Abstract
Obesity has been linked to the increased risk and aggressiveness of many types of carcinoma. A state of chronic inflammation in adipose tissue, resulting in genotoxic stress, may contribute to carcinogenesis and cancer initiation. Evidence that adipose tissue plays a role in cancer aggressiveness is solid and mounting. During cancer progression, tumor cells engage in a metabolic symbiosis with adjacent adipose tissue. Mature adipocytes provide adipokines and lipids to cancer cells, while stromal and immune cells from adipose tissue infiltrate carcinomas and locally secrete paracrine factors within the tumor microenvironment. This review focuses on the cross-talk between adipose tissue and tumor cells that promotes tumor growth and increases cellular lipid metabolism, metastasis, and chemoresistance.
The Cancer-Obesity Link – Epidemiologic Evidence
Obesity results from the expansion of white adipose tissue (WAT), commonly referred to as fat [1]. About 66% of all adults in the United States are overweight and 22% are diagnosed with obesity, as defined as a body mass index (BMI) exceeding 30 kg/m2 [2]. The association of obesity with cardiovascular disease, as well as with glucose intolerance, dyslipidemia, lipotoxicity, and other components of type-2 diabetes and the metabolic syndrome, is well established. In the past few years, it has also become apparent that an estimated 40% of all cancer deaths in the United States are attributable, in large part, to obesity [3]. An association has been established between obesity and increased mortality from cancer of the prostate and stomach in men; the breast (postmenopausal), cervix, and uterus in women; and the kidney (renal cell), colon, esophagus (adenocarcinoma), pancreas, gallbladder, and liver in both sexes [4]. More recently, reports from the Metabolic Syndrome and Cancer Project, a European cohort study of ~ 580,000 adults, also confirmed associations between obesity and risks of colorectal, and thyroid cancers [5]. A link between obesity and cancer is further supported by epidemiological studies that have provided evidence that intentional weight loss and bariatric surgery reduces cancer incidence [6]. Moreover abdominal (central) obesity, resulting from the overgrowth of visceral WAT, has been specifically linked with cancer progression [7]. When considering these associations, it is important to separately consider the stimulatory effects of obesity on carcinogenesis, resulting in higher cancer incidence, and on cancer progression, resulting in increased mortality. For example, while obesity is associated with an increased risk of post-menopausal breast cancer, menopausal status is not a factor in the negative effects of obesity on prognosis [8]. By 2025, the global obesity rate is projected to reach 21% in women which, since 55% of all female cancers currently diagnosed are obesity-associated, is particularly concerning. A number of mechanisms have been proposed to explain the role of obesity in cancer risk and progression. These include chronic inflammation, hyperinsulinemia, and changes in circulating levels of steroid hormones, glucose and lipids, as well as cytokines and growth factors such as IGF-1, leptin and adiponectin [9, 10]. Detailed descriptions of these circulating molecules, and their functions in the setting of cancer, have been previously published [8–12] and are beyond the scope of this review. While diet is important in considering the obesity-cancer link, studies in animal models have shown that WAT overgrowth directly promotes cancer progression irrespective of diet [13, 14].
The Role of Activated Adipose Tissue in Cancer
Adipose tissue (AT) is a complex organ important for the regulation of the body’s energy balance [15]. The primary function of WAT is to store energy as lipids in adipocytes and to release them in response to physiological energy demand [1]. However, in addition to adipocytes, WAT is composed of a number of other cell types within the stromal-vascular fraction, which have diverse functions [11]. Mesenchymal stromal cells of WAT, also termed adipose stromal cells (ASC), are perivascular cells supporting the endothelium and serving as adipocyte progenitors [16, 17]. A heterogeneous palate of innate and adaptive immune cells, including macrophages, dendritic cells, mast cells, eosinophils, neutrophils, and T and B lymphocytes are contained in WAT as well [18]. Collectively, adipocytes and other stromal cells of WAT serve as a source of bioactive molecules regulating important signaling pathways implicated in cancer initiation and progression.
WAT composition and physiology predetermine susceptibility to the metabolic syndrome and its complications. Studies in mouse models have shown that, upon reaching a certain overgrowth threshold, WAT becomes inflamed and fibrotic [19], and it has been proposed that tumorigenesis and cancer progression are promoted by WAT dysfunction and chronic inflammation [10]. Factors, including the anatomic location of AT, sex, age, and metabolic status can alter the tissue milieu and characteristics in ways we are only just beginning to understand [20]. A growing body of evidence indicates that WAT accretion and deregulation in obesity, rather than the lifestyle responsible for obesity onset, is the key determinant of cancer initiation and progression [11].
In addition to storing energy, WAT functions as an endocrine organ, secreting bioactive molecules termed adipokines [9]. Currently, more than 50 different adipokines have been identified, the majority produced by adipocytes [21]. In obesity, with an increase in WAT mass and cellularity, circulating adipokine levels are altered. Leptin and adiponectin are the most thoroughly studied of the adipokines specifically produced by adipocytes. While leptin levels increase in obesity, adiponectin levels decrease, and this altered leptin/adiponectin ratio has been found to correlate with cancer aggressiveness [22]. Other growth factors and cytokines implicated in the progression of obesity-related cancers include TNFα, IL-6, and IGF-1 [12]. Recently, a cancer-promoting role for chemokine CXCL12 (SDF1α), secreted by WAT, has been revealed [23]. Non-peptide WAT-endocrine factors, including steroid hormones and lipids, also modulate processes ranging from extra-cellular matrix (ECM) remodeling to cancer cell signaling and metabolism [21]. Collectively, adipokines promote tumor growth either via oncogenic signaling or through indirect mechanisms, such as angiogenesis and immunomodulation [9, 12, 21].
Since the role of fat tissue in cancer was first investigated, it has been assumed that all of the body’s WAT depots stimulate cancer progression through the systematic circulation of these endocrine factors. However, the observation that cancers that are not surrounded by WAT are not linked with obesity suggests that carcinomas are fueled by direct local exposure to proximal adipose cells. Adipocytes are typically absent within the normal parenchyma of epithelial glands. However, in invasive carcinomas, adipocytes come in direct contact with tumors, especially in the reproductive (prostate, uterus, breast) and digestive organs [4]. Several epithelial cancers (gastric, colon, serous endometrial, and ovarian) disseminate within the abdominal cavity to the omentum, a WAT pad of approximately 20×20×3cm, which drapes from the stomach over the gastrointestinal tract [24]. Tumor-associated WAT has been found to play a role in breast cancer progression [25–27] and in aggressive prostate cancer, extraprostatic extension of the tumor into WAT beyond the confines of the gland is a key determinant of disease aggressiveness [28, 29].
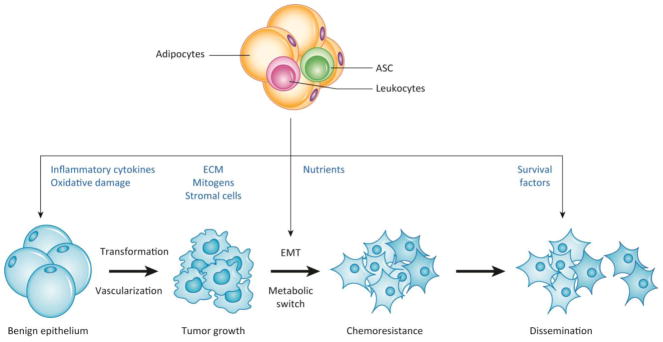
Once the cancer cells become intermingled with adipocytes and other cells of WAT, a crosstalk ensues. Mechanistically, it is mediated by paracrine and contact-dependent signaling from adipocytes, and the adipose stroma [21, 24, 30]. Inflammation-associated changes in WAT and tumor ECM also appear to play a role in cancer initiation and promotion [18]. In obesity, inadequate oxygenation of hypertrophic adipocytes results in cell death, which triggers a dynamic activation of innate and adaptive immune populations. Infiltrating and resident immune cells are a potent source of pro-inflammatory cytokines and chemokines, growth factors, and matrix-degrading enzymes, such as matrix metalloproteases (MMPs), which remodel the tissue and induce chronic low-grade inflammation [19]. In the WAT of obese patients, activated adipocytes, ASC, as well as monocytes/macrophages and other leukocytes infiltrating from WAT, stimulate molecular mechanisms that promote successive stages of cancer progression (Figure 1, Key Figure).
Figure 1 Key Figure.
The roles of cells from adipose tissue in the successive steps of cancer progression
Interaction between AT and epithelium during cancer initiation and progression. WAT secretes factors that promote transformation of benign epithelium, induce the EMT and the switch to increased fatty acid metabolism in cancer cells, and eventually promote metastases and chemoresistance. Both endocrine WAT factors and paracrine factors from WAT-derived cells (adipocytes, ASC, and leukocytes) infiltrating tumors contribute to the steps of cancer progression.
Abbreviations: AT, adipose tissue; WAT, white adipose tissue; ASC, adipose stromal cells; BAT, beige adipose tissue; CAF, cancer associated fibroblasts; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; FA, fatty acids; MMP, matrix metalloproteases; PD-1, Programmed Death-1; ROS, reactive oxygen species; TAM, tumor-associated macrophages
Adipose Tissue Inflammation and Cancer Initiation
Inflammation is a hallmark of cancer that underlies obesity-linked carcinogenesis. Tumors can be considered as wounds that “do not heal” due to chronic inflammation induced by immune cells [31]. In WAT, innate and adaptive immune cells constitute almost half of the population of lipid-free cells in the stromavascular fraction. In healthy WAT, immune cells maintain tissue homeostasis and a immunosuppressive microenvironment by clearing apoptotic cells, regulating angiogenesis, and remodeling the ECM [32]. In obesity, the ability of immune cells to perform these functions is often altered, promoting inflammation, which is critical to the development of WAT fibrosis [18, 19]. The increased tissue stiffness and mechanotransduction of fibrotic tissue may be an important contributor to tumorigenesis [33]. Adipocytes and ASC secrete ECM molecules, including fibronectin, laminin, and collagens. A cleavage product of collagen VI, the most abundant WAT ECM molecule, has been implicated in both breast cancer tumorigenesis and progression [34].
However, while the effect of WAT on cancer progression is well established, and many studies have implicated WAT in tumorigenesis, it is still being debated whether WAT actually promotes cancer initiation and if it does what mechanisms are involved. Because ECM, adipokines, and other WAT-secreted molecules are not known to be directly mutagenic, mechanisms underlying obesity-cancer risk have remained uncertain. It is possible that WAT deregulated in obesity accelerates cancer growth merely by enhancing mitogenic signals in epithelial cells already containing carcinogenic mutations. However, a direct carcinogenic role for WAT cannot be ruled out. Mutations are known to result from oxidative stress which produces reactive oxygen and nitrogen species (ROS/RNS) and other types of metabolites that cause DNA double strand breaks and other lesions [35]. Obesity leads to increased DNA damage and reduced DNA repair in both animal model systems and humans [36, 37]. In obesity-associated inflammation, activated myeloid cells appear to be a major source of ROS [38]. The evolving paradigm is that the inflammatory WAT milieu creates an environment in which ROS production is elevated to a level at which genomic instability ensues. Indeed, obese children have higher levels of double strand breaks, supporting an increased potential for cancer causing mutations in obesity [39, 40]. Until there is direct evidence showing an increase in DNA damage and/or higher genetic instability in tumors at proximity to obese AT, however; the role of WAT-generated ROS in tumor initiation remains hypothetical.
Leukocyte-Cancer Cell Interactions
WAT contains various types of immune cells that play an important role in cancer and become part of the tumor organ [41]. Macrophages are the most highly represented immune cells in WAT and with obesity their numbers increase considerably in both visceral and subcutaneous adipose tissue [18]. While WAT-resident leukocytes may drive cancer by releasing cytokines systemically, it is likely that they are also recruited to tumors from WAT and act locally [42]. It is posited that they are transformed from the activated macrophages found in obese AT to tumor-associated macrophages (TAMs) and accumulate in tumor tissue. In fact, WAT macrophages and TAMs display overlapping phenotypic markers and functions [19]. A change in the macrophage landscape from anti-inflammatory to pro-inflammatory underlies changes in lipid processing and the creation of a low-grade inflammatory state, which contributes to the metabolic syndrome and is observed in both WAT and tumors during cancer progression. Cytokines released by macrophages contribute to tumor malignancy through a variety of mechanisms, including growth promotion, matrix degradation, and tumor angiogenesis. Fibrosis, elevated ECM stiffness, angiogenesis, and regional hypoxia are among the processes driven by both WAT macrophages and TAMs. The presence of “crown-like structures” in mammary WAT, which are conglomerates of macrophages and other immune cells around dead adipocytes, is associated with increased aromatase expression and inflammation, which might increase breast cancer development [43, 44]. A decrease in anti-inflammatory macrophages may also contribute to cancer initiation and promotion.
Lymphocytes also become ‘educated’ and “activated” by inflamed WAT [18]. Pro-inflammatory cytokines released by T lymphocytes include the TNF superfamily, IL-1β, IL-6, CXCL2, MCP-1, COX-2, 5-lipooxygenase, MMPs, VEGF-C, and a number of cell surface adhesion molecules. CD4+ helper T (Th) cells and CD8+ cytotoxic T (Tc) cells, including pro-inflammatory effector Th1 cells and immunoregulatory Th2 cells, as well as Th17, γδT, and the cytotoxic natural killer (NK) cell subsets, regulate the inflammatory tone of WAT and the tumor microenvironment [42]. CD4+ cells direct the proliferation and function of CD8+ Tc cells and antigen-presenting cells (APC), such as macrophages and dendritic cells. CD8+ Tc cells are a critical component of antitumor immune defense that can directly kill tumor cells as well as stimulate APC function. In contrast, regulatory T cells (Tregs) act to suppress CD8+ Tc cells, APCs, and NK cells, through production of immunosuppressive cytokines including IL-10 and TGF-β. These counter-regulatory interactions in the tumor microenvironment suppress immunosurveillance and lead to immune tolerance [42]. Current immunotherapeutic approaches are based on the concept of “exhausted” T cells that arise due to long-term activation. Exhausted T cells express the inhibitory “checkpoint” protein, Programmed Death-1 (PD-1), which incapacitates T cell leading to a failure to kill cancer cells. Monoclonal antibodies blocking PD-1 receptor activation have shown efficacy in cancer treatment [45]. Interestingly, obesity appears to regulate PD-1 activation: TAM-specific expression of PD-L1 (the ligand for PD-1 on T cells) is promoted by HIF-1α in hypoxia [46] and T-cells in obese adipose tissue have high expression of PD-1 [47]. Furthermore, CD4+ T cells expressing PD-1 are increased in the WAT of obese mice [47]. However, until there is an established link between PD-1 expression and obesity in patients, an impact of the adipose microenvironment on the efficacy of immunotherapy remains speculative.
Adipose Stromal Cell-Cancer Cell Interactions
Tumor stroma, composed of a mixture of various non-malignant cell types, has been identified as one of the drivers of cancer progression [11, 48]. While the pool of tumor leukocytes is maintained by hematopoietic progenitors, cancer-associated fibroblasts (CAF), a major component of the tumor stroma, are of mesenchymal origin [49, 50]. This mixed population is partly derived from ASC, which have been found to play an important role in tumor growth in animal models [51]. ASC, which are abundant in WAT and proliferate in obesity, become mobilized, migrate from WAT to tumors, and promote cancer progression [14, 51, 52]. Adipocyte-secreted matricellular protein, SPARC, binding to β1 integrin on the ASC surface, is a molecular trigger of ASC mobilization [53]. Chemokines CXCL1 and CXCL8 signaling upon their receptors, CXCR1 and CXCR2, mediate the homing of ASC to tumors [54, 55]. Poor survival of obese patients with prostate cancer has been linked to ASC trafficking from WAT to tumors, which is mediated by CXCL1 secreted from malignant epithelium and signaling on ASC via its receptor CXCR1 [55]. It was also found that the secretion of CXCL1 by tumors in the setting of obesity is induced by WAT leukocyte-derived IL-22 signaling through IL-22R on cancer cells [56].
In these and other reports, the molecular mechanisms through which tumor-recruited ASC promote cancer progression are now being unraveled. Chemokines secreted by ASC recruit macrophages, which have a tumor promoting role as discussed above [57]. However, ASC within the tumor microenvironment also directly contribute to carcinoma progression. Intratumoral adipocytes, differentiating from infiltrating ASC, stimulate the proliferation of adjacent cancer cells [14]. ASC are a major source of the ECM that drives tumor desmoplasia [33] and they also secrete trophic factors that stimulate vascularization [14, 51]. Moreover, some of the cancer-promoting effects of ASC may be contact-dependent [58]. In a recent study, a role for CXCL12, an ASC-secreted chemokine, in prostate tumor growth and invasiveness has been demonstrated [23]. In addition, roles for ASC in therapy resistance [59] and metastasis [60, 61] have also surfaced. As an illustration of metabolic symbiosis, ASC increase nitric oxide synthesis in cancer cells, leading to their decreased mitochondrial respiration, increased glycolysis, and chemoresistance [62]. Bone marrow mesenchymal stromal cells mute anti-tumor immune response through their effects on T cells [63]. It remains to be determined if ASC have similar immunosuppressive properties. Recently, it has been shown that bone marrow mesenchymal stromal cells induce the epithelial-mesenchymal transition (EMT), an important step in the progression of carcinomas to a chemoresistant and invasive phenotype [64]. The role of ASC in EMT induction and cancer aggressiveness also remains to be proven.
Adipocyte-Cancer Cell Interactions
Breast carcinomas invade adjacent mammary AT, prostate carcinomas invade the peri-glandular AT, and ovarian carcinomas invade omental AT [24, 65]. It is, therefore, not surprising that adipocytes engage in a direct interaction with cancer cells at the invasive front of tumors [28]. An analysis of peritumoral WAT in patients revealed that adipocytes adjacent to malignant cells have smaller lipid droplets than adipocytes further away from the tumor [24]. Adipocytes co-cultured with breast cancer cells also progressively lose lipid droplets and eventually become indistinguishable from ASC [25–27].
With hypoxia, cancer cells undergo metabolic reprogramming to increase lipid utilization [66]. Lipid metabolism has a particular clinical relevance for carcinomas, and increased use of lipids, as opposed to glucose, is a hallmark of cancer aggressiveness [67]. Fatty acids (FA), which produce about twice the energy of glucose, are the primary forms of lipids used by tumors as a source of energy through β-oxidation [68]. In addition, FA derivatives including phospholipids, sterols and sphingolipids, as well as important signaling molecules are integral components of the cancer cell and mitochondrial membrane [69]. Examples of signaling lipid derivatives include sphingosine-1-phosphate and lysophosphatic acid, which regulate the migration, proliferation, and invasion of cancer cells [70]. FA are also used for the lipid modification of proteins. Unsaturated FA have been specifically implicated in cancer cell aggressiveness [71]. Tumor cells synthesize most FAs de novo in spite of a sufficient dietary lipid supply [70] and increased lipogenesis is a hallmark of many aggressive cancers [72]. However, in aggressive cancers endogenous lipogenesis becomes insufficient and cancer cells switch to the uptake of extracellular FA. Indeed, lipogenesis inhibitors reduce the viability of cancer cells only in the absence of exogenous lipids [73].
Adipocytes undergoing lipolysis serve as a source of lipids for cancer cells [74, 75]. Indeed, adipocyte lipolysis-derived FA increase proliferation and invasiveness of breast cancer cells in co-culture settings [27, 76, 77]. In cancer-associated adipocytes, triglycerides are hydrolyzed to release free FA by the sequential action of adipose triglyceride lipase, hormone sensitive lipase and monoacylglycerol lipase [69]. Cancer cells can induce the expression of lipoprotein lipase that processes chylomicrons, triglycerides and phospholipids, and hence enables their uptake of exogenous FAs [78]. The downstream molecular complex that facilitates intercellular cholesterol and FA transport relies on CD36, also known as FA translocase [79]. CD36 is particularly important for the uptake of long chain FA, which are subsequently transported by the FA-binding proteins, FABP3 and FABP4. Overexpression of CD36 is associated with tumor progression and metastasis [80]. Hypoxia, through HIF-1α, increases lipid uptake in cancer cells by inducing the expression of FABP3/4, as well as adipophilin, a lipid droplet structural protein [81]. Cancer cells can transiently store excess triglycerides in lipid droplets and, as energy is needed, will undergo lipolysis to free up FA [25, 82]. In addition to serving as an energy source, imported FAs are utilized for membrane lipid synthesis along with cholesterol [83] and affect cellular signaling. Increased FA load leads to passive mitochondrial uncoupling that reduces lipotoxicity in cancer cells [25]. More aggressive cancer cell lines display higher exogenous FA incorporation into oncogenic signaling lipids, as opposed to oxidative pathways [84]. It has been reported that inhibition of lipolysis, lowering free FA levels, reduces cancer pathogenicity, an observation that may have a therapeutic implication [69]. Other potential therapeutic targets are fatty acid receptors, such as CD36 and fatty acid transport proteins (FABP4/5).
The Role of Adipose Tissue in Therapy Resistance
There is emerging evidence that WAT-derived cells and factors contribute to the therapy resistance of various cancers [59, 62, 85, 86]. This is in part due to the changes in the tumor matrix driven by the adipose microenvironment. In pancreatic tumors from both patients and animal models, obesity-induced fibrotic tissue deposition prevents uniform tumor vascularization, which jeopardizes drug delivery. Interestingly, metformin, a common antidiabetic agent can inhibit desmoplasia in pancreatic cancer by reducing ECM remodeling, EMT, and metastasis [87]. Recent data suggest that ASC and adipocytes in the tumor microenvironment promote EMT, early metastasis, and chemoresistance. While the role of EMT in metastatic dissemination is controversial [88, 89], acquisition of ‘cancer stem cell’ properties and resistance to therapy is undoubtedly a hallmark of EMT [49, 90, 91]. Indeed, acquisition of therapy resistance has been linked with the activation of lipid metabolism in cancer [71, 92], and mounting evidence links the uptake of adipocyte-derived FA to the EMT [25]. Elevated CD36-mediated FA uptake has been reported to promote the EMT in hepatocellular carcinoma [93] and ovarian cancer [80]. CD36-positive cancer cells in tumors which upregulate lipid metabolism have ‘cancer stem cell’ properties and an increased ability to disseminate, which is associated with poor prognosis [92, 94]. CD36-expressing cancer cells also have an increased survival potential and resistance to chemotherapy [74, 92]. In addition, lipolytic changes in the adipose microenvironment, leading to FA release from adipocytes, have been linked to the invasiveness of pancreatic, prostate, and breast cancers [27, 95]. Activation of carnitine palmitoyltransferase I (CPT1), a key β-oxidation gene, by JAK/STAT3 signaling downstream of adipocyte-secreted leptin has been established as a mechanism responsible for breast cancer cell aggressiveness [96]. A recent report shows that breast cancer cells co-cultured with adipocytes are also more radioresistant due to the activation of the Chk1 signaling pathway in tumor cells by IL-6 [26].
Evidence that, in obesity, adipocytes, in response to oxidative stress, secrete protective factors enabling chemotherapy resistance, has also been observed for hematological malignancies [97]. AT creates a microenvironment that supports proliferation and resistance to chemotherapy for disseminating leukemia cells. As in carcinomas, leukemic cells engage in CD36-dependent FA transport from adipocytes, fueling β-oxidation [74]. In addition, recent studies reveal that lipids in adipocytes can sequester cancer drugs, which results in chemotherapy depotentiation [98].
Concluding Remarks
There are many questions raised in this review that remain to be addressed (see outstanding questions). Despite advances in the field, primary tumors eventually progress to aggressive, metastatic phenotypes in many types of cancer. Metastasis and the resistance of cancer to treatment remains an often insurmountable challenge. Because the prevalence of obesity is rising, further insights into the mechanisms that underlie its association with cancer risk and virulence are urgently needed. While obesity is linked with cancer development, patients with high-grade cancer are, in fact, often lipodystrophic. Indeed, advanced stages of many cancers are associated with WAT loss and body wasting (cachexia), which complicates treatment and adversely affects patient survival. This is partly because tumor growth drives lipolysis in adipocytes. As a result, cancer progression tends to eventually result in global WAT mass reduction [99]. It has been proposed that the conversion of WAT into beige/brown adipose tissue (BAT) plays a role in this process [100]. BAT adipocytes have a high capacity for uncoupled thermogenic energy dissipation through the β-oxidation of FA [15]. However, the association of BAT activation with cancer progression that is evident in rodent models has not yet been tested for clinical relevance. At this point, there is a clear need for a better understanding of the changes in WAT, and the resulting changes in other organs, that underlie cancer progression in obesity. Insulin resistance and other manifestations of the metabolic syndrome, involving other organs such as the liver, kidney and muscle, also interfere in cancer development and progression. Importantly, not all obese patients exhibit AT inflammation and metabolic complications. In the future, the comparison of cancer initiation in those with healthy and those with unhealthy obesity will help to further uncouple the relationship between AT overgrowth and metabolic complications.
Outstanding Questions.
How does increased adiposity contribute to carcinogenesis and tumor progression and can the underlying mechanism(s) be blocked?
Could targeting cells from WAT be developed as a combination treatment that improves the efficacy of conventional cancer and immuno therapies?
Could the blockade of fatty acid transfer from adipocytes to cancer cells synergize with conventional therapy?
Is WAT converted to BAT in patients with high-grade tumors and, if so, should different cells/pathways be targeted in advanced cancer patients?
Currently, obese and non-obese cancer patients are given the same treatments, but there is increasing evidence that therapies tailored to obese patients may improve their survival [11]. Targeting the processes through which WAT overgrowth appears to promote cancer initiation and progression could reduce the increased cancer risk and tumor aggressiveness associated with obesity [11, 30, 101]. Recently, experimental therapeutics targeting ASC, adipose endothelium and adipocytes have been designed and their tests in animal models of cancer have shown promise [102–105]. In the future, drugs aimed at the cellular and molecular mechanisms through which WAT cells drive chemoresistance could improve the efficacy of current anticancer treatments.
Trends.
Cancer initiation in obesity can be triggered by chronic inflammation in adipose tissue, which creates localized genotoxic stress leading to carcinogenesis.
Carcinoma growth is fueled by adipokine signaling from adipose-tissue derived cells, which can infiltrate tumors.
Cancer aggressiveness in obesity is supported by fatty acids and other metabolites secreted by adipocytes, as well as by immune system dysfunction.
Pathways mediating the switch of cancer cells to lipid metabolism are among those that could be targeted in combination with conventional therapies.
Acknowledgments
This work was supported by a grant from Bears Care, the charitable beneficiary of the Chicago Bears Football Club and NIH grants CA169604 to E. Lengyel, as well as NIH grants CA180134 (L. Makowski), DK088131 and CA216745 (M.G. Kolonin) and CA195659 (J. DiGiovanni/M.G. Kolonin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ernst Lengyel, Departments of Obstetrics and Gynecology/Section of Gynecologic Oncology, The University of Chicago, Chicago, IL, USA.
Liza Makowski, Department of Medicine - Division of Hematology and Oncology, University of Tennessee Health Science Center Memphis, TN.
John DiGiovanni, Division of Pharmacology and Toxicology, Dell Pediatric Research Institute, The University of Texas at Austin, Austin, TX, USA.
Mikhail G. Kolonin, Institute of Molecular Medicine, University of Texas Health Science Center Houston, TX, USA.
References
- 1.Friedman JM. Obesity: Causes and control of excess body fat. Nature. 2009;459:340–342. doi: 10.1038/459340a. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Shin SW. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:1491–1492. doi: 10.1056/NEJMc1701944. [DOI] [PubMed] [Google Scholar]
- 3.Steele CB, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66:1052–1058. doi: 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eheman C, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schauer DP, et al. Association Between Weight Loss and the Risk of Cancer after Bariatric Surgery. Obesity. 2017;25(Suppl 2):S52–S57. doi: 10.1002/oby.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allott EH, et al. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renehan AG, et al. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Filho G, et al. Associations between adipokines and obesity-related cancer. Front Biosci. 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 10.Park J, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirin O, Kolonin MG. Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discov Today. 2013;11:567–573. doi: 10.1016/j.drudis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Khandekar MJ, et al. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 13.Huffman DM, et al. Cancer progression in the transgenic adenocarcinoma of mouse prostate mouse is related to energy balance, body mass, and body composition, but not food intake. Cancer Res. 2007;67:417–424. doi: 10.1158/0008-5472.CAN-06-1244. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 15.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Z, et al. PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development. 2018;145:1–13. doi: 10.1242/dev.155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traktuev D, et al. A Population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunologic Reviews. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun K, et al. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MJ, et al. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, et al. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550–570. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossmann ME, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 23.Saha A, et al. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Res. 2017;77:5158–5168. doi: 10.1158/0008-5472.CAN-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieman KM, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–15341. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YY, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2:e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochet L, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–68. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 27.Dirat B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 28.Laurent V, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nature Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapoor J, et al. Extraprostatic extension into periprostatic fat is a more important determinant of prostate cancer recurrence than an invasive phenotype. J Urol. 2013;190:2061–2066. doi: 10.1016/j.juro.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 30.Romero IL, et al. Molecular pathways: trafficking of metabolic resources in the tumor microenvironment. Clin Cancer Res. 2015;21:680–686. doi: 10.1158/1078-0432.CCR-14-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 32.Lu P, et al. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo BR, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301–311. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyengar P, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kryston TB, et al. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Savini I, et al. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luperini BC, et al. Gene polymorphisms and increased DNA damage in morbidly obese women. Mutat Res. 2015;776:111–117. doi: 10.1016/j.mrfmmm.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Dang PM, et al. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarpato R, et al. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the gamma-H2AX focus assay and micronucleus test. FASEB J. 2011;25:685–693. doi: 10.1096/fj.10-168427. [DOI] [PubMed] [Google Scholar]
- 40.Azzara A, et al. Different repair kinetic of DSBs induced by mitomycin C in peripheral lymphocytes of obese and normal weight adolescents. Mutat Res. 2016;789:9–14. doi: 10.1016/j.mrfmmm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Olson OC, et al. Obesity and the tumor microenvironment. Science. 2017;358:1130–1131. doi: 10.1126/science.aao5801. [DOI] [PubMed] [Google Scholar]
- 42.Cozzo AJ, et al. Contribution of Adipose Tissue to Development of Cancer. Compr Physiol. 2017;8:237–282. doi: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyengar NM, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res. 2017;10:235–243. doi: 10.1158/1940-6207.CAPR-16-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirakawa K, et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 50.Bhowmick NA, et al. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellows CF, et al. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2461–2468. doi: 10.1158/1055-9965.EPI-11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng C, Kolonin MG. Proteolytic Isoforms of SPARC Induce Adipose Stromal Cell Mobilization in Obesity. Stem Cells. 2015;34:174–190. doi: 10.1002/stem.2192. [DOI] [PubMed] [Google Scholar]
- 54.Klopp AH, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumor microenvironment. Nature Comm. 2016;7:11674–11690. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Kolonin MG. Cytokine signaling regulating adipose stromal cell trafficking. Adipocyte. 2016;5:369–374. doi: 10.1080/21623945.2016.1220452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arendt LM, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen D, et al. Paracrine factors from adipose-mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co-culture model. Cancer Cell Int. 2015;15:42–47. doi: 10.1186/s12935-015-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowicka A, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013;8:e81859. doi: 10.1371/journal.pone.0081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orecchioni S, et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73:5880–5891. doi: 10.1158/0008-5472.CAN-13-0821. [DOI] [PubMed] [Google Scholar]
- 61.Rowan BG, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One. 2014;9:e89595. doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salimian Rizi B, et al. Nitric oxide mediates metabolic coupling of omentum-derived adipose stroma to ovarian and endometrial cancer cells. Cancer Res. 2015;75:456–4571. doi: 10.1158/0008-5472.CAN-14-1337. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerlin L, et al. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mele V, et al. Mesenchymal stromal cells induce epithelial-to-mesenchymal transition in human colorectal cancer cells through the expression of surface-bound TGF-beta. Int J Cancer. 2014;134:2583–2594. doi: 10.1002/ijc.28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi J, et al. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. Am J Clin Pathol. 2008;130:382–388. doi: 10.1309/MX6KKA1UNJ1YG8VN. [DOI] [PubMed] [Google Scholar]
- 66.Palm W, Thompson CB. Nutrient acquisition strategies of mammalian cells. Nature. 2017;546:234–242. doi: 10.1038/nature22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Beloribi-Djefaflia S, et al. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Currie E, et al. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 71.Mukherjee A, et al. Unsaturated Fatty Acids Maintain Cancer Cell Stemness. Cell Stem Cell. 2017;20:291–292. doi: 10.1016/j.stem.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaidi N, et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ros S, et al. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 74.Ye H, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016;19:23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balaban S, et al. Obesity and cancer progression: is there a role of fatty acid metabolism? Biomed Res Int. 2015;2015:274585. doi: 10.1155/2015/274585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Picon-Ruiz M, et al. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b-Mediated Malignant Progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 77.Balaban S, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuemmerle NB, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:62–72. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ladanyi A, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018 doi: 10.1038/s41388-017-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bensaad K, et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9:349–365. doi: 10.1016/j.celrep.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 82.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao CH, et al. Exogenous Fatty Acids Are the Preferred Source of Membrane Lipids in Proliferating Fibroblasts. Cell Chem Biol. 2016;23:483–493. doi: 10.1016/j.chembiol.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louie SM, et al. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta. 2013;1831:1566–1572. doi: 10.1016/j.bbalip.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duong MN, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 2015;17:57–63. doi: 10.1186/s13058-015-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houthuijzen JM, et al. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901–1906. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Incio J, et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borriello L, DeClerck YA. Tumor microenvironment and therapeutic resistance process. Med Sci. 2014;30:445–451. doi: 10.1051/medsci/20143004021. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Pascual G, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 93.Nath A, et al. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hale JS, et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells. 2014;32:1746–1758. doi: 10.1002/stem.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okumura T, et al. Extra-pancreatic invasion induces lipolytic and fibrotic changes in the adipose microenvironment, with released fatty acids enhancing the invasiveness of pancreatic cancer cells. Oncotarget. 2017;8:18280–18295. doi: 10.18632/oncotarget.15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T, et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2017 doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheng X, et al. Adipocytes cause leukemia cell resistance to daunorubicin via oxidative stress response. Oncotarget. 2016;7:73147–73159. doi: 10.18632/oncotarget.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheng X, et al. Adipocytes Sequester and Metabolize the Chemotherapeutic Daunorubicin. Mol Cancer Res. 2017 doi: 10.1158/1541-7786.MCR-17-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25:255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Kir S, Spiegelman BM. Cachexia and Brown Fat: A Burning Issue in Cancer. Trends Cancer. 2016;2:461–463. doi: 10.1016/j.trecan.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cozzo AJ, et al. cMET inhibitor crizotinib impairs angiogenesis and reduces tumor burden in the C3(1)-Tag model of basal-like breast cancer. Springerplus. 2016;5:348. doi: 10.1186/s40064-016-1920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daquinag AC, et al. Non-glycanated Decorin is a Drug Target on Human Adipose Stromal Cells. Molecular Therapy - Oncolytics. 2017;6:1–9. doi: 10.1016/j.omto.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daquinag AC, et al. Targeted Pro-apoptotic Peptides Depleting Adipose Stromal Cells Inhibit Tumor Growth. Mol Ther. 2016;1:34–40. doi: 10.1038/mt.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daquinag AC, et al. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death & Diff. 2015;22:351–363. doi: 10.1038/cdd.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kolonin MG, et al. Reversal of obesity by targeted ablation of adipose tissue. Nature Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]