Abstract
Objective
Cross-sectional, longitudinal, and genetic associations exist between irritability and depression. Prior studies have examined developmental trajectories of irritability, clinical outcomes, and associations with child and familial depression. However, studies have not integrated neurobiological measures. The current study examined developmental trajectories of irritability, clinical outcomes, and cortical structure among preschoolers oversampled for depressive symptoms.
Method
Beginning at 3–5 years old, a sample of 271 children enriched for early depressive symptoms were assessed longitudinally by clinical interview. Latent class mixture models identified trajectories of irritability severity. Risk factors, clinical outcomes, and cortical thickness were compared across trajectory classes. Cortical thickness measures were extracted from three waves of magnetic resonance imaging at 7–12 years of age.
Results
Three trajectory classes were identified among these youth: 53.50% of children exhibited elevated irritability during preschool that declined longitudinally, 30.26% exhibited consistently low irritability, and 16.24% exhibited consistently elevated irritability. Compared to other classes, the elevated irritability class exhibited higher rates of maternal depression, early life adversity, later psychiatric diagnoses and functional impairment. Further, elevated baseline irritability predicted later depression beyond adversity and personal and maternal depression history. The elevated irritability class exhibited thicker cortex in the left superior frontal and temporal gyri and the right inferior parietal lobule.
Conclusion
Irritability manifested with specific developmental trajectories in this sample enriched for early depression. Persistently elevated irritability predicted poor psychiatric outcomes, higher risk for later depression, and reduced overall function later in development. Greater frontal, temporal, and parietal cortical thickness was also found, providing neural correlates of this risk trajectory.
Keywords: irritability, development, latent trajectory, MRI, cortical thickness
Introduction
Associations between irritability and depression are of interest in developmental psychopathology. The unique role that irritability plays in pediatric depression is evident in that, in multiple editions of the Diagnostic and Statistical Manual, irritability is a criterion for pediatric, but not adult, depressive disorders. Indeed, youth irritability predicts subsequent depression, and offspring of depressed mothers face increased risk for irritability and subsequent depression.1–4 Moreover, phenomenological work has begun to elucidate distinct developmental trajectories of irritability in youth,2,5 including associations with maternal depression. However, it is essential to integrate brain development into this research and examine longitudinal outcomes. We present the first study linking trajectories of irritability severity to brain structure differences in a pediatric sample enriched for depression. This adds to a small but growing literature examining the development of irritability, its neural underpinnings, and associations with depression and other clinical outcomes.
It is important to begin longitudinal studies of irritability and depression as early as preschool. While temper outbursts are ubiquitous in preschoolers, normative and non-normative outbursts can be differentiated, providing opportunities for early interventions.6–8 Early intervention may be particularly important in preschoolers also showing symptoms of depression and/or anxiety. The frequency of temper loss generally declines over development, albeit with inter-individual variability.7 Prior population-based work identified several latent trajectories of irritability, where most children showed low irritability that declined across the first nine years of life, but smaller subsamples exhibited chronically elevated or increasing irritability, which were more likely to have a maternal depression history and to develop more internalizing symptoms themselves.2 Another longitudinal study similarly found a small group of children with chronically elevated irritability trajectories exhibiting the worst outcomes, including antisocial personality features in adulthood.5 Similar trajectories have been noted across adolescence/young adulthood when examining irritability related to aggression and violence.9 Critically, irritability relates to parental history of depression and anxiety and to risk for subsequent depression and impairment.3,4 Indeed, across ages, meta-analysis longitudinally associates irritability with risk for depression, anxiety disorders, and global impairment,1,10 while other studies highlight significant genetic associations between depression and irritability.11,12
Complementing these behavioral studies, recent work probes the neural correlates of clinically impairing irritability in youth, though none in a pediatric sample with elevated early symptoms of depression. Irritable youth show dysfunctional frontal, cingulate, and striatal responses to induced frustration13,14 and aberrant frontal and amygdala responses during emotional face processing.15–17 However, few studies examine irritability-related differences in brain structure. Youth with severe irritability (specifically, Disruptive Mood Dysregulation Disorder; DMDD) exhibit smaller gray matter volume (GMV) in pre-supplementary motor area,18 dorsolateral prefrontal cortex (PFC),18,19 superior frontal gyrus (SFG),19 and larger insula volume18 compared to healthy youth.
The current study leveraged a pediatric sample enriched for early depressive symptoms and followed prospectively to examine trajectories of irritability severity from preschool through late childhood/early adolescence. Importantly, this is the first study to examine differences in brain structure as a function of these trajectories; specifically, we measured cortical thickness across three neuroimaging waves during late childhood/early adolescence. Further, we add to the growing literature on irritability by focusing specifically on a sample enriched for early depression. Similar to prior work,2 we expected classes of youth with low and declining or chronically elevated or increasing irritability trajectories across development. We hypothesize that classes with elevated irritability would show higher rates of depressive symptoms relative to others. Also, building on the affective disorders literature, we hypothesized associations between irritability and cortical thickness in PFC regions involved in cognitive control and emotion regulation.
Method
Participants
Participants were drawn from the prospective longitudinal Preschool Depression Study (PDS; N=306, current analyses examined n=271 with 3+ annual clinical assessments available as is necessary for growth curve modeling; Table S1, available online, for demographics by subsample). The PDS has the broad goal exploring clinical and neural outcomes relating to preschool-onset depression. Details of the study have been published previously.20 Briefly, 3- to 5-year-old children and their primary caregivers were recruited from the St. Louis metropolitan area over-sampling for early depressive symptoms. Children and caregivers completed in-depth clinical interviews annually; children participated in three annual neuroimaging scans beginning at 7–12 years of age. Parental written consent and child assent after age 4 were obtained prior to participation and the Institutional Review Board at Washington University in St. Louis approved all experimental procedures.
Diagnostic Assessments
Staff, trained to proficiency by appropriate experts, conducted in-person assessments with participants and parents/guardians from enrollment through the time of the scans (Figure S1, available online, for study flow diagram). Before children were age 8, a semi-structured parent-report interview was used to assess psychiatric symptoms, the Preschool-Age Psychiatric Assessment (PAPA).21 After age 8, the child- and parent-report on the Childhood and Adolescent Psychiatric Assessment (CAPA)22 was collected. Interviews were audiotaped, reviewed for reliability, and calibrated for accuracy.20
PAPA/CAPA data was summarized based on whether children ever received diagnoses of major depressive disorder (MDD), an anxiety disorder (generalized anxiety, separation anxiety, panic disorder, agoraphobia, and/or social phobia), oppositional defiant disorder (ODD) or conduct disorder (CD), or ADHD at any timepoint from baseline through the ninth annual assessment. Further, the presence/absence of fifteen stressors endorsed at baseline was summed plus early exposure to poverty to create a composite early life adversity score (range=0–16; see Supplement 1, available online, for details). Psychotropic medication use was also assessed– as this varied widely across participants and time, a summary variable of use ever was examined.
Measures
Irritability severity was defined as the sum of five items from the depression and conduct problems sections of the PAPA/CAPA (items: losing temper, non-destructive temper tantrums, irritability, touchy or easily annoyed, angry or resentful). See Supplement 1, available online, for full definitions, stem questions, and coding rules for these items. Similar to prior work,3 the presence of symptoms at each assessment was summed (range=0–5) based on PAPA parent-report or if either reporter endorsed an item on the CAPA.23 This measure was reliable across assessments (Cronbach’s alpha=.75).
Parents rated children’s functional impairment at the first scan session on the MacArthur Health and Behavior Questionnaire (HBQ).24 Three HBQ subscales were of interest: Functional Impairment (range=0–16), Global Peer Relationships (range=0–22), and Academic Functioning (range=0–32).
The Family Interview for Genetic Studies25 assessed the presence of affective disorders in first- and second-degree relatives. The presence/absence of maternal depression or anxiety history was examined as a risk factor for internalizing symptoms in the child.
The Kaufman Brief Intelligence Test26 or the Wechsler Abbreviated Scale of Intelligence27 was used to assess IQ at school age (depending on age/study wave). Baseline socioeconomic status was quantified as a family’s income-to-needs ratio,28,29 i.e. total family income divided by the federal poverty level, based on family size, at the time of assessment.
Examining Trajectories of Irritability Across Development
We utilized latent class mixture models implemented in R30 using the lcmm package31,32 to identify latent groups of individuals that shared similar developmental trajectories of irritability severity. In these models, irritability was the dependent variable and age at each assessment (in months) was the independent variable. See Supplement 1, available online, for details.
Demographics and clinical outcomes
Baseline demographic differences (Table 1) across the three latent trajectory classes were characterized using analysis of variance (ANOVA) and chi-squared. Chi-squared tests characterized group differences in maternal depression history and child diagnoses (presence/absence across any of up to eight assessments from baseline through approximately 15 years old). ANOVAs assessed group differences in later functional impairment (HBQ).
Table 1.
Demographics and Clinical Characteristics of the Latent Trajectory Classes
| Low Irritability | Declining Irritability |
High Irritability | |
|---|---|---|---|
| Class N | 82 | 145 | 44 |
| N Assessments (m/sd) | 6.01 (1.36) | 6.14 (1.41) | 5.70 (1.56) |
| Sex (n/% Female)*** | 41 (50.00%) | 81 (55.90%) | 10 (22.70%) |
| Race (n/% white) | 40 (48.80%) | 81 (55.90%) | 28 (63.60%) |
| Age at Baseline (m/sd months)*** | 53.64 (9.91) | 51.72 (8.60) | 59.07 (9.25) |
| Age at Scan 1 (m/sd months) | 125.15 (13.81) | 120.45 (14.84) | 127.08 (15.56) |
| IQ (m/sd) a | 103.52 (13.63) | 106.73 (14.89) | 100.31 (15.47) |
| Baseline Adversity Score (m/sd)* b | 2.36 (1.66) | 2.69 (1.79) | 3.48 (2.40) |
| Baseline Income-to-Needs (m/sd) | 2.31 (1.17) | 2.19 (1.29) | 2.24 (1.34) |
| HBQ Peer Relations (m/sd)* c | 3.61 (0.59) | 3.48 (0.50) | 3.23 (0.77) |
| HBQ Academic Functioning (m/sd)*** c | 4.22 (0.45) | 3.98 (0.58) | 3.34 (0.87) |
| HBQ Global Functioning (m/sd)*** c | 1.96 (2.58) | 4.05 (4.95) | 9.67 (5.52) |
| Maternal MDD (n/%)*** d | 19 (23.20%) | 60 (41.40%) | 29 (69.00%) |
| Child MDD (n/%)*** | 20 (24.40%) | 79 (54.50%) | 39 (88.60%) |
| Child Anxiety (n/%)*** | 37 (45.10%) | 83 (57.20%) | 39 (88.60%) |
| Child ADHD (n/%)*** | 10 (12.20%) | 52 (35.90%) | 32 (72.70%) |
| Child ODD/CD (n/%)*** | 11 (13.40%) | 57 (39.30%) | 35 (79.50%) |
| Psychotropic Medication Use (n/%)*** | 4 (8.30%) | 22 (23.4%) | 16 (61.50%) |
Note: The number of participants in each trajectory class (Class N) as well as the number of times that each child’s symptomology was assessed (N Assessments) is noted here. Demographic characteristics are noted by group as well as early adversity, impairment on the MacArthur Health and Behavior Questionnaire (HBQ), maternal depression history, and rates of child diagnoses across assessments.
Group differences in these factors were tested by ANOVA or chi-squared and significant group differences are indicated here (*p<.05; **p<.01; ***p<.001).
We note here the total sample with available data on each measure and the sample size within each class.
n=220 IQ, n=65 low, n=120 declining, n=35 high irritability
n=269 Adversity, n=80 low, n=145 declining, n= 44 high irritability
n=160 HBQ, n=47 low, n=89 declining, n=24 high irritability
n=269 Maternal MDD, n=82 low, n=145 declining, n=42 high irritability
Baseline Irritability as a Predictor
Additionally, we used logistic regression to test whether baseline irritability predicted ever receiving a depression diagnosis above and beyond standard predictive/risk factors– sex, baseline PAPA depression symptom severity (excluding irritability, including negative mood, anhedonia, somatic changes, guilt, etc.), adversity, and maternal depression history. To examine specificity, we used a matched logistic regression with irritability predicting anxiety diagnosis, beyond sex, baseline anxiety severity (e.g. worrying, avoidance, fear/anxiety of separation, autonomic symptoms, etc.), adversity, and maternal anxiety history.
MRI Acquisition and Structural Analysis
Three waves of neuroimaging data were collected using a 3T TIM TRIO Siemens scanner. Structural images (1mm isotropic voxels) were processed using the longitudinal stream33,34 in FreeSurfer, version 5.3 (http://surfer.nmr.mgh.harvard.edu/). See Supplement 1, available online, for details.
Of the 271 children with estimated irritability trajectories, 139 had 2–3 waves of structural data usable for longitudinal processing. Figure S2, available online, shows the timing of these scans. Differences in cortical thickness were compared across classes controlling for age at the first scan and sex using a “different offset, different slope” general linear model in FreeSurfer (mri_glmfit). Specifically, two standard summary outcome measures from the longitudinal processing model were examined: temporal average (thickness at midpoint of linear fit) and rate of change in thickness across time. Cluster-wise correction was used for multiple comparisons (mri_glmfit-sim --cache 1.3 abs –cwp 0.05 --2spaces) with a vertex-wise/cluster-forming threshold of p<.05 and adjusting for tests in each hemisphere. Interactions with age and sex are presented in Table S2, available online, as we did not have a priori hypotheses about these interactions and power was limited, given the small sample sizes split within class by sex. Exploratory analyses examining potential interactions with quadratic effects of age are also noted in Supplement 1, available online.
Results
Irritability Trajectory Characteristics
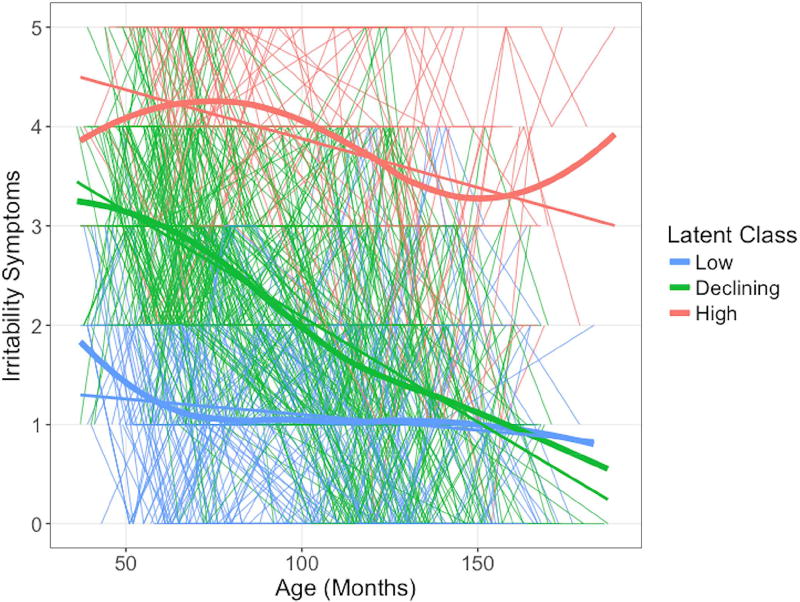
Of the five models tested (Table S3, available online), the three latent classes model (Figure 1) showed the lowest Bayesian information criterion, the lowest Akaike information criterion, and the highest entropy, suggesting the best and most parsimonious fit and best classification of individuals. 14.24% of children showed high irritability during the preschool period, which remained high across development (‘high irritability’ class). 53.14% of children showed preschool-age irritability as elevated as the high irritability class, but decreasing across development (‘declining irritability’ class). This likely represents a normative trajectory across development.7 30.63% of children showed low preschool-age irritability, which remained low (‘low irritability’ class).
Figure 1. Irritability Trajectory Classes.
Note: The low, declining, and high irritability latent trajectory classes are displayed here. Individual participant irritability severity scores (y-axis; PAPA/CAPA sum scores) at each assessment wave are presented in the thin lines (i.e. one line per participant), time locked to the child’s age at assessment indicated in months on the x-axis. Thicker lines display the linear slope and loess fit lines of irritability severity for each trajectory class across age.
The children in the high irritability class were more likely to be male, slightly older at baseline, and had more adverse childhood experiences (Table 1). The trajectory classes did not differ significantly in race, age at scan, IQ, or income-to-needs ratio.
Clinical Associations
Compared to the other classes, children in the high irritability class showed higher rates of maternal depression history (Table 1). These youths were more likely to receive a diagnosis of MDD, anxiety disorder, ADHD, or ODD/CD across clinical assessment waves (Table 1).
Baseline levels of irritability, which was elevated in the high irritability class, predicted later MDD diagnoses (OR=1.37, p=.01), beyond the effects of sex, maternal depression, adversity, and other baseline depressive symptoms (Table S4, available online). Indeed, in AUC analyses predicting who did versus did not develop MDD, baseline irritability alone (AUC=.73, 95% CI=.68–.79) was not significantly different in its discrimination from full baseline MDD symptom severity (AUC=.82, 95% CI=.77–.87). The association between irritability and depression showed specificity, in not predicting later anxiety diagnosis (OR=1.02, p=.89; Table S4, available online).
Brain Structure
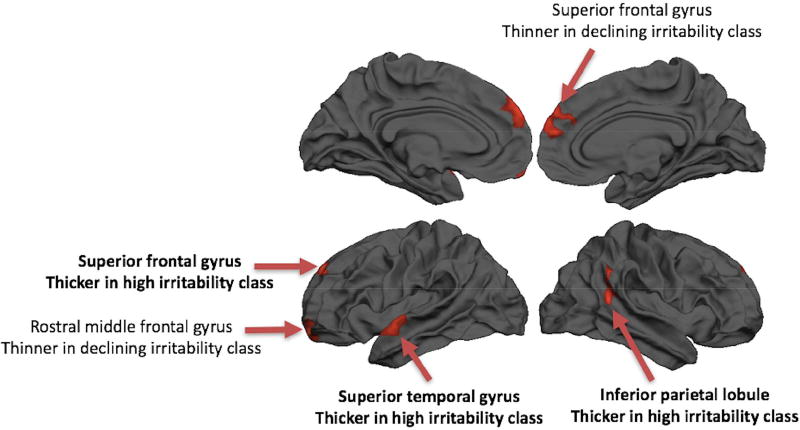
No regions showed significant differences in rate of change of thickness over time as a function of irritability class. Five regions did show overall differences in cortical thickness (temporal average, thickness at midpoint of linear fit) as a function of irritability trajectory classes (Table 2, Figure 2). The left SFG, left superior temporal gyrus (STG), and right inferior parietal lobule (IPL) showed thicker gray matter among children in the high irritability class; these regions showed the same pattern at each wave (Figure S3, available online). The left rostral middle frontal gyrus and right SFG showed a different pattern, specifically, thinner cortex in the declining irritability class compared to the other two classes.
Table 2.
Regions showing differences in cortical thickness by trajectory class.
| Peak Coordinates | Estimated Marginal Means by Class |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | X | Y | Z | Size (mm2) | F | partial η2 | Low | Declining | High | Effect |
| Left Superior Frontal Gyrus | −10 | 48 | 33 | 468 | 9.09 | 0.13 | 3.26 | 3.24 | 3.46 | L=D<H |
| Left Superior Temporal Gyrus | −56 | −4 | −7 | 504 | 5.10 | 0.08 | 3.08 | 3.04 | 3.20 | L=D<H |
| Right Inferior Parietal Lobule | 45 | −48 | 15 | 450 | 8.84 | 0.12 | 2.74 | 2.70 | 2.87 | L=D<H |
| Left Rostral Middle Frontal Gyrus | −16 | 53 | −12 | 438 | 12.03 | 0.16 | 3.06 | 2.89 | 3.09 | D<L=H |
| Right Superior Frontal Gyrus | 8 | 56 | 21 | 446 | 5.44 | 0.08 | 3.26 | 3.14 | 3.23 | D<L=H |
Note: Five regions showed significant trajectory class-related differences in the temporal average of cortical thickness across scan wave. The Talairach coordinates at the peak of thickness differences are indicated (x, y, z) along with the area of each cluster (Size). The average thickness across each cluster was extracted for each participant; the F-statistic and effect size (partial η2) of group differences in mean thickness from a univariate GLM controlling for age and sex is indicated. The estimated marginal means from these models is indicated for each trajectory class as well as a summary of the Bonferroni-corrected significant (p<.05) post-hoc differences between classes (Effect; L=low, D=declining, H=high irritability).
Figure 2. Regions showing differences in cortical thickness by trajectory class.
Note: Five regions showing differences in the temporal average of cortical thickness across wave as a function of irritability trajectory class are displayed. Regions showing greater thickness among the high irritability class are denoted in bold.
Various control analyses were examined to confirm these findings. First, the number of children ever taking psychotropic medications by the time of the first scan differed by trajectory class (Table 1). Yet, differences in cortical thickness across trajectory classes all remained significant controlling for medication use (p<.05). Of these five regions, only left SFG thickness differed significant by medication use, showing thinner cortex in those with prior medication use (Table S5, available online). Medication use was too varied across children and time to examine effects of specific medications here. Additionally, we found no significant differences in cortical thickness comparing participants that had or had not ever been diagnosed with MDD, anxiety, ODD/CD, or ADHD (Table S5, available online), suggesting some specificity to irritability trajectories. These associations between trajectory group also remained significant when controlling for baseline levels of non-irritability depression symptom severity, adverse life events, income-to-needs ratio, and irritability severity at scan. These covariates did not independently associate with thickness in any of these regions (Table S5, available online).
Discussion
This is the first study to relate latent trajectories of irritability symptoms across early development to cortical thickness in a sample of youth enriched for depressive symptoms during the preschool period. We identified three latent trajectories classes based on repeated semi-structured clinical interviews from preschool age through adolescence. The class with chronically elevated irritability showed poor clinical outcomes, specifically increased rates of depression and other psychopathology as well as greater general functional impairment. This trajectory class experienced higher rates of maternal depression and early adversity. Furthermore, during later childhood/early adolescence, these chronically irritable children showed greater SFG, STG, and IPL thickness across three scan waves, relative to other children in the sample. This work builds on the small prior literature examining trajectories and longitudinal outcomes of irritability. Additionally, this work presents novel findings in a sample with elevated early depressive symptoms and in regarding cortical thickness.
While this sample was enriched for children with elevated early depression symptoms, the latent trajectory classes identified resemble prior research. A large community-based study using parent-report questionnaire data from one to nine years of age identified five latent trajectory classes.2 The large sample size in this prior study may have allowed for the identification of smaller trajectory classes that we do not detect here. We do replicate findings that most children show a normative decline in irritability across early childhood,2 though children in the current sample showed higher levels of irritability at baseline, possibly due to the high levels of early depressive symptoms. Further, we replicate a class of children exhibiting chronically high irritability that were more likely to have maternal depression history. We also note a higher proportion of males in this class which may relate to our sample characteristics/acquisition but prior work does also note a higher percentage of males in a high and steady irritability class.2 Our work also identified similar but also fewer classes than work by Hawes et al.5 Similarly, we find the worst psychiatric outcomes among children with early irritability that remains high across development, although this prior study found that most children fell into a chronically low irritability class.5 Notably, a strength and unique feature of the current study was the well-validated clinical interview assessment rather than questionnaire measures and the use of a preschool sample over-sampled for depressive symptoms.
In examining cortical thickness in later childhood/early adolescence, we found that the left SFG, left STG, and right IPL all show elevated thickness in the high irritability class relative to the other trajectory classes. This was stable across three neuroimaging waves. This was also robust when controlling for early depressive symptoms, baseline adversity, and baseline socioeconomic status, indicating some specific to irritability, either in general or in the context of elevated depressive symptoms. Prior work has identified reduced GMV in a similar, but right hemisphere SFG region in children with DMDD, bipolar disorder, or ADHD relative to healthy youth.19 However, ours is the first study to link developmental trajectories of irritability, based on a dimensional measure, to brain structure, and the first to examine this in a sample enriched for early onset depression. We also identify two regions showing thinner cortex in the declining irritability class relative to the other classes, which was not hypothesized and should be explored in future work. Additionally, future work is needed to examine the functional implications of these alterations in SFG, STG, and IPL structure. Given documented neuropsychological aberrations in pediatric irritability (e.g., 35–38), these structural alterations may relate to deficits in attentional or executive control, as well as potentially reward processing or social cognition. Particularly, prior work highlighted a similar SFG region as showing altered reward/loss processing in irritable children13 as well as altered response to negative feedback in the IPL.14
Given the limited number of brain morphometry studies on youth with elevated irritability or on psychiatrically impaired children scanned longitudinally, it is difficult to draw conclusions regarding increased cortical thickness in chronic irritability. Prior cross-sectional research on pediatric psychopathology shows mixed results. Studies of pediatric anxiety disorders39–42 and depression43 suggest increased regional thickness or GMV. Given cross-sectional, longitudinal, and genetic associations among irritability, anxiety, and depression,1,10 increased cortical thickness may be a shared feature among the three symptoms. Other studies suggest thinner cortex associated with ADHD,44,45 conduct disorder,46,47 and also anxiety, though the latter study highlighted weaker associations in older youth.48 Work has also suggested slower patterns of both typical growth and reduction of different subcortical volumes in youth with depression.49 Longitudinal imaging studies suggest that youth with ADHD reach peak cortical thickness later than controls50 and that greater cortical thinning is associated with worse ADHD outcomes.51 The timing of neuroimaging across development may contribute to these mixed results. When interpreting increased cortical thickness, it is important to note the typical developmental thinning in the regions identified here in this age range, though critically, we do not identify any group x time interactions. Thus, further longitudinal studies of irritability, in samples with and without early depression, and other pediatric psychiatric problems are needed to determine the clinical significance and implications of both increased and decreased cortical thickness.
The current study informs the demographic and clinical characteristics of children with high irritability that remains stable across development. Of note, while many children exhibit irritability, only a subset manifest persistent irritability across development even in a sample enriched for early depressive symptoms. This distinct, relatively small subset is worthy of clinical attention given higher risk for later depression and general impairment. Importantly, elevated preschool-age irritability, particularly when persistent, may predict later depressive episodes and poorer clinical outcome over and above risk due to familial depression history and early adversity. Our findings suggest the need to carefully monitor children with irritability, especially those who also have depressive symptoms, and to evaluate familial factors, such as maternal depression and early adversity. The current data suggest that children with persistently elevated irritability may be most in need of early intervention and prevention given the elevated risks of poor outcomes attributable to this potentially modifiable characteristic.
The current study replicates and provides further robust evidence for increased maternal depression and child psychopathology among children with chronically high irritability.2 Because this sample was enriched for early depressive symptoms, the rates of maternal depression and child pathology are above the expected population rates. Nonetheless, the graded increase in risk and manifest illness across the low, declining, and high irritability trajectory groups is marked, mirroring prior findings.2,3 Children in the high irritability class also experienced more early life adversity. Furthermore, we note that early irritability symptoms, which are particularly elevated in the high irritability group, predict later depression above and beyond other risk factors, including maternal depression, adversity, sex, and perhaps most notably even other early depressive symptoms. These data suggest that early irritability may be a robust risk marker and that monitoring these symptoms across development aid prediction of later depression. Future work should continue to confirm the trajectories of irritability across development to highlight additional risk factors and clinical outcomes. Brain imaging beginning earlier in development will also be critical to identifying potential risk markers for sustained irritability and to better understand associated neural development. It will also be important to parse correlates of these neural differences to identify particular behaviors or outcomes associate with regional structure.
While the current approach to retrospectively ascertaining irritability severity from diagnostic interview has been used previously, e.g.,52 and similar approaches have been used with questionnaire measures, e.g.,2,5 future work should utilize measures specifically designed to assess irritability, e.g., the Affective Reactivity Index53 or the Multidimensional Assessment of Preschool Disruptive Behavior.54 These measures may yield more robust assessment of irritability across development and avoid biased recall. Additionally, we may suffer from an omitted variable bias in our analysis and creation of trajectories. As such, the current work should be compared to future studies using prospective and targeted measures to assess the generalizability of these results. Additionally, while the well-validated clinical interview assessments were a strength of the current study, we used only parent-report at the early assessment time points (child-report was not obtained before age 8) and then combined parent- and child-report at later waves. This maximized our use of the available data and should not contribute to systematic differences among trajectory groups. Additionally, while the current study examined three annual waves of high quality neuroimaging, we did not have the data to assess brain structure early in development; earlier scanning could aid in predicting longitudinal outcomes and is an important future step. Additionally, while the over-sampling for depressive symptoms in this sample was useful in examining associations with psychopathology, this may limit generalizability to population samples and thus future work in epidemiologic samples will be needed to replicate these effects.
Additionally, psychotropic medication use differed across trajectory groups, but this did not affect the observed associations between irritability and cortical thickness. Medication use was related on its own to SFG thickness, although we were limited in our ability to parse effects of particular medications. Similarly, we observed differences in IQ between the trajectory groups i.e., compared to the high irritability group, mean IQ was 6.42 points higher in the declining irritability group and 3.21 points higher in the low irritability group. While these differences did not reach statistical significance, and did not correlate with thickness in the regions identified here, we lacked the sample size to test moderation effects by IQ. Given associations between IQ and brain structure,55 it will be important to examine this in future studies, particularly to explore interactions between IQ and irritability on brain development and depression risk. Finally, while the overall sample size was relatively large for neuroimaging studies, the subsamples in each trajectory group too small to assess the age and sex interactions.
Overall, in this sample enriched for early depression, we find evidence for distinct developmental trajectories of irritability across childhood and a unique role for irritability in brain development. Of note, irritability longitudinally predicted later depression in youth above and beyond typical risk factors in this enriched sample. Future studies with larger sample sizes in community samples should examine these phenomena further. Particularly, it will be interesting to explore potential moderation effects of age, sex, and other factors related to brain structure, like psychotropic medication use, SES,29 IQ,55 or cognitive control.5
Supplementary Material
Acknowledgments
This was work was supported by NIMH grants R01MH66031, R01MH084840, R01MH090786, R01MH098454-S, and R01MH064769-06A1. Work by Drs. Pagliaccio, Pine, and Leibenluft was supported by the Intramural Research Program at NIMH.
The authors thank all participants and their families who provided time and effort to making this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Pagliaccio, Pine, Barch, Luby, and Leibenluft report no biomedical financial interests or potential conflicts of interest.
References
- 1.Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, Stringaris A. The Status of Irritability in Psychiatry: A Conceptual and Quantitative Review. JAACAP. 2016;55(7):556–570. doi: 10.1016/j.jaac.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiggins JL, Mitchell C, Stringaris A, Leibenluft E. Developmental trajectories of irritability and bidirectional associations with maternal depression. JAACAP. 2014;53(11):1191–1205. doi: 10.1016/j.jaac.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty LR, Smith VC, Bufferd SJ, et al. Preschool irritability: longitudinal associations with psychiatric disorders at age 6 and parental psychopathology. JAACAP. 2013;52(12):1304–1313. doi: 10.1016/j.jaac.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelan YM, Leibenluft E, Stringaris A, Barker ED. Pathways from maternal depressive symptoms to adolescent depressive symptoms: the unique contribution of irritability symptoms. J Child Psychol Psychiatry. 2015;56(10):1092–1100. doi: 10.1111/jcpp.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawes SW, Perlman SB, Byrd AL, Raine A, Loeber R, Pardini DA. Chronic anger as a precursor to adult antisocial personality features: The moderating influence of cognitive control. J Abnorm Psychol. 2016;125(1):64–74. doi: 10.1037/abn0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan K, Wakschlag LS. More than the terrible twos: The nature and severity of behavior problems in clinic-referred preschool children. Journal of Abnormal Child Psychology. 2000;28(1):33–46. doi: 10.1023/a:1005118000977. [DOI] [PubMed] [Google Scholar]
- 7.Wakschlag LS, Estabrook R, Petitclerc A, et al. Clinical Implications of a Dimensional Approach: The Normal:Abnormal Spectrum of Early Irritability. JAACAP. 2015;54(8):626–634. doi: 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belden AC, Thomson NR, Luby JL. Temper tantrums in healthy versus depressed and disruptive preschoolers: defining tantrum behaviors associated with clinical problems. J Pediatr. 2008;152(1):117–122. doi: 10.1016/j.jpeds.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caprara GV, Paciello M, Gerbino M, Cugini C. Individual differences conducive to aggression and violence: trajectories and correlates of irritability and hostile rumination through adolescence. Aggress Behav. 2007;33(4):359–374. doi: 10.1002/ab.20192. [DOI] [PubMed] [Google Scholar]
- 10.Rice F, Sellers R, Hammerton G, et al. Antecedents of New-Onset Major Depressive Disorder in Children and Adolescents at High Familial Risk. JAMA Psychiatry. 2017;74(2):153–160. doi: 10.1001/jamapsychiatry.2016.3140. [DOI] [PubMed] [Google Scholar]
- 11.Roberson-Nay R, Leibenluft E, Brotman MA, et al. Longitudinal Stability of Genetic and Environmental Influences on Irritability: From Childhood to Young Adulthood. Am J Psychiatry. 2015;172(7):657–664. doi: 10.1176/appi.ajp.2015.14040509. [DOI] [PubMed] [Google Scholar]
- 12.Stringaris A, Zavos H, Leibenluft E, Maughan B, Eley TC. Adolescent irritability: phenotypic associations and genetic links with depressed mood. Am J Psychiatry. 2012;169(1):47–54. doi: 10.1176/appi.ajp.2011.10101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, Phillips ML. Neural substrates of child irritability in typically developing and psychiatric populations. Dev Cogn Neurosci. 2015;14:71–80. doi: 10.1016/j.dcn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deveney CM, Connolly ME, Haring CT, et al. Neural mechanisms of frustration in chronically irritable children. Am J Psychiatry. 2013;170(10):1186–1194. doi: 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoddard J, Tseng WL, Kim P, et al. Association of Irritability and Anxiety With the Neural Mechanisms of Implicit Face Emotion Processing in Youths With Psychopathology. JAMA Psychiatry. 2017;74(1):95–103. doi: 10.1001/jamapsychiatry.2016.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiggins JL, Brotman MA, Adleman NE, et al. Neural Correlates of Irritability in Disruptive Mood Dysregulation and Bipolar Disorders. Am J Psychiatry. 2016;173(7):722–30. doi: 10.1176/appi.ajp.2015.15060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adleman NE, Fromm SJ, Razdan V, et al. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. 2012;53(11):1149–1156. doi: 10.1111/j.1469-7610.2012.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold AL, Brotman MA, Adleman NE, et al. Comparing Brain Morphometry Across Multiple Childhood Psychiatric Disorders. JAACAP. 2016;55(12):1027–1037. doi: 10.1016/j.jaac.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger HL, Ascher B, Angold A. The Preschool Age Psychiatric Assessment: Version 1.4. Durham, NC: Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 2003. [Google Scholar]
- 22.Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) JAACAP. 2000;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. JAACAP. 1992;31(1):78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong JM, Goldstein LH The MacArthur Working Group on Outcome Assessment. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0). MacArthur Foundation Research Network on Psychopathology and Development (David J. Kupfer, Chair) University of Pittsburgh; 2003. [Google Scholar]
- 25.Maxwell ME. Family Interview for Genetic Studies (FIGS): a manual for FIGS. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; Bethesda, MD: 1992. [Google Scholar]
- 26.Kaufman AS, Kaufman NL. Kaufman brief intelligence test. Wiley Online Library; 2004. [Google Scholar]
- 27.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 28.Barch D, Pagliaccio D, Belden A, et al. Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School-Age Depression. AmJPsychiatry. 2016;173(6):625–34. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luby J, Belden A, Botteron K, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 31.Proust-Lima C, Philipps V, Diakite A, Liquet B, Proust MC. Package ‘lcmm’. 2016 [Google Scholar]
- 32.Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. arXiv preprint arXiv:1503.00890. 2015 [Google Scholar]
- 33.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salum GA, Mogg K, Bradley BP, et al. Association between irritability and bias in attention orienting to threat in children and adolescents. JChildPsycholPsychiatry. 2017;58(5):595–602. doi: 10.1111/jcpp.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich BA, Brotman MA, Dickstein DP, Mitchell DG, Blair RJ, Leibenluft E. Deficits in attention to emotional stimuli distinguish youth with severe mood dysregulation from youth with bipolar disorder. JAbnormChildPsychol. 2010;38(5):695–706. doi: 10.1007/s10802-010-9395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. JAACAP. 2011;50(11):1173–1185. doi: 10.1016/j.jaac.2011.07.011. e1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagliaccio D, Wiggins JL, Adleman NE, et al. Behavioral and Neural Sustained Attention Deficits in Disruptive Mood Dysregulation Disorder and Attention-Deficit/Hyperactivity Disorder. JAACAP. 2017;56(5):426–435. doi: 10.1016/j.jaac.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strawn JR, Wehry AM, Chu WJ, et al. Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress Anxiety. 2013;30(9):842–848. doi: 10.1002/da.22089. [DOI] [PubMed] [Google Scholar]
- 40.Gold AL, Steuber ER, White LK, et al. Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology. 2017;42(12):2423–2433. doi: 10.1038/npp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, Phan KL. Neurostructural abnormalities in pediatric anxiety disorders. JAnxietyDisord. 2015;32:81–88. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strawn JR, John Wegman C, Dominick KC, et al. Cortical surface anatomy in pediatric patients with generalized anxiety disorder. JAnxietyDisord. 2014;28(7):717–723. doi: 10.1016/j.janxdis.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds S, Carrey N, Jaworska N, Langevin LM, Yang XR, Macmaster FP. Cortical thickness in youth with major depressive disorder. BMC Psychiatry. 2014;14:83. doi: 10.1186/1471-244X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: age, sex, and clinical correlations. J Atten Disord. 2013;17(8):641–654. doi: 10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- 45.Almeida LG, Ricardo-Garcell J, Prado H, et al. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. JPsychiatrRes. 2010;44(16):1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. BiolPsychiatry. 2012;72(3):207–214. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace GL, White SF, Robustelli B, et al. Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. JAACAP. 2014;53(4):456–465. doi: 10.1016/j.jaac.2013.12.008. e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newman E, Thompson WK, Bartsch H, et al. Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct Funct. 2016;221(6):3013–3025. doi: 10.1007/s00429-015-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle S, Lichter R, Dennison M, et al. Structural brain development and depression onset during adolescence: a prospective longitudinal study. AmJPsychiatry. 2014;171(5):564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- 50.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. PNAS. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. BiolPsychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copeland WE, Brotman MA, Costello EJ. Normative Irritability in Youth: Developmental Findings From the Great Smoky Mountains Study. JAACAP. 2015;54(8):635–642. doi: 10.1016/j.jaac.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stringaris A, Goodman R, Ferdinando S, et al. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. JChildPsycholPsychiatry. 2012;53(11):1109–1117. doi: 10.1111/j.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakschlag LS, Briggs-Gowan MJ, Choi SW, et al. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. JAACAP. 2014;53(1):82–96. doi: 10.1016/j.jaac.2013.10.011. e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menary K, Collins PF, Porter JN, et al. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41(5):597–606. doi: 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.