Abstract
Genetic prion diseases (gPrDs) caused by mutations in the prion protein gene (PRNP) have been classified as genetic Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker disease, or fatal familial insomnia. Mutations in PRNP can be missense, nonsense, and/or octapeptide repeat insertions or, possibly, deletions. These mutations can produce diverse clinical features. They may also show varying ancillary testing results and neuropathological findings. Although the majority of gPrDs have a rapid progression with a short survival time of a few months, many also present as ataxic or parkinsonian disorders, which have a slower decline over a few to several years. A few very rare mutations manifest as neuropsychiatric disorders, with systemic symptoms that include gastrointestinal disorders and neuropathy; these forms can progress over years to decades. In this review, we classify gPrDs as rapid, slow, or mixed types based on their typical rate of progression and duration, and we review the broad spectrum of phenotypes manifested by these diseases.
Human prion protein (PrP) prion diseases (PrDs) are classified into three types based on how they arise: sporadic, genetic, and acquired. By far the most common is sporadic—sporadic Creutzfeldt–Jakob disease (sCJD), accounting for ∼85%–90% of PrD cases (Parchi et al. 1999; Brown and Mastrianni 2010; Puoti et al. 2012). Mutations in the prion protein gene, PRNP, cause genetic PrDs (gPrDs), which account for ∼10%–15% of human PrDs (Masters et al. 1979; Kovács et al. 2005; Ladogana et al. 2005). The most notorious, acquired PrDs account for <1% of all cases of human PrD (Masters et al. 1979; Ladogana et al. 2005; Brown et al. 2006; UK National CJD Surveillance Unit 2015). For a review of sporadic and acquired PrDs, see Will and Ironside (2017).
Historically, gPrDs have been categorized as one of three diseases based on their clinical and neuropathological features: familial CJD (fCJD, Online Mendelian Inheritance in Man [OMIM] catalog #123400), Gerstmann–Sträussler–Scheinker disease (GSS, OMIM #137440), and familial fatal insomnia (FFI, OMIM #600072). Familial CJD (fCJD) typically presents as a rapidly progressive dementia with motor features and a short survival time (usually <1 yr) from onset. Its features are most similar to those of sCJD. Most forms of fCJD also show similar neuropathological features to those of sCJD, including synaptic, granular, or plaque-like patterns of PrPSc (in which Sc stands for scrapie, the prion disease of sheep and goats) deposition, vacuolation (spongiform changes), gliosis, and loss of neurons. Typically, there is a direct relationship between disease duration and the severity of neuropathology (Gambetti et al. 2003a). GSS usually presents as an ataxic disorder, often with parkinsonism and dementia occurring later in the illness. Survival is typically ∼3–10 yr, but some patients have a much shorter course, with symptoms more typical of sCJD. Usually, prominent PrPSc amyloid plaques are found in the cerebellum and cortex (Gambetti et al. 2003a). FFI usually begins with insomnia and dysautonomia, with later development of cognitive impairment and motor features. The median survival time of FFI patients is ∼18 mo. As with GSS, faster forms, more similar to sCJD in their disease course, also can occur, but there can be variability even within families. The neuropathology of FFI is quite distinct, typically with thalamic nerve cell loss and gliosis causing thalamic atrophy, but also similar pathology in the inferior olivary nucleus atrophy, as well as gliosis, but with sparse vacuolation and PrPSc deposition, in the midbrain and hypothalamic gray matter. In cases of long duration, the cortex also is involved (Parchi et al. 1995; Gambetti et al. 2003a). The classification scheme for gPRDs, however, was developed before the identification of PRNP, and we know now that several PRNP mutations do not fit into one of these three disease groups, as we discuss later in this review.
In 1989, mutations in PRNP were first shown to cause gPrDs (Goldgaber et al. 1989; Hsiao et al. 1989; Owen et al. 1989). Yet, it was back in 1930 that Meggendorfer first reported the Backer family, a German kindred with fCJD (Meggendorfer 1930). Affected members presented with rapidly progressive dementia (RPD) and had spongiform changes in the brain. Many decades later, affected descendants of this family were shown to carry the D178N codon 129V PRNP mutation (see below) (Kretzschmar et al. 1995). J. Gerstmann, E. Sträussler, and I. Scheinker reported in 1936 an Austrian kindred, the “H” family, presenting with slowly progressive cerebellar ataxia and who developed dementia late in their illness (Gerstmann et al. 1936). Similar to the Backer family, theirs was an autosomal dominant pattern of inheritance. The H family is now considered the first known GSS family. Decades later, the P102L mutation in PRNP was identified in the family (Hainfellner et al. 1995). The term “FFI” was coined in 1986 by Lugaresi and colleagues (1986) who reported an Italian family with progressive insomnia, dysautonomia, motor dysfunction, and degeneration of the thalamus. In 1992, a PRNP D178N (codon 129M) mutation was found in an Italian FFI kindred, showing this also to be a genetic PrD (Medori et al. 1992). The same D178N mutation resulted in two different phenotypes, either fCJD or FFI, depending on whether the PRNP cis codon 129 polymorphism was methionine or valine: methionine was typically associated with FFI and valine with fCJD (Goldfarb et al. 1992).
Although most PRNP mutations causing gPrD are missense, several insertions, a few nonsense and at least one deletion mutation have also been identified (Beck et al. 2001; Takada et al. 2017). Most insertions are octapeptide repeat insertions (OPRIs) in the PRNP region corresponding to the copper-binding domain of PrP. OPRI mutations can manifest as a gCJD or GSS phenotype (Owen et al. 1990; Brown and Mastrianni 2010; Takada et al. 2017). A patient with a 1-OPRI near codon 129, and distant from the copper-binding domain of typical OPRI mutations, presented with a GSS phenotype and seizures (Hinnell et al. 2011). Recently identified nonsense or stop-codon mutations in PRNP have been reported only in a few kindreds. These patients present with highly variable atypical clinical features for most PrDs, and neuropathological findings reveal amyloid plaques and/or prion amyloid angiopathy (Mead et al. 2013; Guerreiro et al. 2014; Fong et al. 2016).
Prion Protein (PrP) and the Prion Protein Gene (PRNP)
The human PrP gene, PRNP, is located on chromosome 20p13 and has two exons with the entire open reading frame in exon 2. Prion protein genes have been highly conserved across mammals, suggesting that PrP plays an important role in the organism. The canonical PrP sequence consists of 253 amino acids, which is processed posttranslationally, in which a 22-amino-acid, N-terminal signal peptide and a 23-amino-acid, C-terminal peptide are removed. This modification directs the addition of a glycosylphosphatidylinositol anchor, tethering the protein to the cell membrane (Colby and Prusiner 2011). The N-terminal domain of PrP contains an unstable region with repeats of a nonapeptide (Pro-Gln-Gly-Gly-Gly-Gly-Trp-Gly-Gln) followed by four octapeptide repeats (Pro-His-Gly-Gly-Gly-Trp-Gly-Gln) (Fig. 1). PrP can be unglycosylated, monoglycosylated, or diglycosylated at asparagine residues 181 and/or 197 (Capellari et al. 2011; Colby and Prusiner 2011; Yusa et al. 2012).
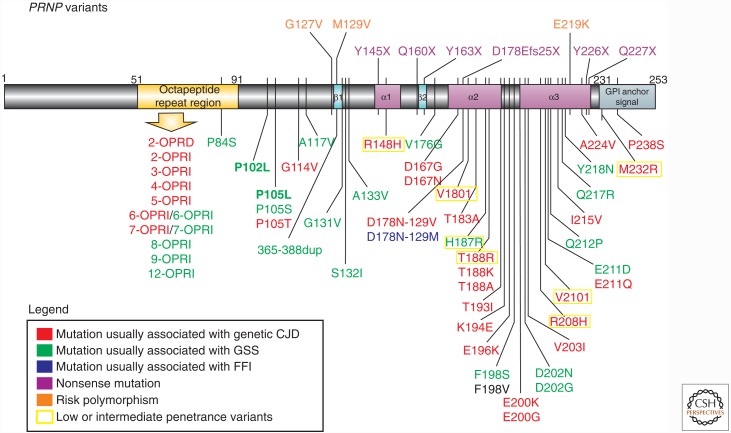
Figure 1.
Schematic of prion protein gene (PRNP) disease–associated variants. Mutations are color coded based on clinicopathological classification as genetic Creutzfeldt–Jakob disease (gCJD), Gerstmann–Sträussler–Scheinker disease (GSS), familial fatal insomnia (FFI), or nonsense mutations. PRNP mutations present in the UCSF cohort are shown in bold. Most mutations are shown below the gene schematic; nonsense mutations and polymorphisms associated with prion disease risk are above the gene schematic. Low- or intermediate-penetrance variants are based on Minikel et al. (2016) (not all low/intermediate-penetrance variants are shown). For the F198V mutation, the clinical presentation was not classifiable as gCJD, GSS, or FFI (see Table 3), and neuropathology was not reported (Zheng et al. 2008). Variants that are most likely benign (largely based on Minikel et al. 2016) are not included (e.g., G54S, P39L, E196A, R208C) (Beck et al. 2010; Minikel et al. 2016). OPRI, Octapeptide repeat insertion; OPRD, octapeptide repeat deletion. (Reprinted from Takada et al. 2017.)
PrP exists in multiple isoforms, two of which are most relevant to PrD, PrPC and PrPSc. Both PrPC and PrPSc isoforms have the same amino acid sequence, but PrPC consists mainly of α-helix, whereas PrPSc has a structure rich in β-sheet (Pan et al. 1993). This conformational difference makes PrPSc insoluble in detergents and relatively resistant to degradation by proteases (Mastrianni 2010; Colby and Prusiner 2011). PrPSc has been shown to be infectious and to self-propagate by acting as a template that induces the conversion of PrPC to PrPSc (Prusiner 1998). Some researchers believe that the accumulation of PrPSc leads to neurodegeneration (Prusiner 2013), whereas others believe that the process of converting PrPC to PrPSc leads to neuronal injury and loss (Mallucci et al. 2007; Moreno et al. 2013). PrPSc propagates as oligomers, which polymerize to form amyloid fibrils (Prusiner 2013). This prion-like pathogenic mechanism may also apply to other neurodegenerative proteinopathies (Colby and Prusiner 2011; Soto 2011; Prusiner 2012; Woerman et al. 2015).
PrPSc in sCJD cases is classified as type 1 or 2; when brain homogenates from patients with PrD are treated with proteinase K (PK) (which digests most of PrPC but leaves behind PrPSc) and run on a Western blot, three bands are shown: diglycosylated, monoglycosylated, and unglycosylated forms of PrPSc. When the molecular weight of the unglycosylated band is 21 kDa, the PrPSc is categorized as type 1; type 2 PrPSc is characterized by a 19-kDa band (Parchi et al. 1996). Only either type 1 or type 2 of PrPSc is found in two-thirds of cases at pathology, but types 1 and 2 are reported to co-occur in about one-third of cases (Parchi et al. 2009). These prion types can be found in many gPrDs, particularly genetic CJD (gCJD), whereas in GSS, there are many more bands, with a prominent band around 7–8 kDa.
At codon 129 in the general population, ∼55% of individuals are homozygous for methionine (MM), 36% are heterozygous (MV), and 9% are homozygous for valine (VV) (1000 Genomes Project Consortium et al. 2012). The polymorphism (rs1799990) affects not only disease susceptibility, but also the clinicopathological features of the genetic, sporadic, and acquired forms of PrD. Homozygosity at codon 129 is a well-established risk factor for sporadic and acquired PrD (Palmer et al. 1991; Mead et al. 2012). For example, in sCJD cohorts from Europe, Australia, Canada, and the United States, 67% are MM, 16% MV, and 17% VV, showing that codon 129 homozygosity is overrepresented (Parchi et al. 1999; Collins et al. 2006).
In gPrD, the cis codon 129 polymorphism usually has greater effect on disease presentation, whereas the trans polymorphism has less or no effect (Capellari et al. 2011). As noted above, the cis codon 129 polymorphism has a strong influence on whether the PRNP D178N mutation presents as gCJD (D178N-129V) or FFI (D178N-129M) (Goldfarb et al. 1992). The codon 129 polymorphism also may influence phenotypes associated with other PRNP mutations (Goldfarb et al. 1992; Capellari et al. 2011). The combination of PrPSc type and PRNP codon 129 polymorphism has been used to molecularly classify the clinicopathological phenotypes of sCJD (for reviews, see Parchi et al. 1999; Brown and Mastrianni 2010; Puoti et al. 2012).
Common PRNP Mutations in Various Cohorts
More than 60 PRNP variants suspected to be pathogenic have been reported in the literature, although several are of questionable pathogenicity. It is highly likely that some variants may be either benign or mutations with low penetrance (Kovács et al. 2005; Minikel et al. 2016). Per data from nine major clinical prion centers, five mutations—namely, E200K, V210I, V180I, D178N, and P102L—are responsible for ∼85% of gPrD cases (Minikel et al. 2016). The first four mutations cause fCJD, whereas P102L is associated with GSS. For as few as four mutations—E200K, D178N, A117V, and P102L—there is very strong evidence of pathogenicity, with the mutation segregating with affected individuals in multiple generations as well as causing spontaneous disease in transgenic mice (Minikel et al. 2016).
Because of misdiagnosis, low penetrance, de novo occurrence, or other factors, many fCJD patients do not have a known family history of PrD (Dagvadorj et al. 2002; Kovács et al. 2005; Krasnianski et al. 2016), and, therefore, we prefer to use the term “genetic CJD (gCJD)” rather than fCJD (Kovács et al. 2005). For the same reason, we prefer the term “gPrD” when referring to any case caused by a PRNP mutation.
A few PRNP mutations have large regional clusters attributable to a founder effect. These include the E200K mutation cluster among Slovakians and another among Sephardic Jews (mostly of Libyan origin but many of whom now are in Israel) (Hsiao et al. 1991a; Lee et al. 1999; Mitrova and Belay 2002); the V180I mutation most commonly found in Japan; and the V210I mutation, which is the most common in Italy (Ladogana et al. 2005; Minikel et al. 2016).
Division into Fast versus Slow Presentations
The historical classification of gPrDs (gCJD, GSS, and FFI), which occurred before the identification of PRNP, has several limitations because nonsense mutations and many OPRIs and an octapeptide repeat deletion (OPRD) do not fit completely into one of these three clinicopathological categories. Therefore, we refer to each PRNP mutation individually and distinguish gPrDs into “Fast” and “Slow” types according to the typical rate of clinical decline and total disease duration. We applied the concept of RPD (dementia with rapid decline and a total disease duration of <3 yr but often <1 yr) (Geschwind 2016) to classifying gPrDs. Fast types of gPrD usually present as RPDs, whereas Slow types have more gradual decline and a total disease duration usually 3 yr or longer. This classification categorizes almost all gCJD, FFI, a few OPRIs (usually those with four or fewer octapeptide repeats) and the single OPRD as Fast, and almost all GSS, most OPRIs (some five to seven OPRI and most octapeptide repeats greater than eight), and all nonsense mutations as Slow. Admittedly, even this classification has limitations. Some OPRIs of the same repeat size can sometimes manifest as Fast and other times as Slow, even rarely within the same family (Goldfarb et al. 1991; Takada et al. 2017). For OPRIs of the same size but in different families, this may result from variations in the pattern of repeats or the location of the repeat within the octapeptide region (Schmitz et al. 2016). We discuss below the features of each mutation starting with the Fast type. We then discuss the Slow type, OPRIs/OPRD, and, lastly, we review the very rare nonsense mutations. To determine the frequency of symptoms, demographics, and test sensitivities for cases of specific PrDs, weighted averages were calculated when data from multiple studies were available.
FAST-TYPE GENETIC PRION DISEASES
Genetic Creutzfeldt–Jakob Disease (gCJD)
The majority of PRNP mutations historically have been associated with gCJD, with clinicopathological features resembling those seen in sCJD (Budka et al. 1995). To our knowledge, 23 missense variants in PRNP have been reported to cause gCJD (P105T, G114V, R148H, D178N [with codon 129 cis V], V180I, T183A, T188A, T188K, T188R, T193I, K194E, E196A, E196K, E200K, E200G, V203I, R208H, V210I, E211Q, I215V, A224V, M232R, and P238S), although for some of these variants, the true pathogenicity is questionable (Gelpi et al. 2015; Minikel et al. 2016; Takada et al. 2017). For example, with some PRNP variants (e.g., M232R), almost no cases have a positive family history despite having older unaffected family members carrying the same PRNP variant. Furthermore, these or other PRNP variants have been found in the Exome Aggregation Consortium (ExAC), or other population genetic data sets, at a much higher than expected frequency for highly penetrant, Mendelian disease–causing variants. Thus, some of these reported mutations are low-penetrance mutations, risk factors for PrD, or even may be benign with no effect on PrD risk (Minikel et al. 2016). The V210I mutation, most frequently reported (and the single most common mutation) in Italy, has an estimated penetrance of only ∼10%. Two of the most commonly reported mutations in Japan, V180I and M232R, even have much lower estimates of penetrance, 1% and 0.1%, respectively (Minikel et al. 2016). Thus, as we discuss below where information is available, the penetrance of each PRNP mutation is variable. Some variants have been reported in only a few patients and are not discussed below, but are only shown in Table 1.
Table 1.
PRNP missense mutations
| PRNP mutation | Codon 129 polymorphism | Number of cases in literaturea | Clinical phenotypes | Age at onset (range)b (yr) | Disease duration (mo or yr) | Pos FHxc | CSF biomarker sensitivity | EEG PSWC | MRI c/w CJDe | Neuropathology | Neuropathological phenotype | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14-3-3 | Total taud | ||||||||||||
| P84S | MV | 1 | Cog (1 yr)→ paranoia and RPD | (60) | (14) mo | 0% (0/1) | N/A | 0% (0/1) | 0% (0/1) | Multic PrP- Plqs w/o NFT No Vac | GSS | Jones et al. 2014 | |
| S97N | cis M | 1 | AD | (72) | N/A | 0% (0/1) | N/A | N/A | 0% (0/1) | N/A | N/A | Zheng et al. 2008 | |
| P102L | MM/MV (most cis M) | ∼221 | Early Cb with late D Some are RPD LE areflexia common |
(27–66) | (7–132) mo | 84–100% | (15%–20%) | 20% | 0%–35% | 25%–30% | Multic PrP-Plqs | GSS | Webb et al. 2008; Higuma et al. 2013; Krasnianski et al. 2016 |
| P102Lf | cis M | 13 | 44 ± 12 (24–57) | 44 ± 13 mo (28–60) | FHx score 0 (n = 1) 1 (n = 2) 2 (n = 6) | 0 % (0/3) | 0% | 0% (0/3) | 33.3% (1/3) | Takada et al. 2017 | |||
| P105L | MV | 13 | D with spastic paraparesis Cb Atx Psych Sxs sometimes |
Mean 44 ± 10 (2nd to 7th decades) | 111 ± 82 mo | 37% | 0% | 0% | 0% | 14% | PrP-Pl, diff PrP (deep CLs) | GSS | Higuma et al. 2013 |
| P105Lf | N/A | 1 | (9) | N/A | FHx score 0 (n = 1) | N/A | N/A | 0% (0/1) | 0% (0/1) | N/A | Takada et al. 2017 | ||
| P105T | cis M | 13 | Usually RPD; Cb Atx freq |
(13–41) | (2–5) yr | 100% (2/2) | 0% | N/A | 0% (0/3) | 25% (1/4) | Vac, PrP-S (all CLs) Unicentric PrP-Plqs (deep CLs) | CJD | Rogaeva et al. 2006; Polymenidou et al. 2011 |
| P105S | MV (cis V) | 1 | Aphasia, frontal- type behav changes, D, late Park | (30) | (10) yr | 0% (0/1) | N/A | N/A | 0% (0/1) | 100% (1/1) | Multic PrP-Plqs (HP), punctate aggregates (Cb), Vac (Pu) | Atypic GSS | Tunnell et al. 2008 |
| G114V | (MM/MV) | 1 | RPD. Onset: Psych Sxs, D, Park, Pyr signs, myoclonus GTCs in some Absent or mild Cb signs |
(18–75) | (1–4) yr | 75% (3 in 4 probands)g | 0% | 0% (0/8) | 42.9% (3/7) predom BG | Mod Vac, G, NL. PrP-S, Type 1 PrPSc (predom monoglycos) | CJD | Rodriguez et al. 2005; Ye et al. 2008; Liu et al. 2010; Beck et al. 2010 | |
| A117V | cis V | 33 | Variable Progressive D w/o Atx LMN SYN with D and Atx |
(20–64) | (1–11 yr) | 100% (4/4) | N/A | N/A | N/A | 0% (0/2) | Ab PrP-Plqs, F Vac, NL, G | GSS | Hsiao et al. 1991b; Mastrianni et al. 1995; Kong et al. 2004 |
| A117Vf | cis V | 6 | 34 ± 14 (14–49) | 46 ± 21 mo (27–78) | FHx score 0 (n = 1) 2 (n = 2) | 0% | 0% | 0% (0/3) | 0% (0/5) | Takada et al. 2017 | |||
| G131V | MM/MV (cis M) | 3 | D with behav changes and late Atx Park |
(36–42) | (9–16) yr | 50% (1/2) | N/A | N/A | 0% (0/1) | 0% (0/1) | PrP-Plqs, NFT (AH, ERC), No Vac | GSS | Panegyres et al. 2001; Jansen et al. 2012 |
| S132I | MM | 2 | RPD | (62) | (18) mo | 100% (1/1) | N/A | N/A | N/A | N/A | diff unicentric and multic PrP-Plqs (neocortex, BG, Cb) Min Vac | GSS | Hilton et al. 2009 |
| A133V | MM | 2 | PSP-like, RPD | (62) | (4) mo | 0% (0/1) | 0% | 0% (0/1) | 0% (0/1) | diff Vac, G, NL multic PrP-Plqs in the Mol, PrP-S (Th) | Atypic GSS | Rowe et al. 2007 | |
| R148H | (MV/MM) | 3 | CJD | (62–82) | (6–18) mo | 0% (0 in 2)g | 50% (1/2) | 100% (1/1) | 50% (1/2) | 100% (1/1) predom BG | 129MM—Similar to sCJDMM1. Vac, G, NL (deeper CLs). PrP-S PrPSc type 1 129MV—similar to sCJDMV2 Vac predom in CLs 5 and 6, Kuru plaques in Cb and WM, PrP-S PrPSc type 2 (predom monoglycos) | CJD | Krebs et al. 2005; Pastore et al. 2005 |
| R148Hf | MM | 1 | (53) | (2) mo | FHx score 0 (n = 1) | N/A | N/A | N/A | 100% (1/1) | Takada et al. 2017 | |||
| D167G | MM | 1 | CJD | N/A | N/A | N/A | N/A | N/A | N/A | N/A | sCJD PrP type 1 | CJD | Bishop et al. 2009 |
| D167N | MM | 1 | RPD with Park and Pyram signs | (33) | (2) yr | 0% (0/1)g | N/A | N/A | 0% (0/1) | 0% (0/1) | N/A | N/A | Beck et al. 2010 |
| V176G | VV | 1 | RPD with behavioral changes, Cb Atx, Pyram signs and myoclonus | (61) | (7) mo | 0% (0/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | Multic PrP-Plqs w/ prominent tau | GSS | Simpson et al. 2013 |
| D178N-129V | MV/VV (cis V) | 209h | Progressive Cog decline, Cb Sxs, myoclonus, EP Sxs | Mean 46 (26–56) | Mean 23 mo (7–60) | 100% (12/12) | N/A | N/A | N/A | N/A | Similar to sCJD VV1 | CJD | Brown et al. 1992a; Goldfarb et al. 1992; Kong et al. 2004 |
| D178N-129Vf | cis V | 8 | 45 ± 6 (37–52) | (18–21) mo | FHx score 2 (n = 4) | 50% (1/2) | 0% (0/1) | 0% (0/2) | 100% (2/2) | Takada et al. 2017 | |||
| D178N-129M | cis M | 106 | Insomnia Sympathetic overactivity |
Mean 52 (∼20–76) | Median 12.6 (4–40) mo | 60%–88% | 14.4 % | 8% | 2.1% | 16.5% | deg of Th (md and av nu) and inf olivary nu, little/no Vac or PrPSc dep | FFI | Collins et al. 2001; Reder et al. 1995; Krasnianski et al. 2016; Zarranz et al. 2005; Sano et al. 2013; Kovács et al. 2005 |
| V180I | cis M | 225 | CJD phenotype, but with slower prog | Mean 77 | Mean 25 mo | 0.7%–6% | 70% | 78.5% | 11% | 99% | Vac PrP-S | CJD | Kong et al. 2004; Higuma et al. 2013 |
| V180If | cis M | 2 | (84) | (21) mo | FHx score 1 (1) 2 (n = 1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | Takada et al. 2017 | ||||
| T183A | cis M | 3 | bvFTD, AD | 45 ± 4 (42–49) | 4 ± 2 yr (2–9) | 100% (2/2) | N/A | N/A | 0% (0/7) | 0% (0/2) | Vac and NL (CLs 4, 5, 6), PrP (Cb, Pu) Small Plq-like PrP, Predom monoglycos PrPSC | CJD | Nitrini et al. 1997; Grasbon-Frodl et al. 2004 |
| H187R | MM/MV/VV | 7 | Early Cog and behavioral Sxs with late Cb Atx Early Cb Atx and D after a few years Case with Psych Sxs in adolescence |
(20–53) | (3–19) yr | 100% (4/4) | 0% (0/2) | 0% (0/4) | 0% (0/4) | G multic-PrP-Plqs in some cases. Curly PrP-G | GSS | Cervenáková et al. 1999; Bütefisch et al. 2000; Hall et al. 2005; Colucci et al. 2006 | |
| H187Rf | cis M | 4 | (30–41) | (12–13) yr | FHx score 2 (1) | 0% (0/1) | 0% (0/1) | 0% (0/1) | 0% (0/1) | Takada et al. 2017 | |||
| T188R | MV/VV (cis V) | 12 | CJD | (55–66) | (14–16) mo | 0% (0/1)g | 50% (1/2) | 100% (1/1) | 50% (1/2) | 50% (1/2) | Vac, NL, A, PrP-S and plaque-like PrP, Type 1 PrPSc | CJD | Tartaglia et al. 2010; Roeber et al. 2008 |
| T188K | MM/MV (cis M) | 3 | CJD | Median 58 (39–76) | (2–13) mo | 8%–37%g | 69% | 12% | 69% | SE PrP-S | CJD | Roeber et al. 2008; Chen et al. 2013; Shi et al. 2015 | |
| T188A | MM | 1 | CJD | (82) | (4) mo | 0% (0/1) | 100% (1/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | Sev G, Vac, Mod NL (predom in OLs) PrP-Neg | CJD | Collins et al. 2000 |
| T193I | MM | CJD | (70) | (10) mo | 0% (0/1) | 100% (1/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | N/A | N/A | Kotta et al. 2006 | |
| E196K | N/A | 13 | CJD | (64–69) | (10–13) mo | 100% (1/1) | N/A | N/A | 0% (0/1) | N/A | N/A | N/A | Peoc’h et al. 2000 |
| F198S | MV/VV | 5 | Cb Atx and D freq Park |
(40–71) | Mean 5 yr (2–12) | 100% (3/3) | N/A | N/A | N/A | 0% (0/1) | Uni- and multic PrP-Plqs | GSS | Farlow et al. 1989; Dlouhy et al. 1992; Ghetti et al. 1995; Kong et al. 2004 |
| F198Sf | cis V | 5 | 55 ± 8 (46–66) | 67 ± 23 mo (34–84) | FHx score 1 (n = 2) 2 (n = 1) | 33.3% (1/3) | 100% (1/1) | 0% (0/4) | 0% (0/3) | Takada et al. 2017 | |||
| F198V | MM | 1 | D with visual hallucinations, myoclonus, and Park Clinical dx of early-onset AD |
(56) | (4) yr | N/A | N/A | N/A | 0% (0/1) | 0% (0/1) | N/A | N/A | Zheng et al. 2008 |
| E200K | MM/MV/VV | 571 | Similar to sCJD Peripheral neuropathy and supranuclear gaze palsy in some |
Mean 60 (33–84) | (1–18) mo | 50% | 85%–100% | 80%–100% | 42%–85% | 50%–88% | Usually sCJD MM1 PrPSc types 1 and 2 | CJD | Spudich et al. 1995; Meiner et al. 1997; Kovács et al. 2005, 2011; Krasnianski et al. 2016 |
| E200Kf | cis M (n = 16) and cis V (n = 1) | 34 | 60 ± 13 (36–84) | 11 ± 17 (1–78) mo | FHx score 0 (n = 2) 1 (n = 10) 2 (n = 12) | 57.1% (4/7) | 37.5% (3/8) | 88.9% (16/18) | Takada et al. 2017 | ||||
| E200G | MV (cis V) | 1 | RPD, Cb Atx, Park ↓sensation in LEs | (57) | (30) mo | 0% (0/1) | 0% (0/1) | 100% (1/1) | 0% (0/1) | 100% (1/1) | SE w/ type 2 PrPSc | CJD | Kim et al. 2013 |
| D202G | MV (cis V) | 1 | Slowly progressive D with Cb Atx Later Pyram and EP signs | (55) | (16) yr | 100% (1/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | 0% (0/1) | N/A | N/A | Heinemann et al. 2008 |
| D202N | VV | 1 | D (AD) with Cb Atx | (73) | (6) yr | N/A | N/A | N/A | N/A | N/A | PrP-Plqs, NFT | GSS | Piccardo et al. 1998 |
| V203I | N/A | 17 | CJD | (69) | (1) mo | 0% (0/1) | N/A | N/A | 100% (1/1) | N/A | N/A | N/A | Peoc’h et al. 2000 |
| R208H | MM/VV | 15 | D with behav changes Park and Pyram signs freq Report of a PSP-like phenotype |
(58–63) | (3–16) mo | 20% (1/5) | 50% (4/8) | 57.1% (4/7) | 25% (2/8) | SE, PrP-S (perineuronal perivacuolar) Type 1 PrP | CJD | Roeber et al. 2005; Capellari et al. 2005; Matej et al. 2012; Vita et al. 2013; Shi et al. 2015 | |
| V210I | cis M | 247 | CJD | Mean 59 (39–82) | Median 5 (2–20) mo | 12%–31% | 90%–100% | 100% | 44%–80% | 15%–33% | Similar to sCJD MM1 | CJD | Kovács et al. 2005; Kong et al. 2004; Breithaupt et al. 2013 Krasnianski et al. 2016 |
| V210If | cis M | 3 | 57 ± 15 (47–74) | (1) mo | FHx score 0 (n = 2) 2 (n = 1) | 100% (1/1) | 33.3% (1/3) | 100% (2/2) | Takada et al. 2017 | ||||
| E211Q | MM | 11 | CJD | (42–81) | (6–32) mo | 100% (2/2) | N/A | N/A | 100% (4/4) | N/A | Vac, G Mi PrP-S types 1 and 2 PrPSc | CJD | Peoc’h et al. 2000, 2012; Ladogana et al. 2001 |
| E211D | VV | 1 | Cb Atx, and late D | (53–68) | (3–13) yr | 50% (1/2) | N/A | N/A | 0% (0/2) | 0% (0/2) | Multic PrP-Plqs, dystrophic neurites, and NFT | GSS | Peoc’h et al. 2000, 2012 |
| Q212P | MM | 2 | Cb Atx w/o D Dx of olivoponto Cb degeneration |
(60) | (8) yr | N/A | N/A | N/A | N/A | N/A | Mod PrP Mi, PrP-Plqs | GSS | Piccardo et al. 1998 |
| I215V | MM | 1 | CJD | (55–76) | (12–15) mo | 0% (0/2) | (1/3) | 100% (2/2) | 50% (1/2) | NL, G, Vac, PrP-Neg | CJD | Muñoz-Nieto et al. 2013 | |
| Q217R | VV/MV (cis V) | 3 | D with Cb Atx Cog decline, stereotypical behav Late Park and apraxia. Clinical dx of bvFTD and CBS |
(45–66) | (5–13) yr | 100% (3/3) | N/A | N/A | 0% (0/1) | 0% (0/1) | Uni- and multic PrP-Plqs, NFT (neocortex) | GSS | Hsiao et al. 1992; Woulfe et al. 2005; Piccardo et al. 1998 |
| Y218N | VV | 1 | Atypic D with AD and bvFTD features Early language and executive impairment No Atx |
(54–61) | (6) yr | 100% (1/1) | N/A | N/A | 0% (0/2) | 0% (0/2) | Uni and multic PrP-Plqs, NFT w/hyperP tau | GSS | Alzualde et al. 2010 |
| A224V | VV (cis V) | 1 | RPD | (48) | (32) mo | 0% (0/1)g | 100% (1/1) | N/A | 100% (1/1) | Diff Vac w/ PrPSc type 1 | CJD | Watts et al. 2015 | |
| M232R | MM | 63 | Similar to sCJD, some with slower prog | Mean 64 (15–81) | Mean 8 (0–32) mo | ∼0% | 55%–75% | 55%–93% | 20%– 100% | 85% | sCJD MM1 | CJD | Shiga et al. 2007; Zheng et al. 2008; Nozaki et al. 2010; Higuma et al. 2013 |
| M232T | MV | Cb Atx, spastic paraparesis and D | N/A | (6) yr | 0% (0/1) | N/A | N/A | N/A | N/A | Multic PrP-Plqs | GSS | Bratosiewicz et al. 2000 | |
| P238S | N/A | CJD | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Windl et al. 1999 | |
A, astrocytosis; Ab, abundant; AD, Alzheimer-type dementia; AH, Ammon horn; atypic, atypical; Atx, ataxia; av, anteroventral; BG, basal ganglia; bvFTD, behavioral variant frontotemporal dementia; c/w, consistent with; Cb, cerebellum; CBS, corticobasal syndrome; CJD, Creutzfeldt–Jakob disease; CLs, cortical layers; Cog, cognitive; CSF, cerebrospinal fluid; D, dementia; deg, degeneration; dep, deposition; diff, diffuse; dx, diagnosis; EEG, electroencephalogram; EP, extrapyramidic; ERC, entorhinal cortex; EP, extrapyramidal; FHx, family history; freq, frequent; G, gliosis; GSS, Gerstmann–Sträussler–Scheinker disease; GTC, generalized tonic clonic seizures; HP, hippocampus; HyperP, hyperphosphorylated; inf, inferior; LE, lower extremities; LMN, lower motor neuron; M, methionine; md, mediodorsal; Mi, mild; Min, minimal; mo, months; Mod, moderate; Mol, molecular layer of the cerebellum; monoglycos, monoglycosylated; MRI, magnetic resonance imaging; multic, multicentric; N/A, not available; Neuropath, Neuropathology; NFT, neurofibrillary tangles; NL, neuronal loss; nu, nuclei; OLs, occipital lobes; Park, parkinsonism; Pos, positive; prog, progression; predom, predominant; PrP-G, granular PrP deposits; PrP-Neg, negative PrP staining; PrP-Plqs, PrP-amyloid plaques; PrP-S, synaptic PrP deposits; PSP, progressive supranuclear palsy; PSWC, periodic sharp wave complexes; Psych, psychiatric; Pu, putamen; Pyram, pyramidal; RPD, rapidly progressive dementia; SE, spongiform (vacuolated) encephalopathy; Sev, severe; Sxs, symptoms; SYN, syndrome; Th, thalamus; Vac, vacuolation; VV, homozygous valine; w/, with; w/o, without; WM, white matter; yr, years.
aBy nine PrD surveillance centers, according to Minikel et al. (2016).
bData on age at onset and duration of disease are shown as mean ± SD (range), unless otherwise indicated.
cPositive family history of dementia with similar clinical features (as of the proband) or PrD. For UCSF family history FHx (family history). Score scale: 0, when there was no positive FHx suspicious for or known PrD; 1, when there was at least one first-degree relative with dementia, encephalopathy, or movement disorder; or 2, in patients who were part of families with known PRNP mutations or who had positive history for clinical or pathology-proven PrDs.
dIn most laboratories, Total tau is considered positive for prion disease if greater than 1150 or 1200 pg/mL.
eAccording to most commonly used European 2009 and UCSF 2011 criteria (Zerr et al. 2009; Vitali et al. 2011).
fIf there are differences between data published in the literature from the more recently published UCSF cohort, this information is provided in the table separately for that mutation.
gThere is evidence of incomplete penetrance, as asymptomatic older carriers also were identified.
hIncluding D178N-129V and D178N-129M.
Notably, a large European study using the EuroCJD database (including Australia, Austria, Canada, France, Germany, Italy, The Netherlands, Slovakia, Spain, Switzerland, and the United Kingdom) reported that 47% of patients with gPrD (i.e., diagnosed with PrD and found to have a PRNP mutation) did not have a positive family history of PrD or other neurological disorder. For GSS alone, only about one-third of subjects had no family history of PrD or other neurological disorder (Kovács et al. 2005). In some cases, more detailed evaluations of the family history reveal dementia or neuropsychiatric illness, which was likely misdiagnosed (Goldman et al. 2004); however, in many cases, negative family histories were due to incomplete penetrance, early death, or less likely de novo mutations or nonpaternity (Mitrova and Belay 2002; Kovács et al. 2005).
Per the EuroCJD database, the mean age of gCJD onset is ∼60 yr but shows great variability, ranging from the second to ninth decades (Kovács et al. 2005). This is ∼7 yr younger than for sCJD (Brown et al. 1986; Parchi et al. 1999; Collins et al. 2006). The median disease duration of gCJD is ∼5 mo, similar to that for sCJD, but also shows great variability—at least 75% of gCJD patients die in <20 mo, but total durations of >8 yr have been reported (Brown et al. 1986; Parchi et al. 1999; Kovács et al. 2002, 2005).
Dementia is reported in ∼95%–98% of patients with gCJD (Meiner et al. 1997; Kovács et al. 2002). Cerebellar symptoms and myoclonus are also quite common, being reported in ∼70% and 60%–70% of patients, respectively (Meiner et al. 1997; Kovács et al. 2002). About half of gCJD patients have extrapyramidal signs and one-quarter have psychiatric symptoms (Kovács et al. 2002). The frequency of these features varies considerably depending on the specific mutation. Regarding ancillary testing, the diagnostic sensitivities of cerebrospinal fluid (CSF) markers, electroencephalogram (EEG), and brain magnetic resonance imaging (MRI) scans are lower in gCJD cases than in sCJD cases overall (Kovács et al. 2002, 2005).
We discuss below the features of the most common gCJD-associated PRNP mutations—E200K, D178N-129V, V210I, V180I, and M232R—along with three recently reported putative mutations—K194E, E200G, and A224V. Additionally, Table 1 summarizes some key features of other Fast gCJD mutations.
E200K
E200K is both the most common gCJD and the most common PRNP mutation worldwide (Lee et al. 1999; Kovács et al. 2005; Minikel et al. 2016). There are at least four ancestral clusters, including the two largest among Sephardic Jews (and their ancestors) and a Slovakian cohort. Other cohorts from Europe (Germany, Sicily, and Austria) and Japan have also been reported (Meiner et al. 1997; Lee et al. 1999; Mitrova and Belay 2002). Most E200K cases have cis codon 129 methionine (E200K-129M) (Kim et al. 2013).
The E200K mutation is highly, but by no means completely, penetrant. Penetrance appears to vary by geographic origin. Among Sephardic E200K mutation carriers, penetrance is 70% at age 70 yr and close to 100% at age 85 (Spudich et al. 1995). Although as many as 50% of E200K patients do not have a family history of PrD (Spudich et al. 1995; Kovács et al. 2005; Higuma et al. 2013), in our experience, however, there sometimes is a family history, but this information has not been shared outside of the immediate family (e.g., between family branches) or there have been misdiagnoses. In some cases, however, there is reduced penetrance of the mutation. In Slovakia, penetrance has been reported to be much lower, ∼60%, than for Sephardic Jews (Mitrova and Belay 2002). A few reports suggest that anticipation occurs in this mutation (Rosenmann et al. 1999; Pocchiari et al. 2013), but a recent study involving several larger international cohorts showed that the anticipation reported by earlier studies was a false signal most likely from ascertainment bias (Minikel et al. 2014).
Overall, the clinical presentation of gCJD with the E200K mutation is similar to that of sCJD (Meiner et al. 1997; Kovács et al. 2005). The mean age of symptom onset is ∼60 yr, a few years less than for sCJD, but there is a wide range (33–84 yr) (Meiner et al. 1997; Mitrova and Belay 2002; Kovács et al. 2005; Begue et al. 2011; Breithaupt et al. 2013; Higuma et al. 2013; Sano et al. 2013; Krasnianski et al. 2016; Takada et al. 2017). The median disease duration is ∼5–6 mo (Kovács et al. 2005; Begue et al. 2011; Breithaupt et al. 2013; Krasnianski et al. 2016; Takada et al. 2017).
In most E200K mutation carriers of Sephardic origin, symptom onset occurs gradually over a few weeks to months; in ∼10% of cases, however, onset is reported as acute, occurring over days. The most common symptom in (and first in about half of) E200K Sephardic and European cohorts is cognitive decline (Meiner et al. 1997; Krasnianski et al. 2016). In the German cohort, other first symptoms are cerebellar ataxia (30%), sensory (15%), and constitutional/vegetative symptoms (e.g., hyperthermia, loss of weight, and hyperhidrosis; 5%) (Krasnianski et al. 2016).
The frequency of various symptoms varies between published E200K cohorts. Based on combined data from several published cohorts from Germany, Israel, Japan, and a European consortium (including Canada and Australia), a majority of patients develop dementia (mean ∼95%), cerebellar ataxia (mean ∼80%), myoclonus (∼74%), pyramidal signs (∼61%) (Meiner et al. 1997; Kovács et al. 2011; Higuma et al. 2013; Krasnianski et al. 2016), and psychiatric symptoms (including hallucinations, depression, delusions, and aggressiveness; 58%) (Kovács et al. 2011; Krasnianski et al. 2016) during the clinical course. Less common signs include extrapyramidal features (mean ∼41%; range 21%–65%) (Meiner et al. 1997; Kovács et al. 2011; Krasnianski et al. 2016), chorea/dystonia (mean ∼33%; range 25%–43%) (Kovács et al. 2011; Krasnianski et al. 2016), and insomnia (mean ∼26%) (Meiner et al. 1997; Kovács et al. 2011; Krasnianski et al. 2016). Insomnia with thalamic degeneration was reported in one case (Meiner et al. 1997).
Although E200K gCJD and sCJD often present similarly, there are differences between the diseases in addition to the earlier median onset of E200K gCJD. Seizures are more common in E200K patients, occurring in ∼20%–35% of cases (Meiner et al. 1997; Krasnianski et al. 2016). This is much higher than in sCJD patients. Supranuclear gaze palsy and peripheral neuropathy, which are very rare in sCJD patients, have been reported in some E200K cases (Bertoni et al. 1992; Neufeld et al. 1992; Meiner et al. 1997; Kovács et al. 2011). Moreover, headaches are found in 20%–30% of patients with the E200K mutation (Meiner et al. 1997; Krasnianski et al. 2016), which is about double reported for sCJD cases (Brown et al. 1979, 1994).
The data on whether or not codon 129 influences the clinical presentation of E200K gCJD are controversial and currently inconclusive (Kim et al. 2013; Minikel et al. 2014). Most E200K patients are cis 129M (Capellari et al. 2011). One study comparing 21 MM and 14 MV (cis M) European cases reported some differences (not statistically analyzed): MM cases have about double rates of myoclonus, behavioral symptoms, and gaze palsy, and MV carriers have at least double rates of rigidity/parkinsonism and polyneuropathy (Kovács et al. 2011).
Investigating the effect of codon 129 cis M versus cis V in the U.S. National Prion Disease Pathology Surveillance Center E200K cohort, cis 129V cases were found to have a mean age at onset of 55 ± 11 yr (range 37–67) and a mean disease duration of 9 ± 4 mo (range 2–17) compared with 60 ± 10 yr (range 40–85) and 5 ± 4.5 mo (range 1–29), respectively, in cis 129M patients (Kim et al. 2013). There was a statistically significant difference only in disease duration, possibly suggesting that cis V cases have a longer duration. A study involving four large international E200K cohorts, however, showed that the codon 129 polymorphism affected disease duration (shorter in 129MM [cis M] than in 129MV [cis M] individuals and shorter in 129M haplotype than in 129V haplotype) but not age of onset or death (Minikel et al. 2014).
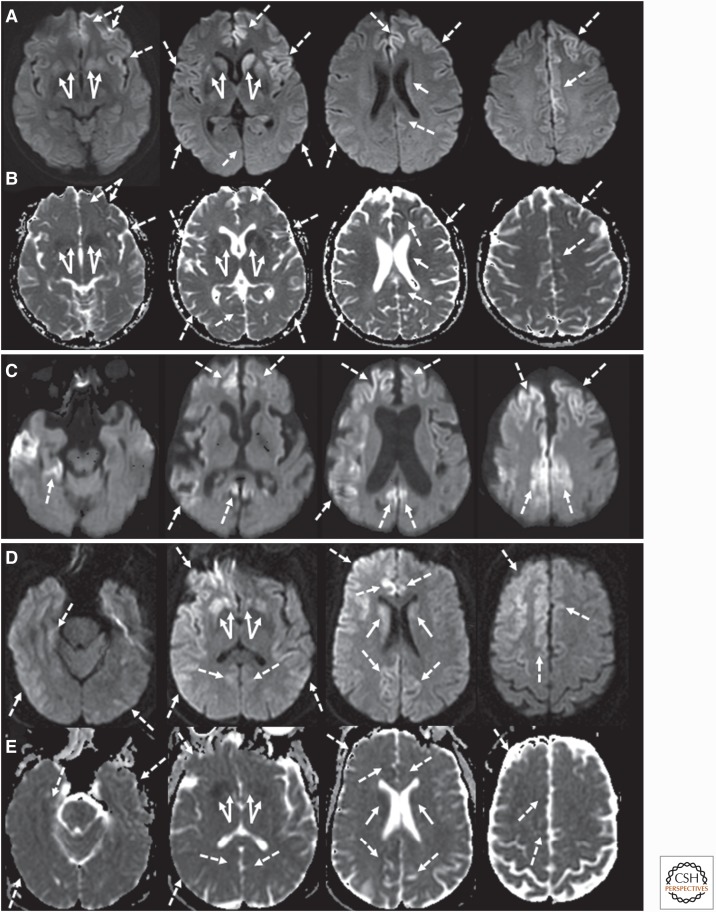
Although data for CSF total tau test are fewer, it appears to be more sensitive than the CSF 14-3-3 test (88.5% vs. 73%, respectively) in E200K carriers (Kovács et al. 2005, 2011; Breithaupt et al. 2013; Higuma et al. 2013; Sano et al. 2013; Krasnianski et al. 2016). The CSF real-time quaking-induced conversion (RT-QuIC) assay in Japanese E200K patients has an average sensitivity of 83% (Higuma et al. 2013; Sano et al. 2013). Periodic sharp-wave complexes (PSWCs) on EEG have been reported in 55% of E200K cases (Kovács et al. 2005, 2011; Breithaupt et al. 2013; Krasnianski et al. 2016). Historically, the frequency of positive MRI scans has been typically underreported because in many studies, more sensitive sequences for prion disease—such as diffusion weighted imaging (DWI) and attenuation diffusion coefficient (ADC) maps—were not used. Furthermore, often only deep nuclei hyperintensities were considered, whereas cortical ribboning (hyperintensity on DWI or fluid-attenuated inversion recovery [FLAIR] MRI) is a more common, but sometimes more subtle, finding. Additionally, deep nuclei and particularly cortical ribboning abnormalties can be difficult to see on T2/FLAIR sequences alone. Nevertheless, deep nuclei hyperintensity on DWI or FLAIR MRI is reported in 40%–77% of E200K patients (Kovács et al. 2005; Krasnianski et al. 2016). If cortical hyperintensities are also taken into account, MRI positivity increases to at least 84%–89% (Breithaupt et al. 2013; Takada et al. 2017). In our cohort of 18 symptomatic E200K cases (a combination of Sephardic Jewish or Slovakian origin) at the UCSF Memory and Aging Center, 89% had restricted diffusion abnormalities—72% had both cortical ribboning and deep nuclei restricted diffusion (bright on DWI and dark on ADC map) (Fig. 2A,B), and ∼17% had predominantly isolated deep nuclei abnormalities (Takada et al. 2017).
Figure 2.
Brain magnetic resonance images (MRIs) in Fast forms of gPrD. (A) Diffusion weighted imaging (DWI) MRI of a genetic Creutzfeldt–Jakob disease (gCJD) 47-yr-old E200K patient 3 mo after onset shows diffuse cortical hyperintensity (cortical ribboning; dashed arrows) of the bilateral frontal and insula (left to right) and temporoparietal cortices. There was also DWI hyperintensity in the bilateral striata (left to right, solid arrows). (B) Attenuation diffusion coefficient (ADC) map of the same gCJD E200K case showed hypointensity in most of the regions that were hyperintense on DWI, confirming reduced diffusion, consistent with prion disease. (C) DWI MRI of an 85-yr-old V180I case 11 mo after onset shows diffuse cortical atrophy and cortical hyperintensity (cortical ribboning; dashed arrows) of the bilateral frontal and cingulate (right to left) and the right temporal and parietal lobes. There was no clear abnormality in the deep nuclei. (D) DWI in a 49-year-old gCJD A224V case 11 mo after the onset shows diffuse cortical ribboning (dashed arrows) greater on the right than the left and hyperintensity in the bilateral deep nuclei (solid arrows) also greater in the right. (E) The ADC map of the same gCJD A224V case showed hypointensity in most of the regions that were bright on DWI, confirming restricted diffusion, which is consistent with CJD/prion disease. Orientation is radiological (left brain is right side of figure). (Reprinted from Takada et al. 2017.)
Overall, the neuropathological findings in E200K cases are close to those found in sCJD MM1 patients, including vacuolation, astrocytic gliosis, and loss of neurons. In E200K patients, however, neuronal loss is more severe in the neocortex, basal ganglia, and thalami (Kovács et al. 2011; Higuma et al. 2013). Most E200K-129M cases have PrPSc type 1, but cases with PrPSc types 1 and 2 have been reported. PrPSc type 2 was reported in the few E200K-129V cases (Puoti et al. 2000; Kim et al. 2013). PrPSc deposition is predominantly synaptic in almost all E200K cases, but perineuronal and intraneuronal deposits also have been reported (Kovács et al. 2011). Curiously, plaque-like deposits are more frequent in codon 129 MV than MM cases (Mitrova and Belay 2002; Kovács et al. 2011). Regarding glycosylation of PrPSc, the diglycosylated form appears to be increased and the unglycosylated PrPSc decreased in E200K mutation carriers compared with sCJD cases (Kovács et al. 2011).
D178N-129V
The D178N mutation with valine at the cis codon 129 is usually associated with the CJD phenotype, whereas cis methionine at codon 129 is associated with the FFI phenotype (Goldfarb et al. 1992). There are exceptions, however, as mentioned in the “Introduction” and discussed in “FFI” below. D178N-129MM presents clinicopathologically as CJD, and even within families, both phenotypes (FFI and CJD) have appeared (Zerr et al. 1998; Zarranz et al. 2005).
The mean age of onset of CJD cases caused by D178N-129V mutation is 46 yr, which ranges from the second to eighth decades (Brown et al. 1992a; Goldfarb et al. 1992), and the mean disease duration is 23 mo (range 7–60 mo), which is longer compared with sCJD (Brown et al. 1992a; Goldfarb et al. 1992). One study suggests that the codon 129VV genotype might be associated with earlier onset and shorter disease duration than the 129MV genotype (Goldfarb et al. 1992), which would be consistent with the notion that codon 129 homozygosity is not only a risk factor but also may affect the clinical course of disease.
Some of the strongest clinical data on the D178N mutation is from a series of 43 cases in 1992. These data were gathered, however, before the effect of cis codon 129 on the D178N mutation was known (Goldfarb et al. 1992); thus, it is not clear if all of these cases were D178N-129V. Nevertheless, given their phenotype, it is likely that most were 129V (Brown et al. 1992a). Cognitive decline (particularly memory) was the initial symptom in most of the cases (95%), and dementia occurred in almost all of the patients. At disease onset, behavioral changes were reported in 30% of the cases and cerebellar symptoms in 21%. During the disease course, cerebellar symptoms developed in 86%, myoclonus in 74%, extrapyramidal symptoms in 67%, pyramidal symptoms and signs in about half of patients, and seizures in 12% of cases (Brown et al. 1992a). Few, if any, had PSWCs on EEG (Brown et al. 1992a; Nozaki et al. 2010). To our knowledge, there are no sufficiently large cohort data on MRI and CSF findings in D178N-129V cases.
The neuropathological findings of D178N-129V cases are similar to those reported in sCJD VV1—spongiosis and neuronal loss are more severe in the neocortex, whereas they are very mild in the thalamus and spared in the cerebellum. PrPSc immunostaining reveals weakly stained synaptic-type deposits. PrPSc type 1 with decreased unglycosylated form is usually observed in this mutation (Gambetti et al. 2003b).
Low-Penetrance Mutations
The role of V180I, V210I, and M232R in causing PrD is controversial; they are likely low- to very low-penetrance mutations, as mentioned above (Minikel et al. 2016).
V180I
The V180I mutation has mostly been reported in Japan (Nozaki et al. 2010; Minikel et al. 2016), although there is one Korean case (Yang et al. 2010) and a few Caucasian cases (Nixon et al. 2000; Chasseigneaux et al. 2006; Takada et al. 2017). Most cases have no positive family history of PrD or other neurodegenerative disease. It is estimated that this mutation has very low penetrance, ∼1% (Minikel et al. 2016). Most V180I cases begin in late adulthood and progress slowly; the mean age of onset is ∼76.5 yr, and the mean duration of disease is ∼25 mo (Yang et al. 2010; Higuma et al. 2013; Qina et al. 2014). A positive family history for PrD or other neurodegenerative disease is low at ∼6% (Higuma et al. 2013; Qina et al. 2014). The most common symptoms in decreasing order among Japanese V180I cases are dementia (100%), extrapyramidal signs (53%), psychiatric disorder (52%), pyramidal signs (50%), akinetic mutism (54%), myoclonus (35%), and cerebellar dysfunction (34%).
Regarding ancillary testing in the Japanese V180I cases, MRI is positive in 99%, PSWCs on EEG are reported in 7%, CSF 14-3-3 protein are positive in 87%, total tau in 91%, and PrPSc by RT-QuIC assay in 68%. The majority of these reported Japanese cases are codon 129MM (75.5% of 184), with the remainder being 129MV (Qina et al. 2014). Neuropathology shows vacuolation in the cortex and weakly staining PrPSc deposits of the synaptic type (Higuma et al. 2013). The three known Caucasian V180I carriers (all cis 129 M) all presented with generally similar clinicopathological phenotypes to the Japanese cases (Nixon et al. 2000; Chasseigneaux et al. 2006; Takada et al. 2017).
V210I
The V210I mutation, the most common in Italy (Ladogana et al. 2005; Minikel et al. 2016), is not fully Mendelian; it is either a risk factor or of low penetrance, ∼10% (Minikel et al. 2016). Clinically, V210I cases present similarly to sCJD MM1 cases (Kovács et al. 2005; Capellari et al. 2011). The mean age at onset is ∼60 yr (range 39–82) (Kovács et al. 2005; Krasnianski et al. 2016), and the median disease duration is ∼5 mo (range 2–20) (Krasnianski et al. 2016). In the EuroCJD database, only ∼12% of 69 patients with the V210I mutation had a family history for PrD or other neurological disorders (Kovács et al. 2005), although a smaller more recent study from Germany found 13 of 91 gPrD cases to be V201I, and of these 13, 31% had a positive family history (Krasnianski et al. 2016). The most common initial symptom in the German cohort of 13 cases was dementia (38%), followed by ataxia, vertigo, and visual disturbances (each 15%), and then sleep and sensory (each 8%) problems. Symptoms throughout the disease course in decreasing frequency included ataxia (100%), dementia, extrapyramidal signs and myoclonus (each 92%), visual/oculomotor difficulty (85%), sensory problems (77%), pyramidal signs (71%), vertigo (61.5%), headache (42%), hemianopsia, and seizures (each 38.5%) (Krasnianski et al. 2016).
Regarding ancillary testing, MRIs are reported as positive in only 15%–33% of cases, however, DWI and ADC map, which are much more sensitive than T2/FLAIR sequences, apparently were not included in most cases (Kovács et al. 2005; Breithaupt et al. 2013; Krasnianski et al. 2016). The presence of PSWCs on EEG is more variable, only being reported in 44%–80% of cases (Kovács et al. 2005; Breithaupt et al. 2013; Krasnianski et al. 2016). In contrast, among three study cohorts (EUROCJD, Germany/Argentina, and Germany) two CSF biomarkers are highly positive: 14-3-3 positivity occurred in ∼90%–100% of cases (Kovács et al. 2005; Breithaupt et al. 2013; Krasnianski et al. 2016), and CSF total tau is 100% in the German cohort (Krasnianski et al. 2016).
M232R
The M232R mutation is one of the four most common gPrD mutations reported in Japan and primarily has been reported in CJD cases from Japan, China, and Korea (Zheng et al. 2008; Choi et al. 2009; Nozaki et al. 2010; Minikel et al. 2016). Its pathogenicity as a fully penetrant Mendelian mutation is unlikely, as it primarily has been reported in CJD cases with no family history and identified in patients with dementia but in whom neuropathology showed no evidence of PrD. Moreover, it has been reported in older healthy individuals and in individuals with some other pathologically proven neurodegenerative disease. It is likely either a risk factor or a low-penetrance mutation (Shiga et al. 2007; Beck et al. 2012; Higuma et al. 2013; Minikel et al. 2016).
The clinical presentation of M232R is very similar to that of sCJD, but Japanese researchers have reported two different clinicopathological subtypes, which they have termed as a “Rapid” (∼75% of cases) and a “Slow” (25% of cases) type (Shiga et al. 2007). Age at onset in the Rapid versus Slow group showed considerable overlap (mean of 66.2 ± 7.7 SE years vs. 57.6 ± 15.4 SE years in the Rapid vs. Slow group, respectfully). Between the Rapid and Slow subtypes, however, the disease durations until either onset of akinetic-mutism (3.1 ± 1.5 mo Rapid vs. 20.6 ± 4.4 mo Slow) (Shiga et al. 2007) or until death (12.5 ± 10.9 mo Rapid vs. 49.9 ± 53.8 for Slow) were quite different (Higuma et al. 2013).
MRI findings consistent with CJD are reported in ∼85% of patients and are similar in both Rapid and Slow groups (Shiga et al. 2007; Nozaki et al. 2010). Positivities for other biomarkers such as PSWCs on EEG (100% in the Rapid type vs. 20% in the Slow type), CSF 14-3-3 (75% Rapid vs. 55% Slow), CSF total tau (93% Rapid vs. 55% Slow), and CSF RT-QuIC for PrPSc (78% Rapid vs. 60% Slow) are all higher in the Rapid type (Higuma et al. 2013).
Interestingly, the neuropathological findings in the M232R Rapid type group are similar to those in sCJD MM1 patients, with type 1 PrPSc and synaptic-type deposits, whereas in the Slow type group, the findings are similar to sCJD MM2-cortical cases, with type 2 PrPSc, perivacuolar deposits, and spongiform changes (Parchi et al. 1999; Shiga et al. 2007; Higuma et al. 2013). These findings suggest that M232R is within the spectrum of sCJD and may just be a risk factor.
T188R
To our knowledge, only two patients with the T188R mutation have been reported. One presented with visual symptoms and later dementia and ataxia, whereas the other developed personality changes followed by rapid cognitive decline. They survived 14 and 16 mo, and neither had a positive family history (Roeber et al. 2008; Tartaglia et al. 2010). The pathogenic role of the T188R mutation is not yet clear (Minikel et al. 2016), although the substitution of threonine with a highly basic amino acid, arginine, may result in structural destabilization. Cell culture studies show that PrPSc from the T188R mutation has increased resistance to proteinase K digestion, a hallmark of prion diseases (Prusiner 1998), further suggesting the mutation might be pathogenic (Lorenz et al. 2002). Even if it is a causative mutation, however, and not just a risk factor, it is not 100% penetrant (Tartaglia et al. 2010).
K194E
Only a single case of the novel K194E PRNP mutation has been reported. The mutation occurred in a 71-year-old Caucasian man with progressive memory loss, psychiatric symptoms, and motor problems (parkinsonism and gait instability) who died 11 mo after onset with a positive MRI and EEG (Takada et al. 2017). The pathogenic role of the K194E mutation in CJD is not yet clear, but the mutation location at the end of helix 2 of PrP might affect the correct folding of PrPC. Furthermore, this mutation is a rare genetic variation as it was not identified in the 1000 Genomes Project database (http://www.internationalgenome.org/) (1000 Genomes Project Consortium et al. 2012) or in the 60,000 exomes in the Exome Aggregation Consortium database (Minikel et al. 2016; E Minikel, pers. comm.). Lastly, PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) predicts this variation to be “possibly damaging,” MutationTaster (http://www.mutationtaster.org/) predicts it to be “disease causing,” and the SIFT algorithm (http://sift.jcvi.org/) suggests it is “damaging.”
E200G
An E200G (codon 129MV, cis V) mutation was recently reported in a symptomatic 57-yr-old Caucasian woman without a family history of PrD who initially developed gait problems, followed 6 mo later by dysphasia. She eventually developed parkinsonism and cerebellar ataxia and died 30 mo after symptom onset. Her MRI results and CSF total tau level were consistent with CJD. Additionally, her neuropathology was consistent with CJD, showed PrPSc type 2, and was similar to E200K cases, displaying a small amount of unglycosylated PrPSc on Western blot. Further supporting the mutation to be familial, the patient’s father died at age 50 from an RPD diagnosed as alcohol-related dementia, which was likely a misdiagnosis (Kim et al. 2013).
A224V
A novel A224V mutation (codon 129VV) was identified in a single 48-yr-old patient with rapid onset of short-term memory difficulties, followed by gait and other motor problems, who died 32 mo after onset (the patient’s long survival time was likely due to an extraordinarily high level of care). Brain MRI, CSF neuron-specific enolase (NSE), and total tau (6300 pg/mL; >1200 consistent with CJD) were all positive. Neuropathology was consistent with CJD with type 1 PrPSc. Although the family history was negative for PrD, the patient’s father, in his mid-80s, had slowly progressive clinical Alzheimer’s disease (AD) and the same mutation but with codon 129 MV. Thus, the mutation, if pathogenic, is likely not 100% penetrant, although in animal models, it did shorten the incubation period, suggesting an effect on PrPSc (Watts et al. 2015).
Additional mutations causing various forms of fast-type PrD, particularly gCJD, are summarized in Table 1.
Fatal Familial Insomnia (FFI)
D178N-129M
The D178N-129M mutation (FFI) is the third most common PRNP mutation causing gPrD worldwide. In Germany, it is the most common gPrD (Gambetti et al. 2003b; Krasnianski et al. 2016). Lugaresi and colleagues first used the term “fatal familial insomnia” in 1986 to describe the illness of an Italian family whose affected members presented with progressive insomnia, dysautonomia, and motor dysfunction gradually leading to death (Lugaresi et al. 1986). After the discovery of the PRNP gene, sequencing in three symptomatic family members showed the D178N mutation in conjunction with a cis codon 129M (Goldfarb et al. 1992). As noted above, the 129 codon polymorphism strongly affects the clinicopathological phenotype of the D178N mutation; codon 129V cis usually results in a CJD phenotype, whereas codon 129M cis results in a FFI phenotype (Reder et al. 1995; Krasnianski et al. 2008). Some D178N-129M cases, however, present as CJD, and some D178N-129V present as FFI. Different presentations also can occur even within the same family (Zerr et al. 1998; Zarranz et al. 2005). FFI (D178N-129M) has been reported in about 30 families with the mean age at symptom onset of ∼52 yr (range, ∼20–76 yr), and disease duration averages from ∼13.5 mo (range 4–40) (Reder et al. 1995; Padovani et al. 1998; Collins et al. 2001; Kovács et al. 2005; Zarranz et al. 2005; Sano et al. 2013; Krasnianski et al. 2016). Disease duration appears to be influenced by the codon 129 trans polymorphism; FFI cases homozygous for methionine at codon 129 (MM) have a much shorter duration of ∼12 (± 4 SD) mo whereas heterozygous cases (MV) have a longer duration of ∼21 (± 15 SD) mo (Padovani et al. 1998; Gambetti et al. 2003b). A positive family history for PrD is reported in ∼60%–88% of FFI (D178N-129M) cases (Kovács et al. 2005; Shi et al. 2015; Krasnianski et al. 2016; Minikel et al. 2016).
The key symptoms of FFI include marked disruption of the normal sleep–wake cycle (disorganization of the EEG sleep patterns), sympathetic over activity, endocrine dysfunction (attenuation of the normal circadian oscillations), and profoundly impaired attention (Gambetti et al. 2003b; Krasnianski et al. 2016) linked to thalamic dysfunction. Nevertheless, clinical heterogeneity is observed; some mutation carriers present with only moderate insomnia and do not have abnormal EEG sleep study results despite having characteristic thalamic gliosis neuropathologically (Zerr et al. 1998; Taniwaki et al. 2000; Collins et al. 2001). Dysautonomia is one of the earlier features in most cohorts, although in D178N-129M patients from the Basque region, psychiatric symptoms and abnormal gait are the most common presenting features (Zarranz et al. 2005). One recent study of the German FFI cohort found hallucinations to be more common in FFI than in other gPrDs (Krasnianski et al. 2016). Sometimes FFI (D178N-129M) presents as early-onset dementia, including with a clinical phenotype similar to early-onset AD (Guerreiro et al. 2009).
The diagnosis of FFI is made based on the key features noted above, polysomnography and ultimately genetic testing. Other testing can be helpful. FDG-PET scans can show thalamic hypoactivity or gliosis both in symptomatic patients and in presymptomatic carriers several months before onset. As patients become more symptomatic, the cingulate cortex becomes hypometabolic followed by other cortical areas (Cortelli et al. 2006; Haïk et al. 2008; Krasnianski et al. 2014). EEG usually shows reduced sleep spindles and K complexes and only rarely shows PSWCs in the very late stage of disease. Polysomnographic studies, without which abnormal sleep patterns can go undetected, typically show reduction in rapid eye movement and deep sleep (Zarranz et al. 2005; Krasnianski et al. 2008). Some endocrine abnormalities, including persistently elevated cortisol levels and variable thyroid and gonadotropin hormone levels, also have been reported (Collins et al. 2001). MRI infrequently (∼16%) shows classic CJD findings (Kovács et al. 2005; Krasnianski et al. 2016). CSF biomarkers 14-3-3 and total tau have low sensitivities, 8%–18% and ∼8%, respectively (Kovács et al. 2005; Sano et al. 2013; Krasnianski et al. 2016). One study of 12 patients in Japan, however, found CSF RT-QuIC sensitivity to be 83% (Sano et al. 2013). A distinct and possibly invariable neuropathological feature of FFI is severe thalamic degeneration, particularly the anteroventral, mediodorsal, and pulvinar nuclei, with 80%–90% neuronal loss and significant astrogliosis. Often there is atrophy of the inferior olivary nuclei and astrogliosis in the midbrain gray matter and hypothalamus. Unlike most other prion diseases, there is usually little to no PrPSc deposition or vacuolation in these regions noted above (Lugaresi et al. 1986; Medori et al. 1992; Gambetti et al. 2003b). In cases of longer duration, more than ∼10 mo, the cortex can become involved with vacuolation, and to a lesser extent astrogliosis and neuron loss; these changes often spread from focal to more widespread cortical involvement the longer the disease duration (Gambetti et al. 2003b).
SLOW TYPE GENETIC PRION DISEASES
Gerstmann–Sträussler–Scheinker (GSS)
GSS generally is defined by neuropathological findings rather than by its clinical manifestations. It is characterized neuropathologically by the presence of multicentric PrPSc amyloid plaques (Budka et al. 1995; Ghetti et al. 1995; Kong et al. 2004). The classic phenotype is slowly progressive cerebellar ataxia, with cognitive decline and parkinsonism later in the disease course. Age of onset is variable, usually between the third and seventh decades (Hsiao et al. 1989; Piccardo et al. 1998). Often in GSS, cognitive decline is the first or an early sign; however, less commonly it may present rapidly, similar to classic sCJD. Clinical heterogeneity can occur even within families (Hsiao et al. 1989; Hainfellner et al. 1995; Piccardo et al. 1998; Webb et al. 2008).
At least 16 missense mutations (P84S, P102L, P105L, P105S, A117V, G131V, S132I, V176G, H187R, F198S, D202N, E211D, Q212P, Q217R, Y218N, and M232T) have been associated with GSS; however, the pathogenicity of some of these variants is unclear (Hsiao et al. 1989, 1992; Minikel et al. 2016). GSS phenotypes also have been reported with several PRNP OPRI mutations, particularly the longer insertion mutations (≥∼8-OPRI) (Paucar et al. 2013; Takada et al. 2017), but also with shorter insertion mutations as well (discussed below) (Rossi et al. 2000; Hinnell et al. 2011; Jansen et al. 2011; Vitali et al. 2011). Worldwide, P102L is the most common GSS mutation (Minikel et al. 2016).
The mean age of symptom onset is ∼52.5 yr (ranging from the third to the ninth decades) (Kovács et al. 2002, 2005; Takada et al. 2017), and the mean disease duration is close to 60 mo (ranging from <1 to >10 yr), which is much longer compared with gCJD (Kovács et al. 2002, 2005). Cerebellar ataxia or gait disturbance (72%) and cognitive decline (82%) are common, whereas extrapyramidal signs (36%), psychiatric symptoms (21%), and myoclonus (15%) are less frequent. A positive family history is reported in 69%–100% of cases (Kovács et al. 2005; Krasnianski et al. 2016).
Ancillary testing for many biomarkers is much less sensitive in GSS than in sCJD. About half of GSS cases have a positive CSF 14-3-3 protein, and <10% of cases have PSWCs on EEG (Kovács et al. 2005). Brain MRI typically shows global or cerebellar atrophy. The EuroCJD study, however, reported basal ganglia FLAIR or DWI hyperintensities in 30% of cases; other studies noted FLAIR or DWI abnormalities, such as cortical ribboning or deep nuclei hyperintensities (typical for sCJD) to be very uncommon (Vitali et al. 2011; Krasnianski et al. 2016). White-matter hyperintensities have occasionally been reported in GSS as well as sCJD and other gPrDs (such as gCJD owing to the E196K mutation), although it is not often clear whether the white matter changes result from the primary prion disease or to other comorbidities (Webb et al. 2008; Schelzke et al. 2011; Simpson et al. 2013).
P102L
P102L is the most common mutation reported in GSS, and patients from various kindreds around the world have been found to have the mutation. Typically, symptoms begin at a mean age of 52 yr (range ∼24–66 yr), and the mean disease duration is 52 mo (range ∼7–132 mo) (Kovács et al. 2005; Webb et al. 2008; Higuma et al. 2013; Sano et al. 2013; Krasnianski et al. 2016; Takada et al. 2017).
Family history is positive in 84%–100% of cases, but penetrance is estimated to be 100% (Higuma et al. 2013; Krasnianski et al. 2016; Minikel et al. 2016). The P102L mutation is most commonly associated with methionine at cis codon 129, and disease onset is earlier in patients homozygous for methionine at codon 129 (MM) than in heterozygous (MV) individuals (Webb et al. 2008).
With 70%–90% cases presenting with slowly progressive cerebellar dysfunction during the course, almost all cases of P102L develop such symptoms during their clinical course (Webb et al. 2008; Higuma et al. 2013; Krasnianski et al. 2016). Cognitive symptoms usually are mild initially and become more severe later in the disease course, although some patients begin with or have early cognitive deficits. In ∼20%–30% of patients with the P102L mutation, neuropsychiatric symptoms with cognitive decline were reported to be the most prominent early in the disease course (Webb et al. 2008; Higuma et al. 2013; Krasnianski et al. 2016). A minority of P102L cases present as RPD, similar to sCJD. In fact, among 57 Japanese P102L cases, 21% presented with an sCJD-like RPD presentation with dementia being early and prominent (Higuma et al. 2013). Additionally, a recent study noted that ∼40% of the cases showed cognitive symptoms at onset (about the same percentage had cerebellar symptoms) (Takada et al. 2017).
Regarding other symptoms occurring during the disease course, one of the largest P102L studies, with 84 cases, from nine distinct families found ∼80% of patients had lower motor neuron signs, such as areflexia and muscle weakness. They also found that ∼70% had sensory symptoms such as dysaesthesia and hyperaesthesia, often with a sensorimotor axonal neuropathy. Parkinsonism and psychiatric features (delusions, paranoia, visual hallucinations, and personality change) occurred in almost 50% of the patients, but apraxia, myoclonus seizures, and dystonia are infrequently reported (Webb et al. 2008). In the majority of P102L cases, symptoms are slowly progressive, eventually leading to akinetic-mutism (Kong et al. 2004; Webb et al. 2008). Some patients with rare cis 129V polymorphism presented with psychiatric symptoms and seizures (Bianca et al. 2003; Webb et al. 2008).
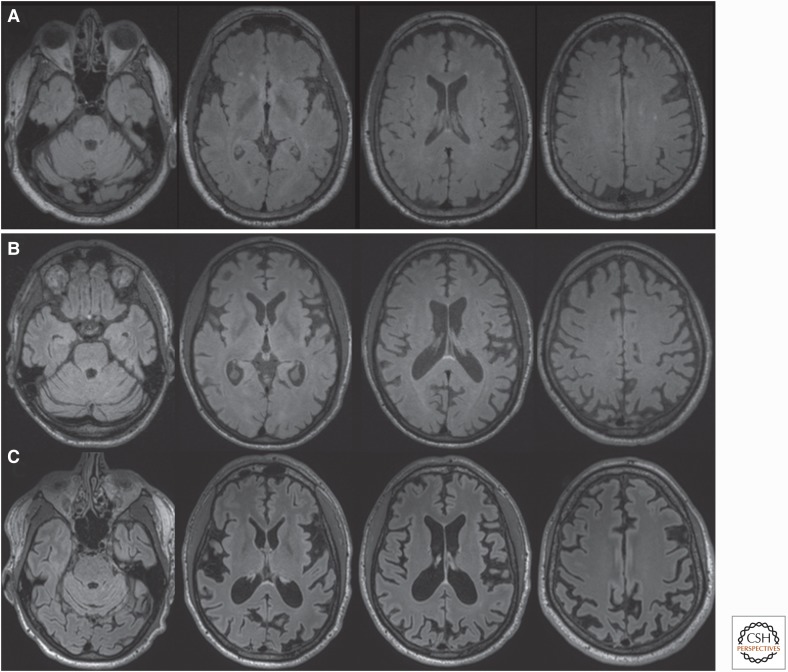
In one cohort in which 15 symptomatic P102L patients had brain MRIs, five patients within 1–3 yr after onset who had no or mild cognitive and psychiatric features had reportedly normal MRIs. Among patients with significant cognitive or psychiatric features, however, three had generalized atrophy and one had only cerebellar atrophy. Four cases had multiple white matter lesions. None had any features often found in sporadic and some gPrDs, such as T2-weighted hyperintensity or restricted diffusion in the cortex or deep nuclei (Webb et al. 2008). Among a few cohorts, ∼30% or more of the scans reveal T2-weighted white matter hyperintensities, mostly consistent with small vessel ischemic vascular disease (Webb et al. 2008; Takada et al. 2017). T2-weighted basal ganglia hyperintensities are reported in ∼25%–40% of P102L patients, although from much of the literature it is not clear if these are more typical slow progressors or the rapid, CJD-like presentations (Higuma et al. 2013; Krasnianski et al. 2016; Takada et al. 2017). Among Japanese patients with a rapid clinical phenotype resembling CJD, 80% have MRI abnormalities like those found in CJD (Higuma et al. 2013). A typical MRI showing mild cerebellar atrophy of a slow progressor with a P102L mutation is shown in Figure 3A.
Figure 3.
Brain magnetic resonance images (MRIs) in Slow forms of genetic prion disease (gPrD). (A) Fluid-attenuated inversion recovery (FLAIR) brain MRI in a 57-yr-old patient with P102L gPrD possibly 16.5 yr after onset (precise age of onset unclear because of comorbidities) shows mild cerebellar atrophy but no cortical atrophy or typical T2-weighted or diffusion weighted imaging (DWI) hyperintensites (cortical ribboning or deep nuclei hyperintensity), but only several punctate hyperintensities in the white matter consistent with small vessel ischemic vascular disease. DWI/attenuation diffusion coefficient (ADC) map sequences showed no restricted diffusion (not shown). (B) FLAIR brain MRI of with 37-yr-old gPrD 6-octapeptide repeat insertion (OPRI) patient showing diffuse cortical atrophy only 7 mo after reported clinical onset; the amount of atrophy suggests that the disease began years before obvious clinical onset. DWI and ADC map sequences showed no restricted diffusion (not shown). (C) Repeat brain MRI on the same 6-OPRI patient 25 mo later, 32 mo after clinical onset, shows the progression of atrophy. DWI and ADC map sequences still showed no restricted diffusion (not shown). Orientation is radiological. (Reprinted from Takada et al. 2017.)
Regarding other ancillary testing, EEG PSWCs are infrequent in P102L and reported in about one-quarter of cases (range 0%–33%), with the CJD-like cases appearing somewhat more likely to have these EEG abnormalities (Higuma et al. 2013; Sano et al. 2013; Krasnianski et al. 2016; Takada et al. 2017). CSF biomarker positivity is also low. About 20% of P102L cases (range 0%–50%) are positive for 14-3-3, and ∼13% (range 0%–100%) have elevated tau (Higuma et al. 2013; Sano et al. 2013; Krasnianski et al. 2016; Takada et al. 2017). Japanese rapidly progressive (CJD-like) P102L cases, however, uniformly have elevated t-tau and 14-3-3. The same study found RT-QuIC to have a sensitivity of 80% among P102L cases who manifested with the GSS phenotype and 100% among those with a CJD-like phenotype (Higuma et al. 2013). Neuropathology shows multicentric PrP amyloid plaques, but synaptic-type PrP deposition and vacuolation are only rarely seen (Webb et al. 2008; Higuma et al. 2013).
A117V
The A117V mutation was initially reported in European families (British-Irish, French-Alsatian, American [one with German descent], and Hungarian). Patients carrying this mutation showed variable age at onset (20–64 yr) and disease duration (1–11 yr) (Tateishi et al. 1990; Hsiao et al. 1991b; Mastrianni et al. 1995; Kovács et al. 2001). Cognitive impairment is a common early or main clinical presentation, but ataxia is less common (Tateishi et al. 1990; Hsiao et al. 1991b; Mastrianni et al. 1995). Some patients with the A117V mutation also develop weakness (lower motor neuron deficits) and parkinsonism (Tranchant et al. 1992; Kovács et al. 2001). To our knowledge, MRI findings are only reported in four A117V patients, who showed diffuse or asymmetric cortical atrophy but no T2 or DWI abnormalities (Kovács et al. 2001; Takada et al. 2017). The minimal data on EEG and CSF biomarkers (14-3-3, total tau, and NSE) show these to be negative in A117V (Kovács et al. 2001; Takada et al. 2017). Neuropathology shows many PrP amyloid plaques in the cortex and cerebellum, as well as focal areas of vacuolation, neuronal loss, and gliosis. In one patient with lower motor neuron weakness, intraneuronal vacuolation, and loss of spinal motor neurons accompanied by neurogenic muscular atrophy were found (Kovács et al. 2001).
F198S
The F198S mutation was first reported in a large American family and is sometimes referred to as the “Indiana kindred” (Dlouhy et al. 1992; Hsiao et al. 1992). Symptoms begin between the fourth and seventh decades, typically in the mid-50s. The codon 129 polymorphism appears to affect the age at onset, as valine homozygous (VV) cases are affected 10 yr earlier than heterozygous (MV) individuals (Dlouhy et al. 1992; Ghetti et al. 1995; Takada et al. 2017). The mean disease duration is ∼5 yr (range 2–12) (Farlow et al. 1989; Ghetti et al. 1995; Takada et al. 2017). Patients develop memory problems and gait ataxia early in the disease course and progress to dementia. Other cerebellar deficits (e.g., clumsiness, dysarthria, and eye movement abnormalities) are common, as development of a rigid parkinsonism (Farlow et al. 1989; Ghetti et al. 1995). Brain MRI reported only in a few cases revealed cerebellar atrophy and reduced T2 signal intensity in the basal ganglia (Farlow et al. 1989). EEG usually does not show PSWCs, and DWI MRI is usually normal. CSF biomarker data are sparse, but some cases have shown elevated 14-3-3 and/or total tau (Takada et al. 2017). Gross neuropathology reveals cortical and cerebellar atrophy, and microscopic analysis shows unicentric and multicentric PrPSc amyloid plaques of unicentric or multicentric forms in many areas of the brain with concurrent neuronal loss and gliosis. Vacuolation is minimal, however (Ghetti et al. 1995).
H187R
The H187R mutation has been identified in a few families (Cervenáková et al. 1999; Bütefisch et al. 2000; Hall et al. 2005; Colucci et al. 2006). Age at onset is reported to range from 20 to 53 yr, and disease duration from 3 to 19 yr (Cervenáková et al. 1999; Bütefisch et al. 2000; Hall et al. 2005). Clinically, the H187R mutation is reported to present similarly to GSS. Early symptoms vary considerably, even within a family. Some patients develop cerebellar ataxia, followed after a few years by cognitive impairment, whereas other patients begin the illness with behavioral and cognitive problems including disinhibition and executive dysfunction, and develop dementia and cerebellar dysfunction years later (Bütefisch et al. 2000; Hall et al. 2005). One report of H187R mutation carriers noted neuropsychiatric symptoms, including kleptomania, pyromania, depression, as well as suicidal ideation and attempts during adolescence, with dementia occurring years later (Hall et al. 2005). Other features such as pyramidal signs, seizures, and myoclonus are observed frequently (Bütefisch et al. 2000).
On brain MRI, there usually is cerebellar atrophy. Cortical atrophy may also be present, but to our knowledge T2/FLAIR hyperintensities or reduced diffusion in the deep nuclei or cortex have not been identified (Bütefisch et al. 2000). CSF biomarkers, such as 14-3-3, are usually normal, and EEG does not show PSWCs and may be normal (Bütefisch et al. 2000; Hall et al. 2005). Given the relatively slow course of the H187R mutation, the absence of these positive biomarkers is not unexpected.
Although the clinical presentation of the H187R mutation can be similar to GSS, the characteristic multicentric PrPSc amyloid plaques are found only in some H187R cases (Bütefisch et al. 2000; Hall et al. 2005; Colucci et al. 2006). Vacuolation is minimal or absent, whereas astrocytic gliosis is seen more consistently (Bütefisch et al. 2000; Hall et al. 2005; Colucci et al. 2006). PrPSc immunohistochemistry shows granular deposits with a distinct “curly” shape and sparse synaptic-type deposits (Bütefisch et al. 2000; Colucci et al. 2006).
P105L
To our knowledge, about 10 families with the P105L mutation have been reported, with nine from Japan (at least five of which apparently are unrelated), as well as one British family and one de novo American case (Beck et al. 2010; Higuma et al. 2013; Mano et al. 2016; Takada et al. 2017). It is suspected that all Japanese families have a common founder, however (Mano et al. 2016). Age of onset seems to range from ∼33 to 50 yr (Beck et al. 2010) with ∼10% of patients presenting before age 40. Among eight Japanese cases, the mean age at onset was ∼44 yr, and the mean disease duration was ∼9 yr (Higuma et al. 2013). More than half of cases live more than 5 yr (Kovács et al. 2002). In the Japanese literature, PrD attributable to the P105L mutation often is referred to as “variant,” “spastic,” or “paraspastic” GSS because patients often show dementia with spastic paraparesis rather than cerebellar ataxia typical of other forms of GSS (Yamada et al. 1999; Kovács et al. 2002). Although initial reports suggested clinical presentations were spastic paraparesis and dementia, subsequently more clinical heterogeneity has been found, with variations even within families (Yamada et al. 1999; Higuma et al. 2013; Mano et al. 2016). Some cases, including three Japanese families, recently reported, present with atypical parkinsonism and/or cerebellar features followed by progressive dementia (Mano et al. 2016). Clinical symptoms vary considerably, particularly between families. Among 20 Japanese patients, 100% had dementia; ∼90% had parkinsonism; approximately one-half had parkinsonism, emotional lability, and/or spastic paraparesis; and approximately one-third had ataxia and/or tremor. Other less common features were myoclonus, insomnia, and least commonly chorea, seizure, or neuropathy (Higuma et al. 2013; Mano et al. 2016). Sensory and psychiatric symptoms occurring for years before onset of other neurological features also have been reported (Shiraishi et al. 2002; Beck et al. 2010). A family history for prion or neuropsychiatric disease, although common, is not always present.
Regarding ancillary testing, only one of 16 cases (6.25%) who had brain MRI performed showed abnormalities consistent with prion disease (cortical ribboning), although frontal predominant atrophy has been reported in several cases (Yamada et al. 1999; Kovács et al. 2002; Mano et al. 2016). None of at least 15 Japanese cases whom had EEG showed PSWCs, and CSF 14-3-3 and total tau were all negative in the cases tested (Higuma et al. 2013; Mano et al.). RT-QuIC was positive in CSF in one of two cases analyzed (Higuma et al. 2013).
Neuropathology usually shows findings consistent GSS including lack of vacuolation but the presence of PrP amyloid plaques and diffuse PrP deposits in deep layers of the cerebral cortex (Higuma et al. 2013).
A recent paper presented a very young-onset case with a de novo P105L mutation with early cognitive impairment (Takada et al. 2017). At age 9, the patient began to have poor performance in school, followed by handwriting problems by age 10, seizures and gait ataxia by age 11, and becoming wheelchair bound by age 12. He had no family history for PrD or any other neurodegenerative disorders. Whole-exome sequencing revealed the young patient had a de novo P105L mutation that was not found in his parents. His brain MRI scans ∼1–2 mo after onset reportedly were unremarkable, but EEGs showed high-amplitude posterior dominant slow wave as well as bursts and spikes with diffuse background slowing (Takada et al. 2017).
INSERTION AND DELETION MUTATIONS (MIXED FAST AND SLOW TYPES)
Octapeptide Repeat Insertions (OPRI) and Deletion (OPRD)
Between codons 51 and 91 in wild-type PRNP, there is an octapeptide repeat domain consisting of a nonapeptide (27 bp, R1) followed by four octapeptide (24 bp) repeats (R2, R2, R3, and R4). The four octapeptides are identical, but their nucleotide sequences have small variations (Palmer et al. 1993; Gambetti et al. 2003b). Insertions and possibly deletions in this octapeptide repeat region of PRNP are thought to occur by replication slippage or recombination between identical 24-bp repeats (Beck et al. 2001; Chen et al. 2005).
OPRIs do not appear to directly influence PrP conformation, but functional studies suggest that two or more extra octapeptide repeats make PrPC more resistant to protease and more prone to aggregate (Priola and Chesebro 1998; Moore et al. 2006), which can facilitate PrPSc formation (Moore et al. 2006). Octapeptide repeat expansions also affect copper binding to PrP, resulting in increased interactions between PrPC and PrPSc, and possibly reducing PrPC stability and resistance to polymerization (Leliveld et al. 2006; Hodak et al. 2009; Stevens et al. 2009).
OPRIs/OPRD are usually classified based on the number of additional repeats, but the exact position of the insertion/deletion can also vary. There also may be minor nucleotide sequence variations between these mutations (Gambetti et al. 2003a; Croes et al. 2004). OPRIs with two to 12 extra octapeptide repeats and a 2-octapeptide deletion have been reported to cause gPrD, showing significant clinical and neuropathological heterogeneity (Goldfarb et al. 1991; Gambetti et al. 2003b; Croes et al. 2004; Takada et al. 2017). One octapeptide insertion or deletion is not thought to be pathogenic, as 1-OPRI and 1-OPRD have also been found in healthy individuals (Palmer et al. 1993; Beck et al. 2001, 2010; Capellari et al. 2002; Gambetti et al. 2003b; Yu et al. 2004). Among all octapeptide mutations, there is significant phenotypic heterogeneity, but the molecular basis for this appears in part to depend on the size of the octapeptide changes and the codon 129 polymorphism (Croes et al. 2004; Jansen et al. 2011).
Considering the effect of the size of the OPRI, some studies suggest roughly three groups exist: (1) Insertions of 2- to 4-OPRIs usually manifest with an sCJD phenotype, later age at onset, and shorter clinical duration, whereas >4-OPRIs appear to have a longer disease course; (2) insertions of 5- to 7-OPRIs tend to frequently present as CJD with a prolonged clinical course in early to mid-adulthood, and clinical presentation is variable and includes cognitive, psychiatric, motor dysfunction (cerebellar ataxia, pyramidal dysfunction, parkinsonism, dystonia, chorea, tremor), and seizures; and (3) 8-OPRIs or more are reportedly more frequently associated with the GSS phenotype, including widespread PrPSc amyloid deposition (Owen et al. 1989; Gambetti et al. 2003b; Croes et al. 2004). Amyloid plaques are more commonly found in the cerebellum of individuals with 8- or 9-OPRIs in comparison to 4- to 7-OPRIs (Vital et al. 1998; Mead et al. 2006; Moore et al. 2006). The categorization of OPRIs by repeat insertion size into three distinct clinicopathological groups, however, is not always accurate. A recent paper from the UCSF cohort suggests that there is great variability even within OPRI mutations and families. There is clearly great heterogeneity in age of onset and duration of OPRIs. Furthermore, there are exceptions to the notion that OPRIs greater than seven present with GSS (Table 2) (Takada et al. 2017). Regarding factors influencing age of onset, a meta-analysis of 55 OPRI cases from 22 different families (of which 38 patients from 17 families had codon 129 data) found a significant inverse relationship between the number of OPRIs and onset age (whether or not they adjusted for codon 129) and disease duration. Longer repeats had earlier age of onset and shorter survival. When, however, the investigators included from these families an additional 40 cases presumed to have OPRI based on clinical features, but which did not have genetic testing or pathology, only the inverse relation between the number of OPRIs and age of onset, but not survival, remained statistically significant. Codon 129 MM cases had an earlier age at onset and VV cases had later onset than MV carriers (Croes et al. 2004). In contrast, another study reports that 129 polymorphism does not affect the age at onset in some OPRIs cases (Kovács et al. 2005). Some individual octapeptide mutations are discussed in more detail below.
Table 2.
Octapeptide repeat insertions and deletions
| PRNP mutation | Codon 129 polymorphism | Number of cases in literature | Clinical phenotypes | Age at onset (range)a (yr) | Disease duration (range)a (mo or yr) | Pos FHxb | CSF biomarker sensitivity | EEG PSWC | MRI c/w CJDd | Neuropathology | Neuropathologic phenotype | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14-3-3 | Total tauc | ||||||||||||
| 2-OPRD | MM (1) Unknown (1) | 2 | RPD RPD, Sz, Myo |
(62–86) | (18–23) mo | 0% (0/2) | N/A | N/A | N/A | N/A | CJD | Beck et al. 2001; Capellari et al. 2002 | |
| 2-OPRI | MM (1) MV (1) VV (1) Unknown (1) | 4 | RPD D Cbr Atx |
Mean 63.3 ± 7.9 (58–75) | Mean 6.8 ± 6.4 (0.25–13) yr | 50% (2/4) | N/A | N/A | N/A | 0% (0/1) | CJD | Goldfarb et al. 1993; van Harten et al. 2000; Croes et al. 2004 | |
| 3-OPRI | VV (1) MM (1) | 2 | RPD | (68–69) | (4 mo–3 yr) | 0% (0/1) | 50% (1/2) | N/A | 50% (1/2) | 100% (1/1) | CJD | Grasbon-Frodl et al. 2004; Nishida et al. 2004 | |
| 4-OPRI | MM (9) VV (1) MV (cis V, 1) | 11 | MM; RPD, Myo, Cbr Atx VV; Dep, Behav MV; RPD |
MM Mean 60 ± 13.6 (39–85) VV 82 MV 38 | MM Mean 33.8 ± 32.7 (2–76) mo VV 4 mo MV 8 mo | MM 12.5% (1/8) VV 0% (0/1) MV 100% (1/1) | MM100% (4/4) VV N/A MV 100% (1/1) | N/A | MM22% (2/9) VV 100% (1/1) MV 0% (0/1) | MM 20% (1/5) VV N/A MV 100% (1/1) | MM CJD (7/7) VV N/A MV CJD (1/1) | Laplanche et al. 1995; Kaski et al. 2011; Sanchez-Valle et al. 2012 | |
| 5-OPRI | MM (6) MV (cis M, 3) Unknown (8) | 17 | Cog, motor | Mean 45.7 ± 11.0 (26–63), | Mean 76 ± 51.8 (10 mo–14.5 yr) | 100% (15/15) | 100% (1/1) | N/A | 25% (2/8) | 0% (0/8) | Vac, Neu loss, kuru-like PrPSc | Goldfarb et al. 1991; Cochran et al. 1996; Skworc et al. 1999; Beck et al. 2005; Mead et al. 2007 | |
| 5-OPRIe | MM (1) | 1 | Visuosp | 39 | 19 yr | FHx score 2 | N/A | N/A | 0% | 0% | N/A | Takada et al. 2017 | |
| 6-OPRI | 63 | Cog D, Front, Cbr Atx |
MM (30) 31.4 MV (10) 41.7 | MM (19) 11.4 yr, MV (8) 8.9 yr | N/A | N/A | 0% | 0% | Mead et al. 2006 | ||||
| 6-OPRIe | cis M (3) cis V (2) | 5 | Cog, Cbr Atx | cis M 35 ± 2.6 (32–37) Cis V (47–51) | cis M 5.9 ± 3 (3–9) yr Cis V (5–10) mo | FHx score 2 (n = 5) | 0% (0/2) | 0% (0/2) | 0% (0/2) | 0% (0/5) | CJD (4/4) | Takada et al. 2017 | |
| 7-OPRI | cis M/cis V | 16 | Cog, Behav motor |
35 ± 12.4 (18–59) | 8.4 ± 4.9 (0.6–17) yr | 86% (6/7) | N/A | N/A | 33.3% (1/3) | 50% (1/2) | cis M no PrP-Plqs cis V Uni, multicentric PrP-Plqs | Goldfarb et al. 1991; Tateishi et al. 1991; Brown et al. 1992b; Dermaut et al. 2000; Lewis et al. 2003; Cannella et al. 2007; Mauro et al. 2008; Wang et al. 2007; Guo et al. 2008; Jansen et al. 2011 | |
| 8-OPRI | cis M (4) | 11 | Psy, D | Mean 28 (21–34) | Mean 3.8 (1–7) yr | 100% (11/11) | N/A | N/A | 0% (0/3) | 0% (0/2) | Kuru, multicentric PrP-Plqs | GSS | Laplanche et al. 1999 |
| 8-OPRIe | MM | 1 | Dep, Cbr Atx | 22 | >5 yrf | FHx score 0 | N/A | N/A | N/A | 0% | N/A | Takada et al. 2017 | |
| 9-OPRI | cis M (2) Unknown (1) | 3 | Cbr Atx Fall, Cog Behav, Cog |
47 ± 13 (32–55) | (7 mo–2.5 yr) | N/A | N/A | N/A | 0% (0/1) | 0% (0/1) | numerous small PrP Plqs | Owen et al. 1992; Duchen et al. 1993; Krasemann et al. 1995 | |
| 9-OPRIe | VV (1) | 1 | Visuosp, Cog | 47 | 21 mo | FHx score 0 | N/A | 100% (1/1) | 100% (1/1) | 100% (1/1) | CJD+GSS | Takada et al. 2017 | |
| 12-OPRI | N/A | 3 | Cog, Behav, Cbr Atx, Sz | 44 ± 1 (43–45) | 8 ± 1.7 (7–10) yr | 100% (3/3) | 0% (0/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | multicentric PrP-Plqs | GSS | Kumar et al. 2011 |
AD, Alzheimer-type dementia; Atx, ataxia; Behav, behavioral changes; Cbr, cerebellar; Cog, cognitive; D, dementia; Dep, depression; FHx, family history; Front, frontal lobe dysfunction; M, methionine; mo, months; Myo, myoclonus; N/A, not available; Neu, neuronal; OPRD, octapeptide repeat deletion; OPRI, octapeptide repeat insertion mutation; Path, pathology; PrP-Plqs, PrP-amyloid plaques; PSWC, periodic sharp wave complexes; Psy, psychiatric changes; RPD, rapidly progressive dementia; Sz, seizures; V, valine; Vac, vacuolation; Visuosp, visuospatial; yr, years.
aData on age at onset and duration of disease are shown as mean ± SD (range), unless otherwise indicated.
bPositive family history of dementia with similar clinical features (as of the proband) or PrD. For UCSF family history (FHx) Score scale: 0 when there was no positive FMH suspicious for or known PrD; 1 when there was at least one first-degree relative with dementia, encephalopathy, or movement disorder; or 2 in patients who were part of families with known PRNP mutations, or had positive history for clinical or path-proven PrDs.
cIn most laboratories, Total tau is considered positive for prion disease if greater than 1150 or 1200 pg/mL.
dAccording to most commonly used European 2009 and UCSF 2011 criteria (Zerr et al. 2009; Vitali et al. 2011).
eIf there are differences between data published in the literature from the more recently published UCSF cohort, this information is provided in the table separately for that mutation.
fSome patients still alive, so duration at last follow-up.
2-OPRD
Two octapeptide deletions in the octapeptide repeat region were reported in two unrelated patients. An 86-yr-old woman with no family history of neurodegenerative disease or early death had progressive tremors for 20 yr, beginning in the head and progressing to involve her entire body; she developed RPD with pyramidal and extrapyramidal signs and died 23 mo later without autopsy. MRI reportedly showed T2-weighted white matter hyperintensity and atrophy. Her clinical diagnosis was possible CJD (WHO 1998; Beck et al. 2001). The second 2-OPRD case was a 62-yr-old male presenting with dizziness and behavioral changes, progressing to RPD 6 mo later, followed by myoclonus, with gait difficulty at 16 mo and death 18 mo after onset. This patient had methionine homozygosity at codon 129 (MM), and his neuropathology was similar to that seen in sCJD MM. Regarding his family history, his mother died in her 80s with a clinical Alzheimer’s diagnosis, and other family members died young, although none had known dementia (Capellari et al. 2002).
2-OPRI
Goldfarb et al. reported a 58-yr-old woman with RPD of 3 mo duration. Autopsy showed CJD, and the patient was MM at codon 129. Her mother carried the same mutation but had onset of a slowly progressive dementia at age 75 and a disease duration >13 yr (Goldfarb et al. 1993). Two nonrelated Dutch patients with this mutation were also reported (van Harten et al. 2000; Croes et al. 2004). One (129MV cis V) had onset at age 59 and was mute 10 yr later (Croes et al. 2004), whereas the other (129VV cis V) developed gradually progressive memory problems at age 61, with gait ataxia later in the disease course. The patient died 7 yr after symptom onset. Brain MRI showed extensive white-matter abnormalities and severe cerebral atrophy (van Harten et al. 2000). Neuropathological findings were not reported in either case. Both patients had first-degree relatives with dementia, but no PRNP genetic testing was performed. To our knowledge, the 2-OPRI mutation has not been found in healthy individuals, further supporting its pathogenicity (Beck et al. 2001; Capellari et al. 2002).
3-OPRI
At least three 3-OPRI cases have been reported in the literature. The 3-OPRI mutation in association with codon 129 VV was identified in a 69-year-old male diagnosed both clinically and neuropathologically with CJD who died 4 mo after onset. His EEG did not show PSWCs, but CSF 14-3-3 was positive (Grasbon-Frodl et al. 2004).
Nishida et al. (2004) reported a Japanese man with symptom onset at age 68, diagnosed with probable CJD, who had a 3-yr disease duration and no family history of PrD. CSF 14-3-3 and tau testing were negative, but EEG showed periodic synchronous discharges with generalized slowing. Brain MRI scans 6 mo after onset revealed diffuse cortical atrophy and widespread DWI cortical hyperintensities and bilateral striatal hyperintensity, compatible with CJD. PRNP testing revealed the 3-OPRI mutation with codon 129MM, but an autopsy was not performed (Nishida et al. 2004).
The 3-OPRI mutation with the 129MM genotype was also identified in a Chinese woman who was asymptomatic into her late thirties. This has raised a question about the pathogenicity of this mutation (Yu et al. 2004), but as noted above, shorter insertions usually result in a late age of onset (Croes et al. 2004).
4-OPRI
About 14 4-OPRI cases have been reported in the literature, most presenting similar to sCJD. About 12 of these 4-OPRI cases, including 10 from a UK series, with the codon 129 MM had a mean symptom onset of ∼60 yr (range 39–85) and a median duration of disease of ∼14 mo (range 2–77) (Croes et al. 2004; Kaski et al. 2011). The most common clinical symptomatology resembles sCJD, with RPD, myoclonus (80%), and cerebellar ataxia (50%). The phenotype is diverse, however, and even a presentation resembling AD has been reported (Kaski et al. 2011). In the UK study, among the few cases with CSF testing, 14-3-3 and S100β had 100% sensitivity (4/4 and 3/3 cases, respectively), but only 22% (2/9) of patients had PSWCs on EEG (Kaski et al. 2011). In the same UK study, brain MRI findings, performed in half of subjects, had variable findings, two showed atrophy, one was normal, one had a subdural, and only one had hyperintensity (presumably T2-weighted) in the unilateral caudate and cortex, consistent with MRI findings in CJD (Kaski et al. 2011). The neuropathological findings were distinct from those found in sCJD. There were no synaptic PrPSc deposits, but instead a fine network thought to be within axons and dendrites. There were no PrP amyloid plaques, but somewhat similar to cerebellar staining in 5-OPRI and 6-OPRI (discussed below), there were PrPSc deposits in the dendrites of Purkinje cells extending perpendicularly to the surface, resulting in a “tigroid” appearance of the cerebellum. Type 1 and 2 PrPSc have both been found, but not concomitantly, in 4-OPRI cases (Kaski et al. 2011). The UK study found that all of their cases were homozygous for a risk allele that was previously shown to be a risk factor for PrD, and the investigators suggested that patients might need to have both the 4-OPRI and homozygosity of the risk allele to develop PrD (Kaski et al. 2011).
Laplanche et al. reported a 4-OPRI patient with codon 129 VV who developed depression and behavioral disorders at age 82 and died 4 mo later without autopsy. Her family history was negative, and EEG showed pseudoperiodic triphasic waves (Laplanche et al. 1995). Sánchez-Valle et al. reported a 38-year-old patient with the 4-OPRI mutation (codon 129 MV, cis V) with an 8-mo disease duration, positive CSF protein 14-3-3, and cortical and basal ganglia hyperintensities on brain MRI consistent with CJD. Neuropathological evaluation revealed extensive spongiform changes in deep cortical layers and basal ganglia, neuronal loss and astroglial and microglial proliferation, and diffuse synaptic PrPSc deposit in all of the affected brain areas, similar to sCJD. The proband’s mother had the same mutation but was VV at codon 129 and was cognitively normal at age 74 (Sanchez-Valle et al. 2012). Given that there have been older persons with this mutation who are unaffected clinically, this mutation probably has low penetrance (Rossi et al. 2000) or might require additional genetic risk factors for development of disease (Kaski et al. 2011).
5-OPRI
The 5-OPRI mutation typically presents over several to many years as a slowly progressive dementia with cerebellar and other motor dysfunction. Seventeen 5-OPRI cases from South African, English, Northern Irish, Japanese, German, and Ukrainian families had a mean age at onset of 45.7 yr (± 11.0 SD, range 26–63) and a mean disease duration of 76 mo (± 51.8 SD, range 4–174). Onset is usually with dementia, followed by ataxia, myoclonus, and pyramidal and extrapyramidal symptoms (Mead et al. 2007). Among nine of the 17 cases with known codon 129 polymorphism, this polymorphism appeared to have an effect on age of onset. The mean age at onset for 129 MM cases was about 16 yr earlier (n = 6, 42.3 yr±12.4 SD, range 34–63) than for 129 MV cases (cis M, n = 3, 58.0 yr ± 5.2 SD, range 52–61), whereas clinical duration was not different. MRI reportedly did not show DWI signal changes but showed mild to moderate cerebral and cerebellar atrophy. EEG findings were quite varied, including normal, diffuse background slowing, PSWCs, or nonperiodic spike and wave complexes. Neuropathology revealed severe spongiform change and neuronal loss in the cortex and cerebellum with extensive kuru-like and synaptic PrPSc deposition (Goldfarb et al. 1991; Cochran et al. 1996; Skworc et al. 1999; Beck et al. 2005; Mead et al. 2007).
We recently reported an atypical 5-OPRI case with codon 129MM who had an onset at age 39, within the range reported in the literature, but a long duration of >19 yr (Takada et al. 2017) (compared with a mean of 8 yr and range of ∼10 mo–16 yr in the literature for OPRIs) (Table 2) (Depaz et al. 2012). A very unusual French family initially thought to have familial AD was reported in which the father had dementia onset at age 55, died at 60, and ultimately was shown to have both PrD and AD Braak stage VI pathology. He had four affected children with ages of onset of 30, 42, 44, and 46 yr old. All had onset of biparietal cognitive syndromes, reminiscent of posterior cortical atrophy due to AD, including acalculia and apraxia in all four, agraphia and visuospatial deficits each in three, and short-term memory and attentional deficits each in two. Disease duration ranged from 5 to 16 yr. They all eventually developed rigid syndromes, often with ataxia, seizures, and/or severe myoclonus. Two of the children and the father had neuropathological analyses. The youngest child had two brain biopsies negative for PrD, but brain autopsy showed PrD. Both children had mild spongiosis and gliosis in the caudate, thalamus, and cerebellum, with more pronounced pathology in the frontal, temporal, hippocampal, and entorhinal cortices, but no kuru or GSS-like amyloid plaques. Both the youngest daughter and the father had atypical elongated synaptic deposits of PrPSc (in the cortex and molecular layer of the cerebellum in the daughter and only in the cerebellum in the father). Interestingly, both had AD copathology—the father with Braak stage VI and the daughter with neurofibrillary tangles, Aβ amyloid plaques, and Aß deposits limited to the entorhinal cortex (Depaz et al. 2012).
Related to this pathological finding of concomitant AD pathology in PrD, the relationship between PrPSc, β-amyloid (Aβ), and tau proteins remains to be elucidated. One autopsy study from our center on 266 serial sCJD cases reported that 17% of the sCJD cases had AD-like pathology and that the age of disease onset in most of these cases were much younger than the typical one for AD (Tousseyn et al. 2015). Another study examining neuropathology of contaminated human growth hormone–induced iatrogenic CJD cases found much higher Aβ pathology than expected for age of the subjects. The investigators suggested that this finding supported the notion that Aβ was transmitted from the growth hormone (Jaunmuktane et al. 2015). Another interpretation, however, is that the PrP prions induce Aβ pathology. Given that nonsense PRNP mutations (discussed below) also showed an amyloid and/or tau pathology, even in young cases, supports the idea of an interaction between PrP prions and both Aβ and tau. It is possible that PrPSc might disrupt the normal metabolism of Aβ or tau protein, leading to copathology of PrD and AD.
6-OPRI
The 6-OPRI mutation is one of the best characterized forms of OPRIs because of the relatively larger number of well-described cases. The first autopsied case of human PrD in the 1890s was likely a 6-OPRI case (Mead et al. 2006). A detailed study of a very large and extended family with the 6-OPRI mutation in southeast England reported that the family members presented with progressive frontoparietal dysfunction (aggressive or apathetic behavioral change) with later cerebellar ataxia, pyramidal tract signs, and/or myoclonus. Several members had chorea and were diagnosed with Huntington’s disease (HD) during life. Differential diagnoses also included AD, Parkinson’s disease, Pick’s disease, frontotemporal dementia, and progressive myoclonic epilepsy (Mead et al. 2006). The mean age of onset was 34.9 yr (± 6.93 SD, range 20–53, n = 63), and the mean age of death was 45.1 yr (± 7.3 SD, range 30–65, n = 73). Brain CT or MRI scans showed only generalized cerebral and cerebellar atrophy (Mead et al. 2006), and a later published report found no FLAIR or DWI abnormalities in nine of the cases studied (De Vita et al. 2013). EEG in affected family members showed slow background or low-amplitude activity. Basic CSF examinations of the few patients tested (n = 3) were normal, but CSF biomarkers (total tau, NSE, and 14-3-3) were not reported. There was early age at onset in patients with longer disease duration. Codon 129 appeared to play a role in the age of onset, with 129 MM patients having ∼10 yr earlier onset (n = 30, mean age of onset 31.4 yr±5.7 SD) than in those with 129 MV (cis M) (n = 10, 41.7 yr±5.3). Disease duration, however, was not statistically significantly different between 129 MM (n = 19, 11.4 yr) and 129 MV cases (n = 8, 8.9 yr) (Mead et al. 2006). These findings suggest that as in 5-OPRI cases, the trans codon 129 polymorphism might affect the age at onset, but not the disease duration.
A Japanese 6-OPRI family (codon 129 MM), with a different order of repeats than the British family above, had similar clinical presentation, although an average of 5 yr earlier age of onset (average ∼30 yr, range 26–34) and more homogeneity of symptoms and progression (Ishida et al. 1995). Neuropathology in the British and Japanese families was similar, with variable spongiosis and astrocytosis between cases and brain regions. Most cases showed many patches of PrPSc deposition in the molecular layer of the cerebellum (Ishida et al. 1995; Mead et al. 2006).
Our center has followed a family of Ashkenazi Jewish ancestry that includes three 6-OPRI siblings (Table 2) with 129 cis M (MM, MV, M?) with early ages at onset (32, 36, and 37 yr) and prolonged clinical courses (>3 yr, ∼9 yr, and ∼6 yr, respectively). Their father died at age 47 after a 10-yr history of progressive dementia. Two full siblings presented with several years of memory loss and were initially diagnosed with AD before motor symptoms developed (Boxer et al. 2007), whereas the younger half-sibling presented with more rapid cognitive decline and only began developing motor dysfunction a few years after symptom onset (Takada et al. 2017). MRIs in all three siblings showed diffuse cortical and cerebellar atrophy without FLAIR and diffusion abnormalities. Two other 6-OPRI cases from our cohort, but with 129 cis V, had a later age of onset (51 and 47 yr) and short clinical courses (5 and 10 mo) than the codon 129 cis M cases above (Table 2). MRI also only showed atrophy without typical CJD findings, but autopsy revealed CJD pathology in these 6-OPRI cases (n = 4) (Fig. 3B,C) (Takada et al. 2017). Two 6-OPRI cases from one French family presented with memory loss and developed cerebellar signs, myoclonic jerks, and seizures. Both had onset at age 38, and disease duration was 4–10 yr (Vital et al. 1998). Other published reports of 6-OPRI cases also showed variable degrees of PrPSc deposits and spongiosis without amyloid PrPSc plaques (Vital et al. 1998; Mead et al. 2006). Other families or cases have been reported in Austria and Spain (Mead et al. 2006).
7-OPRI
There is marked phenotypic variability in 7-OPRI mutation carriers, but most present with relatively early onset of a slowly progressive cognitive and behavioral syndrome and eventually develop motor features (parkinsonism). In some cases, motor features are present early but in most cases become common during the disease course. A review of 16 published cases showed a mean age of onset of 35 (± 12.4 SD, range 18–59) and a mean disease duration of 8.4 yr (± 4.9 SD, range 0.6–17) (Goldfarb et al. 1991; Tateishi 1991; Brown et al. 1992b; Dermaut et al. 2000; Lewis et al. 2003; Cannella et al. 2007; Wang et al. 2007; Guo et al. 2008; Mauro et al. 2008; Jansen et al. 2011). A Dutch pedigree with six symptomatic members reportedly showed a GSS phenotype, two of whom were tested and had a 7-OPRI mutation, one with codon 129 VV and the other with codon 129 MV (cis V) (Jansen et al. 2011). Clinical information, available only in four family members, showed slowly progressive cognitive decline, personality changes, psychiatric features (depression with anxiety, panic attacks), apraxia, and parkinsonism. Mean age at onset was 52.2 yr (range 49–59), and mean disease duration was 2.4 yr (range 0.6–5.4). Neuropathology on three autopsied cases showed numerous unicentric or multicentric amyloid PrP plaques and diffuse synaptic PrP staining throughout the cerebrum and cerebellum. PrP amyloid plaques were most abundant in the molecular layer of the cerebellum, which also showed mild to moderate vacuolation. There was variable vacuolation, neuronal loss, and astrocytic gliosis in all areas of the cerebral cortex and basal ganglia. Aβ staining showed diffuse plaques and a few core plaques in the frontal cortex but less in other cortical regions. Staining for phosphorylated tau revealed diffuse puncta with sparse neuropil threads in all areas, including the striatum and molecular layer of the cerebellum (Jansen et al. 2011).
Dermaut et al. reported a small Belgian family with a 7-OPRI mutation (cis codon 129 M) with an age at onset of 24–32 and a duration of 11–17 yr, beginning with progressive cognitive decline, behavioral features including psychosis, and followed by motor features (pyramidal, extrapyramidal, cerebellar, and myoclonus) and seizures. There were several pathological findings that the investigators felt were different from those of typical GSS. First, neuropathology showed elongated PrPSc deposits in the molecular layer of the cerebellum, whereas in GSS, amyloid PrP plaques are usually multicentric with an amyloid core. Second, there was degeneration of Purkinje cells, which are usually spared in GSS. Third, there was both cerebellar and cortical pathology, whereas they noted that GSS pathology was usually isolated to the cerebellum (Dermaut et al. 2000), although this latter point probably is not universal for GSS, which often shows pathology in the cerebellum, cortex, brainstem, and even spinal cord (Ghetti et al. 1995).
8-OPRI
The 8-OPRI mutation was identified in five generations of a French family with codon 129 cis M (Laplanche et al. 1999). Eleven family members had the phenotype of GSS; the mean age at onset was 28 yr (range 21–34), and the mean disease duration was 3.8 yr (range 1–7). Onset included prominent psychiatric features (mania or mania-like symptoms being the most frequent) followed by ataxia, dysarthria, and dementia at later stages. Pathology showed cerebellar atrophy, kuru-type, and multicentric PrP plaques with minimal spongiosis.
Another 8-OPRI pedigree (codon 129 cis M) was reported to present as an HD phenocopy, with many features similar to the French pedigree, with similar age of onset (mean 29.7 yr, range 23–41) and a syndrome consisting of personality change, cognitive decline, motor disturbance with chorea, dysarthria, and ataxia with basal ganglia atrophy (Moore et al. 2001). A reported Dutch family (codon 129 cis V, trans V and M) with six affected family members in three generations had a range of onset from 21 to 54 yr and duration from 5 mo to 6 yr. The first symptoms in all of the patients included personality changes, gait problems, and memory difficulties followed by hypokinesia and/or rigidity. Cognitive deficits were both cortical and subcortical (Van Gool et al. 1995). We reported a young onset 8-OPRI case (codon 129 MM) with onset of depression at age 22, followed by mild gait ataxia. About 5 yr after onset, he reportedly had preserved cognitive function but had cortical atrophy on imaging, perhaps suggesting a GSS-like phenotype (Takada et al. 2017).
9-OPRI
Only a few cases carrying 9-OPRI mutations have been reported. The first report of a 9-OPRI case, (129 cis M) by Owen et al., was a patient who developed gait ataxia, self neglect, and bladder incontinence at age 53 and died ∼2.5 yr later. Unfortunately, ancillary testing (CSF, EEG, or brain imaging) was not reported (Owen et al. 1992). Another report also described a patient with onset at age 53 and death ∼2.5 yr later. She was first seen at age 55 with a 2-yr history of frequent falls, with progressive behavioral changes and memory impairment, and 6 mo of intermittent urinary incontinence. Her examination revealed a Mini Mental Status Examination (MMSE) score of 14/30, disorientation, ideomotor apraxia, several frontal release signs including grasping and utilization behaviors, gait and limb ataxia, limb myoclonus, axial rigidity, and brisk reflexes with upgoing toes. Her CSF and EEG were reportedly normal, but it is unclear if any CSF biomarkers (e.g., S100B, 14-3-3, t-tau, NSE) were tested. Brain CT only revealed diffuse cerebral and cerebellar atrophy. She died suddenly 2.5 yr after onset. The family history for neuropsychiatric disease was unclear as her parents died in their 50s from a stroke and heart disease, and there were many early deaths in the family from nondementia or unreported causes. Most grandparents lived into their 70s, only one with late-onset dementia. Interestingly, numerous small PrP plaques were revealed postmortem in decreasing amounts in the cerebellum, basal ganglia, and cerebral cortex, which differed from the typical amyloid plaques of GSS. There was no vacuolation without spongiosis and only mild astrocytic gliosis, but there were several features reportedly reminiscent of AD (Duchen et al. 1993). Another patient (129 MM) presented about 20 yr younger than the other two cases, at age ∼32, with behavioral changes (withdrawal) and an inability to do household work, and within 1 yr, showed worsening cognitive impairment (inability to recognize bills and dyscalculia), dysarthria, gait disturbances, vision change (concentric narrowing), and headaches. Later, she developed agraphia and eventually a tremor and worsening ataxia. Her CSF was reportedly nonspecific, EEG showed bilateral slow wave activity, and generalized cortical atrophy was present on MRI. She was alive 6 yr into her disease course. Her mother died at 41 yr with severe dementia and spastic paraparesis, and her maternal grandmother and great-grandmother died around the same age with dementia (Krasemann et al. 1995).
We reported a 9-OPRI codon 129VV case with a CJD phenotype, although somewhat early onset for CJD (age 47), with a rapidly progressive dementia, cortical ribboning on MRI, PSWCs on EEG, and positive CSF total tau and NSE. He died after a 21-mo course with neuropathology interestingly showing mixed pathology of CJD and GSS (Takada et al. 2017). Thus, in the few 9-OPRI cases reported, there has been varied clinical presentation and neuropathology.
12-OPRI
Insertion of 12 octapeptides was reported in three affected individuals, a father and two daughters, from an American family. Patients had a frontotemporal dementia-like presentation with onset at 43–45 yr and disease durations of 7–10 yr (Kumar et al. 2011). Their initial symptoms were cognitive decline and frontotemporal dementia-like behavioral changes. Late in the illness, they developed gait ataxia and generalized tonic-clonic seizures. Brain MRI in the proband (daughter) showed severe bilateral frontal atrophy greater than posterior atrophy but no cortical ribboning or deep nuclei hyperintensities on T2-weighted or DWI sequences. Generalized spikes and sharp waves, but no PSWCs, were present on the proband’s EEG, and CSF showed elevated NSE and t-tau but negative protein 14-3-3. Curiously, her CSF Aβ amyloid level was low, in the range consistent with AD. Neuropathology of two sisters showed multicentric PrPSc amyloid plaques in the cerebellar cortex consistent with GSS along with widespread tau-positive neurofibrillary tangles in the neocortex (Kumar et al. 2011).
Nonrepeat Octapeptide Insertion
Recently, a Canadian patient was reported to have a 24-bp nucleotide insertion (1 OPRI) between nucleotides 388 and 389 (codons 129 and 130) of PRNP, but downstream from the octapeptide repeat domain. Onset at age 28 began with night terrors, followed by the first signs of cognitive decline at age 31, and status epilepticus at age 34. He later developed gait ataxia, slurred speech, and disinhibition. Only mild cortical and cerebellar atrophy without cortical ribboning or deep nuclei hyperintensities were seen on FLAIR MRI (unclear if DWI and ADC map were performed). EEG revealed diffuse slowing and multifocal epileptiform discharges, and CSF was positive for 14-3-3 and total tau. Brain biopsy revealed microspongiosis, loss of neurons, and PrP-positive multicentric plaques, consistent with GSS. His parents did not carry the insertion, suggesting a de novo mutation (Hinnell et al. 2011).
PRNP Nonsense Mutations
Nonsense mutations have only been reported in a few kindreds, but show highly variable and atypical manifestations, including Alzheimer’s dementia or behavioral variant frontotemporal dementia (bvFTD) phenotypes, sensory and autonomic peripheral nervous system involvement, and/or chronic diarrhea, and prolonged disease courses. Neuropathology shows PrPSc amyloid plaques and/or PrPSc cerebral amyloid angiopathy, frequently combined with tau pathology in the brain (Guerreiro et al. 2014). Pathogenic nonsense mutations reported to date are located in the C-terminal region in codons 145, 160, 163, 178, 226, and 227 (Table 3) (Ghetti et al. 1996; Jansen et al. 2010; Matsuzono et al. 2013; Guerreiro et al. 2014; Fong et al. 2016; Minikel et al. 2016).
Table 3.
PRNP nonsense mutations
| PRNP mu- tation | Codon 129 polymorphism | Number of cases in literature | Clinical phenotypes | Age at onset (range)a (yr) | Disease duration (range)a (mo or yr) | Pos FHxb | CSF biomarker sensitivity | EEG PSWC | MRI c/w CJDd | Neuropathology | Neuropathological phenotype | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14-3-3 | Total tauc | ||||||||||||
| Q145X | MM | 1 | Cog | 38 | 21 yr | 0% (0/1) | N/A | N/A | N/A | N/A | NFT, PrP-angio | atypic | Ghetti et al. 1996 |
| Q160X | MM (4) MV (3) Unknown (4) | 11 | D Cog, Dep Cog, dysauto, neuropathy |
42.1 ± 8.4 (32–59) | 9.6 ± 4.9 (4–21) yr | 100% (11/11) | 0% (0/1) | 0% (0/1) | 0% (0/4) | 0% (0/4) | NFT, PrP-angio, AD pathology | atypic | Owen et al. 1989; Finckh et al. 2000; Jayadev et al. 2011; Fong et al. 2016 |
| Q163X | cis V (10) | 10 | Dysauto, neuropathy, Cog | 33.0 ± 3.4 (30–38) | 26.8 ± 8.0 (15–33) yr | 100% (10/10) | 100% (1/1) | 100%d (1/1) | 0% (0/3) | 0% (0/2) | NFT, PrP-angio, PrP-plaqs, Sp, Vac | atypic | Mead et al. 2013 |
| Y226X | MV (2) Unknown (1) | 3 | D Park, Cog |
55.3 ± 17.0 (39–73) | 3.5 ± 2.3 (1.5–6) yr | 100% (3/3) | 100% (1/1) | N/A | 100% (1/1) | 0% (0/1) | PrP-angio, PrP-plaqs | atypic | Jansen et al. 2010 |
| 2 bp Del 178 | Unknown (3) | 3 | Dysauto, cog, neuropathy | 42.0 ± 14.0 (26–52) | 5.5 ± 6.4 (1–10) yr | 100% (3/3) | 100% (2/2) | 100% (1/1) | N/A | 0% (0/2) | N/A | atypic | Matsuzono et al. 2013 |
AD, Alzheimer-type dementia; atypic, atypical; Cog, cognitive; c/w, consistent with; D, dementia; Del, deletion; Dep, depression; Dysauto, dysautonomia; FHx, family history; M, methionine; mo, months; months; N/A, not available; NFT, neurofibrillary tangles; Park, parkinsonism; PrP-angio, PrP amyloid angiopathy; PrP-Plqs, PrP-amyloid plaques; PSWC, periodic sharp wave complexes; Sp, spongiosis; V, valine; Vac, vacuolation; yr, years.
aData on age at onset and duration of disease are shown as mean±SD (range), unless otherwise indicated.
bPositive family history of dementia with similar clinical features (as of the proband) or PrD.
cIn most laboratories, Total tau is considered positive for prion disease if greater than 1150 or 1200 pg/mL.
dAccording to most commonly used European 2009 and UCSF 2011 criteria (Zerr et al. 2009; Vitali et al. 2011).
Three families with a Q160X mutation, causing a premature translation stop resulting in the production of C-terminally truncated PrP, have been reported. One is an Austrian family (codon 129 cis M) that includes three affected members, two brothers and their father with early-onset dementia. The younger brother (129 MM) developed RPD at age 32, and the older brother (129MV) developed a more slowly progressive dementia at age 48. At 6 and 8 yr after onset, neither had motor features. Brain imaging of the younger brother showed severe atrophy, whereas the older brother had mild cerebellar atrophy. Their father had developed dementia at age 48 and died after 12 yr, at age 60, with diffuse cortical atrophy (Finckh et al. 2000).
In a second Q160X family (also codon 129 cis M), the daughter (proband, 129 MV) developed short-term memory loss at age 39 after some mild head trauma without loss of consciousness, followed by 3 yr of progressive behavioral changes consistent with depression, and then 5 yr of progressive language impairment, mild parkinsonism (including an asymmetric essential-like tremor), and loss of activities of daily living (ADLs). She died after an 8-yr course, at age 47, with a clinical diagnosis of AD. Her MMSE at 3 yr from onset was 14/30. Ancillary testing 3 yr after onset reportedly showed an unremarkable EEG and brain MRI. The proband’s mother (129 MM) reportedly had onset 20 yr later than her daughter, at age 59, with personality changes including depression, significant short-term memory loss, and poor job performance. She had, however, an unexplained history of weight loss and intermittent diarrhea. Two years after onset of her cognitive and behavioral changes, she developed severe postprandial diarrhea with significant weight loss. Four years after onset, neurological examination revealed an MMSE of 15/30 with impairments mostly in memory, but an otherwise unremarkable neurological examination. Basic laboratory examinations, brain CT, and EEG were apparently unremarkable, and she was diagnosed with AD. Over the next 4 yr, her cognitive function worsened with development of significant language impairment and loss of ADLs. Her gastrointestinal symptoms and weight loss lasted until her death at age 67, 8 yr after onset and 20 yr later than her daughter’s age at death. The daughter’s brain neuropathology revealed severe PrPSc amyloid neuritic plaques and tau-positive neurofibrillary tangles in the neocortex and hippocampus with PrP positive amyloid angiopathy, but no vacuolation or Aβ plaques. There were also PrPSc positive amyloid angiopathy and extensive PrPSc deposits in gray matter of the neocortex and limbic system, with more sparse deposits in the cerebellum. The mother had similar brain PrP and tau pathology but had positive Aβ plaques, meeting Braak stage VI for neurofibrillary tangles and CERAD plaque stage “frequent,” having both PrD and AD pathology. The mother’s general autopsy did not reveal gastrointestinal pathology despite her symptoms (Jayadev et al. 2011).
We recently reported a third family with a single case of a 31-yr-old with Q160X mutation (codon 129MM) and a 4-yr history of a frontal dementia syndrome, cyclical gastric upset (diarrhea and vomiting), and peripheral neuropathy. Brain MRI showed global atrophy. He died suddenly and unexpectedly ∼6 yr into his course (autopsy pending). One of his parents carries the same mutation but is codon 129 MV and is asymptomatic (Fong et al. 2016). The wide range of age at onset of the Q160X mutation, all codon 129 cis M—27 (129MM), 32 (129MM), 48 (129MV), 39 (129MV), and 59 (129MM) yrs—may suggest that the codon 129 genotype does not affect the age at disease onset in these mutation carriers. The multiple proteinopathy at relatively early ages is a remarkable feature of some of these cases.
The Y163X-129V mutation was identified in a large British family with onset usually in the fourth decade (range 40–70). Affected family members presented with chronic diarrhea followed by a predominant sensory and autonomic axonal polyneuropathy. Cognitive decline and seizures appeared to occur at about the fifth or sixth decades. Neuropathological assessment revealed the presence of PrPSc not only in the central and peripheral nervous systems but also in the gut, lymphoreticular system, heart, arteries and veins, liver, kidney, and lung. As PrP staining is not found in peripheral tissues in the case of sporadic PrDs, this is a unique feature of the Y163X-129V mutation. Neuropathological findings revealed PrP amyloid plaques, amyloid angiopathy, and tau pathology with activated microglia in addition to mild spongiosis and vacuolation of deeper cortical laminae (Mead et al. 2013).
Even fewer cases of the other nonsense mutations have been reported. A Japanese woman with the Y145X-129M mutation developed memory problems at age 38 and was bed-bound by age 50. She died at age 59 after a 21-yr course, with severe cerebral atrophy on imaging and a clinical AD diagnosis. Neuropathological examination showed PrPSc-positive amyloid angiopathy and neurofibrillary pathology (Ghetti et al. 1996). A Dutch patient with a Y226X-129V mutation had onset of cognitive impairment (memory) at age 54, developed dementia (memory and visuospatial), auditory and visual hallucinations, and parkinsonism, and died after a 27-mo course. Neuropathology showed PrPSc cerebral amyloid angiopathy. Her mother had been diagnosed with probable CJD with onset at age 73 and death in 18 mo without autopsy. Another Dutch patient developed a slowly progressive hypokinetic rigid syndrome with cognitive decline at age 39. This patient was diagnosed clinically with bvFTD and parkinsonism and was found to have a Q227X-129V PRNP mutation. Brain pathology showed PrP positive amyloid plaques and severe neurofibrillary pathology (Jansen et al. 2010).
Lastly, a 2-bp deletion at codon 178 resulting in a frameshift mutation with premature stop codon was identified in a Japanese kindred who presented with severe dysautonomia, sensory neuropathy, and cognitive impairment (Matsuzono et al. 2013).
CONCLUDING REMARKS
The phenotypes associated with PRNP mutations are very heterogeneous. Certain mutations such as octapeptide repeat insertions/deletions and stop codon mutations can have atypical presentations for PrD and do not fit well into the historical clinicopathological classifications of gPrD, CJD, GSS, or FFI. PRNP mutations show great heterogeneity regarding age at onset, illness duration, penetrance, pathology, and other clinical features. These disorders often are mistaken for other neurodegenerative diseases, such as AD, bvFTD, and even HD. The nonsense mutations are particularly diagnostically challenging, as they manifest early-onset dementia and sometimes with prominent peripheral symptoms, such as peripheral neuropathy or chronic diarrhea, not usually associated with PrD.
Because many gPrDs present very differently than the most common human PrD, sCJD, physicians may not consider the possibility of a PrD in the diagnostic differential of such cases. gPrD patients often have much earlier ages at onset and a longer disease course than sCJD patients, and ancillary testing is often negative. Thus, making a correct diagnosis of gPrD depends on considering PrD in a diagnostic differential and being aware that even seemingly sporadic disease can be gPrD. As almost half of gPrD patients lack a positive family history, the absence of family history alone should not dissuade one from PRNP genetic counseling and testing, particularly in early-onset or atypical dementia cases (Goldman et al. 2004; Kovács et al. 2005). When considering ordering PRNP testing, the protocol used for HD, including counseling, is recommended (MacLeod et al. 2013; Goldman 2015). Genetic counseling allows persons at risk for gPrD to make informed decisions regarding life planning and to explore potential options, such as preimplantation genetic diagnosis and in vitro fertilization to prevent passing on the mutation (Uflacker et al. 2014).
Although there are currently no cures for human PrDs, gPrDs may be more amenable to future treatments than sCJD because through genetic testing of those at risk, one can identify presymptomatic persons and begin treatment before symptom onset. For similar reasons, studying presymptomatic gPrDs might teach us about the earliest features and changes occurring in other human PrDs.
ACKNOWLEDGMENTS
We thank our patients and their families for allowing us to learn from them and share this information. We also thank Stacy Metcalf at UCSF for her contribution to collecting and analyzing data from the UCSF gPrD cohort.
DISCLOSURES
Dr. Geschwind has the following disclosures: Dr Geschwind serves on the board of directors for San Francisco Bay Area Physicians for Social Responsibility and on the editorial board of Dementia and Neuropsychologia and serves or has served as a consultant for Best Doctors, Inc; Advance Medical; Grand Rounds, Second Opinion, the Gerson Lehrman Group, Inc; MEDACorp; Franciscan Hospitals; Kendall Brill Kelly; Guidepoint Global, LLC; Lewis Brisbois Bisgaard and Smith, LLP; Biohaven Pharmaceuticals, Inc.; Lundbeck, Inc; NeuroPhage Pharmaceuticals; and Quest Diagnostics. Related to his work on this review, he receives research support for his work on prion diseases from the Michael J. Homer Family Fund and the National Institute on Aging (R01 AG031189). He also receives or has received research support from CurePSP, Quest Diagnostics, Alliance BioSecure, and the Tau Consortium.
REFERENCES
- 1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. 2012. An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzualde A, Indakoetxea B, Ferrer I, Moreno F, Barandiaran M, Gorostidi A, Estanga A, Ruiz I, Calero M, van Leeuwen FW, et al. 2010. A novel PRNP Y218N mutation in Gerstmann–Sträussler–Scheinker disease with neurofibrillary degeneration. J Neuropathol Exp Neurol 69: 789–800. [DOI] [PubMed] [Google Scholar]
- Beck JA, Mead S, Campbell TA, Dickinson A, Wientjens DP, Croes EA, Van Duijn CM, Collinge J. 2001. Two-octapeptide repeat deletion of prion protein associated with rapidly progressive dementia. Neurology 57: 354–356. [DOI] [PubMed] [Google Scholar]
- Beck G, Kawano T, Naba I, Nishimura T, Sawada J, Hazama T. 2005. A case with a 120 base pair insertional mutation in the prion protein gene: The first case in Japan. J Neurol Neurosurg Psychiatry 76: 756–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JA, Poulter M, Campbell TA, Adamson G, Uphill JB, Guerreiro R, Jackson GS, Stevens JC, Manji H, Collinge J, et al. 2010. PRNP allelic series from 19 years of prion protein gene sequencing at the MRC Prion Unit. Hum Mutat 31: E1551–E1563. [DOI] [PubMed] [Google Scholar]
- Beck J, Collinge J, Mead S. 2012. Prion protein gene M232R variation is probably an uncommon polymorphism rather than a pathogenic mutation. Brain 135: e209; author reply e210. [DOI] [PubMed] [Google Scholar]
- Begue C, Martinetto H, Schultz M, Rojas E, Romero C, D’Giano C, Sevlever G, Somoza M, Taratuto AL. 2011. Creutzfeldt–Jakob disease surveillance in Argentina, 1997–2008. Neuroepidemiology 37: 193–202. [DOI] [PubMed] [Google Scholar]
- Bertoni JM, Brown P, Goldfarb LG, Rubenstein R, Gajdusek DC. 1992. Familial Creutzfeldt–Jakob disease (codon 200 mutation) with supranuclear palsy. JAMA 268: 2413–2415. [PubMed] [Google Scholar]
- Bianca M, Bianca S, Vecchio I, Raffaele R, Ingegnosi C, Nicoletti F. 2003. Gerstmann–Sträussler–Scheinker disease with P102L–V129 mutation: A case with psychiatric manifestations at onset. Ann Genet 46: 467–469. [DOI] [PubMed] [Google Scholar]
- Bishop MT, Pennington C, Heath CA, Will RG, Knight RS. 2009. PRNP variation in UK sporadic and variant Creutzfeldt Jakob disease highlights genetic risk factors and a novel non-synonymous polymorphism. BMC Med Genet 10: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Rabinovici GD, Kepe V, Goldman J, Furst AJ, Huang SC, Baker SL, O’Neil J P, Chui H, Geschwind MD, et al. 2007. Amyloid imaging in distinguishing atypical prion disease from Alzheimer disease. Neurology 69: 283–290. [DOI] [PubMed] [Google Scholar]
- Bratosiewicz J, Barcikowska M, Cervenakowa L, Brown P, Gajdusek DC, Liberski PP. 2000. A new point mutation of the PRNP gene in Gerstmann–Sträussler–Scheinker case in Poland. Folia Neuropathol 38: 164–166. [PubMed] [Google Scholar]
- Breithaupt M, Romero C, Kallenberg K, Begue C, Sanchez-Juan P, Eigenbrod S, Kretzschmar H, Schelzke G, Meichtry E, Taratuto A, et al. 2013. Magnetic resonance imaging in E200K and V210I mutations of the prion protein gene. Alzheimer Dis Assoc Disord 27: 87–90. [DOI] [PubMed] [Google Scholar]
- Brown K, Mastrianni JA. 2010. The prion diseases. J Geriatr Psychiatry Neurol 23: 277–298. [DOI] [PubMed] [Google Scholar]
- Brown P, Cathala F, Sadowsky D, Gajdusek DC. 1979. Creutzfeldt–Jakob disease in France: II. Clinical characteristics of 124 consecutive verified cases during the decade 1968–1977. Ann Neurol 6: 430–437. [DOI] [PubMed] [Google Scholar]
- Brown P, Cathala F, Castaigne P, Gajdusek DC. 1986. Creutzfeldt–Jakob disease: Clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol 20: 597–602. [DOI] [PubMed] [Google Scholar]
- Brown P, Goldfarb LG, Kovanen J, Haltia M, Cathala F, Sulima M, Gibbs CJ Jr., Gajdusek DC. 1992a. Phenotypic characteristics of familial Creutzfeldt–Jakob disease associated with the codon 178Asn PRNP mutation. Ann Neurol 31: 282–285. [DOI] [PubMed] [Google Scholar]
- Brown P, Goldfarb LG, McCombie WR, Nieto A, Squillacote D, Sheremata W, Little BW, Godec MS, Gibbs CJ Jr, Gajdusek DC. 1992b. Atypical Creutzfeldt–Jakob disease in an American family with an insert mutation in the PRNP amyloid precursor gene. Neurology 42: 422–427. [DOI] [PubMed] [Google Scholar]
- Brown P, Gibbs CJ Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC. 1994. Human spongiform encephalopathy: The National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 35: 513–529. [DOI] [PubMed] [Google Scholar]
- Brown P, Brandel JP, Preece M, Sato T. 2006. Iatrogenic Creutzfeldt–Jakob disease: The waning of an era. Neurology 67: 389–393. [DOI] [PubMed] [Google Scholar]
- Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K, et al. 1995. Neuropathological diagnostic criteria for Creutzfeldt–Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 5: 459–466. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Gambetti P, Cervenáková L, Park KY, Hallett M, Goldfarb LG. 2000. Inherited prion encephalopathy associated with the novel PRNP H187R mutation: A clinical study. Neurology 55: 517–522. [DOI] [PubMed] [Google Scholar]
- Cannella M, Martino T, Simonelli M, Ciammola A, Gradini R, Ciarmiello A, Gianfrancesco F, Squitieri F. 2007. De novo seven extra repeat expanded mutation in the PRNP gene in an Italian patient with early onset dementia. J Neurol Neurosurg Psychiatry 78: 1411–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellari S, Parchi P, Wolff BD, Campbell J, Atkinson R, Posey DM, Petersen RB, Gambetti P. 2002. Creutzfeldt–Jakob disease associated with a deletion of two repeats in the prion protein gene. Neurology 59: 1628–1630. [DOI] [PubMed] [Google Scholar]
- Capellari S, Cardone F, Notari S, Schinina ME, Maras B, Sita D, Baruzzi A, Pocchiari M, Parchi P. 2005. Creutzfeldt–Jakob disease associated with the R208H mutation in the prion protein gene. Neurology 64: 905–907. [DOI] [PubMed] [Google Scholar]
- Capellari S, Strammiello R, Saverioni D, Kretzschmar H, Parchi P. 2011. Genetic Creutzfeldt–Jakob disease and fatal familial insomnia: Insights into phenotypic variability and disease pathogenesis. Acta Neuropathol 121: 21–37. [DOI] [PubMed] [Google Scholar]
- Cervenáková L, Buetefisch C, Lee HS, Taller I, Stone G, Gibbs CJ Jr, Brown P, Hallett M, Goldfarb LG. 1999. Novel PRNP sequence variant associated with familial encephalopathy. Am J Med Genet 88: 653–656. [DOI] [PubMed] [Google Scholar]
- Chasseigneaux S, Haïk S, Laffont-Proust I, De Marco O, Lenne M, Brandel JP, Hauw JJ, Laplanche JL, Peoc’h K. 2006. V180I mutation of the prion protein gene associated with atypical PrPSc glycosylation. Neurosci Lett 408: 165–169. [DOI] [PubMed] [Google Scholar]
- Chen JM, Chuzhanova N, Stenson PD, Ferec C, Cooper DN. 2005. Meta-analysis of gross insertions causing human genetic disease: Novel mutational mechanisms and the role of replication slippage. Hum Mutat 25: 207–221. [DOI] [PubMed] [Google Scholar]
- Chen C, Shi Q, Zhou W, Zhang XC, Dong JH, Hu XQ, Song XN, Liu AF, Tian C, Wang JC, et al. 2013. Clinical and familial characteristics of eight Chinese patients with T188K genetic Creutzfeldt–Jakob disease. Infect Genet Evol 14: 120–124. [DOI] [PubMed] [Google Scholar]
- Choi BY, Kim SY, Seo SY, An SS, Kim S, Park SE, Lee SH, Choi YJ, Kim SJ, Kim CK, et al. 2009. Mutations at codons 178, 200–129, and 232 contributed to the inherited prion diseases in Korean patients. BMC Infect Dis 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran EJ, Bennett DA, Cervenáková L, Kenney K, Bernard B, Foster NL, Benson DF, Goldfarb LG, Brown P. 1996. Familial Creutzfeldt–Jakob disease with a five-repeat octapeptide insert mutation. Neurology 47: 727–733. [DOI] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB. 2011. Prions. Cold Spring Harb Perspect Biol 3: a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Boyd A, Fletcher A, Byron K, Harper C, McLean CA, Masters CL. 2000. Novel prion protein gene mutation in an octogenarian with Creutzfeldt–Jakob disease. Arch Neurol 57: 1058–1063. [DOI] [PubMed] [Google Scholar]
- Collins S, McLean CA, Masters CL. 2001. Gerstmann–Sträussler–Scheinker syndrome, fatal familial insomnia, and kuru: A review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 8: 387–397. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, Pocchiari M, Almonti S, Cuadrado-Corrales N, de Pedro-Cuesta J, et al. 2006. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt–Jakob disease. Brain 129: 2278–2287. [DOI] [PubMed] [Google Scholar]
- Colucci M, Moleres FJ, Xie ZL, Ray-Chaudhury A, Gutti S, Bütefisch CM, Cervenáková L, Wang W, Goldfarb LG, Kong Q, et al. 2006. Gerstmann–Sträussler–Scheinker: A new phenotype with ‘curly’ PrP deposits. J Neuropathol Exp Neurol 65: 642–651. [DOI] [PubMed] [Google Scholar]
- Cortelli P, Perani D, Montagna P, Gallassi R, Tinuper P, Provini F, Avoni P, Ferrillo F, Anchisi D, Moresco RM, et al. 2006. Pre-symptomatic diagnosis in fatal familial insomnia: Serial neurophysiological and 18FDG-PET studies. Brain 129: 668–675. [DOI] [PubMed] [Google Scholar]
- Croes EA, Theuns J, Houwing-Duistermaat JJ, Dermaut B, Sleegers K, Roks G, Van den Broeck M, van Harten B, van Swieten JC, Cruts M, et al. 2004. Octapeptide repeat insertions in the prion protein gene and early onset dementia. J Neurol Neurosurg Psychiatry 75: 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj A, Petersen RB, Lee HS, Cervenáková L, Shatunov A, Budka H, Brown P, Gambetti P, Goldfarb LG. 2002. Spontaneous mutations in the prion protein gene causing transmissible spongiform encephalopathy. Ann Neurol 52: 355–359. [DOI] [PubMed] [Google Scholar]
- De Vita E, Ridgway GR, Scahill RI, Caine D, Rudge P, Yousry TA, Mead S, Collinge J, Jager HR, Thornton JS, et al. 2013. Multiparameter MR imaging in the 6-OPRI variant of inherited prion disease. AJNR Am J Neuroradiol 34: 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaz R, Haïk S, Peoc’h K, Seilhean D, Grabli D, Vicart S, Sarazin M, DeToffol B, Remy C, Fallet-Bianco C, et al. 2012. Long-standing prion dementia manifesting as posterior cortical atrophy. Alzheimer Dis Assoc Disord 26: 289–292. [DOI] [PubMed] [Google Scholar]
- Dermaut B, Cruts M, Backhovens H, Lübke U, Van Everbroeck B, Sciot R, Dom R, Martin JJ, Van Broeckhoven C, Cras P. 2000. Familial Creutzfeldt–Jakob disease in a patient carrying both a presenilin 1 missense substitution and a prion protein gene insertion. J Neurol 247: 364–368. [DOI] [PubMed] [Google Scholar]
- Dlouhy SR, Hsiao K, Farlow MR, Foroud T, Conneally PM, Johnson P, Prusiner SB, Hodes ME, Ghetti B. 1992. Linkage of the Indiana kindred of Gerstmann–Sträussler–Scheinker disease to the prion protein gene. Nat Genet 1: 64–67. [DOI] [PubMed] [Google Scholar]
- Duchen LW, Poulter M, Harding AE. 1993. Dementia associated with a 216 base pair insertion in the prion protein gene. Clinical and neuropathological features. Brain 116: 555–567. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Yee RD, Dlouhy SR, Conneally PM, Azzarelli B, Ghetti B. 1989. Gerstmann–Sträussler–Scheinker disease. I. Extending the clinical spectrum. Neurology 39: 1446–1452. [DOI] [PubMed] [Google Scholar]
- Finckh U, Müller-Thomsen T, Mann U, Eggers C, Marksteiner J, Meins W, Binetti G, Alberici A, Sonderegger P, Hock C, et al. 2000. High frequency of mutations in four different disease genes in early-onset dementia. Ann N Y Acad Sci 920: 100–106. [DOI] [PubMed] [Google Scholar]
- Fong JC, Rojas JC, Bang J, Legati A, Rankin KP, Forner S, Miller ZA, Karydas AM, Coppola G, Grouse CK, et al. 2016. Genetic prion disease caused by PRNP Q160X mutation presenting with an orbitofrontal syndrome, cyclic diarrhea, and peripheral neuropathy. J Alzheimers Dis 55: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. 2003a. Sporadic and familial CJD: Classification and characterisation. Br Med Bull 66: 213–239. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Parchi P, Chen SG. 2003b. Hereditary Creutzfeldt–Jakob disease and fatal familial insomnia. Clin Lab Med 23: 43–64. [DOI] [PubMed] [Google Scholar]
- Gelpi E, Colom-Cadena M, Budka H. 2015. Molecular genetics of Creutzfeldt–Jakob disease and Gerstmann–Sträussler–Scheinker disease. eLS 1–11. 10.1002/9780470015902.a0024442 [DOI] [Google Scholar]
- Gerstmann J, Sträussler E, Scheinker I. 1935. Über eine eigenartige hereditär–familiäre Erkrankung des Zentralnervensystems. Z Gesamte Neurolog Psychiat 154: 736–762. [DOI] [PubMed] [Google Scholar]
- Geschwind MD. 2016. Rapidly progressive dementia. Continuum (Minneap Minn) 22: 510–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti B, Dlouhy SR, Giaccone G, Bugiani O, Frangione B, Farlow MR, Tagliavini F. 1995. Gerstmann–Sträussler–Scheinker disease and the Indiana kindred. Brain Pathol 5: 61–75. [DOI] [PubMed] [Google Scholar]
- Ghetti B, Piccardo P, Spillantini MG, Ichimiya Y, Porro M, Perini F, Kitamoto T, Tateishi J, Seiler C, Frangione B, et al. 1996. Vascular variant of prion protein cerebral amyloidosis with tau-positive neurofibrillary tangles: The phenotype of the stop codon 145 mutation in PRNP. Proc Natl Acad Sci 93: 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb LG, Brown P, McCombie WR, Goldgaber D, Swergold GD, Wills PR, Cervenáková L, Baron H, Gibbs CJ Jr, Gajdusek DC. 1991. Transmissible familial Creutzfeldt–Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the PRNP gene. Proc Natl Acad Sci 88: 10926–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb LG, Petersen RB, Tabaton M, Brown P, LeBlanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, et al. 1992. Fatal familial insomnia and familial Creutzfeldt–Jakob disease: Disease phenotype determined by a DNA polymorphism. Science 258: 806–808. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Brown P, Little BW, Cervenáková L, Kenney K, Gibbs CJ Jr, Gajdusek DC. 1993. A new (two-repeat) octapeptide coding insert mutation in Creutzfeldt–Jakob disease. Neurology 43: 2392–2394. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Goldfarb LG, Brown P, Asher DM, Brown WT, Lin S, Teener JW, Feinstone SM, Rubenstein R, Kascsak RJ, et al. 1989. Mutations in familial Creutzfeldt–Jakob disease and Gerstmann–Sträussler–Scheinker’s syndrome. Exp Neurol 106: 204–206. [DOI] [PubMed] [Google Scholar]
- Goldman JS. 2015. Genetic testing and counseling in the diagnosis and management of young-onset dementias. Psychiatr Clin North Am 38: 295–308. [DOI] [PubMed] [Google Scholar]
- Goldman JS, Miller BL, Safar J, de Tourreil S, Martindale JL, Prusiner SB, Geschwind MD. 2004. When sporadic disease is not sporadic: The potential for genetic etiology. Arch Neurol 61: 213–216. [DOI] [PubMed] [Google Scholar]
- Grasbon-Frodl E, Schmalzbauer R, Weber P, Krebs B, Windl O, Zerr I, Kretzschmar HA. 2004. A novel three extra-repeat insertion in the prion protein gene (PRNP) in a patient with Creutzfeldt–Jakob disease. Neurogenetics 5: 249–250. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Vaskov T, Crews C, Singleton A, Hardy J. 2009. A case of dementia with PRNP D178Ncis-129M and no insomnia. Alzheimer Dis Assoc Disord 23: 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Brás J, Wojtas A, Rademakers R, Hardy J, Graff-Radford N. 2014. Nonsense mutation in PRNP associated with clinical Alzheimer’s disease. Neurobiol Aging 35: 2656.e13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YJ, Wang XF, Han J, Zhang BY, Zhao WQ, Shi Q, Wan YZ, Gao C, Li JM, Wang DX, et al. 2008. A patient with Creutzfeldt–Jakob disease with an insertion of 7 octa-repeats in the PRNP gene: Molecular characteristics and clinical features. Am J Med Sci 336: 519–523. [DOI] [PubMed] [Google Scholar]
- Haïk S, Galanaud D, Linguraru MG, Peoc’h K, Privat N, Faucheux BA, Ayache N, Hauw JJ, Dormont D, Brandel JP. 2008. In vivo detection of thalamic gliosis: A pathoradiologic demonstration in familial fatal insomnia. Arch Neurol 65: 545–549. [DOI] [PubMed] [Google Scholar]
- Hainfellner JA, Brantner-Inthaler S, Cervenáková L, Brown P, Kitamoto T, Tateishi J, Diringer H, Liberski PP, Regele H, Feucht M, et al. 1995. The original Gerstmann–Sträussler–Scheinker family of Austria: Divergent clinicopathological phenotypes but constant PrP genotype. Brain Pathol 5: 201–211. [DOI] [PubMed] [Google Scholar]
- Hall DA, Leehey MA, Filley CM, Steinbart E, Montine T, Schellenberg GD, Bosque P, Nixon R, Bird T. 2005. PRNP H187R mutation associated with neuropsychiatric disorders in childhood and dementia. Neurology 64: 1304–1306. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Krasnianski A, Meissner B, Grasbon-Frodl EM, Kretzschmar HA, Zerr I. 2008. Novel PRNP mutation in a patient with a slow progressive dementia syndrome. Med Sci Monit Intl Med J Exp Clin Res 14: CS41–CS43. [PubMed] [Google Scholar]
- Higuma M, Sanjo N, Satoh K, Shiga Y, Sakai K, Nozaki I, Hamaguchi T, Nakamura Y, Kitamoto T, Shirabe S, et al. 2013. Relationships between clinicopathological features and cerebrospinal fluid biomarkers in Japanese patients with genetic prion diseases. PLoS One 8: e60003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DA, Head MW, Singh VK, Bishop M, Ironside JW. 2009. Familial prion disease with a novel serine to isoleucine mutation at codon 132 of prion protein gene (PRNP). Neuropathol Appl Neurobiol 35: 111–115. [DOI] [PubMed] [Google Scholar]
- Hinnell C, Coulthart MB, Jansen GH, Cashman NR, Lauzon J, Clark A, Costello F, White C, Midha R, Wiebe S, et al. 2011. Gerstmann–Sträussler–Scheinker disease due to a novel prion protein gene mutation. Neurology 76: 485–487. [DOI] [PubMed] [Google Scholar]
- Hodak M, Chisnell R, Lu W, Bernholc J. 2009. Functional implications of multistage copper binding to the prion protein. Proc Natl Acad Sci 106: 11576–11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, Westaway D, Ott J, Prusiner SB. 1989. Linkage of a prion protein missense variant to Gerstmann–Sträussler syndrome. Nature 338: 342–345. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Meiner Z, Kahana E, Cass C, Kahana I, Avrahami D, Scarlato G, Abramsky O, Prusiner SB, Gabizon R. 1991a. Mutation of the prion protein in Libyan Jews with Creutzfeldt–Jakob disease. N Engl J Med 324: 1091–1097. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Cass C, Schellenberg GD, Bird T, Devine-Gage E, Wisniewski H, Prusiner SB. 1991b. A prion protein variant in a family with the telencephalic form of Gerstmann–Sträussler–Scheinker syndrome. Neurology 41: 681–684. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Dlouhy SR, Farlow MR, Cass C, Da Costa M, Conneally PM, Hodes ME, Ghetti B, Prusiner SB. 1992. Mutant prion proteins in Gerstmann–Sträussler–Scheinker disease with neurofibrillary tangles. Nat Genet 1: 68–71. [DOI] [PubMed] [Google Scholar]
- Ishida S, Sugino M, Koizumi N, Shinoda K, Ohsawa N, Ohta T, Kitamoto T, Tateishi J. 1995. Serial MRI in early Creutzfeldt-Jacob disease with a point mutation of prion protein at codon 180. Neuroradiology 37: 531–534. [DOI] [PubMed] [Google Scholar]
- Jansen C, Parchi P, Capellari S, Vermeij AJ, Corrado P, Baas F, Strammiello R, van Gool WA, van Swieten JC, Rozemuller AJ. 2010. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol 119: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, Voet W, Head MW, Parchi P, Yull H, Verrips A, Wesseling P, Meulstee J, Baas F, van Gool WA, et al. 2011. A novel seven-octapeptide repeat insertion in the prion protein gene (PRNP) in a Dutch pedigree with Gerstmann–Sträussler–Scheinker disease phenotype: Comparison with similar cases from the literature. Acta Neuropathol 121: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, Parchi P, Capellari S, Ibrahim-Verbaas CA, Schuur M, Strammiello R, Corrado P, Bishop MT, van Gool WA, Verbeek MM, et al. 2012. Human prion diseases in the Netherlands (1998–2009): Clinical, genetic and molecular aspects. PloS ONE 7: e36333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, Launchbury F, Linehan J, Richard-Loendt A, Walker AS, et al. 2015. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525: 247–250. [DOI] [PubMed] [Google Scholar]
- Jayadev S, Nochlin D, Poorkaj P, Steinbart EJ, Mastrianni JA, Montine TJ, Ghetti B, Schellenberg GD, Bird TD, Leverenz JB. 2011. Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann Neurol 69: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Odunsi S, du Plessis D, Vincent A, Bishop M, Head MW, Ironside JW, Gow D. 2014. Gerstmann–Sträussler–Scheinker disease: Novel PRNP mutation and VGKC-complex antibodies. Neurology 82: 2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaski DN, Pennington C, Beck J, Poulter M, Uphill J, Bishop MT, Linehan JM, O’Malley C, Wadsworth JD, Joiner S, et al. 2011. Inherited prion disease with 4-octapeptide repeat insertion: Disease requires the interaction of multiple genetic risk factors. Brain 134: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Kim MO, Cali I, Oehler A, Fong JC, Wong K, See T, Katz JS, Gambetti P, Bettcher BM, Dearmond SJ, et al. 2013. Genetic CJD with a novel E200G mutation in the prion protein gene and comparison with E200K mutation cases. Acta Neuropathol Commun 1: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Surewicz WK, Petersen RB, Zou W, Chen SG, Gambetti P, Parchi P, Capellari S, Goldfarb L, Montagna P, et al. 2004. Inherited prion diseases. In Prion biology and diseases (ed. Prusiner SB), pp. 673–775. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Kotta K, Paspaltsis I, Bostantjopoulou S, Latsoudis H, Plaitakis A, Kazis D, Collinge J, Sklaviadis T. 2006. Novel mutation of the PRNP gene of a clinical CJD case. BMC Infect Dis 6: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács GG, Ertsey C, Majtényi C, Jelencsik I, László L, Flicker H, Strain L, Szirmai I, Budka H. 2001. Inherited prion disease with A117V mutation of the prion protein gene: A novel Hungarian family. J Neurol Neurosurg Psychiatry 70: 802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács GG, Trabattoni G, Hainfellner JA, Ironside JW, Knight RS, Budka H. 2002. Mutations of the prion protein gene phenotypic spectrum. J Neurol 249: 1567–1582. [DOI] [PubMed] [Google Scholar]
- Kovács GG, Puopolo M, Ladogana A, Pocchiari M, Budka H, van Duijn C, Collins SJ, Boyd A, Giulivi A, Coulthart M, et al. 2005. Genetic prion disease: The EUROCJD experience. Hum Genet 118: 166–174. [DOI] [PubMed] [Google Scholar]
- Kovács GG, Seguin J, Quadrio I, Hoftberger R, Kapas I, Streichenberger N, Biacabe AG, Meyronet D, Sciot R, Vandenberghe R, et al. 2011. Genetic Creutzfeldt–Jakob disease associated with the E200K mutation: Characterization of a complex proteinopathy. Acta Neuropathol 121: 39–57. [DOI] [PubMed] [Google Scholar]
- Krasemann S, Zerr I, Weber T, Poser S, Kretzschmar H, Hunsmann G, Bodemer W. 1995. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res Mol Brain Res 34: 173–176. [DOI] [PubMed] [Google Scholar]
- Krasnianski A, Bartl M, Sanchez Juan PJ, Heinemann U, Meissner B, Varges D, Schulze-Sturm U, Kretzschmar HA, Schulz-Schaeffer WJ, Zerr I. 2008. Fatal familial insomnia: Clinical features and early identification. Ann Neurol 63: 658–661. [DOI] [PubMed] [Google Scholar]
- Krasnianski A, Sanchez Juan P, Ponto C, Bartl M, Heinemann U, Varges D, Schulz-Schaeffer WJ, Kretzschmar HA, Zerr I. 2014. A proposal of new diagnostic pathway for fatal familial insomnia. J Neurol Neurosurg Psychiatry 85: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnianski A, Heinemann U, Ponto C, Kortt J, Kallenberg K, Varges D, Schulz-Schaeffer WJ, Kretzschmar HA, Zerr I. 2016. Clinical findings and diagnosis in genetic prion diseases in Germany. Eur J Epidemiol 31: 187–196. [DOI] [PubMed] [Google Scholar]
- Krebs B, Lederer RM, Windl O, Grasbon-Frodl EM, Zerr I, Kretzschmar HA. 2005. Creutzfeldt–Jakob disease associated with an R148H mutation of the prion protein gene. Neurogenetics 6: 97–100. [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Neumann M, Stavrou D. 1995. Codon 178 mutation of the human prion protein gene in a German family (Backer family): Sequencing data from 72-year-old celloidin-embedded brain tissue. Acta Neuropathol 89: 96–98. [DOI] [PubMed] [Google Scholar]
- Kumar N, Boeve BF, Boot BP, Orr CF, Duffy J, Woodruff BK, Nair AK, Ellison J, Kuntz K, Kantarci K, et al. 2011. Clinical characterization of a kindred with a novel 12-octapeptide repeat insertion in the prion protein gene. Arch Neurol 68: 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladogana A, Almonti S, Petraroli R, Giaccaglini E, Ciarmatori C, Liu QG, Bevivino S, Squitieri F, Pocchiari M. 2001. Mutation of the PRNP gene at codon 211 in familial Creutzfeldt–Jakob disease. Am J Med Genet 103: 133–137. [DOI] [PubMed] [Google Scholar]
- Ladogana A, Puopolo M, Poleggi A, Almonti S, Mellina V, Equestre M, Pocchiari M. 2005. High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology 64: 1592–1597. [DOI] [PubMed] [Google Scholar]
- Laplanche JL, Delasnerie-Lauprêtre N, Brandel JP, Dussaucy M, Chatelain J, Launay JM. 1995. Two novel insertions in the prion protein gene in patients with late-onset dementia. Hum Mol Genet 4: 1109–1111. [DOI] [PubMed] [Google Scholar]
- Laplanche JL, Hachimi KH, Durieux I, Thuillet P, Defebvre L, Delasnerie-Lauprêtre N, Peoc’h K, Foncin JF, Destée A. 1999. Prominent psychiatric features and early onset in an inherited prion disease with a new insertional mutation in the prion protein gene. Brain 122: 2375–2386. [DOI] [PubMed] [Google Scholar]
- Lee HS, Sambuughin N, Cervenáková L, Chapman J, Pocchiari M, Litvak S, Qi HY, Budka H, del Ser T, Furukawa H, et al. 1999. Ancestral origins and worldwide distribution of the PRNP 200K mutation causing familial Creutzfeldt–Jakob disease. Am J Hum Genet 64: 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliveld SR, Dame RT, Wuite GJ, Stitz L, Korth C. 2006. The expanded octarepeat domain selectively binds prions and disrupts homomeric prion protein interactions. J Biol Chem 281: 3268–3275. [DOI] [PubMed] [Google Scholar]
- Lewis V, Collins S, Hill AF, Boyd A, McLean CA, Smith M, Masters CL. 2003. Novel prion protein insert mutation associated with prolonged neurodegenerative illness. Neurology 60: 1620–1624. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jia L, Piao Y, Lu D, Wang F, Lv H, Lu Y, Jia J. 2010. Creutzfeldt–Jakob disease with PRNP G114V mutation in a Chinese family. Acta Neurol Scand 121: 377–383. [DOI] [PubMed] [Google Scholar]
- Lorenz H, Windl O, Kretzschmar HA. 2002. Cellular phenotyping of secretory and nuclear prion proteins associated with inherited prion diseases. J Biol Chem 277: 8508–8516. [DOI] [PubMed] [Google Scholar]
- Lugaresi E, Medori R, Montagna P, Baruzzi A, Cortelli P, Lugaresi A, Tinuper P, Zucconi M, Gambetti P. 1986. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med 315: 997–1003. [DOI] [PubMed] [Google Scholar]
- MacLeod R, Tibben A, Frontali M, Evers-Kiebooms G, Jones A, Martinez-Descales A, Roos RA, Editorial C, Working Group ‘Genetic Testing Counselling’ of the European Huntington Disease N. 2013. Recommendations for the predictive genetic test in Huntington’s disease. Clin Genet 83: 221–231. [DOI] [PubMed] [Google Scholar]
- Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, Brandner S, Jefferys JG, Collinge J. 2007. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53: 325–335. [DOI] [PubMed] [Google Scholar]
- Mano KK, Matsukawa T, Mitsui J, Ishiura H, Tokushige S, Takahashi Y, Sato NS, Nakamoto FK, Ichikawa Y, Nagashima Y, et al. 2016. Atypical parkinsonism caused by Pro105Leu mutation of prion protein: A broad clinical spectrum. Neurol Genet 2: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Harris JO, Gajdusek DC, Gibbs CJ Jr., Bernoulli C, Asher DM. 1979. Creutzfeldt–Jakob disease: Patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol 5: 177–188. [DOI] [PubMed] [Google Scholar]
- Mastrianni JA. 2010. The genetics of prion diseases. Genet Med 12: 187–195. [DOI] [PubMed] [Google Scholar]
- Mastrianni JA, Curtis MT, Oberholtzer JC, Da Costa MM, DeArmond S, Prusiner SB, Garbern JY. 1995. Prion disease (PrP-A117V) presenting with ataxia instead of dementia. Neurology 45: 2042–2050. [DOI] [PubMed] [Google Scholar]
- Matej R, Kovacs GG, Johanidesova S, Keller J, Matejckova M, Novakova J, Sigut V, Keller O, Rusina R. 2012. Genetic Creutzfeldt–Jakob disease with R208H mutation presenting as progressive supranuclear palsy. Move Disord 27: 476–479. [DOI] [PubMed] [Google Scholar]
- Matsuzono K, Ikeda Y, Liu W, Kurata T, Deguchi S, Deguchi K, Abe K. 2013. A novel familial prion disease causing pan-autonomic-sensory neuropathy and cognitive impairment. Eur J Neurol 20: e67–e69. [DOI] [PubMed] [Google Scholar]
- Mauro C, Giaccone G, Piscosquito G, Lavorgna A, Nigro M, Di Fede G, Leonardi A, Coppola C, Formisano S, Tagliavini F, et al. 2008. A novel insertional mutation in the prion protein gene: Clinical and bio-molecular findings. J Neurol Neurosurg Psychiatry 79: 1395–1398. [DOI] [PubMed] [Google Scholar]
- Mead S, Poulter M, Beck J, Webb TE, Campbell TA, Linehan JM, Desbruslais M, Joiner S, Wadsworth JD, King A, et al. 2006. Inherited prion disease with six octapeptide repeat insertional mutation—Molecular analysis of phenotypic heterogeneity. Brain 129: 2297–2317. [DOI] [PubMed] [Google Scholar]
- Mead S, Webb TE, Campbell TA, Beck J, Linehan JM, Rutherfoord S, Joiner S, Wadsworth JD, Heckmann J, Wroe S, et al. 2007. Inherited prion disease with 5-OPRI: Phenotype modification by repeat length and codon 129. Neurology 69: 730–738. [DOI] [PubMed] [Google Scholar]
- Mead S, Uphill J, Beck J, Poulter M, Campbell T, Lowe J, Adamson G, Hummerich H, Klopp N, Rückert IM, et al. 2012. Genome-wide association study in multiple human prion diseases suggests genetic risk factors additional to PRNP. Hum Mol Genet 21: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead S, Gandhi S, Beck J, Caine D, Gallujipali D, Carswell C, Hyare H, Joiner S, Ayling H, Lashley T, et al. 2013. A novel prion disease associated with diarrhea and autonomic neuropathy. N Engl J Med 369: 1904–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medori R, Tritschler HJ, LeBlanc A, Villare F, Manetto V, Chen HY, Xue R, Leal S, Montagna P, Cortelli P, et al. 1992. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med 326: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggendorfer F. 1930. Klinische und genealogische Beobachtunge bei einem Fall von spastischer Pseudosklerose Jakobs. Z Gesamte Neurol Psychiatr 128: 337–341. [Google Scholar]
- Meiner Z, Gabizon R, Prusiner SB. 1997. Familial Creutzfeldt–Jakob disease. Codon 200 prion disease in Libyan Jews. Medicine (Baltimore) 76: 227–237. [DOI] [PubMed] [Google Scholar]
- Minikel EV, Zerr I, Collins SJ, Ponto C, Boyd A, Klug G, Karch A, Kenny J, Collinge J, Takada LT, et al. 2014. Ascertainment bias causes false signal of anticipation in genetic prion disease. Am J Hum Genet 95: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, McLean CY, Tung JY, Yu LP, Gambetti P, et al. 2016. Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med 8: 322ra329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrova E, Belay G. 2002. Creutzfeldt–Jakob disease with E200K mutation in Slovakia: Characterization and development. Acta Virol 46: 31–39. [PubMed] [Google Scholar]
- Moore RC, Xiang F, Monaghan J, Han D, Zhang Z, Edstrom L, Anvret M, Prusiner SB. 2001. Huntington disease phenocopy is a familial prion disease. Am J Hum Genet 69: 1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Herzog C, Errett J, Kocisko DA, Arnold KM, Hayes SF, Priola SA. 2006. Octapeptide repeat insertions increase the rate of protease-resistant prion protein formation. Protein Sci 15: 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, et al. 2013. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 5: 206ra138. [DOI] [PubMed] [Google Scholar]
- Muñoz-Nieto M, Ramonet N, López-Gastón JI, Cuadrado-Corrales N, Calero O, Diaz-Hurtado M, Ipiens JR, Ramón y Cajal S, de Pedro-Cuesta J, Calero M. 2013. A novel mutation I215V in the PRNP gene associated with Creutzfeldt–Jakob and Alzheimer's diseases in three patients with divergent clinical phenotypes. J Neurol 260: 77–84. [DOI] [PubMed] [Google Scholar]
- Neufeld MY, Josiphov J, Korczyn AD. 1992. Demyelinating peripheral neuropathy in Creutzfeldt–Jakob disease. Muscle Nerve 15: 1234–1239. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Sodeyama N, Toru Y, Toru S, Kitamoto T, Mizusawa H. 2004. Creutzfeldt–Jakob disease with a novel insertion and codon 219 Lys/Lys polymorphism in PRNP. Neurology 63: 1978–1979. [DOI] [PubMed] [Google Scholar]
- Nitrini R, Rosemberg S, Passos-Bueno MR, da Silva LS, Iughetti P, Papadopoulos M, Carrilho PM, Caramelli P, Albrecht S, Zatz M, et al. 1997. Familial spongiform encephalopathy associated with a novel prion protein gene mutation. Ann Neurol 42: 138–146. [DOI] [PubMed] [Google Scholar]
- Nixon R, Camicioli R, Jamison K, Cervenáková L, Mastrianni JA. 2000. The PRNP-V180I mutation is associated with abnormally glycosylated PrPCJD and intracellular PrP accumulations. Presented at XIVth International Congress of Neuropathology Scientific Programme. Brain Pathol 10: 670. [Google Scholar]
- Nozaki I, Hamaguchi T, Sanjo N, Noguchi-Shinohara M, Sakai K, Nakamura Y, Sato T, Kitamoto T, Mizusawa H, Moriwaka F, et al. 2010. Prospective 10-year surveillance of human prion diseases in Japan. Brain 133: 3043–3057. [DOI] [PubMed] [Google Scholar]
- Owen F, Poulter M, Lofthouse R, Collinge J, Crow TJ, Risby D, Baker HF, Ridley RM, Hsiao K, Prusiner SB. 1989. Insertion in prion protein gene in familial Creutzfeldt–Jakob disease. Lancet 1: 51–52. [DOI] [PubMed] [Google Scholar]
- Owen F, Poulter M, Shah T, Collinge J, Lofthouse R, Baker H, Ridley R, McVey J, Crow TJ. 1990. An in-frame insertion in the prion protein gene in familial Creutzfeldt–Jakob disease. Brain Res Mol Brain Res 7: 273–276. [DOI] [PubMed] [Google Scholar]
- Owen F, Poulter M, Collinge J, Leach M, Lofthouse R, Crow TJ, Harding AE. 1992. A dementing illness associated with a novel insertion in the prion protein gene. Brain Res Mol Brain Res 13: 155–157. [DOI] [PubMed] [Google Scholar]
- Padovani A, D’Alessandro M, Parchi P, Cortelli P, Anzola GP, Montagna P, Vignolo LA, Petraroli R, Pocchiari M, Lugaresi E, et al. 1998. Fatal familial insomnia in a new Italian kindred. Neurology 51: 1491–1494. [DOI] [PubMed] [Google Scholar]
- Palmer MS, Dryden AJ, Hughes JT, Collinge J. 1991. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt–Jakob disease. Nature 352: 340–342. [DOI] [PubMed] [Google Scholar]
- Palmer MS, Mahal SP, Campbell TA, Hill AF, Sidle KC, Laplanche JL, Collinge J. 1993. Deletions in the prion protein gene are not associated with CJD. Hum Mol Genet 2: 541–544. [DOI] [PubMed] [Google Scholar]
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, et al. 1993. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci 90: 10962–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panegyres PK, Toufexis K, Kakulas BA, Cernevakova L, Brown P, Ghetti B, Piccardo P, Dlouhy SR. 2001. A new PRNP mutation (G131V) associated with Gerstmann–Sträussler–Scheinker disease. Arch Neurol 58: 1899–1902. [DOI] [PubMed] [Google Scholar]
- Parchi P, Castellani R, Cortelli P, Montagna P, Chen SG, Petersen RB, Manetto V, Vnencak-Jones CL, McLean MJ, Sheller JR, et al. 1995. Regional distribution of protease-resistant prion protein in fatal familial insomnia. Ann Neurol 38: 21–29. [DOI] [PubMed] [Google Scholar]
- Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, Farlow M, Dickson DW, Sima AA, Trojanowski JQ, et al. 1996. Molecular basis of phenotypic variability in sporadic Creutzfeldt–Jakob disease. Ann Neurol 39: 767–778. [DOI] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, et al. 1999. Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46: 224–233. [PubMed] [Google Scholar]
- Parchi P, Strammiello R, Notari S, Giese A, Langeveld JP, Ladogana A, Zerr I, Roncaroli F, Cras P, Ghetti B, et al. 2009. Incidence and spectrum of sporadic Creutzfeldt–Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: An updated classification. Acta Neuropathol 118: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore M, Chin SS, Bell KL, Dong Z, Yang Q, Yang L, Yuan J, Chen SG, Gambetti P, Zou WQ. 2005. Creutzfeldt–Jakob disease (CJD) with a mutation at codon 148 of prion protein gene: Relationship with sporadic CJD. Am J Pathol 167: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucar M, Xiang F, Moore R, Walker R, Winnberg E, Svenningsson P. 2013. Genotype-phenotype analysis in inherited prion disease with eight octapeptide repeat insertional mutation. Prion 7: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoc'h K, Manivet P, Beaudry P, Attane F, Besson G, Hannequin D, Delasnerie-Laupretre N, Laplanche JL. 2000. Identification of three novel mutations (E196K, V203I, E211Q) in the prion protein gene (PRNP) in inherited prion diseases with Creutzfeldt–Jakob disease phenotype. Hum Mutat 15: 482. [DOI] [PubMed] [Google Scholar]
- Peoc'h K, Levavasseur E, Delmont E, De Simone A, Laffont-Proust I, Privat N, Chebaro Y, Chapuis C, Bedoucha P, Brandel JP, et al. 2012. Substitutions at residue 211 in the prion protein drive a switch between CJD and GSS syndrome, a new mechanism governing inherited neurodegenerative disorders. Hum Mol Genet 21: 5417–5428. [DOI] [PubMed] [Google Scholar]
- Piccardo P, Dlouhy SR, Lievens PM, Young K, Bird TD, Nochlin D, Dickson DW, Vinters HV, Zimmerman TR, Mackenzie IR, et al. 1998. Phenotypic variability of Gerstmann–Sträussler–Scheinker disease is associated with prion protein heterogeneity. J Neuropathol Exp Neurol 57: 979–988. [DOI] [PubMed] [Google Scholar]
- Pocchiari M, Poleggi A, Puopolo M, D’Alessandro M, Tiple D, Ladogana A. 2013. Age at death of Creutzfeldt–Jakob disease in subsequent family generation carrying the E200K mutation of the prion protein gene. PLoS One 8: e60376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Prokop S, Jung HH, Hewer E, Peretz D, Moos R, Tolnay M, Aguzzi A. 2011. Atypical prion protein conformation in familial prion disease with PRNP P105T mutation. Brain Pathol 21: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priola SA, Chesebro B. 1998. Abnormal properties of prion protein with insertional mutations in different cell types. J Biol Chem 273: 11980–11985. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1998. Prions. Proc Natl Acad Sci 95: 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 2012. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 336: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 2013. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47: 601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti G, Rossi G, Giaccone G, Awan T, Lievens PM, Defanti CA, Tagliavini F, Bugiani O. 2000. Polymorphism at codon 129 of PRNP affects the phenotypic expression of Creutzfeldt–Jakob disease linked to E200K mutation. Ann Neurol 48: 269–270. [PubMed] [Google Scholar]
- Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. 2012. Sporadic human prion diseases: Molecular insights and diagnosis. Lancet Neurol 11: 618–628. [DOI] [PubMed] [Google Scholar]
- Qina T, Sanjo N, Hizume M, Higuma M, Tomita M, Atarashi R, Satoh K, Nozaki I, Hamaguchi T, Nakamura Y, et al. 2014. Clinical features of genetic Creutzfeldt–Jakob disease with V180I mutation in the prion protein gene. BMJ Open 4: e004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder AT, Mednick AS, Brown P, Spire JP, Van Cauter E, Wollmann RL, Cervenáková L, Goldfarb LG, Garay A, Ovsiew F, et al. 1995. Clinical and genetic studies of fatal familial insomnia. Neurology 45: 1068–1075. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Peoc'h K, Haik S, Bouchet C, Vernengo L, Manana G, Salamano R, Carrasco L, Lenne M, Beaudry P, et al. 2005. A novel mutation (G114V) in the prion protein gene in a family with inherited prion disease. Neurology 64: 1455–1457. [DOI] [PubMed] [Google Scholar]
- Roeber S, Krebs B, Neumann M, Windl O, Zerr I, Grasbon-Frodl EM, Kretzschmar HA. 2005. Creutzfeldt–Jakob disease in a patient with an R208H mutation of the prion protein gene (PRNP) and a 17-kDa prion protein fragment. Acta Neuropathol 109: 443–448. [DOI] [PubMed] [Google Scholar]
- Roeber S, Grasbon-Frodl EM, Windl O, Krebs B, Xiang W, Vollmert C, Illig T, Schroter A, Arzberger T, Weber P, et al. 2008. Evidence for a pathogenic role of different mutations at codon 188 of PRNP. PLoS One 3: e2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Zadikoff C, Ponesse J, Schmitt-Ulms G, Kawarai T, Sato C, Salehi-Rad S, St George-Hyslop P, Lang AE. 2006. Childhood onset in familial prion disease with a novel mutation in the PRNP gene. Arch Neurol 63: 1016–1021. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Kahana E, Korczyn AD, Kahana I, Chapman J, Gabizon R. 1999. Preliminary evidence for anticipation in genetic E200K Creutzfeldt–Jakob disease. Neurology 53: 1328–1329. [DOI] [PubMed] [Google Scholar]
- Rossi G, Giaccone G, Giampaolo L, Iussich S, Puoti G, Frigo M, Cavaletti G, Frattola L, Bugiani O, Tagliavini F. 2000. Creutzfeldt–Jakob disease with a novel four extra-repeat insertional mutation in the PrP gene. Neurology 55: 405–410. [DOI] [PubMed] [Google Scholar]
- Rowe DB, Lewis V, Needham M, Rodriguez M, Boyd A, McLean C, Roberts H, Masters CL, Collins SJ. 2007. Novel prion protein gene mutation presenting with subacute PSP-like syndrome. Neurology 68: 868–870. [DOI] [PubMed] [Google Scholar]
- Sanchez-Valle R, Yague J, Turon A, Arostegui JI, Nos C, Rey MJ, Ferrer I, Gelpi E. 2012. Inherited prion disease with 4-octapeptide repeat insertion linked to valine at codon 129. Brain 135: e212. [DOI] [PubMed] [Google Scholar]
- Sano K, Satoh K, Atarashi R, Takashima H, Iwasaki Y, Yoshida M, Sanjo N, Murai H, Mizusawa H, Schmitz M, et al. 2013. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS One 8: e54915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelzke G, Eigenbrod S, Romero C, Varges D, Breithaupt M, Taratuto AL, Kretzschmar HA, Zerr I. 2011. Genetic prion disease with codon 196 PRNP mutation: Clinical and pathological findings. Neurobiol Aging 32: 756.e1-9. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Dittmar K, Llorens F, Gelpi E, Ferrer I, Schulz-Schaeffer WJ, Zerr I. 2016. Hereditary human prion diseases: An update. Mol Neurobiol. 10.1007/s12035-016-9918-y. [DOI] [PubMed] [Google Scholar]
- Shi Q, Zhou W, Chen C, Zhang BY, Xiao K, Zhang XC, Shen XJ, Li Q, Deng LQ, Dong JH, et al. 2015. The features of genetic prion diseases based on Chinese surveillance program. PLoS One 10: e0139552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga Y, Satoh K, Kitamoto T, Kanno S, Nakashima I, Sato S, Fujihara K, Takata H, Nobukuni K, Kuroda S, et al. 2007. Two different clinical phenotypes of Creutzfeldt–Jakob disease with a M232R substitution. J Neurol 254: 1509–1517. [DOI] [PubMed] [Google Scholar]
- Shiraishi A, Mizusawa H, Yamada M. 2002. Early and persistent sensory-psychiatric symptoms in an inherited prion disease with a PrP P105L mutation. J Neurol 249: 1740–1741. [DOI] [PubMed] [Google Scholar]
- Simpson M, Johanssen V, Boyd A, Klug G, Masters CL, Li QX, Pamphlett R, McLean C, Lewis V, Collins SJ. 2013. Unusual clinical and molecular-pathological profile of Gerstmann–Sträussler–Scheinker disease associated with a novel PRNP mutation (V176G). JAMA Neurol 70: 1180–1185. [DOI] [PubMed] [Google Scholar]
- Skworc KH, Windl O, Schulz-Schaeffer WJ, Giese A, Bergk J, Nagele A, Vieregge P, Zerr II, Poser S, Kretzschmar HA. 1999. Familial Creutzfeldt–Jakob disease with a novel 120-bp insertion in the prion protein gene. Ann Neurol 46: 693–700. [PubMed] [Google Scholar]
- Soto C. 2011. Prion hypothesis: The end of the controversy? Trends Biochem Sci 36: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Mastrianni JA, Wrensch M, Gabizon R, Meiner Z, Kahana I, Rosenmann H, Kahana E, Prusiner SB. 1995. Complete penetrance of Creutzfeldt–Jakob disease in Libyan Jews carrying the E200K mutation in the prion protein gene. Mol Med 1: 607–613. [PMC free article] [PubMed] [Google Scholar]
- Stevens DJ, Walter ED, Rodriguez A, Draper D, Davies P, Brown DR, Millhauser GL. 2009. Early onset prion disease from octarepeat expansion correlates with copper binding properties. PLoS Pathog 5: e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada LT, Kim MO, Cleveland RW, Wong K, Forner SA, Gala II, Fong JC, Geschwind MD. 2017. Genetic prion disease: Experience of a rapidly progressive dementia center in the United States and a review of the literature. Am J Med Genet B Neuropsychiatr Genet 174: 36–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki Y, Hara H, Doh-Ura K, Murakami I, Tashiro H, Yamasaki T, Shigeto H, Arakawa K, Araki E, Yamada T, et al. 2000. Familial Creutzfeldt–Jakob disease with D178N-129M mutation of PRNP presenting as cerebellar ataxia without insomnia. J Neurol Neurosurg Psychiatry 68: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia MC, Thai JN, See T, Kuo A, Harbaugh R, Raudabaugh B, Cali I, Sattavat M, Sanchez H, DeArmond SJ, et al. 2010. Pathologic evidence that the T188R mutation in PRNP is associated with prion disease. J Neuropathol Exp Neurol 69: 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi J. 1991. [Recent advances in the research of Creutzfeldt–Jakob disease (CJD) and Gerstmann-Sträussler syndrome (GSS)]. Rinsho Shinkeigaku 31: 1306–1308. [PubMed] [Google Scholar]
- Tateishi J, Kitamoto T, Doh-ura K, Sakaki Y, Steinmetz G, Tranchant C, Warter JM, Heldt N. 1990. Immunochemical, molecular genetic, and transmission studies on a case of Gerstmann–Sträussler–Scheinker syndrome. Neurology 40: 1578–1581. [DOI] [PubMed] [Google Scholar]
- Tousseyn T, Bajsarowicz K, Sanchez H, Gheyara A, Oehler A, Geschwind M, DeArmond B, DeArmond SJ. 2015. Prion disease induces Alzheimer disease-like neuropathologic changes. J Neuropathol Exp Neurol 74: 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchant C, Doh-ura K, Warter JM, Steinmetz G, Chevalier Y, Hanauer A, Kitamoto T, Tateishi J. 1992. Gerstmann–Sträussler–Scheinker disease in an Alsatian family: Clinical and genetic studies. J Neurol Neurosurg Psychiatry 55: 185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnell E, Wollman R, Mallik S, Cortes CJ, Dearmond SJ, Mastrianni JA. 2008. A novel PRNP-P105S mutation associated with atypical prion disease and a rare PrPSc conformation. Neurology 71: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uflacker A, Doraiswamy PM, Rechitsky S, See T, Geschwind M, Tur-Kaspa I. 2014. Preimplantation genetic diagnosis (PGD) for genetic prion disorder due to F198S mutation in the PRNP gene. JAMA Neurol 71: 484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK National CJD Surveillance Unit. 2015. Variant Creutzfeldt–Jakob disease worldwide current data (April 2015). Western General Hospital, Edinburgh, Scotland. [Google Scholar]
- Van Gool WA, Walstra GJ, Teunisse S, Van der Zant FM, Weinstein HC, Van Royen EA. 1995. Diagnosing Alzheimer’s disease in elderly, mildly demented patients: The impact of routine single photon emission computed tomography. J Neurol 242: 401–405. [DOI] [PubMed] [Google Scholar]
- van Harten B, van Gool WA, Van Langen IM, Deekman JM, Meijerink PH, Weinstein HC. 2000. A new mutation in the prion protein gene: A patient with dementia and white matter changes. Neurology 55: 1055–1057. [DOI] [PubMed] [Google Scholar]
- Vita MG, Gaudino S, Di Giuda D, Sauchelli D, Alboini PE, Gangemi E, Bizzarro A, Scaricamazza E, Capellari S, Parchi P, et al. 2013. R208H-129VV haplotype in the prion protein gene: phenotype and neuroimaging of a patient with genetic Creutzfeldt–Jakob disease. J Neurol 260: 2650–2652. [DOI] [PubMed] [Google Scholar]
- Vital C, Gray F, Vital A, Parchi P, Capellari S, Petersen RB, Ferrer X, Jarnier D, Julien J, Gambetti P. 1998. Prion encephalopathy with insertion of octapeptide repeats: The number of repeats determines the type of cerebellar deposits. Neuropathol Appl Neurobiol 24: 125–130. [DOI] [PubMed] [Google Scholar]
- Vitali P, Maccagnano E, Caverzasi E, Henry RG, Haman A, Torres-Chae C, Johnson DY, Miller BL, Geschwind MD. 2011. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 76: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Guo YJ, Zhang BY, Zhao WQ, Gao JM, Wan YZ, Li F, Han J, Wang DX, Dong XP. 2007. Creutzfeldt–Jakob disease in a Chinese patient with a novel seven extra-repeat insertion in PRNP. J Neurol Neurosurg Psychiatry 78: 201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Serban A, Patel S, Oehler A, Bhardwaj S, Guan S, Greicius MD, Miller BL, DeArmond SJ, et al. 2015. Modulation of Creutzfeldt–Jakob disease prion propagation by the A224V mutation. Ann Neurol 78: 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Poulter M, Beck J, Uphill J, Adamson G, Campbell T, Linehan J, Powell C, Brandner S, Pal S, et al. 2008. Phenotypic heterogeneity and genetic modification of P102L inherited prion disease in an international series. Brain 131: 2632–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 1998. Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies: Report of a WHO consultation Geneva, Switzerland 9–11 February 1998. In World Health Organization: Emerging and other communicable diseases, surveillance and control. World Health Organization, Geneva. [Google Scholar]
- Will RG, Ironside JW. 2017. Sporadic and infectious human prion diseases. Cold Spring Harb Perspect Med 7: a024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windl O, Giese A, Schulz-Schaeffer W, Zerr I, Skworc K, Arendt S, Oberdieck C, Bodemer M, Poser S, Kretzschmar HA. 1999. Molecular genetics of human prion diseases in Germany. Hum Genet 105: 244–252. [DOI] [PubMed] [Google Scholar]
- Woerman AL, Stohr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC, Ohyama T, Patel S, Widjaja K, Oehler A, et al. 2015. Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci 112: E4949–E4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woulfe J, Kertesz A, Frohn I, Bauer S, St George-Hyslop P, Bergeron C. 2005. Gerstmann–Sträussler–Scheinker disease with the Q217R mutation mimicking frontotemporal dementia. Acta Neuropathol 110: 317–319. [DOI] [PubMed] [Google Scholar]
- Yamada M, Itoh Y, Inaba A, Wada Y, Takashima M, Satoh S, Kamata T, Okeda R, Kayano T, Suematsu N, et al. 1999. An inherited prion disease with a PrP P105L mutation: Clinicopathologic and PrP heterogeneity. Neurology 53: 181–188. [DOI] [PubMed] [Google Scholar]
- Yang TI, Jung DS, Ahn BY, Jeong BH, Cho HJ, Kim YS, Na DL, Geschwind MD, Kim EJ. 2010. Familial Creutzfeldt–Jakob disease with V180I mutation. J Korean Med Sci 25: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Han J, Shi Q, Zhang BY, Wang GR, Tian C, Gao C, Chen JM, Li CJ, Liu Z, et al. 2008. Human prion disease with a G114V mutation and epidemiological studies in a Chinese family: A case series. J Med Case Rep 2: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SL, Jin L, Sy MS, Mei FH, Kang SL, Sun GH, Tien P, Wang FS, Xiao GF. 2004. Polymorphisms of the PRNP gene in Chinese populations and the identification of a novel insertion mutation. Eur J Hum Genet 12: 867–870. [DOI] [PubMed] [Google Scholar]
- Yusa S, Oliveira-Martins JB, Sugita-Konishi Y, Kikuchi Y. 2012. Cellular prion protein: From physiology to pathology. Viruses 4: 3109–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Digon A, Atarés B, Rodríguez-Martínez AB, Arce A, Carrera N, Fernández-Manchola I, Fernández-Martínez M, Fernández-Maiztegui C, Forcadas I, et al. 2005. Phenotypic variability in familial prion diseases due to the D178N mutation. J Neurol Neurosurg Psychiatry 76: 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr I, Giese A, Windl O, Kropp S, Schulz-Schaeffer W, Riedemann C, Skworc K, Bodemer M, Kretzschmar HA, Poser S. 1998. Phenotypic variability in fatal familial insomnia (D178N-129M) genotype. Neurology 51: 1398–1405. [DOI] [PubMed] [Google Scholar]
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, et al. 2009. Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132: 2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Longfei J, Jing Y, Xinqing Z, Haiqing S, Haiyan L, Fen W, Xiumin D, Jianping J. 2008. PRNP mutations in a series of apparently sporadic neurodegenerative dementias in China. Am J Med Genet B Neuropsychiatr Genet 147B: 938–944. [DOI] [PubMed] [Google Scholar]