Abstract
Most broadly neutralizing antibodies to HIV-1 have in common an extreme degree of somatic hypermutation (SHM), which correlates with their ability to neutralize multiple viral strains. However, achieving such extreme SHM by immunization remains a challenge. Here, we discuss how antigenic variation during HIV-1 infection may work to exacerbate SHM by permitting multiple iterative cycles of affinity maturation in germinal centers, and speculate on how this could be recapitulated through vaccination.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
After almost 30 years of combined efforts to develop an effective HIV-1 vaccine that we so desperately need, it has now become clear that protective vaccines would require eliciting antibodies capable of neutralizing most, if not all, HIV-1 strains (Burton et al. 2012). Although these “broadly neutralizing” HIV-1 antibodies (bnAbs) can develop naturally in infected individuals, this happens only rarely (1%–2% of cases), in part owing to long and complex B-cell maturation pathways (Mouquet 2014). These pathways often result in extreme degrees of somatic hypermutation (SHM). As many as 30% of nucleotides in the bnAb V regions differ from their germline configuration, with mutations scattered not only in the immunoglobulin (Ig) variable loops but also in ordinarily more conserved framework regions (Klein et al. 2013). Within a single B-cell clonotype, the number of mutations usually correlates with neutralization potency and breadth (Mouquet 2014). Adding to the complexity, numerous bnAbs are also characterized by uncommon gene modifications (i.e., nucleotide insertions and deletions [indels]), which are critical for their neutralization activity (Kepler et al. 2014). Thus, “extreme SHM” appears to be a necessary feature of HIV-1 bnAbs. Although multiple factors account for failure to generate an HIV-1 vaccine so far, our inability to induce by vaccination antibodies that even approach the mutational burdens associated with HIV-1 bnAbs remains an important challenge.
SHM takes place in germinal centers (GCs), clusters of proliferating B cells that emerge within B-cell follicles of secondary lymphoid organs on exposure to antigen, either by infection or immunization (Victora and Nussenzweig 2012). GC B cells express activation-induced cytidine deaminase (AID), which inserts point mutations (and, more rarely, indels) throughout the V regions of the Ig heavy and light chains. This generates a panel of mutated B cells that are then subjected to selection based on their affinity for antigen, held as immune complexes on the surface of GC-resident follicular dendritic cells (FDCs). Iterative rounds of selection and proliferation of somatically mutated clonal variants result in a population that is, on average, enriched for higher-affinity binders, and that accumulates somatic mutations over time (Victora and Nussenzweig 2012). The lifetime of a GC reaction can vary over a long range, from 1 to 2 weeks for soluble protein boosting to several months or longer for certain infections. The factor(s) controlling GC longevity remain poorly defined (Mesin et al. 2016).
The extraordinary degree of gene modification necessary to shape bnAbs is not only indicative of the extent of SHM, but also of the strength of GC selection, which must ensure that multiple affinity-enhancing mutations are maintained while avoiding the presumably much more frequent mutations that either reduce affinity or prevent proper expression of the Ig. The challenge for developing an HIV-1 vaccine capable of eliciting bnAbs is therefore double: In addition to the ability to “fish out” rare bnAb precursors from a sea of (likely higher-affinity) competitors, a successful vaccination strategy must also be able to shepherd these clones through repetitive rounds of GC selection, again in the face of fierce competition, until the mutational load required for broad neutralization is reached. Although this challenge is common to natural HIV-1 infection and HIV-1 vaccination, the means by which broad neutralization is (or can be) achieved in each of these settings is likely to differ.
HOW DOES HIV-1 INFECTION LEAD TO EXTREME SHM AND bnAb DEVELOPMENT?
Landmark studies in infected individuals who develop bnAbs have shown that mutations/indels are progressively incorporated into Ig genes by coevolution between bnAb-prone B-cell lineages and the viral population as it escapes immune pressure (Mouquet 2014; Moore et al. 2015). Thus, evolution of bnAb-expressing B cells occurs through iterative cycles of affinity maturation toward rapidly evolving antigenic variants of the HIV-1 glycoprotein. Although the rates of antibody evolution in these individuals can fluctuate according to several variables (e.g., available B-cell lineages, disease phase, size, and diversity of the viral population, etc.), they globally tend to slow down during the course of infection, as determined using hypermutation as a molecular clock (Wu et al. 2015). This observation likely reflects the rarefaction of AID mutational hotspots over time caused by the progressive increase in already mutated target sites (Dosenovic et al. 2015), even if HIV-1 infection could potentially lead to overall higher AID expression in “hyperactive” GC B cells.
The effects of competition between different B-cell clones on the priming, maturation, and selection of clones harboring bnAb specificities remain largely unknown. Even if envelope glycoproteins expressed by transmitted/founder viruses possess specific antigenic properties that preferentially stimulate bnAb progenitors (McGuire et al. 2014), how these rare precursors are maintained in the GC for long enough to acquire extensive SHM in the presence of competition from strain-specific clones is not clear. Part of the answer may come from recent work in mice showing that competition between B-cell clones does not invariably lead to extensive loss of diversity (Kuraoka et al. 2016; Tas et al. 2016). Early GCs can contain up to hundreds of B-cell clones with different V(D)J recombinations, and GCs also lose diversity at different rates so that lower affinity variants are not always efficiently eliminated. Thus, several clones can evolve in parallel within the same GC, and less competitive variants can be maintained in the response and continue to evolve, allowing for greater plasticity in following a trajectory that may ultimately lead to broad antibody neutralization. However, GCs must ultimately select clones that are broad over those that are strain-specific. By imposing immune pressure on HIV-1, T-cell and antibody responses create antigenic drift, which can result in hundreds or thousands of distinct viral strains coexisting within a single infected individual. When presented with such a heterogeneous viral population, avidity effects may come into play: Theoretically, B-cell clones binding to conserved epitopes will have a relative advantage in competing for limiting T follicular helper (TFH) help when compared to clones that bind to a small subset of antigenic variants, even if the latter bind with higher affinity. This process could be facilitated by the intervention of additional B-cell lineages that positively influence viral evolution by creating the antigenic variants needed to sustain bnAb lineage maturation (Moore et al. 2015). This coevolution configures a “Red Queen” scenario (Nourmohammad et al. 2016), in which HIV-1 evolves to escape neutralization by antibodies that, in turn, evolve to neutralize the escape variants. Constant “running to stay in place” would thus explain the extreme degree of mutations found in HIV-1 bnAbs simply as a matter of constantly presenting the immune system with a slightly different target than what it can efficiently respond to.
IS ANTIGENIC VARIATION SUFFICIENT TO INDUCE EXTREME SHM?
A key question is whether the highly mutated antibodies that arise during HIV-1 infection are simply a result of the immune system being confronted with the right sequence and/or diversity of variant antigens at the right times during antibody evolution (the alternative being that there is something unique about the biology of GCs during HIV-1 infection that allows for higher-than-normal SHM). Recently, a series of groundbreaking studies showed that mice engineered to express human Ig genes associated with bnAb potential, when immunized successively with a series of progressively divergent antigens, develop antibodies that neutralize a broad array of HIV-1 isolates (Briney et al. 2016; Escolano et al. 2016; Tian et al. 2016). These antibodies showed substantial SHM burdens, even if not quite at the level found in bnAbs. Importantly, consecutive immunizations with the same antigen led only to a fraction of the SHM elicited by sequential immunization (Briney et al. 2016; Escolano et al. 2016; Tian et al. 2016), providing strong evidence that the extent of SHM can be controlled by the nature of the epitopes presented to the immune system.
On the other hand, sequential exposure to divergent forms of an antigen over extended periods does not invariably lead to extreme SHM, as exemplified by the human response to influenza. Although over the years the human population is repeatedly exposed, by infection and/or vaccination, to progressively drifting variants of the influenza hemagglutinin (HA), antibodies (including bnAbs) cloned from HA-exposed individuals display only a fraction of the SHM burden seen in HIV-1-infected patients (Corti and Lanzavecchia 2013; Neu et al. 2016). A caveat of this argument is that the seasonal drift in influenza HA is very narrow compared to the wider antigenic variation observed even within a single HIV-1-infected individual. Thus, the year-to-year antigenic variation in influenza could arguably be more akin to repeated immunization with the same antigen than to the HIV-1 scenario. Thus, achieving extreme SHM may require making the antigenic distance between sequential immunogens “just right.” Although a distance that is too short may lead to inefficient recruitment of B cells to GCs by original antigenic sin–type phenomena, too large a distance may lead to preferential recruitment of strain-specific naïve B cells to GCs over cross-reactive memory cells, as we have proposed previously for immunization with different subtypes of influenza (Victora and Wilson 2015).
Besides rapid antigenic variation, HIV-1 infection is characterized by a series of unique pathological features, including chronic immune activation, abnormalities in B-cell development and function, and specific alterations in GC and TFH-cell biology (Vinuesa 2012; Mouquet 2014). TFH cells are highly permissive to HIV-1 infection (Kohler et al. 2016), and constitute an important source of viral replication within GCs (Perreau et al. 2013). This is facilitated by long-term trapping of infectious HIV-1 particles in immune complexes on FDCs (Heath et al. 1995), creating an anatomical environment conducive to the establishment and maintenance of a viral reservoir. As observed in chronic SIV infection (Petrovas et al. 2012), HIV-1 propagation in GCs may lead to an accumulation of local TFH cells, which, in turn, could expand GC B cells and enhance the production of virus-specific serum Ig (Lindqvist et al. 2012). Although HIV-1 likely interferes with proper TFH-cell support for bnAb development (Crotty 2014; Pissani and Streeck 2014), it is also plausible that some of these alterations may contribute to lengthening the time bnAb precursors and their clonal descendants spend in GCs beyond what is normally achievable by vaccination or other infections. If this is the case, strategies to achieve high mutational loads by immunization may benefit from the ability to manipulate the biology of GCs, potentially by use of adjuvants or other approaches to extend GC lifetime, as we shall see below.
HOW CAN THE SHM LOAD INDUCED BY VACCINATION BE INCREASED?
In theory, the SHM burden on a B-cell clone can be boosted either by increasing the rate of mutations per unit time or by lengthening the time a clone spends hypermutating (the GC “dwell time”). Increasing SHM rates by increasing AID expression or activity, though attractive in principle, is problematic given that SHM and selection must be tightly balanced for GC B cells to avoid mutations that are deleterious to Ig function (Kepler and Perelson 1993; Oprea and Perelson 1997). Indeed, increasing AID expression experimentally leads to stunted GCs that are less capable of sustaining prolonged SHM and affinity maturation (Teng et al. 2008; Robbiani et al. 2009). AID-induced mutations can also be concentrated by increasing the number of cell cycles a B cell undergoes over a given period of time. When strong, positive selection is induced experimentally, B cells undergo several rounds of accelerated proliferation in the GC dark zone, accompanied by a higher degree of SHM than normally observed (Victora et al. 2010; Gitlin et al. 2014, 2015). Because negative selection of damaged B-cell receptors (BCRs) can, in principle, act between each cell cycle in the dark zone, accelerated cell cycling is more likely to yield a high degree of SHM than simply increasing AID activity per incorporated nucleotide. Recent work from our (Victora) laboratory has shown that accelerated diversification of a clone by SHM can be seen also under physiological conditions, when single GC cells undergo “clonal bursts” that lead to massive accumulation of clonal variants over a short period of time (Tas et al. 2016). Understanding how the occurrence of these bursts is regulated may help improve our ability to increase SHM by vaccination.
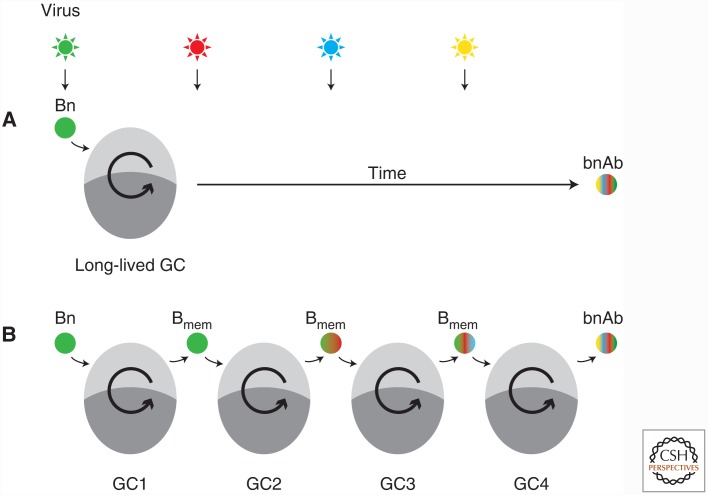
Perhaps more tractable than increasing the rate of mutations over time is to lengthen the time a B-cell clone spends in GCs. B-cell clones can extend their GC dwell time by remaining in the same GC for an extended period (Fig. 1A) and/or by entering different GC reactions successively, passing through a memory B-cell (Bmem) intermediate (which we refer to as “GC hopping”; Fig. 1B).
Figure 1.
Alternative scenarios by which extended germinal center (GC) residency can lead to broadly neutralizing antibody (bnAb) development. (A) An unmutated naïve B cell (Bn) bnAb precursor enters a long-lived GC. Exposure to successive antigenic variants of the virus drives affinity maturation of the clone within the same GC reaction, until breadth is achieved. (B) GC hopping: A bnAb-prone clone enters into a GC and exits as a memory B cell (Bmem), being recalled to a second GC on exposure to a mutated antigenic variant of the virus. Successive cycles of entry and exit drive the acquisition of breadth. The two models are not mutually exclusive and, in both cases, extreme somatic hypermutation (SHM) is achieved by prolonged GC “dwell time.” However, only the second scenario can currently be elicited by vaccination with protein subunits.
GCs induced by immunization are generally short-lived, disappearing after 3 or 4 weeks (Takahashi et al. 1998). GCs can be made to last longer (and, presumably, accumulate a larger number of mutations) by varying the way in which the antigen is presented to the immune system. For example, immunization of mice with nanoparticles containing antigen and Toll-like receptor (TLR) ligands generate GCs that last for at least a few months (Pulendran et al. 1995). Long-lived GCs are also a common feature of virus-induced immune responses (Adachi et al. 2015). Given the chronic immune activation associated with HIV-1 infection, it is conceivable that a clone could remain associated with the same GC over months or years, fueled by stimulation through innate receptors, and shifting its reactivity pattern as the virus escapes the immune response by mutation. It is unclear whether such an extreme scenario can ever be recapitulated by immunization, emphasizing the need for development of novel adjuvants and antigen formulations aimed at extending GC duration. Refreshing TFH cells help by adding novel CD4 T-cell epitopes to B-cell antigens that are driving ongoing GCs may provide an alternative approach (Shulman et al. 2013).
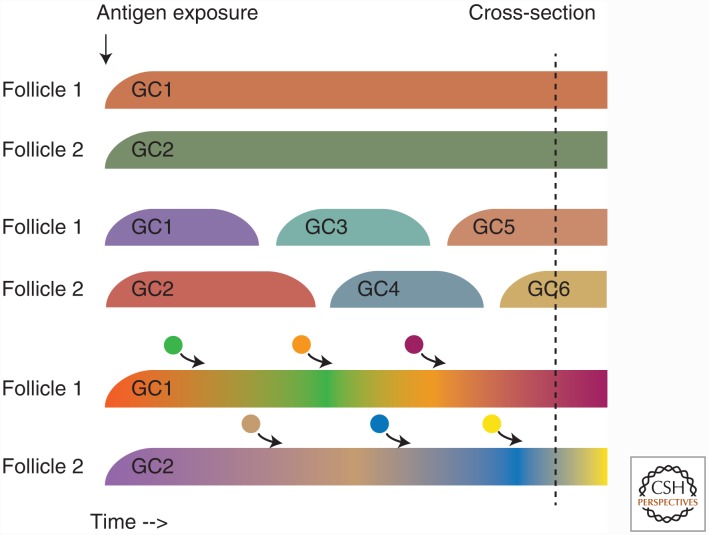
An unresolved question in this sense is what exactly the nature of a long-lived GC response is (Fig. 2). GCs present several months after initial antigenic exposure could represent either long-lasting GCs (Fig. 2A) or recurring GCs that wax and wane independently and asynchronously (Fig. 2B). Although these two scenarios are undistinguishable by cross-sectional analysis at a given end point, only the first scenario would allow for a B-cell clone to continuously mutate and evolve within the same GC over a long period of time. Because GCs are, in principle, open to continuous entry of B and T cells (Schwickert et al. 2009; Bergqvist et al. 2013; Shulman et al. 2013), an intermediate scenario could potentially exist in which clonal diversity in long-lived GCs is molded by constant re-seeding by naïve or Bmem cells. This is especially likely in the setting of a mutating chronic virus, where novel T-cell and B-cell epitopes capable of recruiting naïve cells into the reaction are constantly emerging.
Figure 2.
Nature of the “long-lived” germinal center (GC) response. A response in which GCs are observable several months after induction could result from different GC formation and disappearance kinetics. The figure illustrates three possible models. (A) Long-lived GCs: Individual GCs appear simultaneously close to the induction of the response and last until the GC reactions resolve several months later. Somatic hypermutation (SHM) load increases and clonal diversity decreases continuously as the reaction progresses. (B) Recurrent GCs: Individual GCs are short-lived, but as a GC disappears, a new one appears in the same follicle but with a different clonal composition, and possibly lower SHM load. (C) Long-lived GCs as in A are constantly being seeded by B-cell clones recruited from the outside, either from the memory or naïve pools. Diversity remains high throughout the course of the response. Importantly, cross-sectional analysis at a given time point does not allow for a clear distinction between these models.
GC dwell time can also be increased by successive GC hopping (Fig. 1B). The ability of B-cell clones to return to GCs has become a topic of debate, as reviewed in Pape and Jenkins (2017). Emerging evidence suggests that unswitched (IgM+) Bmem cells have a relative advantage in accessing GCs when compared to class-switched Bmem cells (Kurosaki et al. 2015) (although access of IgG+ cells to GCs has been inferred from sequencing data [Andrews et al. 2015; McHeyzer-Williams et al. 2015]). Recent studies propose that Bmem cells derive predominantly from the pre-GC or early GC reaction, before substantial SHM has occurred (Weisel et al. 2016), and from GC cells with lower affinity for antigen (Shinnakasu et al. 2016). These constraints create a theoretical barrier to accumulation of mutations and affinity maturation by GC hopping, because each passage through the GC would lead to only modest gains in SHM load and affinity. Whether the Bmem cells that are actually recruited into recall GCs derive from the majority of IgM+, low-affinity, low-SHM cells or from a minority of cells that arise later in the GC reaction has not been determined, although experimental data suggest that B-cell clones specific for escape mutants of antigenically evolving pathogens reside in the class-switched memory compartment (Purtha et al. 2011). Resolving these questions may aid in the design of vaccination strategies based on sequential immunization with closely related antigens (Briney et al. 2016; Escolano et al. 2016; Tian et al. 2016).
CONCLUDING REMARKS
Although we have made great strides in understanding how HIV-1 bnAbs acquire the ability to focus on the rare conserved epitopes of the HIV-1 envelope glycoprotein, the exact process by which they achieve the extreme SHM levels necessary for broad neutralization remains elusive. Understanding this process may help outline a strategy for generating highly mutated antibodies by vaccination. If successive recall of Bmem cells to GCs using the right sequence of antigens is sufficient for extreme SHM, this means that, in principle, we have the necessary tools for a successful HIV-1 vaccine. If, on the other hand, prolonged residency of bnAb precursor clones in hyperactive and/or long-lived GC is required, more investment will need to be made in refining our control over GCs reactions, so that bnAb precursors and their progeny are not eliminated from long-lived GCs by competition from strain-specific clones.
ACKNOWLEDGMENTS
G.D.V. is supported by National Institutes of Health Grant 5DP5OD012146. H.M. is supported by the G5 Institut Pasteur Program and the Milieu Intérieur Program (ANR-10-LABX-69-01).
REFERENCES
*Reference is also in this collection.
- Adachi Y, Onodera T, Yamada Y, Daio R, Tsuiji M, Inoue T, Kobayashi K, Kurosaki T, Ato M, Takahashi Y. 2015. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J Exp Med 212: 1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, et al. 2015. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 7: 316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist P, Stensson A, Hazanov L, Holmberg A, Mattsson J, Mehr R, Bemark M, Lycke NY. 2013. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol 6: 122–135. [DOI] [PubMed] [Google Scholar]
- Briney B, Sok D, Jardine JG, Kulp DW, Skog P, Menis S, Jacak R, Kalyuzhniy O, de Val N, Sesterhenn F, et al. 2016. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell 166: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Lanzavecchia A. 2013. Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31: 705–742. [DOI] [PubMed] [Google Scholar]
- Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, et al. 2015. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knockin mice. Cell 161: 1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolano A, Steichen JM, Dosenovic P, Kulp DW, Golijanin J, Sok D, Freund NT, Gitlin AD, Oliveira T, Araki T, et al. 2016. Sequential immunization elicits broadly neutralizing anti-HIV-1 antibodies in Ig knockin mice. Cell 166: 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, Shulman Z, Nussenzweig MC. 2014. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 509: 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, Mayer CT, Oliveira TY, Shulman Z, Jones MJ, Koren A, Nussenzweig MC. 2015. T cell help controls the speed of the cell cycle in germinal center B cells. Science 349: 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath SL, Tew JG, Szakal AK, Burton GF. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377: 740–744. [DOI] [PubMed] [Google Scholar]
- Kepler TB, Perelson AS. 1993. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today 14: 412–415. [DOI] [PubMed] [Google Scholar]
- Kepler TB, Liao HX, Alam SM, Bhaskarabhatla R, Zhang R, Yandava C, Stewart S, Anasti K, Kelsoe G, Parks R, et al. 2014. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe 16: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, et al. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, Meditz AL, McCarter M, Levy DN, et al. 2016. Germinal center T follicular helper cells are highly permissive to HIV-1 and alter their phenotype during virus replication. J Immunol 196: 2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G. 2016. Complex antigens drive permissive clonal selection in germinal centers. Immunity 44: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, Ise W. 2015. Memory B cells. Nat Rev Immunol 15: 149–159. [DOI] [PubMed] [Google Scholar]
- Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 122: 3271–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Dreyer AM, Carbonetti S, Lippy A, Glenn J, Scheid JF, Mouquet H, Stamatatos L. 2014. HIV antibodies. Antigen modification regulates competition of broad and narrow neutralizing HIV antibodies. Science 346: 1380–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. 2015. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 16: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Ersching J, Victora GD. 2016. Germinal center B cell dynamics. Immunity 10.1016/j.immuni.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Williamson C, Morris L. 2015. Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol 23: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H. 2014. Antibody B cell responses in HIV-1 infection. Trends Immunol 35: 549–561. [DOI] [PubMed] [Google Scholar]
- Neu KE, Henry Dunand CJ, Wilson PC. 2016. Heads, stalks and everything else: How can antibodies eradicate influenza as a human disease? Curr Opin Immunol 42: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourmohammad A, Otwinowski J, Plotkin JB. 2016. Host-pathogen coevolution and the emergence of broadly neutralizing antibodies in chronic infections. PLoS Genet 12: e1006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea M, Perelson AS. 1997. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J Immunol 158: 5155–5162. [PubMed] [Google Scholar]
- *.Pape KA, Jenkins MMK. 2017. Do memory B cells form secondary germinal centers? It depends. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo C. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. 2012. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest 122: 3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissani F, Streeck H. 2014. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol 35: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. 1995. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature 375: 331–334. [DOI] [PubMed] [Google Scholar]
- Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. 2011. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208: 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, Klein IA, Stone G, Eisenreich TR, et al. 2009. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell 36: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Alabyev B, Manser T, Nussenzweig MC. 2009. Germinal center reutilization by newly activated B cells. J Exp Med 206: 2907–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, Kurosaki Y. 2016. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 17: 861–869. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. 2013. T follicular helper cell dynamics in germinal centers. Science 341: 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. V: Affinity maturation develops in two stages of clonal selection. J Exp Med 187: 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. 2016. Visualizing antibody affinity maturation in germinal centers. Science 351: 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, Papavasiliou FN. 2008. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity 28: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Cheng C, Chen X, Duan H, Cheng HL, Dao M, Sheng Z, Kimble M, Wang L, Lin S, et al. 2016. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell 166: 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. 2012. Germinal centers. Annu Rev Immunol 30: 429–457. [DOI] [PubMed] [Google Scholar]
- Victora GD, Wilson PC. 2015. Germinal center selection and the antibody response to influenza. Cell 163: 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG. 2012. HIV and T follicular helper cells: A dangerous relationship. J Clin Invest 122: 3059–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. 2016. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 44: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang Z, Schramm CA, Joyce MG, Kwon YD, Zhou T, Sheng Z, Zhang B, O’Dell S, McKee K, et al. 2015. Maturation and diversity of the VRC01-antibody lineage over 15 years of chronic HIV-1 infection. Cell 161: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]