or by modulating the hypoxia-inducible factor 1-α activity. Jhang et al. (208) used the multifunctional silver nanoparticles to alleviate allergic asthma. Silver NP attenuated MUC5AC hypersecretion via blocking of HIF-1α and Th-2 cytokines. Furthermore, Yu et al. showed that CS stimulates MUC5AC expression in airway epithelial cells via induction of HIF-1α by EGFR facilitated promotion of PI3K and ERK signaling pathways (209).
In addition to hypoxia, smoking modulates MUC5AC expression in various pathological settings. Molecular mechanism for smoking-mediated mucin production inhibition is an area of high clinical relevance for both benign and malignant pathologies. CS, a complex mixture of volatile (acrolein, naphthalene and aldehydes) and non-volatile [aflatoxin B1, benzo(α)pyrene, phorbol ester analogs] components have been strongly associated with differential mucins induction. Among various mucins, MUC5AC is the predominant mucin produced from goblet cells in human airways, and its expression is regulated by neutrophil elastase, air pollutants and bacterial products and smoking in various benign and malignant pathologies. Among lung toxicants, total particulate matter and acrolein increased the percentage of MUC5AC positive cells and induced a profound effect on cellular differentiation, while formaldehyde and acetaldehyde had partial effect on MUC5AC production (210). CS not only induces MUC5AC by itself but also synergize with pro-inflammatory cytokines including TNF-α, TGF-α, LPS and amphiregulin (211). Additionally, elevated MUC5AC levels have also been associated with lung tumors from smokers. Overexpression of MUC5AC showed significant association with increased probability of postoperative recurrence (23). Smoking generated reactive oxygen species result in activation of TNF-α-converting enzyme that causes increased TGF-α shedding leading to EGFR phosphorylation and MUC5AC induction in human airway epithelial (NCI-H292) (212,213). Iwashita et al. reported that an increased expression of Ca2+-activated chloride channel (CLCA1) in airway epithelium of smokers (with or without chronic obstructive pulmonary disease [COPD]) is a critical contributor in its pathogenesis of COPD due to its significant positive correlation with MUC5AC expression (214). EGFR and chloride channel CLCA1 are speculated to affect MUC5AC production as a part of a single complex signaling pathway (215). Additionally, MUC5AC transcriptional upregulation in response to tobacco smoke is also mediated by transcription factor AP-1 via JNK and Src-dependent fashion (216). MUC5AC expression in long bronchial biopsies from long-term smoker is associated with HER3 expression but not with neutrophil infiltration (217). Furthermore, CS-exposed lungs produce growth differentiation factor 15 that activates PI3K pathway to promote MUC5AC production (218). Di et al. suggest that CS exposure in lung relevant model results in increased SP1 nuclear translocation and binding to MUC5AC promoter region to induce MUC5AC gene transcription (219). Similar to lung pathologies, CS-mediated inflammation in middle ear epithelial cells results in increased expression of MUC5AC via EGFR signaling implicating in otitis media with effusion (220). Focused efforts have been made to inhibit smoke-induced MU5AC production. Both in vitro and in vivo studies on Rbamipide, an amino acid derivative of 2-(1H)-quinolinone [(2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl] propionic acid, OPC-12759], found to inhibit CS-induced TNF-α release and MUC5AC production (221). Further studies by the same group identified that PPAR-γ agonist ‘Rosiglitazone’ inhibit MUC5AC production by upregulating phosphatase and tensin homolog (PTEN) signaling and downregulating AKT expression (222). Anti-inflammatory and antioxidant enzyme-Heme oxygenase-1 (HO-1) was found to significantly attenuate airway mucus hypersecretion in animals and humans exposed to CS. Long-acting muscarinic antagonist, aclidinium suppresses CS-induced MUC5AC overexpression in human airway epithelial cells (223). Wang et al. observed that cigarette smoking significantly modulates expression of GAD67 gene (rate-limiting enzyme in GABA synthesis) in small and large airway epithelium of smokers. Additionally, a significant positive correlation was revealed between GAD67 gene expression and MUC5AC expression, thus providing a novel therapeutic target to curb manifestations of mucus overproduction due to smoking (224). Monzon et al. underscored therapeutic utility of targeting monocyte chemoattractant protein-1 to attenuate CS-induced mucus hypersecretion in COPD patients. They observed an association of monocyte chemoattractant protein-1 expression with increased MUC5AC expression in airway epithelium of smokers. Additionally, these observations were corroborated in vitro where monocyte chemoattractant protein-1 induced MUC5AC expression by initiating interaction of its receptor CCR2B with G(q) subunits in caveolae and subsequent PLCβ, PKC and ERK1/2 activation (225). NRG1β/ErbB3 signaling via the MAPK and PI3K signal pathways mediates induction of MUC5AC in CS-exposed airway epithelial cells (16HBE) (226). Fu et al. (227) reported that Ly-6 protein (Lynx1) agonists or mimetic could be utilized to attenuate MUC5AC production in CS-induced pathologies, due to its ability to negatively regulate α7nAChR downstream signaling mediated by inhibition of Src activation. Overall in the past decade, regulation of MUC5AC under conditions of physiologic stress, inflammation, infection and oncogenesis is well elucidated across different studies by multiple groups. Considering the current research scenario, therapeutic intervention of this molecule is possible by regulating its positive regulators to alleviate manifestations of its overproduction in diseases.
Therapeutic antibodies against MUC5AC
Development of resistance to different therapeutic modalities poses the biggest challenge in targeting devastating malignancies. In order to therapeutically target pancreatic cancer, a chimeric immunoglobulin (IgG1) called NPC-1C has been developed, which targets a glycan variant of MUC5AC. During preclinical characterization of NPC-1C antibody, 48% of pancreatic cancer tissues reacted compared with no reactivity in normal pancreatic tissues. Furthermore, the potential therapeutic utility was highlighted as NPC-1C, which not only mediated pancreatic cancer cells killing but also caused 2- to 3-fold reduction in CFPAC-1 xenograft tumor growth compared with normal saline and IgG controls (95). The mAb NPC-1 was, however, able to kill more than 40% pancreatic cancer cells. It was also capable to reduce and in many cases eliminate the growth of established tumor in the animals (228). Yamazoe et al. assessed MUC5AC as a potential immunotherapeutic target for pancreatic cancer. Two peptide epitopes [MUC5AC-A02-1398 (FLNDAGACV) and MUC5AC-A24-716 (TCQPTCRSL)] elicited cytotoxic T-lymphocytes bearing cytotoxicity specifically against HLA-A*0201- and A*2402-positive target cells, respectively (229). Gold et al. observed high specificity of anti-MUC5AC PAM4 antibody (clivatuzumab) for PDAC in relative to the normal pancreas, non-malignant pancreatic lesions and cancers of other tissues. Interestingly, PAM4 antibody specifically reacted with an epitope present within the N-terminal cysteine-rich subdomain2 (Cys2) of MUC5AC (230,231). These studies highlight the clinical utility of PAM4 antibody for early stage diagnosis and subsequent therapy of pancreatic cancer.
Conclusions and perspectives
Extensive research efforts in last decade established mucins as one of the clinically relevant molecules. Under physiological conditions, mucin glycoproteins primarily function to provide protection against various ambient stimuli, lubrication and for the establishment of homeostasis at the epithelial surfaces lining the internal organs of the body. Mucins are impacted by various pathologies including inflammatory, benign and malignant and in turn impact the disease progression. Significant alterations in the levels and glycosylation of secretory mucin MUC5AC have been observed both in benign and malignant pathologies. Involved in protection and lubrication of various exposed surfaces such as conjunctiva, lungs, stomach and endocervix under physiological conditions by forming polymeric gels over epithelial surfaces, MUC5AC plays a critical role in the innate mucosal defense. Lack of appropriate models mimicking the pathological contexts hindered understanding of the exact contextual roles played by MUC5AC. However, with the advent of genetically engineered mouse models, our understanding of the mechanisms associated with MUC5AC-mediated processes are getting refined, but they are far from being clearly elucidated. For an example, despite information about MUC5AC expression in endocervix, nothing is indicated about its role in the reproductive process. Besides, the differences in anatomy and physiology of rodents and humans also pose as a disadvantage in fully understanding the exact functions and exploiting them to therapeutic benefit. Much progress has been made to elucidate the major domains, structure and assembly of MUC5AC. However, not much has been done to understand exact roles of these domains in various pathological contexts. Moreover, information about molecular interacting partners of MUC5AC needs extensive research. It has been hypothesized that by its cysteine-rich D-domains and vWF-like domains, MUC5AC may interact with other proteins having similar domains. As a component of mucus, through ionic or hydrophobic interactions, it might even interact with other mucins and play critical role in maintaining stability of the mucus layer. However, this warrants further investigations. As MUC5AC is a secretory mucin, identification of its associated molecules to clarify the nature of MUC5AC barrier should better be done without disrupting the mucus gel. The knowledge gained will help to devise strategies for better access and facilitated uptake of pharmaceuticals and gene therapy agents across MUC5AC polymeric gel barrier. Looking at the aberrant alterations of MUC5AC in multiple pathological states, significant efforts are invested to comprehend mechanisms of its regulation, utility as early-stage diagnostic marker, prognostic marker and its functions in different contexts. Substantial strides are carried out to characterize promoter of MUC5AC and understand the transcriptional, epigenetic regulation of MUC5AC in cell- and tissue-specific manner and explain how its aberrant alterations in its expression occur during inflammatory, benign and neoplastic progression. The biggest challenge that remained is the procurement, processing and absolute quantification of MUC5AC levels in samples from healthy versus diseased states. Therefore, the lack of current understanding regarding the levels to which altered MUC5AC levels should be titrated has impacted appropriate therapeutic targeting of this mucin, rendering mixed outcomes. Furthermore, some studies recently highlighted post-transcriptional regulation of MUC5AC mediated by miRNAs in different pathologies providing an underexplored field with a huge promise to explore further. Another area that demands extensive attention is the characterization of post-translational modifications such as differential glycosylation patterns associated with MUC5AC, specifically during different disease states in comparison with the healthy individuals. The knowledge gained from here can be employed to devise better diagnostic markers and disease-specific therapeutic modalities. Interestingly, extensive efforts are currently being made to utilize MUC5AC in conjunction with other markers as an early stage diagnostic marker of cholangiocarcinoma and PDAC. Interestingly, recent studies have linked genetic variants of MUC5AC to be involved in predisposition to cystic fibrosis and GCs. Functional variations in MUC5AC owing to variations in domain structure and regulatory regions resulting in different pathologies are also emerging. Interestingly, not much has been investigated concerning aberrant assembly of MUC5AC due to genetic polymorphisms, stress conditions such as hypoxia and smoking, which may predispose individuals to various disease conditions and malignancies. MUC5AC has contrary roles in gastric and pancreatic cancer. It would be interesting to comprehend how the pathological context and associated microenvironment affects the various roles played by MUC5AC. Future studies should focus on understanding the mechanisms related to roles played by MUC5AC in various malignancies. Furthermore, it will be important to take cues from the mechanisms associated with clearance of overexpressed and hypersecreted MUC5AC in airway diseases. These cues could be of help in developing therapeutic strategies for clearance of MUC5AC in malignancies where its’ presence has poor prognosis. Finally, the promise and the driving objective of research on MUC5AC is the opportunity to discover novel diagnostic markers for different cancers and new therapies that can normalize its levels under different pathological conditions.
Funding
The authors on this manuscript are supported, in parts, by the grants from National Institutes of Health (Cancer Center Support Grant P30 CA036727, EDRN CA200466, UO1 CA210240, SPORE P50 CA127297 R44 CA224619 and R41CA213718).
Conflict of Interest Statement: The authors declare no conflict of interests.
Abbreviations
- CK
cystine knot
- CS
cigarette smoke
- CRC
colorectal cancer
- COPD
Chronic obstructive pulmonary disease
- DNA
deoxyribonucleic acid
- GC
gastric cancer
- GDPH
Gly-Asp-Pro-His
- IPMN
intraductal papillary mucinous neoplasm
- mRNA
messenger RNA
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PI3K
phosphoinositide 3 kinase
- PTS
proline, threonine and serine
- TRR
tandem repeat regions
- UC
ulcerative colitis
- VEGF
vascular endothelial growth factor
- VILI
ventilator-induced lung injury
References
- 1. Hollingsworth M.A., et al. (2004)Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer, 4, 45–60. [DOI] [PubMed] [Google Scholar]
- 2. Corfield A.P., et al. (2001)Mucins in the gastrointestinal tract in health and disease. Front. Biosci., 6, D1321–D1357. [DOI] [PubMed] [Google Scholar]
- 3. Corfield A.P. (2015)Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta, 1850, 236–252. [DOI] [PubMed] [Google Scholar]
- 4. Kaur S., et al. (2013)Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol., 10, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thornton D.J., et al. (2008)Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol., 70, 459–486. [DOI] [PubMed] [Google Scholar]
- 6. Macha M.A., et al. (2015)Emerging potential of natural products for targeting mucins for therapy against inflammation and cancer. Cancer Treat. Rev., 41, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rachagani S., et al. (2009)Current status of mucins in the diagnosis and therapy of cancer. Biofactors, 35, 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kufe D.W. (2009)Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer, 9, 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bafna S., et al. (2010)Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene, 29, 2893–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desseyn J.L., et al. (2000)Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol., 17, 1175–1184. [DOI] [PubMed] [Google Scholar]
- 11. Meezaman D., et al. (1994)Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5). J. Biol. Chem., 269, 12932–12939. [PubMed] [Google Scholar]
- 12. Guyonnet D.V., et al. (1995)Characterization of the human mucin gene MUC5AC: a consensus cysteine-rich domain for 11p15 mucin genes?. Biochem. J., 305, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rose M.C., et al. (2006)Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev., 86, 245–278. [DOI] [PubMed] [Google Scholar]
- 14. Williams O.W., et al. (2006)Airway mucus: from production to secretion. Am. J. Respir. Cell Mol. Biol., 34, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curran D.R., et al. (2010)Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol., 42, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alos L., et al. (2005)Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am. J. Surg. Pathol., 29, 806–813. [DOI] [PubMed] [Google Scholar]
- 17. Handra-Luca A., et al. (2005)MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am. J. Surg. Pathol., 29, 881–889. [DOI] [PubMed] [Google Scholar]
- 18. Kusafuka K., et al. (2012)Mucin-rich salivary duct carcinoma with signet-ring cell feature ex pleomorphic adenoma of the submandibular gland: a case report of an unusual histology with immunohistochemical analysis and review of the literature. Med. Mol. Morphol., 45, 45–52. [DOI] [PubMed] [Google Scholar]
- 19. Zhang S., et al. (1998)Selection of tumor antigens as targets for immune attack using immunohistochemistry: protein antigens. Clin. Cancer Res., 4, 2669–2676. [PubMed] [Google Scholar]
- 20. Kasashima S., et al. (2007)Expression of aberrant mucins in lobular carcinoma with histiocytoid feature of the breast. Virchows Arch., 450, 397–403. [DOI] [PubMed] [Google Scholar]
- 21. Pereira M.B., et al. (2001)Immunohistochemical study of the expression of MUC5AC and MUC6 in breast carcinomas and adjacent breast tissues. J. Clin. Pathol., 54, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamada N., et al. (2010)Expression of MUC5AC, an early marker of pancreatobiliary cancer, is regulated by DNA methylation in the distal promoter region in cancer cells. J. Hepatobiliary Pancreat. Sci., 17, 844–854. [DOI] [PubMed] [Google Scholar]
- 23. Yu C.J., et al. (1996)Overexpression of MUC5 genes is associated with early post-operative metastasis in non-small-cell lung cancer. Int. J. Cancer, 69, 457–465. [DOI] [PubMed] [Google Scholar]
- 24. Yu C.J., et al. (2005)Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer, 47, 59–67. [DOI] [PubMed] [Google Scholar]
- 25. Hoorens P.R., et al. (2011)Genome wide analysis of the bovine mucin genes and their gastrointestinal transcription profile. BMC Genomics, 12, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldus S.E., et al. (2002)Correlation of MUC5AC immunoreactivity with histopathological subtypes and prognosis of gastric carcinoma. Ann. Surg. Oncol., 9, 887–893. [DOI] [PubMed] [Google Scholar]
- 27. Xiao L.J., et al. (2012)Clinicopathological and prognostic significance of MUC-2, MUC-4 and MUC-5AC expression in Japanese gastric carcinomas. Asian Pac. J. Cancer Prev., 13, 6447–6453. [DOI] [PubMed] [Google Scholar]
- 28. Choi J.S., et al. (2009)Mucinous gastric carcinomas: clinicopathologic and molecular analyses. Cancer, 115, 3581–3590. [DOI] [PubMed] [Google Scholar]
- 29. Zhang C.T., et al. (2015)Prognostic value of Muc5AC in gastric cancer: a meta-analysis. World J. Gastroenterol., 21, 10453–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sasaki M., et al. (1999)Expression of MUC2, MUC5AC and MUC6 apomucins in carcinoma, dysplasia and non-dysplastic epithelia of the gallbladder. Pathol. Int., 49, 38–44. [DOI] [PubMed] [Google Scholar]
- 31. Vandenhaute B., et al. (1997)Mucin gene expression in biliary epithelial cells. J. Hepatol., 27, 1057–1066. [DOI] [PubMed] [Google Scholar]
- 32. Chang H.J., et al. (2004)Phenotypic alterations of mucins and cytokeratins during gallbladder carcinogenesis. Pathol. Int., 54, 576–584. [DOI] [PubMed] [Google Scholar]
- 33. Xiong L., et al. (2012)Expressive levels of MUC1 and MUC5AC and their clinicopathologic significances in the benign and malignant lesions of gallbladder. J. Surg. Oncol., 105, 97–103. [DOI] [PubMed] [Google Scholar]
- 34. Dursun N., et al. (2012)Mucinous carcinomas of the gallbladder: clinicopathologic analysis of 15 cases identified in 606 carcinomas. Arch. Pathol. Lab. Med., 136, 1347–1358. [DOI] [PubMed] [Google Scholar]
- 35. Park S.Y., et al. (2009)Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol. Rep., 22, 649–657. [DOI] [PubMed] [Google Scholar]
- 36. Boonla C., et al. (2005)MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J. Gastroenterol., 11, 4939–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abe T., et al. (2015)Prognostic evaluation of mucin-5AC expression in intrahepatic cholangiocarcinoma, mass-forming type, following hepatectomy. Eur. J. Surg. Oncol., 41, 1515–1521. [DOI] [PubMed] [Google Scholar]
- 38. Boonla C., et al. (2003)Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer, 98, 1438–1443. [DOI] [PubMed] [Google Scholar]
- 39. Danese E., et al. (2014)Assessment of bile and serum mucin5AC in cholangiocarcinoma: diagnostic performance and biologic significance. Surgery, 156, 1218–1224. [DOI] [PubMed] [Google Scholar]
- 40. Silsirivanit A., et al. (2013)CA-S27: a novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci., 104, 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balagué C., et al. (1995)In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology, 109, 953–964. [DOI] [PubMed] [Google Scholar]
- 42. Yonezawa S., et al. (1999)Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol. Int., 49, 45–54. [DOI] [PubMed] [Google Scholar]
- 43. Jinfeng M., et al. (2003)Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int. J. Gastrointest. Cancer, 34, 9–18. [DOI] [PubMed] [Google Scholar]
- 44. Higashi M., et al. (2015)Mucin expression in endoscopic ultrasound-guided fine-needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas, 44, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takikita M., et al. (2009)Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res., 69, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takano Y., et al. (2012)Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas. Hepatobiliary Pancreat. Dis. Int., 11, 424–428. [DOI] [PubMed] [Google Scholar]
- 47. Buisine M.P., et al. (2001)Mucin gene expression in intestinal epithelial cells in Crohn’s disease. Gut, 49, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kocer B., et al. (2002)Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol. Int., 52, 470–477. [DOI] [PubMed] [Google Scholar]
- 49. Kesari M.V., et al. (2015)Immunohistochemical study of MUC1, MUC2 and MUC5AC in colorectal carcinoma and review of literature. Indian J. Gastroenterol., 34, 63–67. [DOI] [PubMed] [Google Scholar]
- 50. Yu X.W., et al. (2007)[Expression and clinical significance of Mucin and E-cadherin in colorectal tumors]. Ai Zheng., 26, 1204–1210. [PubMed] [Google Scholar]
- 51. Buisine M.P., et al. (2003)Developmental patterns of mucin gene expression in human fetal small intestinal xenografts maintained in severe-combined immunodeficient mice. Pediatr. Res., 53, 898–904. [DOI] [PubMed] [Google Scholar]
- 52. Wang J., et al. (2014)Expression profile of mucins in ovarian mucinous tumors: distinguishing primary ovarian from metastatic tumors. Int. J. Gynecol. Pathol., 33, 166–175. [DOI] [PubMed] [Google Scholar]
- 53. Musrap N., et al. (2014)Proteomic analysis of cancer and mesothelial cells reveals an increase in Mucin 5AC during ovarian cancer and peritoneal interaction. J. Proteomics, 103, 204–215. [DOI] [PubMed] [Google Scholar]
- 54. Gipson I.K., et al. (1997)Mucin genes expressed by human female reproductive tract epithelia. Biol. Reprod., 56, 999–1011. [DOI] [PubMed] [Google Scholar]
- 55. Riethdorf L., et al. (2000)Differential expression of MUC2 and MUC5AC in benign and malignant glandular lesions of the cervix uteri. Virchows Arch., 437, 365–371. [DOI] [PubMed] [Google Scholar]
- 56. Mitsuhashi A., et al. (2004)Correlation between MUC5AC expression and the prognosis of patients with adenocarcinoma of the uterine cervix. Ann. Surg. Oncol., 11, 40–44. [DOI] [PubMed] [Google Scholar]
- 57. Stojnev S., et al. (2014)Prognostic significance of mucin expression in urothelial bladder cancer. Int. J. Clin. Exp. Pathol., 7, 4945–4958. [PMC free article] [PubMed] [Google Scholar]
- 58. Lopez-Beltran A., et al. (2011)Flat urothelial carcinoma in situ of the bladder with glandular differentiation. Hum. Pathol., 42, 1653–1659. [DOI] [PubMed] [Google Scholar]
- 59. Lopez B.A., et al. (2014)Microcystic urothelial carcinoma: morphology, immunohistochemistry and clinical behaviour. Histopathology, 64, 872–879. [DOI] [PubMed] [Google Scholar]
- 60. Marko C.K., et al. (2014)The ocular surface phenotype of Muc5ac and Muc5b null mice. Invest. Ophthalmol. Vis. Sci., 55, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gipson I.K. (2004)Distribution of mucins at the ocular surface. Exp. Eye Res., 78, 379–388. [DOI] [PubMed] [Google Scholar]
- 62. Lin J., et al. (2001)Characterization of mucins in human middle ear and Eustachian tube. Am. J. Physiol. Lung Cell. Mol. Physiol., 280, L1157–L1167. [DOI] [PubMed] [Google Scholar]
- 63. Kerschner J.E. (2007)Mucin gene expression in human middle ear epithelium. Laryngoscope, 117, 1666–1676. [DOI] [PubMed] [Google Scholar]
- 64. Elsheikh M.N., et al. (2006)Up-regulation of MUC5AC and MUC5B mucin genes in nasopharyngeal respiratory mucosa and selective up-regulation of MUC5B in middle ear in pediatric otitis media with effusion. Laryngoscope, 116, 365–369. [DOI] [PubMed] [Google Scholar]
- 65. Ferretti V., et al. (2015)Muc5ac mucin expression during rat skin development. Eur. J. Histochem., 59, 2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lin H., et al. (2015)Lentiviral shRNA against KCa3.1 inhibits allergic response in allergic rhinitis and suppresses mast cell activity via PI3K/AKT signaling pathway. Sci. Rep., 5, 13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ji C., et al. (2009)[The expression of mucins gene in the human nasal polyps and allergic rhinitis]. Lin Chung. Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 23, 923–925, 929. [PubMed] [Google Scholar]
- 68. Kim D.H., et al. (2004)Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg., 130, 747–752. [DOI] [PubMed] [Google Scholar]
- 69. Ding G.Q., et al. (2007)The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am. J. Rhinol., 21, 359–366. [DOI] [PubMed] [Google Scholar]
- 70. Mao Y.J., et al. (2015)Increased expression of MUC5AC and MUC5B promoting bacterial biofilm formation in chronic rhinosinusitis patients. Auris Nasus Larynx, 42, 294–298. [DOI] [PubMed] [Google Scholar]
- 71. Val S., et al. (2015)Middle ear response of Muc5ac and Muc5b mucins to nontypeable haemophilus influenzae. JAMA Otolaryngol. Head Neck Surg., 141, 997–1005. [DOI] [PubMed] [Google Scholar]
- 72. Ubell M.L., et al. (2010)Mucin gene polymorphisms in otitis media patients. Laryngoscope, 120, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Voynow J.A., et al. (2006)Regulation of mucin genes in chronic inflammatory airway diseases. Am. J. Respir. Cell Mol. Biol., 34, 661–665. [DOI] [PubMed] [Google Scholar]
- 74. Kirkham S., et al. (2002)Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J., 361, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guo X., et al. (2011)Mucin variable number tandem repeat polymorphisms and severity of cystic fibrosis lung disease: significant association with MUC5AC. PLoS One, 6, e25452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Niv Y., et al. (2011)The role of mucin in GERD and its complications. Nat. Rev. Gastroenterol. Hepatol., 9, 55–59. [DOI] [PubMed] [Google Scholar]
- 77. Song S., et al. (2011)Induction of MUC5AC mucin by conjugated bile acids in the esophagus involves the phosphatidylinositol 3-kinase/protein kinase C/activator protein-1 pathway. Cancer, 117, 2386–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sue S., et al. (2016)Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis. J. Gastroenterol., 51, 949–960. [DOI] [PubMed] [Google Scholar]
- 79. Niv Y. (2010)H. pylori/NSAID—negative peptic ulcer—the mucin theory. Med. Hypotheses, 75, 433–435. [DOI] [PubMed] [Google Scholar]
- 80. Shi D., et al. (2014)The changes in MUC5AC expression in gastric cancer before and after Helicobacter pylori eradication. Clin. Res. Hepatol. Gastroenterol., 38, 235–240. [DOI] [PubMed] [Google Scholar]
- 81. Perrais M., et al. (2014)Helicobacter pylori urease and flagellin alter mucin gene expression in human gastric cancer cells. Gastric Cancer, 17, 235–246. [DOI] [PubMed] [Google Scholar]
- 82. Banks P.A., et al. (2010)The management of acute and chronic pancreatitis. Gastroenterol. Hepatol. (NY)., 6, 1–16. [PMC free article] [PubMed] [Google Scholar]
- 83. Ohtani H., et al. (2011)Autoimmune pancreatitis and biliary intraepithelial neoplasia of the common bile duct: a case with diagnostically challenging but pathogenetically significant association. Pathol. Int., 61, 481–485. [DOI] [PubMed] [Google Scholar]
- 84. Strobel O., et al. (2010)Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology, 138, 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Borralho P., et al. (2007)Aberrant gastric apomucin expression in ulcerative colitis and associated neoplasia. J. Crohns. Colitis, 1, 35–40. [DOI] [PubMed] [Google Scholar]
- 86. Forgue-Lafitte M.E., et al. (2007)Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int. J. Cancer, 121, 1543–1549. [DOI] [PubMed] [Google Scholar]
- 87. Guo X., et al. (2014)Genome reference and sequence variation in the large repetitive central exon of human MUC5AC. Am. J. Respir. Cell Mol. Biol., 50, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Escande F., et al. (2001)Human mucin gene MUC5AC: organization of its 5’-region and central repetitive region. Biochem. J., 358, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van de Bovenkamp J.H., et al. (1998)Molecular cloning of human gastric mucin MUC5AC reveals conserved cysteine-rich D-domains and a putative leucine zipper motif. Biochem. Biophys. Res. Commun., 245, 853–859. [DOI] [PubMed] [Google Scholar]
- 90. Buisine M.P., et al. (1998)Genomic organization of the 3’-region of the human MUC5AC mucin gene: additional evidence for a common ancestral gene for the 11p15.5 mucin gene family. Biochem. J., 332, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lidell M.E., et al. (2006)Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem. J., 399, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sheehan J.K., et al. (2004)Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem., 279, 15698–15705. [DOI] [PubMed] [Google Scholar]
- 93. Ermund A., et al. (2017)The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem. Biophys. Res. Commun., 492, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ho J.J., et al. (2002)Secretion of MUC5AC mucin from pancreatic cancer cells in response to forskolin and VIP. Biochem. Biophys. Res. Commun., 294, 680–686. [DOI] [PubMed] [Google Scholar]
- 95. Patel S.P., et al. (2013)Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunol. Immunother., 62, 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yoshii N., et al. (2002)Expression of mucin core proteins in extramammary Paget’s disease. Pathol. Int., 52, 390–399. [DOI] [PubMed] [Google Scholar]
- 97. Hata H., et al. (2014)MUC5AC expression correlates with invasiveness and progression of extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol., 28, 727–732. [DOI] [PubMed] [Google Scholar]
- 98. Sotozono M., et al. (1996)Novel monoclonal antibody, SO-MU1, against human gastric MUC5AC apomucin. J. Immunol. Methods, 192, 87–96. [DOI] [PubMed] [Google Scholar]
- 99. Thornton D.J., et al. (1996)Respiratory mucins: identification of core proteins and glycoforms. Biochem. J., 316, 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carlstedt I., et al. (1995)‘Soluble’ and ‘insoluble’ mucins–identification of distinct populations. Biochem. Soc. Trans., 23, 845–851. [DOI] [PubMed] [Google Scholar]
- 101. Nollet S., et al. (2002)Mapping of two new epitopes on the apomucin encoded by MUC5AC gene: expression in normal GI tract and colon tumors. Int. J. Cancer, 99, 336–343. [DOI] [PubMed] [Google Scholar]
- 102. Nollet S., et al. (2004)Mapping of SOMU1 and M1 epitopes on the apomucin encoded by the 5’ end of the MUC5AC gene. Hybrid. Hybridomics, 23, 93–99. [DOI] [PubMed] [Google Scholar]
- 103. Bara J., et al. (1998)Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int. J. Cancer, 75, 767–773. [DOI] [PubMed] [Google Scholar]
- 104. Reis C.A., et al. (2000)Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J. Histochem. Cytochem., 48, 377–388. [DOI] [PubMed] [Google Scholar]
- 105. Lidell M.E., et al. (2008)Mapping of the 45M1 epitope to the C-terminal cysteine-rich part of the human MUC5AC mucin. FEBS J., 275, 481–489. [DOI] [PubMed] [Google Scholar]
- 106. Decaens C., et al. (1993)Biochemical characterization of a rat oncofetal colonic antigen defined by a monoclonal antibody raised against gastric surface epithelium. Biochem. J., 293, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lesuffleur T., et al. (1995)Characterization of a mucin cDNA clone isolated from HT-29 mucus-secreting cells. The 3’ end of MUC5AC?J. Biol. Chem., 270, 13665–13673. [DOI] [PubMed] [Google Scholar]
- 108. Bara J., et al. (1983)Immunohistological study of precancerous mucus modification in human distal colonic polyps. Cancer Res., 43, 3885–3891. [PubMed] [Google Scholar]
- 109. Shekels L.L., et al. (1995)Mouse gastric mucin: cloning and chromosomal localization. Biochem. J., 311, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ho J.J., et al. (1991)Characterization of new pancreatic cancer-reactive monoclonal antibodies directed against purified mucin. Cancer Res., 51, 372–380. [PubMed] [Google Scholar]
- 111. Goso Y., et al. (2003)Characterization of rat gastric mucins using a monoclonal antibody, RGM23, recognizing surface mucous cell-type mucins. J. Biochem., 133, 453–460. [DOI] [PubMed] [Google Scholar]
- 112. Ho S.B., et al. (1995)Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology, 109, 735–747. [DOI] [PubMed] [Google Scholar]
- 113. Luka J., et al. (2011)Development of a serum biomarker assay that differentiates tumor-associated MUC5AC (NPC-1C ANTIGEN) from normal MUC5AC. J. Biomed. Biotechnol., 2011, 934757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Asker N., et al. (1998)Human MUC5AC mucin dimerizes in the rough endoplasmic reticulum, similarly to the MUC2 mucin. Biochem. J., 335, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. de Bolós B.C., et al. (1995)MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology, 109, 723–734. [DOI] [PubMed] [Google Scholar]
- 116. López-Ferrer A., et al. (2000)Role of fucosyltransferases in the association between apomucin and Lewis antigen expression in normal and malignant gastric epithelium. Gut, 47, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. López-Ferrer A., et al. (2002)The expression of human FUT1 in HT-29/M3 colon cancer cells instructs the glycosylation of MUC1 and MUC5AC apomucins. Glycoconj. J., 19, 13–21. [DOI] [PubMed] [Google Scholar]
- 118. Lindén S., et al. (2004)Effects of pH on Helicobacter pylori binding to human gastric mucins: identification of binding to non-MUC5AC mucins. Biochem. J., 384, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Magalhães A., et al. (2016)Muc5ac gastric mucin glycosylation is shaped by FUT2 activity and functionally impacts Helicobacter pylori binding. Sci. Rep., 6, 25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Raclawska D.S., et al. (2016)Mucins and their sugars. Critical mediators of hyperreactivity and inflammation. Ann. Am. Thorac. Soc., 13, S98–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hennebicq-Reig S., et al. (1997)O-Glycosylation and cellular differentiation in a subpopulation of mucin-secreting HT-29 cell line. Exp. Cell Res., 235, 100–107. [DOI] [PubMed] [Google Scholar]
- 122. Wu Y.M., et al. (2009)Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J. Proteome Res., 8, 1876–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pan S., et al. (2014)Quantitative glycoproteomics analysis reveals changes in N-glycosylation level associated with pancreatic ductal adenocarcinoma. J. Proteome Res., 13, 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Haab B.B., et al. (2010)Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann. Surg., 251, 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cao Z., et al. (2013)Specific glycoforms of MUC5AC and endorepellin accurately distinguish mucinous from nonmucinous pancreatic cysts. Mol. Cell. Proteomics, 12, 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pinto R., et al. (2012)Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell. Mol. Med., 16, 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kerschner J.E., et al. (2010)MUC5AC expression in human middle ear epithelium of patients with otitis media. Arch. Otolaryngol. Head Neck Surg., 136, 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Buisine M.P., et al. (2000)Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J. Histochem. Cytochem., 48, 1667–1676. [DOI] [PubMed] [Google Scholar]
- 129. Buisine M.P., et al. (2000)Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. I. Stomach. A relationship to gastric carcinoma. J. Histochem. Cytochem., 48, 1657–1666. [DOI] [PubMed] [Google Scholar]
- 130. Nam S.Y., et al. (2005)Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig. Dis. Sci., 50, 2110–2120. [DOI] [PubMed] [Google Scholar]
- 131. Navabi N., et al. (2013)Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect. Immun., 81, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vilkin A., et al. (2007)Gallbladder inflammation is associated with increase in mucin expression and pigmented stone formation. Dig. Dis. Sci., 52, 1613–1620. [DOI] [PubMed] [Google Scholar]
- 133. Kim S.M., et al. (2014)Decreased Muc5AC expression is associated with poor prognosis in gastric cancer. Int. J. Cancer, 134, 114–124. [DOI] [PubMed] [Google Scholar]
- 134. Inaguma S., et al. (2011)GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene, 30, 714–723. [DOI] [PubMed] [Google Scholar]
- 135. Lakshmanan I., et al. (2016)MUC5AC interactions with integrin β4 enhances the migration of lung cancer cells through FAK signaling. Oncogene, 35, 4112–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. López-Ferrer A., et al. (2001)Mucins as differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. Am. J. Respir. Cell Mol. Biol., 24, 22–29. [DOI] [PubMed] [Google Scholar]
- 137. Copin M.C., et al. (2001)Mucinous bronchioloalveolar carcinomas display a specific pattern of mucin gene expression among primary lung adenocarcinomas. Hum. Pathol., 32, 274–281. [DOI] [PubMed] [Google Scholar]
- 138. Nishiumi N., et al. (2003)Use of 11p15 mucins as prognostic factors in small adenocarcinoma of the lung. Clin. Cancer Res., 9, 5616–5619. [PubMed] [Google Scholar]
- 139. Awaya H., et al. (2004)Expression of MUC1, MUC2, MUC5AC, and MUC6 in atypical adenomatous hyperplasia, bronchioloalveolar carcinoma, adenocarcinoma with mixed subtypes, and mucinous bronchioloalveolar carcinoma of the lung. Am. J. Clin. Pathol., 121, 644–653. [DOI] [PubMed] [Google Scholar]
- 140. Wakata K., et al. (2015)A favourable prognostic marker for EGFR mutant non-small cell lung cancer: immunohistochemical analysis of MUC5B. BMJ Open, 5, e008366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yu C.J., et al. (1997)Sialomucin expression is associated with erbB-2 oncoprotein overexpression, early recurrence, and cancer death in non-small-cell lung cancer. Am. J. Respir. Crit. Care Med., 155, 1419–1427. [DOI] [PubMed] [Google Scholar]
- 142. Yu C.J., et al. (1998)Quantitative analysis of mRNA encoding MUC1, MUC2, and MUC5AC genes: a correlation between specific mucin gene expression and sialomucin expression in non-small cell lung cancer. Am. J. Respir. Cell Mol. Biol., 18, 643–652. [DOI] [PubMed] [Google Scholar]
- 143. Chari R., et al. (2007)Effect of active smoking on the human bronchial epithelium transcriptome. BMC Genomics, 8, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ilhan O., et al. (2010)Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk. J. Gastroenterol., 21, 345–352. [DOI] [PubMed] [Google Scholar]
- 145. Pinto-de-Sousa J., et al. (2002)Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch., 440, 304–310. [DOI] [PubMed] [Google Scholar]
- 146. Lee H.S., et al. (2001)MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer, 92, 1427–1434. [DOI] [PubMed] [Google Scholar]
- 147. Wang J.Y., et al. (2003)Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J. Surg. Oncol., 83, 253–260. [DOI] [PubMed] [Google Scholar]
- 148. Zhang H.K., et al. (2004)Expression of mucins and E-cadherin in gastric carcinoma and their clinical significance. World J. Gastroenterol., 10, 3044–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Handra-Luca A., et al. (2006)Human pancreatic mucinous cystadenoma is characterized by distinct mucin, cytokeratin and CD10 expression compared with intraductal papillary-mucinous adenoma. Histopathology, 48, 813–821. [DOI] [PubMed] [Google Scholar]
- 150. Ban S., et al. (2006)Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am. J. Surg. Pathol., 30, 1561–1569. [DOI] [PubMed] [Google Scholar]
- 151. Nissim S., et al. (2012)Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas, 41, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lüttges J., et al. (2002)The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am. J. Surg. Pathol., 26, 466–471. [DOI] [PubMed] [Google Scholar]
- 153. Nadig S.N., et al. (2012)Clinical implications of mucinous nonneoplastic cysts of the pancreas. Pancreas, 41, 441–446. [DOI] [PubMed] [Google Scholar]
- 154. Kim G.E., et al. (2002)Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology, 123, 1052–1060. [DOI] [PubMed] [Google Scholar]
- 155. Matsuyama M., et al. (2012)Evaluation of pancreatic intraepithelial neoplasia and mucin expression in normal pancreata. J. Hepatobiliary Pancreat. Sci., 19, 242–248. [DOI] [PubMed] [Google Scholar]
- 156. Yamasaki H., et al. (2004)Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int. J. Oncol., 24, 107–113. [PubMed] [Google Scholar]
- 157. Ohuchida K., et al. (2006)Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int. J. Cancer, 118, 405–411. [DOI] [PubMed] [Google Scholar]
- 158. Giorgadze T.A., et al. (2006)Diagnostic utility of mucin profile in fine-needle aspiration specimens of the pancreas: an immunohistochemical study with surgical pathology correlation. Cancer, 108, 186–197. [DOI] [PubMed] [Google Scholar]
- 159. Wang Y., et al. (2007)Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound-guided fine-needle aspiration specimens of the pancreas. Int. J. Cancer, 121, 2716–2722. [DOI] [PubMed] [Google Scholar]
- 160. Yue T., et al. (2009)The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol. Cell. Proteomics, 8, 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yue T., et al. (2011)Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS One, 6, e29180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kaur S., et al. (2017)A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am. J. Gastroenterol., 112, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Buisine M.P., et al. (1996)Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology, 110, 84–91. [DOI] [PubMed] [Google Scholar]
- 164. Bartman A.E., et al. (1999)Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int. J. Cancer, 80, 210–218. [DOI] [PubMed] [Google Scholar]
- 165. Renaud F., et al. (2015)MUC5AC hypomethylation is a predictor of microsatellite instability independently of clinical factors associated with colorectal cancer. Int. J. Cancer, 136, 2811–2821. [DOI] [PubMed] [Google Scholar]
- 166. Walsh M.D., et al. (2013)Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod. Pathol., 26, 1642–1656. [DOI] [PubMed] [Google Scholar]
- 167. Biemer-Hüttmann A.E., et al. (2000)Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin. Cancer Res., 6, 1909–1916. [PubMed] [Google Scholar]
- 168. Renaud F., et al. (2016)The serrated neoplasia pathway of colorectal tumors: identification of MUC5AC hypomethylation as an early marker of polyps with malignant potential. Int. J. Cancer, 138, 1472–1481. [DOI] [PubMed] [Google Scholar]
- 169. Krishn S.R., et al. (2016)Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett., 374, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Krishn S.R., et al. (2017)Mucins and associated O-glycans based immunoprofile for stratification of colorectal polyps: clinical implication for improved colon surveillance. Oncotarget, 8, 7025–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tatsumi N., et al. (2006)Cytokeratin 7/20 and mucin core protein expression in ulcerative colitis-associated colorectal neoplasms. Virchows Arch., 448, 756–762. [DOI] [PubMed] [Google Scholar]
- 172. Terada T. (2013)An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int. J. Clin. Exp. Pathol., 6, 613–621. [PMC free article] [PubMed] [Google Scholar]
- 173. Melis M., et al. (2010)Gene expression profiling of colorectal mucinous adenocarcinomas. Dis. Colon Rectum, 53, 936–943. [DOI] [PubMed] [Google Scholar]
- 174. Kocer B., et al. (2006)Humoral immune response to MUC5AC in patients with colorectal polyps and colorectal carcinoma. BMC Gastroenterol., 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Losi L., et al. (2004)Relationship between MUC5AC and altered expression of MLH1 protein in mucinous and non-mucinous colorectal carcinomas. Pathol. Res. Pract., 200, 371–377. [DOI] [PubMed] [Google Scholar]
- 176. Nishida T., et al. (2014)Predictors of lymph node metastasis in T1 colorectal carcinoma: an immunophenotypic analysis of 265 patients. Dis. Colon Rectum, 57, 905–915. [DOI] [PubMed] [Google Scholar]
- 177. Bara J., et al. (2003)Abnormal expression of gastric mucin in human and rat aberrant crypt foci during colon carcinogenesis. Tumour Biol., 24, 109–115. [DOI] [PubMed] [Google Scholar]
- 178. Che T.C., et al. (2010)Early lesions induced in rat colon epithelium by N-methyl-N’-nitro-N-nitrosoguanidine. Tissue Cell, 42, 190–194. [DOI] [PubMed] [Google Scholar]
- 179. Yamazoe S., et al. (2010)RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J. Exp. Clin. Cancer Res., 29, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hoshi H., et al. (2011)Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int. J. Oncol., 38, 619–627. [DOI] [PubMed] [Google Scholar]
- 181. Hoshi H., et al. (2013)MUC5AC protects pancreatic cancer cells from TRAIL-induced death pathways. Int. J. Oncol., 42, 887–893. [DOI] [PubMed] [Google Scholar]
- 182. Zhu X., et al. (2016)Abrogation of MUC5AC expression contributes to the apoptosis and cell cycle arrest of colon cancer cells. Cancer Biother. Radiopharm., 31, 261–267. [DOI] [PubMed] [Google Scholar]
- 183. Escande F., et al. (2004)The mouse secreted gel-forming mucin gene cluster. Biochim. Biophys. Acta, 1676, 240–250. [DOI] [PubMed] [Google Scholar]
- 184. Ehre C., et al. (2012)Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc. Natl. Acad. Sci. USA, 109, 16528–16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Inatomi T., et al. (1997)Cloning of rat Muc5AC mucin gene: comparison of its structure and tissue distribution to that of human and mouse homologues. Biochem. Biophys. Res. Commun., 236, 789–797. [DOI] [PubMed] [Google Scholar]
- 186. Hasnain S.Z., et al. (2011)Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med., 208, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Koeppen M., et al. (2013)Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol., 6, 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Evans C.M., et al. (2015)The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun., 6, 6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Floyd A.M., et al. (2012)Mucin deficiency causes functional and structural changes of the ocular surface. PLoS One, 7, e50704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rachagani S., et al. (2012)Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy. J. Hematol. Oncol., 5, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Thai P., et al. (2008)Regulation of airway mucin gene expression. Annu. Rev. Physiol., 70, 405–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chen Y., et al. (2014)IL-1β induction of MUC5AC gene expression is mediated by CREB and NF-κB and repressed by dexamethasone. Am. J. Physiol. Lung Cell. Mol. Physiol., 306, L797–L807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Qin Y., et al. (2016)Interleukin-13 stimulates MUC5AC expression via a STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. Int. Immunopharmacol., 40, 106–114. [DOI] [PubMed] [Google Scholar]
- 194. Krishn S.R., et al. (2015)Advances in miRNA-mediated mucin regulation. Curr. Pharmacol. Rep., 1, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhong T., et al. (2011)MiR-146a negatively regulates neutrophil elastase-induced MUC5AC secretion from 16HBE human bronchial epithelial cells. Mol. Cell. Biochem., 358, 249–255. [DOI] [PubMed] [Google Scholar]
- 196. Skrzypek K., et al. (2013)Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal., 19, 644–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Teng Y., et al. (2015)miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem. Biophys. Res. Commun., 457, 58–64. [DOI] [PubMed] [Google Scholar]
- 198. Kato S., et al. (2006)MUC5AC mucin gene regulation in pancreatic cancer cells. Int. J. Oncol., 29, 33–40. [PubMed] [Google Scholar]
- 199. Kageyama-Yahara N., et al. (2014)Gli regulates MUC5AC transcription in human gastrointestinal cells. PLoS One, 9, e106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Perrais M., et al. (2002)Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J. Biol. Chem., 277, 32258–32267. [DOI] [PubMed] [Google Scholar]
- 201. Komatsu H., et al. (2014)A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PLoS One, 9, e87875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Vincent A., et al. (2007)Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene, 26, 6566–6576. [DOI] [PubMed] [Google Scholar]
- 203. Zhou X., et al. (2012)Hypoxia induces mucin expression and secretion in human bronchial epithelial cells. Transl. Res., 160, 419–427. [DOI] [PubMed] [Google Scholar]
- 204. Young H.W., et al. (2007)Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5’ elements. Am. J. Respir. Cell Mol. Biol., 37, 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Evans C.M., et al. (2009)Mucus hypersecretion in asthma: causes and effects. Curr. Opin. Pulm. Med., 15, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Yang J., et al. (2013)Study on TRPV1-mediated mechanism for the hypersecretion of mucus in respiratory inflammation. Mol. Immunol., 53, 161–171. [DOI] [PubMed] [Google Scholar]
- 207. Kim Y.J., et al. (2014)Hypoxia-mediated mechanism of MUC5AC production in human nasal epithelia and its implication in rhinosinusitis. PLoS One, 9, e98136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jang S., et al. (2012)Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int. J. Nanomedicine, 7, 1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yu H., et al. (2012)Regulation of cigarette smoke-mediated mucin expression by hypoxia-inducible factor-1α via epidermal growth factor receptor-mediated signaling pathways. J. Appl. Toxicol., 32, 282–292. [DOI] [PubMed] [Google Scholar]
- 210. Haswell L.E., et al. (2010)Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: a potential model of goblet cell hyperplasia. Toxicol. In Vitro., 24, 981–987. [DOI] [PubMed] [Google Scholar]
- 211. Baginski T.K., et al. (2006)Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am. J. Respir. Cell Mol. Biol., 35, 165–174. [DOI] [PubMed] [Google Scholar]
- 212. Takeyama K., et al. (2001)Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am. J. Physiol. Lung Cell. Mol. Physiol., 280, L165–L172. [DOI] [PubMed] [Google Scholar]
- 213. Shao M.X., et al. (2004)Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am. J. Physiol. Lung Cell. Mol. Physiol., 287, L420–L427. [DOI] [PubMed] [Google Scholar]
- 214. Iwashita H., et al. (2012)Increased human Ca²⁺-activated Cl⁻ channel 1 expression and mucus overproduction in airway epithelia of smokers and chronic obstructive pulmonary disease patients. Respir. Res., 13, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Hegab A.E., et al. (2007)Niflumic acid and AG-1478 reduce cigarette smoke-induced mucin synthesis: the role of hCLCA1. Chest, 131, 1149–1156. [DOI] [PubMed] [Google Scholar]
- 216. Gensch E., et al. (2004)Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J. Biol. Chem., 279, 39085–39093. [DOI] [PubMed] [Google Scholar]
- 217. O’Donnell R.A., et al. (2004)Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax, 59, 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Wu Q., et al. (2012)Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun., 18, 617–626. [DOI] [PubMed] [Google Scholar]
- 219. Di Y.P., et al. (2012)Cigarette smoke induces MUC5AC protein expression through the activation of Sp1. J. Biol. Chem., 287, 27948–27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cho J.G., et al. (2009)Effects of cigarette smoking on mucin production in human middle ear epithelial cells. Int. J. Pediatr. Otorhinolaryngol., 73, 1447–1451. [DOI] [PubMed] [Google Scholar]
- 221. Lee S.Y., et al. (2006)The inhibitory effects of rebamipide on cigarette smoke-induced airway mucin production. Respir. Med., 100, 503–511. [DOI] [PubMed] [Google Scholar]
- 222. Lee S.Y., et al. (2006)Peroxisome proliferator-activated receptor-gamma inhibits cigarette smoke solution-induced mucin production in human airway epithelial (NCI-H292) cells. Am. J. Physiol. Lung Cell. Mol. Physiol., 291, L84–L90. [DOI] [PubMed] [Google Scholar]
- 223. Cortijo J., et al. (2011)Aclidinium inhibits cholinergic and tobacco smoke-induced MUC5AC in human airways. Eur. Respir. J., 37, 244–254. [DOI] [PubMed] [Google Scholar]
- 224. Wang G., et al. (2010)Smoking-mediated up-regulation of GAD67 expression in the human airway epithelium. Respir. Res., 11, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Monzon M.E., et al. (2011)MCP-1/CCR2B-dependent loop upregulates MUC5AC and MUC5B in human airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol., 300, L204–L215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yu H., et al. (2011)Regulation of cigarette smoke-induced mucin expression by neuregulin1β/ErbB3 signalling in human airway epithelial cells. Basic Clin. Pharmacol. Toxicol., 109, 63–72. [DOI] [PubMed] [Google Scholar]
- 227. Fu X.W., et al. (2012)The ly-6 protein, lynx1, is an endogenous inhibitor of nicotinic signaling in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol., 303, L661–L668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Arlen M., et al. (2012)The use of specific monoclonal antibodies to target immunogenic tumor membrane proteins in patients with recurrent pancreatic and colon cancer. Curr. Drug Deliv., 9, 52–56. [DOI] [PubMed] [Google Scholar]
- 229. Yamazoe S., et al. (2011)Identification of HLA-A*0201- and A*2402-restricted epitopes of mucin 5AC expressed in advanced pancreatic cancer. Pancreas, 40, 896–904. [DOI] [PubMed] [Google Scholar]
- 230. Gold D.V., et al. (2013)Mapping PAM4 (clivatuzumab), a monoclonal antibody in clinical trials for early detection and therapy of pancreatic ductal adenocarcinoma, to MUC5AC mucin. Mol. Cancer, 12, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Liu D., et al. (2015)Identification of PAM4 (clivatuzumab)-reactive epitope on MUC5AC: a promising biomarker and therapeutic target for pancreatic cancer. Oncotarget, 6, 4274–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]