Abstract
Background and Objective
Postranslational modification of proteins can lead to the production of autoantibodies and loss of immune tolerance. This process has been hypothesised to be a critical factor in the pathogenesis of rheumatoid arthritis. The objective of this study was to demonstrate that inflamed human gingival tissue provides an extrasynovial source of malondialdehyde-acetaldehyde adducts, citrullinated and carbamylated proteins all of which are considered to be linked to the development of rheumatoid arthritis. Identification of such modified proteins in inflamed gingiva may explain, in part, how inflammation of the periodontal tissues may influence the development of rheumatoid arthritis.
Material and Methods
Gingival biopsies of healthy, mild and moderate periodontitis were triple stained with antibodies against malondialdehyde-acetaldehyde adducts, citrullinated and carbamylated proteins.
Results
Assessment of healthy gingival tissue revealed negligible staining for carbamylated, MAA, or citrullinated proteins. Mild periodontitis was positive for all three modifications. Furthermore, there was an increase in staining intensity for carbamylated, citrullinated and MAA-modified proteins in moderate periodontitis. Negative staining results were observed for the isotype controls
Conclusion
This study provides evidence for the presence of citrullinated, carbamylated and MAA adduct modified proteins in inflamed periodontal tissues. The potential for these proteins to play a role in autoimmunity in a multi-system inflammatory syndromic disease model now needs to be determined.
Keywords: citrullination, carbamylation, malondialdehyde-acetaldehyde adduct, triple Immunofluorescence staining immunohistochemistry
Introduction
The relationship between periodontitis and rheumatoid arthritis (RA) has received considerable attention in recent years. It has been proposed that these two diseases are interrelated through common pathogenic mechanisms (1, 2). Many studies have demonstrated that the relationship may be bi-directional in that periodontitis can influence clinical RA parameters and, conversely, RA can influence the manifestation of periodontitis (3). Interestingly, treatments for both conditions can influence each other (4, 5).
RA is an autoimmune disease characterized by the presence of auto-antibodies. The recognition that autoantibody production to citrullinated proteins plays a role in its development and/or progression of the disease has been a significant advance in understanding the pathophysiology of RA (6–8). ACPA have a high predictive value for the onset of RA several years before the disease is evident clinically and are also associated with more severe and worse clinical outcomes (6, 7). In addition to citrullination, a process known as carbamylation can also lead to post-translational modification of proteins resulting in the production of autoantibodies that are elevated in patients with RA (9). Most recently, it has been noted, that malondialdehyde-acetaldehyde (MAA) adduct formation, as a result of inflammation-associated oxidative stress, is increased in RA patients and that the antibody response against these post-translationally modified proteins are intricately associated with ACPAs and potentially act as another factor leading to tolerance loss and the robust autoimmune response observed in RA (10).
All three of these responses, citrullination, carbamylation and malondialdehyde-acetaldehyde adduct formation, can arise due to inflammatory reactions occurring outside of the synovium (11). Since the development of periodontitis is a gradual progression, initially involving the development of gingivitis with subsequent extensive inflammatory-mediated tissue damage leading to periodontitis, we and others have proposed that the inflamed periodontium associated with gingivitis and periodontitis may be an initiating source of autoantibody production and the loss of immune tolerance (12). To date, all of the focus of this concept of induction of autoimmunity and loss of tolerance as a linking feature for periodontitis and RA has been towards citrullination. Here, we propose that not only does citrullination occur in inflamed periodontal tissues, but these tissues can also act as a significant source of protein carbamylation and MAA adduct formation. Therefore, the aim of this study was to identify the presence of all three postranslational protein modifications in inflamed human periodontal tissues and confirm the periodontium as a source of extra-synovial citrullination, carbamylation and MAA adduct formation.
Materials and Methods
Gingival Tissue Biopsies
Human ethics approval was obtained from the University of Adelaide and all patients signed informed consent for the use of the excised tissues. General inclusion criteria included dentate patients (at least 20 teeth) willing to participate in the study. General exclusion criteria included patients who would not give informed consent, aggressive periodontitis, obvious endodontic infections or other sources of oral infection, pregnant or lactating females, patients with a significant medical history indicating evidence of known systemic modifiers of periodontal disease such as type I and II diabetes mellitus, osteoporosis, disorders of cellular immunity (e.g. AIDS, cyclic neutropenia, or other known specific leukocyte defects which we know predispose to periodontitis) and medications known to influence the periodontal tissues (e.g. calcium channel blockers, phenytoin and immunomodulatory medications such as cyclosporine). Smokers were also excluded.
Biopsies of inflamed periodontal tissues (n=6) were obtained following periodontal surgery as part of routine patient management protocols at the University of Adelaide Periodontal Clinic.
Chronic periodontitis was classified and graded using clinical (attachment loss and pocket depth) and radiographic assessments as mild or moderate (13). Accordingly mild periodontitis cases (n = 3; age matched = 65 years old) were identified as having probing depths between >3mm & <5 mm, bleeding on probing, radiographic evidence of bone loss of ≥2 mm & ≤3 mm and clinical attachment loss of 1 mm - 2 mm. Moderate periodontitis cases (n=3; age matched = 58 years old) were identified as having between >5 mm & <7 mm probing depths, bleeding on probing, radiographic evidence of bone loss of ≥3 mm & ≤5 mm and clinical attachment loss of 3mm - 4 mm. Control healthy tissues (n=3; age matched = 56 years old) were obtained following routine crown lengthening procedures. The excised tissues were immediately fixed in 10% buffered formalin for 24 hours prior to processing and paraffin embedding. The Sections were cut for haematoxylin and eosin staining and unstained sections were cut at 7 μm for triple immunofluorescence staining.
Triple Staining Immunohistochemistry
Slides containing the gingival sections were dewaxed, dehydrated and rinsed in phosphate buffered saline (PBS). Primary antibodies were diluted 1:200 Mouse anti-peptidyl-citrulline (clone F95, EMD Millipore Corporation, Temecula, CA, USA) and 1:2000 Rabbit anti-malondialdehyde-acetaldehyde adducts (10) and incubated with the gingival tissue sections overnight at room temperature. Sections were washed and incubated with mouse secondary 594 and rabbit secondary 488 diluted at 1:400. Sections were again washed and incubated overnight in the dark with the third primary antibody, rabbit anti-carbamyl-lysine (Cell Biolabs, Inc. San Diego, CA, and USA) diluted at 1:8000. After washing in PBS, sections were incubated with biotinylated anti-rabbit (Vector Laboratories, Burlingame, CA, USA) for 30 minutes followed by Streptavidin, Alexa Fluor® 405 conjugate (Thermo Fisher Scientific, Waltham, MA, USA) diluted at 1:400. After incubation for one hour at room temperature, the slides were washed, mounted, and imaged using a Leica SP5 spectral scanning confocal microscope. Sections were also stained with haematoxylin and eosin to determine the level of inflammation as described previously (14).
Results
Hematoxylin and eosin sections confirmed clinical diagnosis (Figure 1). Mild periodontitis showed a slight increase in polymorphonuclear leukocyte (PMN) infiltration, moderate periodontitis show a marked increase in PMN infiltration together with architectural changes to the gingival connective tissue. Gingival tissue samples from 3 healthy and 6 periodontitis subjects were stained for the presence of citrullinated, carbamylated and MAA modified proteins. Representative immunostaining of inflamed periodontal tissues is shown in Figures 2A–2C as follows: carbamylated proteins (blue staining, top right), MAA-modified proteins (green staining, bottom left) and citrullinated proteins (red staining, bottom right), and merged images of all three protein (top left). Assessment of healthy gingival tissue (Figure 2A) revealed negligible staining for carbamylated, MAA, or citrullinated proteins. Mild periodontitis was positive for all three modifications (Figure 2B). Furthermore, there was an increase in staining intensity for carbamylated, citrullinated and MAA-modified proteins in moderate periodontitis (Figure 2C). Negative staining results were observed for the isotype controls (Figure 2D).
Figure 1.
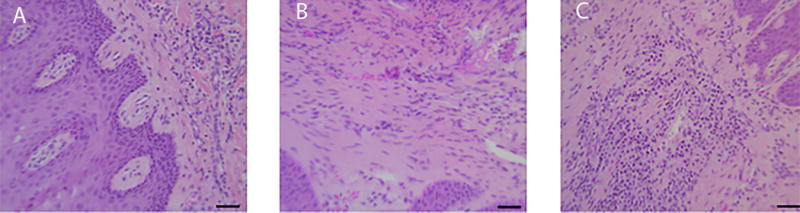
Hematoxylin and eosin stained gingival sections of healthy (A), mild (B) and moderate periodontitis (C) showing increasing inflammatory changes. Magnification 40×
Figure 2.
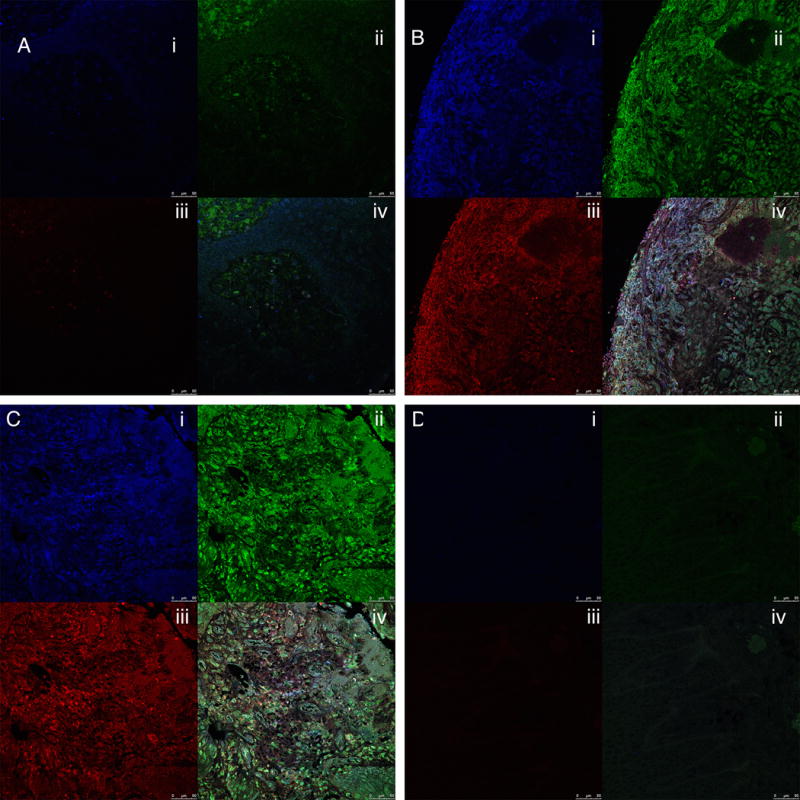
Immunostaining of human gingival connective tissue for carbamylated, citrullinated and MAA-modified proteins. 2A; healthy gingival tissue, 2B; mild periodontitis, 2C; moderate periodontitis. 2D Isotype control showing no background signal. (i) = Carbamylated proteins (blue staining), (ii) = MAA-modified proteins (green staining, bottom left); (iii) = citrullinated proteins (red staining), and (iv) = merged images of staining for all three modified proteins. Magnification: 40×; Bar = 50μm.
These sections correspond to the connective tissue fields shown in Figure 1.
Discussion
Evidence for the relationship between periodontitis and RA is accruing at a considerable pace. However, the precise mechanisms that are associated with, or drive, this relationship are far from clear. A number of hypotheses have been presented over the years to explain how these two diseases might be interconnected (1, 12). These have included immune/inflammatory dysregulation, common pathways of osteoclast activation and vascular damage and citrullination leading to tolerance loss (1). Of these, citrullination has received the most attention. In particular extrasynovial citrullination and other posttranslational modifications of proteins are considered a very important step in the etiopathogenesis of RA and potential sites for these protein modifications to occur include the lungs of smokers, inflamed periodontal tissues and other sites of chronic inflammation.
To date, the focus of periodontal citrullination has been largely targeted towards the role of the periodontal pathogen, P gingivalis, and its ability to citrullinate proteins through its own peptidyl arginine deiminase (PPAD) (15). However, while this is an attractive hypothesis, neither P gingivalis nor PPAD have been demonstrated in the early inflammatory lesion associated with the development of periodontitis (16, 17). Conversely, inflammation is the hallmark pathologic feature of both gingivitis and periodontitis and, thus, we propose that it is the inflammation (not bacteria) occurring very early in the development of gingivitis and periodontitis that has the capability to induce posttranslational protein modification, autoantibody production and subsequent exacerbation of the RA lesion (14).
In this study, for the first time, we demonstrate the co-existence of three important postranslationally modified proteins that are considered influential in subsequent development of an autoantibody repertoire specific for the clinical manifestation of RA. Several studies have identified citrullinated proteins in inflamed periodontal tissues (14, 18). In addition, one preliminary report has demonstrated the presence of carbamylated proteins in inflamed human gingiva (19). These findings correlate well with a report demonstrating that induction of autoimmunity to citrullinated proteins can be associated with citrullination of proteins within inflamed periodontal tissues (20). In particular these proteins appear to be closely associated with the inflammatory cell infiltrate within the gingival connective tissues of mild and moderate periodontitis samples. This observation is consistent with other studies that have identified posttranslational protein modification in the presence of inflammatory cell infiltrates, particularly neutrophils. The findings of the present study expand these earlier observations and confirm that significant post-translational modification of proteins occurs in inflamed periodontal tissues and has the potential to lead to significant autoantibody formation. If this is the case, then the possibility exists that auto-antibodies may be produced in inflamed periodontal tissues. If a later event such as joint inflammation results in further protein modifications then, in a primed individual, the subsequent antibody response could be very robust. This concept supports the two-hit model proposed for the development of chronic inflammatory disorders such as RA (21). Furthermore, such a response is in keeping with recent observations that experimental animals with a pre-existing chronic infection or periodontitis develop experimental arthritis at a faster and more pronounced rate than non-primed animals (22, 23).
In conclusion, this study provides evidence for the presence of citrullinated, carbamylated and MAA adduct modified proteins in inflamed periodontal tissues. The potential for these proteins to play a role in autoimmunity in a multi-system inflammatory syndromic disease model now needs to be determined.
Footnotes
PROF. P MARK BARTOLD (Orcid ID : 0000-0002-5695-3877)
References
- 1.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: A review. J Periodontol. 2005;76:2066–2074. doi: 10.1902/jop.2005.76.11-S.2066. [DOI] [PubMed] [Google Scholar]
- 2.de Pablo P, Chapple ILC, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, White S, Bartold PM. Periodontal Disease and Rheumatoid Arthritis: A Systematic Review. Journal of dental research. 2013;92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- 4.Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis Semin Arthritis Rheu. 2014;44:113–122. doi: 10.1016/j.semarthrit.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Payne JB, Golub LM, Thiele GM, Mikuls TR. The Link Between Periodontitis and Rheumatoid Arthritis: A Periodontist’s Perspective. Current oral health reports. 2015;2:20–29. doi: 10.1007/s40496-014-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroot EJJA, de Jong BAW, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis and rheumatism. 2000;43:1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Rantapaa-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis and rheumatism. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 8.Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunological reviews. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele GM, Duryee MJ, Anderson DR, et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis & rheumatology. 2015;67:645–655. doi: 10.1002/art.38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immun. 2016;137:28–34. doi: 10.1016/j.jaci.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–318. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Periodontology. Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol. 2015;86:835–838. doi: 10.1902/jop.2015.157001. [DOI] [PubMed] [Google Scholar]
- 14.Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. Journal of periodontal research. 2013;48:252–261. doi: 10.1111/jre.12002. [DOI] [PubMed] [Google Scholar]
- 15.Gully N, Bright R, Marino V, et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS One. 2014;9:e100838. doi: 10.1371/journal.pone.0100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Laboratory investigation; a journal of technical methods and pathology. 1976;34:235–249. [PubMed] [Google Scholar]
- 17.Bartold PM, Van Dyke TE. Host modulation: Controlling the inflammation to control the infection. Periodontology 2000. 2017 doi: 10.1111/prd.12169. Accepted for publication May 18, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Nesse W, Dijkstra PU, Abbas F, et al. Increased Prevalence of Cardiovascular and Autoimmune Diseases in Periodontitis Patients: A Cross-Sectional Study. J Periodontol. 2010;81:1622–1628. doi: 10.1902/jop.2010.100058. [DOI] [PubMed] [Google Scholar]
- 19.Bright R, Proudman SM, Rosenstein ED, Bartold PM. Is there a link between carbamylation and citrullination in periodontal disease and rheumatoid arthritis? Medical Hypotheses. 2015;84:570–576. doi: 10.1016/j.mehy.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 20.de Pablo P, Dietrich T, Chapple ILC, et al. The autoantibody repertoire in periodontitis: a role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Annals of the rheumatic diseases. 2014;73:580–586. doi: 10.1136/annrheumdis-2012-202701. [DOI] [PubMed] [Google Scholar]
- 21.Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. Journal of dental research. 2006;85:102–105. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 22.Ramamurthy HS, Greenwald RA, Celiker MY, Shi EY. Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metalloproteinases. J Periodontol. 2005;76:229–233. doi: 10.1902/jop.2005.76.2.229. [DOI] [PubMed] [Google Scholar]
- 23.Cantley MD, Haynes DR, Marino V, Bartold PM. Pre-existing periodontitis exacerbates experimental arthritis in a mouse model. Journal of clinical periodontology. 2011;38:532–541. doi: 10.1111/j.1600-051X.2011.01714.x. [DOI] [PubMed] [Google Scholar]


