Abstract
Purpose: Half of US states mandate women be notified if they have dense breasts on their mammogram, yet guidelines and data on supplemental screening modalities are limited. Breast density (BD) refers to the extent that breast tissue appears radiographically dense on mammograms. High BD reduces the sensitivity of screening mammography and increases breast cancer risk. The aim of this study was to determine the potential impact of California’s 2013 BD notification legislation on breast cancer screening patterns. Methods: We conducted a cohort study of women aged 40 to 74 years who were members of a large Northern California integrated health plan (approximately 3.9 million members) in 2011-2015. We calculated pre- and post-legislation rates of screening mammography and magnetic resonance imaging (MRI). We also examined whether women with dense breasts (defined as BI-RADS density c or d) had higher MRI rates than women with nondense breasts (defined as BI-RADS density a or b). Results: After adjustment for race/ethnicity, age, body mass index, medical facility, neighborhood median income, and cancer history, there was a relative 6.6% decrease (relative risk [RR] 0.934, confidence interval [CI] 0.92-0.95) in the rate of screening mammography, largely driven by a decrease among women <50 years. While infrequent, there was a relative 16% increase (RR 1.16, CI 1.07-1.25) in the rate of screening MRI, with the greatest increase among the youngest women. In the postlegislation period, women with extremely dense breasts (BI-RADS d) had 2.77 times (CI 1.93-3.95) the odds of a MRI within 9 months of a screening mammogram compared with women with nondense breasts (BI-RADS b). Conclusions: In this setting, MRI rates increased in the postlegislation period. In addition, women with higher BD were more likely to have supplementary MRI. The decrease in mammography rates seen primarily among younger women may have been due to changes in national screening guidelines.
Keywords: breast density, breast cancer, notification legislation, cancer screening, supplementary screening, breast MRI, mammography, California
Introduction
Breast cancer is the second leading cause of cancer deaths among women.1 Breast density (BD), which is the “relative amount of radiopaque epithelial and stromal tissue elements compared with the amount of radiolucent fatty elements seen at mammography,”2 is an established breast cancer risk factor.2-7 Besides increasing cancer risk, high BD may mask lesions on mammography, decreasing its sensitivity as a screening tool.8,9 BD is classified according to the Breast Imaging Reporting and Data System (BI-RADS) developed by the American College of Radiology. BI-RADS has 4 BD categories—from least to most dense: (a) the breasts are almost entirely fatty, (b) there are scattered areas of fibroglandular density, (c) the breasts are heterogeneously dense, which may obscure small masses, and (d) the breasts are extremely dense, which lowers the sensitivity of mammography.2 The categories are often dichotomized: a and b considered “nondense,” and c and d considered “dense.”2
In 2009, Connecticut became the first state to pass legislation requiring patients with dense breasts on mammograms be notified. The goal was to increase awareness and improve decision making regarding supplemental breast ultrasound and magnetic resonance imaging (MRI).10 However, while BD legislation has been passed in at least 25 other US states as of April 2016 (California in April 2013), it is unclear how notification affects breast cancer detection and patient morbidity. Since 43% of US women 40 to 74 years old have dense breasts, such legislation could have substantial public health impact.11
Only a few studies have examined changing clinical practices after BD legislation.12,13 Such information may influence guidelines and determine whether California legislation will continue beyond 2019, the date for its repeal unless it is extended. Thus, this study’s purpose was to examine the impact of the California BD notification legislation on rates of screening mammography and MRI, focusing on the 2 years before and 2 years after BD legislation. The authors hypothesized that the postlegislation screening MRI rate would increase from the prelegislation rate.
Methods
Study Setting and Population
This retrospective cohort study analyzed women aged 40 to 74 years enrolled in Kaiser Permanente Northern California (KPNC) in 2011-2015. KPNC is an integrated health care system serving 3.9 million enrollees that reflects the region’s underlying population, although it slightly underrepresents socioeconomic extremes. Study eligibility criteria included 2 years of continuous KPNC enrollment without more than two 45-day gaps in membership. Women with a double mastectomy before the screening mammogram or MRI were excluded. These criteria were chosen because of the recommended breast cancer screening age range at KPNC, and are similar to criteria for Healthcare Effectiveness Data and Information Set (HEDIS) measures. The study was approved by the KPNC Institutional Review Board in December 2015 (approval number CN-15-2492-H). Informed consent and Privacy Rule authorization requirements were waived.
Databases
Information was collected from The Permanente Medical Group (TPMG) Breast Cancer Tracking System (BCTS) and patient medical records. Collected information included KPNC facility where the screening mammogram (hereafter referred to as mammogram) or screening MRI (hereafter referred to as MRI) was performed, patient age at end of each 2-year period, body mass index (BMI) closest to end of each 2-year period, race/ethnicity, neighborhood median family income (using census block address) at end of each 2-year period, double mastectomy and breast cancer history prior to mammogram or MRI, and BD (BI-RADS fifth edition). Screening mammograms were designated with Current Procedural Terminology (CPT) code 77057. Screening MRIs utilized codes 77058 and 77059, and were classified as “screening” by BCTS clinicians. For mastectomy history, International Classification of Diseases, 9th revision (ICD-9) codes for bilateral mastectomy procedure (85.42, 85.44, 85.46, 85.48) and 2 unilateral mastectomy procedures done on different days (85.41, 85.43, 85.45, 85.47) as well as CPT codes (19180, 19182, 19200, 19220, 19240, 19303-7) were used.
Screening Rates
For most of 2011-2015, screening mammography in KPNC was recommended every 1 to 2 years for women aged 40 to 74 years, while screening MRI was recommended as an adjunct for those at high genetic risk. After August 2014, KPNC guidelines were modified to mammography every 1 to 2 years for women aged 50 to 74 years, while decision making between the patient and physician was recommended for women aged 40 to 49 years. In these guidelines, BD category did not affect screening recommendations. To calculate changes in screening practices, a 2-year prelegislation period from April 2011 to March 2013 was compared with the 2-year postlegislation period from April 2013 to March 2015. Women were only counted once for each 2-year period.
Statistical Methods
Crude rates of mammography and MRI were compared in pre- and post-legislation periods using chi-square tests. To determine screening trends after adjusting for race, age, BMI, neighborhood median income, medical facility, and cancer history, Poisson regression estimates were used to calculate relative risk comparing mammography and MRI rates in both periods. A separate Poisson model with an interaction term for legislation period and age was included to determine age group–specific rate change from pre- to postlegislation period. Nesting of patients within medical facilities was accounted for using a generalized estimating equation with a robust sandwich covariance estimator. Bivariate comparisons of BD frequencies by demographic characteristics were analyzed using chi-square tests. To isolate the effect of legislation on the likelihood of supplemental MRI, multivariable logistic regression was used to estimate an odds ratio of MRI within 9 months of mammogram in the prelegislation period versus the postlegislation period. When BD data became available during the post-legislation period, multivariable logistic regression was used to estimate an odds ratio of MRI within 9 months of mammogram among each BI-RADS BD category. The logistic regression model similarly adjusted for race, age, BMI, neighborhood median income, medical facility, and cancer history, and used a robust sandwich covariance estimator to allow for repeated subjects within medical facilities. Statistical analyses were done using SAS 9.3 (SAS Institute, Cary, NC). An alpha of 0.05 was considered statistically significant.
Results
Mammogram and MRI Screening Rates
The number of eligible women increased from the prelegislation period (April 2011 to March 2013) to the postlegislation period (April 2013 to March 2015), from 614 663 to 631 478. The number of women with a mammogram who met inclusion criteria decreased in the postlegislation period, from 506 400 to 487 365 while the number of women with an MRI increased from 743 to 872.
Table 1 shows total and demographic-specific counts and crude rates of mammograms and MRIs. There was a decrease in mammogram rate from 82.4% (95% CI 82.3%-82.5%) prelegislation to 77.2% (95% CI 77.1%-77.3%) postlegislation (P < 0.001). This decreasing trend was statistically significant for all race, age, BMI, and neighborhood median income–specific rates, except women aged 70 to 74 years (significant increase, P < 0.001). During the same period, the rate of MRI increased from 12.1 per 10 000 women (95% CI 11.2-13.0) to 13.8 per 10 000 women (95% CI 12.9-14.7) (P = 0.008). There were statistically significant increases in MRI rates for Asian and white women, women aged 42 to 44 years, women with normal BMI, and women living in neighborhoods with a median family income of $60 000 to <$90 000 or more than $120 000.
Table 1.
Total and demographic-specific counts and crude rates of screening mammograms and MRIs.
| No. (Percent) of Women With Screening Mammogram |
No. (Rate per 10 000 Women) With Screening MRI |
|||||
|---|---|---|---|---|---|---|
| Pre (N = 614 663) | Post (N = 631 478) | P a | Pre (N = 614 663) | Post (N = 631 478) | P a | |
| Total no. (percent or rate) | 506 400 (82.4) | 487 365 (77.2) | <.001 | 743 (12.1) | 872 (13.8) | .008 |
| Age (years) | ||||||
| 42-44 | 45 259 (77.3) | 35 091 (60.7) | <.001 | 67 (11.4) | 98 (17.0) | .01 |
| 45-49 | 78 735 (79.9) | 62 723 (64.2) | <.001 | 142 (14.4) | 152 (15.6) | .51 |
| 50-54 | 89 192 (82.0) | 83 589 (77.8) | <.001 | 165 (15.2) | 182 (16.9) | .30 |
| 55-59 | 89 610 (83.1) | 87 624 (80.9) | <.001 | 142 (13.2) | 166 (15.3) | .18 |
| 60-64 | 85 205 (84.5) | 84 582 (83.0) | <.001 | 96 (9.5) | 119 (11.7) | .14 |
| 65-69 | 70 631 (86.7) | 77 862 (84.7) | <.001 | 88 (10.8) | 97 (10.6) | .88 |
| 70-74 | 47 768 (81.3) | 55 894 (84.0) | <.001 | 43 (7.3) | 58 (8.7) | .38 |
| Race | ||||||
| White | 278 286 (83.0) | 263 802 (78.2) | <.001 | 488 (14.6) | 577 (17.1) | .009 |
| Asian | 85 536 (83.4) | 85 771 (77.8) | <.001 | 94 (9.2) | 146 (13.2) | .005 |
| Black | 38 838 (81.9) | 36 856 (76.6) | <.001 | 37 (7.8) | 29 (6.0) | .30 |
| Hispanic | 73 865 (83.9) | 72 904 (77.6) | <.001 | 82 (9.3) | 83 (8.8) | .73 |
| Native American | 2270 (76.4) | 2209 (73.7) | .02 | 2 (6.7) | 2 (6.7) | .99 |
| BMI (kg/m2) | ||||||
| <18.5 | 5835 (77.4) | 6165 (74.1) | <.001 | 16 (21.2) | 20 (24.0) | .71 |
| 18.5-24.9 | 164 016 (86.1) | 157 829 (80.7) | <.001 | 345 (18.1) | 427 (21.8) | .01 |
| 25-29.9 | 152 900 (86.7) | 147 060 (81.5) | <.001 | 205 (11.6) | 235 (13.0) | .23 |
| 30-39.9 | 135 563 (85.8) | 130 707 (80.1) | <.001 | 156 (9.9) | 160 (9.8) | .95 |
| 40+ | 32 778 (81.8) | 31 214 (75.3) | <.001 | 18 (4.5) | 17 (4.1) | .79 |
| Median incomeb ($) | ||||||
| <30 000 | 10 015 (77.6) | 9759 (72.8) | <.001 | 9 (7.0) | 11 (8.2) | .72 |
| 30 000-<60 000 | 91 178 (79.8) | 87 275 (74.6) | <.001 | 91 (8.0) | 86 (7.4) | .60 |
| 60 000-90 000 | 160 976 (82.3) | 155 281 (76.7) | <.001 | 185 (9.5) | 233 (11.5) | .05 |
| 90 000<120 000 | 131 901 (83.4) | 128 204 (78.3) | <.001 | 217 (13.7) | 251 (15.3) | .23 |
| 120 000+ | 103 708 (84.6) | 99 928 (79.6) | <.001 | 225 (18.3) | 275 (21.9) | .05 |
Abbreviations: MRI, magnetic resonance imaging; Pre, prelegislation (April 2011 to March 2013); Post, postlegislation (April 2013 to March 2015); BMI, body mass index.
Chi-square test to compare pre- and post-legislation rates. Values in boldface indicate statistical significance.
Median income = census block median family income.
Table 2 lists relative risks (RRs) comparing mammogram and MRI rates in the postlegislation period relative to the prelegislation period. After adjustment for race/ethnicity, age, BMI, neighborhood median income, medical facility, and cancer history, there was a relative 6.6% decrease (RR 0.934, CI 0.92-0.95) in the mammogram rate and a relative 16% increase (RR 1.16, CI 1.07-1.25) in the MRI rate (Ps < 0.001). Age group specific change in mammography rates, determined using an interaction term between legislative period and age, varied substantially; there was a modest, if any, reduction in rates among women aged 55 years and older. In contrast, the increase in MRI screening rates postlegislation appeared to be greatest among women in their early 40s.
Table 2.
Multivariable Relative Risks Comparing Postlegislation With Prelegislation Screening Mammogram and MRIa Rates.
| Screening Mammogram |
Screening MRI |
|||
|---|---|---|---|---|
| Rate Ratio (95% CI) | P a | Rate Ratio (95% CI) | P a | |
| Model 1: Post-/pre-legislationb | 0.93 (0.92-0.95) | <.001 | 1.16 (1.07-1.25) | <.001 |
| Model 2: Post-/pre-legislation among age groupsc | ||||
| 42-44 | 0.79 (0.77-0.80) | <.001 | 1.51 (1.30-1.75) | <.001 |
| 45-49 | 0.80 (0.78-0.83) | <.001 | 1.08 (0.79-1.49) | 0.61 |
| 50-54 | 0.95 (0.93-0.96) | <.001 | 1.13 (0.84-1.52) | 0.43 |
| 55-59 | 0.97 (0.96-0.99) | 0.002 | 1.16 (1.01-1.34) | 0.04 |
| 60-64 | 0.98 (0.97-0.99) | 0.005 | 1.22 (0.97-1.55) | 0.09 |
| 65-69 | 0.98 (0.97-0.99) | <.001 | 1.00 (0.82-1.23) | 0.97 |
| 70-74 | 1.03 (1.02-1.04) | <.001 | 1.14 (0.74-1.76) | 0.55 |
Abbreviations: MRI, magnetic resonance imaging.
Values in boldface indicate statistical significance.
Calculated from Poisson regression model parameters, where model adjusts for post-/pre-legislation period, age, race, body mass index, neighborhood income, medical facility, cancer history. Generalized estimating equation model accounts for nesting within medical facilities.
Poisson regression generalized estimating equation model with same variables as above plus interaction terms interacting post-/pre-legislative period with age group (years). Relative risks show age group–specific relative change post-/pre-legislation.
Breast Density Patterns
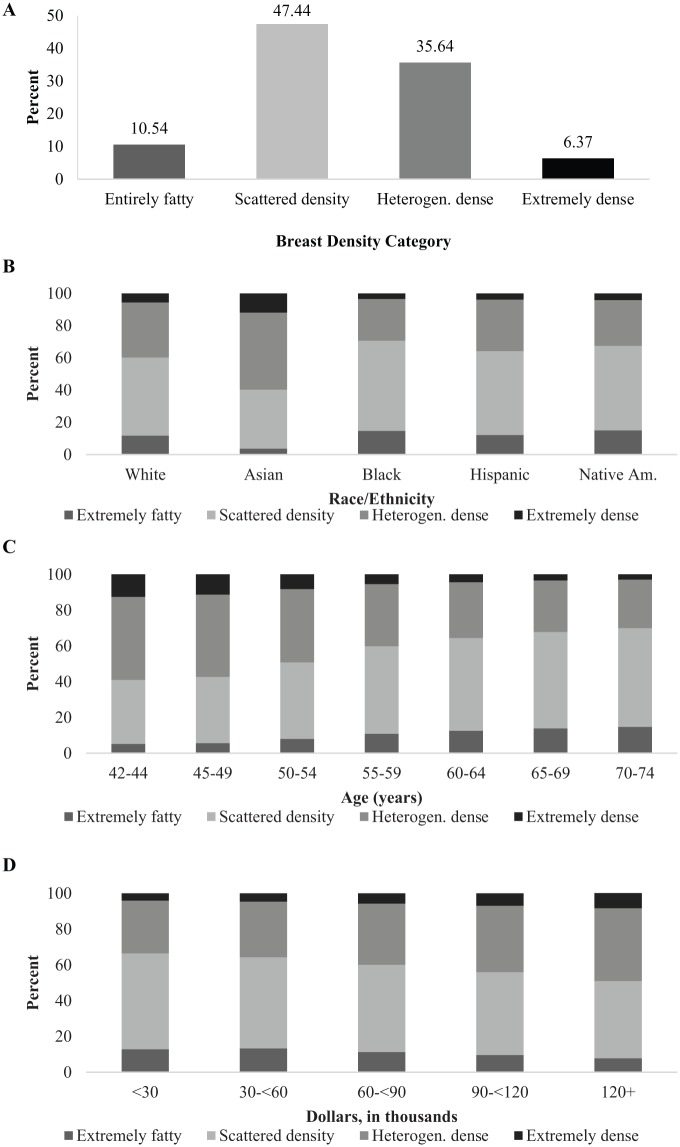
Figure 1a-d shows, respectively, frequencies of BD categories overall, by race/ethnicity, by age, and by neighborhood median income. When BD categories were collapsed into 2 groups, the overall distribution became 58% not dense and 42% dense. Categorical frequencies stratified by age and BMI (not shown) revealed inverse relationships between each variable with BD, whereas BD frequencies stratified by neighborhood median income revealed a direct relationship. BD patterns were significantly different across race, age, BMI, and neighborhood median income (Ps < 0.001).
Figure 1.
Distribution of breast density categories (April 2013 to March 2015): (a) overall; (b) by race/ethnicity; (c) by age; and (d) Neighborhood median income. Heterogen. dense, heterogenously dense; Native Am, Native American.
MRI Within 9 Months of Screening Mammogram
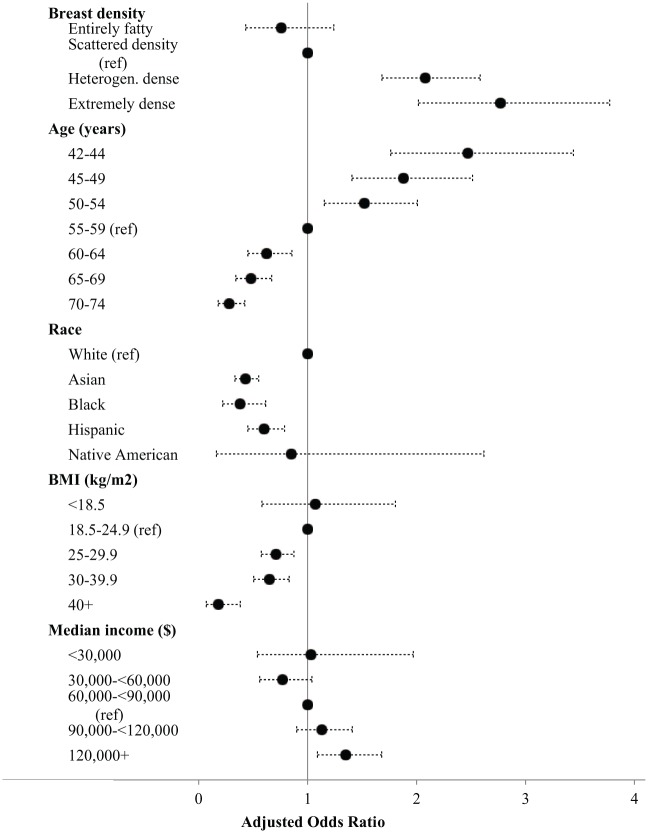
Table 3 shows select adjusted odds ratios (ORs) for having MRI within 9 months of mammogram. There was a 46% increase (OR 1.46, CI 1.30-1.64) in the odds of MRI within 9 months of mammogram pre- to postlegislation. ORs decreased with increasing age and BMI, increased with increasing neighborhood median income, and were lower in non-white women. In the postlegislation period, women who had dense breasts had 2.08 times (CI 1.769-2.46) or 2.77 times (CI 1.93-3.97) (category c or d, respectively) the odds of having a MRI within 9 months of a mammogram compared with women in category b (also shown in Figure 2).
Table 3.
Selected Odds Ratios Indicating Odds of Having Screening MRI Within 9 Months of Screening Mammogram.
| Model 1a: Data From Pre and Postlegislation Periods |
Model 2b: Data From Postlegislation Period Only |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P c | Odds Ratio (95% CI) | P c | |
| Post vs pre period | 1.46 (1.30-1.64) | <.001 | NA | |
| Breast density | NA | |||
| Entirely fatty | 0.76 (0.49-1.20) | .24 | ||
| Scattered density | 1.00 (Reference) | — | ||
| Heterogeneously dense | 2.08 (1.76-2.46) | <.001 | ||
| Extremely dense | 2.77 (1.93-3.97) | <.001 | ||
| Age (years) | ||||
| 42-44 | 2.25 (1.83-2.76) | <.001 | 2.47 (1.91-3.20) | <.001 |
| 45-49 | 1.76 (1.38-2.26) | <.001 | 1.88 (1.33-2.65) | <.001 |
| 50-54 | 1.57 (1.29-1.92) | <.001 | 1.52 (1.10-2.11) | 0.01 |
| 55-59 | 1.00 (Reference) | — | 1.00 (Reference) | — |
| 60-64 | 0.58 (0.43-0.78) | <.001 | 0.62 (0.46-0.84) | 0.002 |
| 65-69 | 0.45 (0.32-0.63) | <.001 | 0.48 (0.31-0.73) | <.001 |
| 70-74 | 0.25 (0.19-0.32) | <.001 | 0.28 (0.19-0.42) | <.001 |
| Race | ||||
| White | 1.00 (Reference) | — | 1.00 (Reference) | — |
| Asian | 0.43 (0.37-0.53) | <.001 | 0.43 (0.33-0.56) | <.001 |
| Black | 0.54 (0.42-0.69) | <.001 | 0.38 (0.24-0.62) | <.001 |
| Hispanic | 0.61 (0.52-0.70) | <.001 | 0.60 (0.48-0.75) | <.001 |
| Native American | 0.51 (0.15-1.67) | .26 | 0.85 (0.25-2.87) | .79 |
| BMI (kg/m2) | ||||
| <18.5 | 1.35 (0.81-2.25) | .26 | 1.07 (0.56-2.02) | .84 |
| 18.5-24.9 | 1.00 (Reference) | — | 1.00 (Reference) | — |
| 25-29.9 | 0.62 (0.55-0.70) | <.001 | 0.71 (0.60-0.85) | <.001 |
| 30-39.9 | 0.49 (0.40-0.60) | <.001 | 0.65 (0.51-0.83) | <.001 |
| 40+ | 0.18 (0.10-0.32) | <.001 | 0.18 (0.10-0.32) | <.001 |
| Median incomed ($) | ||||
| <30 000 | 0.87 (0.51-1.47) | .60 | 1.03 (0.54-1.97) | .92 |
| 30 000-<60 000 | 0.86 (0.63-1.17) | .33 | 0.77 (0.56-1.04) | .09 |
| 60 000-<90 000 | 1.00 (Reference) | — | 1.00 (Reference) | — |
| 90 000-<120 000 | 1.19 (1.07-1.34) | .002 | 1.13 (0.90-1.41) | .30 |
| 120 000+ | 1.49 (1.25-1.77) | <.001 | 1.35 (1.09-1.68) | .006 |
Abbreviations: MRI, magnetic resonance imaging; BMI, body mass index.
Logistic regression model adjusting for age, race, BMI, neighborhood median income, medical facility, and cancer history. Odds ratio estimates for medical facility and cancer history not shown.
Logistic regression model adjusting for breast density, age, race, BMI, neighborhood median income, medical facility, cancer history. Generalized estimating equation model allowed for nesting of individuals within medical facility. Odds ratio estimates for medical facility and cancer history not shown.
Values in boldface indicate statistical significance.
Median income = census block median family income.
Figure 2.
Adjusted odds ratiosa and confidence bounds for having screening magnetic resonance imaging (MRI) within 9 months of screening mammogram in the postlegislative period. Ref, reference.
a Odds ratios estimated by logistic regression controlled for all variables in the figure plus medical center and cancer history.
Discussion
To our knowledge, this is the first study to quantitatively examine how breast cancer screening practices have changed since the California BD notification legislation went into effect. KPNC screening mammography rates were consistently higher than average commercial, Medicaid, and Medicare screening rates reported to HEDIS.14 The study’s distribution of BD categories stratified by demographic variables resemble what has been reported for the US female population.11,15,16 In the 2 years after BD notification legislation went into effect, screening mammography decreased slightly, although this was largely limited to women younger than 50 years. In contrast, rates of MRIs appeared to increase for most age groups, but was greatest among the youngest women. Higher BD was associated with supplemental MRI, suggesting that observed increases in MRI screening rates could have been associated with BD legislation. Because we did not have prelegislation BD, we could not examine whether increases in screening MRIs from the pre- to post-legislation period were limited to women with dense breasts.
Other studies found increased supplementary screening subsequent to BD legislation. At a single community hospital in New Jersey, Sobotka and Hinrichs12 found follow-up breast ultrasound utilization increased 176% to 336% over 1 year. Meanwhile, Mason et al13 at Baylor University in Texas found a 23-fold increase in supplementary MRIs in the 2-year period after BD legislation went into effect. In contrast to these 2 facility-specific studies, our study encompassed an entire region of California served by a single health plan.
Our study had additional limitations. It was conducted in an insured population, and results may not be generalizable to other settings. The study was limited to a 4-year period based on available data, but this may have been too short a time period to expect substantial behavior change, especially since studies have demonstrated low awareness of BD legislation among both physicians and patients.4,17 Information on other predictors of screening (such as individual-level socioeconomic status, MRI access, and physician training) was unavailable. Technology updates and increased acceptability of screening MRI among physicians could contribute to increased screening MRI rates. There may have been misclassification of screening versus diagnostic imaging, which depends on the radiologist’s interpretation of provided clinical information. Lastly, while BI-RADS for BD is used routinely in clinical care, it relies on radiologists’ visual estimation and can be subjective.18
An unexpected result was the decrease in KPNC screening mammography rates. While the age at initiation of breast cancer screening began shifting from age 40 to 50 years due to US Preventive Services Task Force (USPSTF) recommendations in 2009,19 KPNC guidelines continued to recommend screening for women in their 40s until 2014. These recommendation changes may explain reduced screening mammography among women aged 40 to 49 years, but little if any decrease among women 50 years and older.
In their latest guidelines, the USPSTF and the National Comprehensive Cancer Network state there is insufficient evidence to establish screening recommendations for women with dense breasts.15,20 Although some studies have examined BD awareness and legislation,4,12,21 research about effects on clinical practice is limited. More studies based on clinical algorithms and cost-effectiveness analyses of supplemental screening methods are needed, so that evidence-based policies and recommendations can guide health providers as they counsel patients with dense breasts.
Acknowledgments
The authors would like to acknowledge the Kaiser Permanente Oakland Medical Center Internal Medicine Residency Program, University of California Berkeley School of Public Health Interdisciplinary Program, Kaiser Permanente’s Division of Research, TPMG BCTS, and specifically Dr Joan Lo, Dr Thomas Baudendistel, Dr Anke Hemmerling, Dr Phuoc Le, Debbie Postlethwaite, Mary Anne Armstrong, Mary Callahan, Jane Bethard-Tracy, Dr Sherry Butler, and Dr Veronica Shim.
Author Biographies
Stephanie Lynn Chau, MD, MPH is an outpatient Internal Medicine physician at Kaiser Oakland Medical Center. Her research interests include breast cancer screening, preventive medicine and health disparities.
Amy Alabaster, MPH, is a consulting data analyst with the Kaiser Permanente Northern California (KPNC) Division of Research. In her role within the Biostatistical Consulting Unit, she provides research support for KPNC clinicians.
Karin Luikart, RN, MSN, is a Nurse Coordinator for the Breast Cancer Tracking System (BCTS) in the Genetics Department at KPNC. In her role within BCTS she provides data to KPNC clinicians to support accreditation, quality improvement, and research.
Leslie Manace Brenman MD, MPhil is Sub-Chief for Genetics and Screening & Tracking at KPNC, Medical Director for Prenatal Screening, BCTS, and Multispecialty Neurofibromatosis Program for KPNC, and Assistant Professor at the University of California, San Francisco. In her practice and research, she focuses on adult and prenatal genetics, delivery science, and ethical, legal, and social implications (ELSI) in medical genetics.
Laurel A. Habel, PhD is a cancer epidemiologist and the Associate Director of Cancer Research in the Division of Research at KPNC. She has conducted several studies of mammographic density, including studies of mammographic density and recurrence and other outcomes in women with early breast cancer, a study of the association of breast cancer risk factors and mammographic density in a multiethnic cohort of women transitioning through menopause, and a large genome wide association study (GWAS) study of mammographic density.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the KPNC Community Benefit Program and its KPNC Graduate Medical Education Research funds.
References
- 1. American Cancer Society. What are the key statistics about breast cancer? http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics. Updated 2015. Accessed December 2, 2015.
- 2. Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics. 2015;35:302-315. doi: 10.1148/rg.352140106. [DOI] [PubMed] [Google Scholar]
- 3. Desreux J, Bleret V, Lifrange E. Should we individualize breast cancer screening? Maturitas. 2012;73:202-205. doi: 10.1016/j.maturitas.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4. Rhodes DJ, Radecki Breitkopf C, Ziegenfuss JY, Jenkins SM, Vachon CM. Awareness of breast density and its impact on breast cancer detection and risk. J Clin Oncol. 2015;33:1143-1150. doi: 10.1200/JCO.2014.57.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ray KM, Price ER, Joe BN. Breast density legislation: mandatory disclosure to patients, alternative screening, billing, reimbursement. AJR Am J Roentgenol. 2015;204:257-260. doi: 10.2214/AJR.14.13558. [DOI] [PubMed] [Google Scholar]
- 6. Price ER, Hargreaves J, Lipson JA, et al. The California Breast Density Information Group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology. 2013;269:887-892. doi: 10.1148/radiol.13131217. [DOI] [PubMed] [Google Scholar]
- 7. Freer PE, Slanetz PJ, Haas JS, et al. Breast cancer screening in the era of density notification legislation: summary of 2014 Massachusetts experience and suggestion of an evidence-based management algorithm by multi-disciplinary expert panel. Breast Cancer Res Treat. 2015;153:455-464. doi: 10.1007/s10549-015-3534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kerlikowske K, Zhu W, Tosteson AN, et al. ; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang AT, Vachon CM, Brandt KR, Ghosh K. Breast density and breast cancer risk: a practical review. Mayo Clin Proc. 2014;89:548-557. doi: 10.1016/j.mayocp.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 10. Haas JS, Kaplan CP. The divide between breast density notification laws and evidence-based guidelines for breast cancer screening: legislating practice. JAMA Intern Med. 2015;175:1439-1440. doi: 10.1001/jamainternmed.2015.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sobotka J, Hinrichs C. Breast density legislation: discussion of patient utilization and subsequent direct financial ramifications for insurance providers. J Am Coll Radiol. 2015;12:1011-1015. doi: 10.1016/j.jacr.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 13. Mason C, Yokubaitis K, Howard E, Shah Z, Wang J. Impact of Henda’s law on the utilization of screening breast magnetic resonance imaging. Proc (Bayl Univ Med Cent). 2015;28:7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breast cancer screening. NCQA Report Cards Web site. http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2015-table-of-contents/breast-cancer. Updated 2015. Accessed June 21, 2016.
- 15. Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:268-278. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Habel LA, Capra AM, Oestreicher N, et al. Mammographic density in a multiethnic cohort. Menopause. 2007;14:891-899. doi: 10.1097/gme.0b013e318032569c. [DOI] [PubMed] [Google Scholar]
- 17. Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California Breast Density Law on primary care physicians. J Am Coll Radiol. 2015;12:256-260. doi: 10.1016/j.jacr.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winkler NS, Raza S, Mackesy M, Birdwell RL. Breast density: clinical implications and assessment methods. Radiographics. 2015;35:316-324. doi: 10.1148/rg.352140134. [DOI] [PubMed] [Google Scholar]
- 19. U.S. Preventive Services Task Force. Breast cancer: screening. November 2009. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening. Updated 2016. Accessed April 13, 2016.
- 20. National Comprehensive Cancer Network. Breast cancer screening and diagnosis. NCCN Clinical Practice Guidelines in Oncology. 2015. (Version 1.2015). [Google Scholar]
- 21. Trinh L, Ikeda DM, Miyake KK, et al. Patient awareness of breast density and interest in supplemental screening tests: comparison of an academic facility and a county hospital. J Am Coll Radiol. 2015;12:249-255. doi: 10.1016/j.jacr.2014.10.027. [DOI] [PubMed] [Google Scholar]