Abstract
Circumstantial evidence based on ultrastructural correlation, specific labeling, and subcellular fractionation studies indicates that at least the early steps of monoterpene biosynthesis occur in plastids. (4S)-Limonene synthase, which is responsible for the first dedicated step of monoterpene biosynthesis in mint species, appears to be translated as a preprotein bearing a long plastidial transit peptide. Immunogold labeling using polyclonal antibodies raised to the native enzyme demonstrated the specific localization of limonene synthase to the leucoplasts of peppermint (Mentha × piperita) oil gland secretory cells during the period of essential oil production. Labeling was shown to be absent from all other plastid types examined, including the basal and stalk cell plastids of the secretory phase glandular trichomes. Furthermore, in vitro translation of the preprotein and import experiments with isolated pea chloroplasts were consistent in demonstrating import of the nascent protein to the plastid stroma and proteolytic processing to the mature enzyme at this site. These experiments confirm that the leucoplastidome of the oil gland secretory cells is the exclusive location of limonene synthase, and almost certainly the preceding steps of monoterpene biosynthesis, in peppermint leaves. However, succeeding steps of monoterpene metabolism in mint appear to occur outside the leucoplasts of oil gland cells.
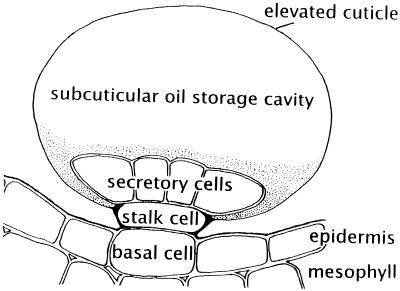
Peppermint (Mentha × piperita), spearmint (Mentha spicata), and other essential oil plants of the Lamiaceae produce and accumulate monoterpenes in anatomically specialized glandular trichomes (Fahn, 1979). The mints bear two types of nonphotosynthetic glandular trichomes, a small capitate type with a limited capacity to store secreted material, and a peltate type containing a basal cell, a stalk cell, and eight secretory cells arranged in a disc (Fig. 1) (Amelunxen, 1965; Amelunxen et al., 1969; Fahn, 1979). The latter type develops a large oil-storage space at the apex of the glandular trichome, where the thick cuticle separates from the secretory cells to produce a subcuticular pocket and is therefore thought to be responsible for the production of the bulk of the monoterpenoid essential oil (Amelunxen et al., 1969).
Figure 1.
Schematic diagram of a peppermint leaf peltate glandular trichome illustrating the placement of these epidermal structures and the relationship of the disc of secretory cells to the stalk and basal cells and to the subcuticular storage space.
Biochemical studies with isolated peppermint peltate glandular trichomes have revealed that the secretory cells are not only responsible for the secretion of monoterpenes into the oil-storage space, but also serve as the actual site of monoterpene biosynthesis (Gershenzon et al., 1992; McCaskill et al., 1992). Plant essential oil- and resin-secreting glands often share a syndrome of specialized ultrastructural features, including numerous amoeboid leucoplasts and abundant smooth ER (Schnepf, 1974; Dell and McComb, 1978; Fahn, 1988). There have been relatively few ultrastructural studies of Lamiaceae oil glands, and most of those that have been done have stressed the possible role of the extensive ER or the densely staining cytoplasm in monoterpene biosynthesis (Amelunxen, 1965; Schnepf, 1974; Bosabalidis and Tsekos, 1982). Recent papers by Bourett et al. (1994) and Ascensao et al. (1997) have discussed the possible role of leucoplasts. Oil gland leucoplasts are nonpigmented plastids, often with few internal membranes (Carde, 1984), that have been implicated in monoterpene biosynthesis by a survey of the secretory structures of nearly 50 plant species that demonstrated a close correlation between the presence of such leucoplasts and the accumulation of monoterpenes (Cheniclet and Carde, 1985).
Related evidence for the role of leucoplasts and other plastids in monoterpene formation derives from studies showing that isolated plastids are capable of monoterpene biosynthesis when supplied with exogenous precursors (Gleizes et al., 1983; Pauly et al., 1986; Mettal et al., 1988; Pérez et al., 1990; Soler et al., 1992). Finally, the labeling patterns of several monoterpenes derived from exogenous [13C]Glc are consistent with their origin via the mevalonate-independent pathway (Eisenreich et al., 1997; Adam et al., 1998), an isoprenoid biosynthetic pathway known to operate in some Eubacteria and exclusively in the plastids of a variety of phylogenetically divergent plants and green algae (Lichtenthaler et al., 1997). Although there is considerable circumstantial evidence to indicate that at least the early steps of monoterpene biosynthesis are associated with gland cell plastids, there is as yet no direct evidence for the localization of relevant biosynthetic enzymes to these structures that would reveal the details of subcellular pathway organization.
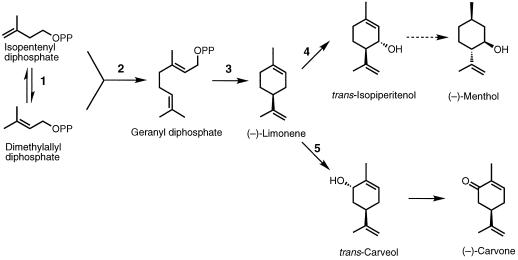
(−)-Limonene synthase catalyzes the cyclization of geranyl diphosphate, the universal C10 precursor of the monoterpenes, to (−)-4S-limonene (Croteau, 1991, 1993) (Fig. 2). This olefin serves as the common progenitor of the characteristic monoterpenes in mints, including menthol in peppermint and carvone in spearmint, following a series of enzymatic redox transformations (Croteau and Gershenzon, 1994) (Fig. 2). The conversion of geranyl diphosphate to limonene is one of the simplest of all terpenoid cyclization reactions, and this synthase (cyclase) serves as a model for the general enzyme type (Wise and Croteau, 1999). The operationally soluble (−)-4S-limonene synthase from spearmint has been purified and characterized (Alonso et al., 1992; Rajaonarivony et al., 1992), and has been employed to prepare polyclonal antibodies that were shown to cross-react with the enzyme from other mint species (Alonso et al., 1993).
Figure 2.
Pathway for the conversion of C5 isoprenoid units via geranyl diphosphate and limonene to the principal essential oil components (−)-menthol (peppermint) and (−)-carvone (spearmint). The responsible enzymes are: isopentenyl diphosphate isomerase (1); geranyl diphosphate synthase (2); 4S-limonene synthase (3); 4S-limonene-3-hydroxylase (4); and 4S-limonene-6-hydroxylase (5). The broken arrow indicates five enzymatic steps. OPP, Diphosphate moiety.
A limonene synthase cDNA has been isolated from spearmint and functionally expressed in Escherichia coli (Colby et al., 1993); the limonene synthase cDNA from peppermint was recently isolated and shown to be 93% identical to the spearmint clone at the deduced amino acid level (B.M. Lange and R.B. Croteau, unpublished data). Evaluation of the encoded sequence of the 72.4-kD protein with respect to the physical properties of the 65-kD native enzyme suggests that limonene synthase is translated as a preprotein bearing what appears to be a substantial N-terminal plastid-targeting peptide (Colby et al., 1993). Deletion of the presumptive transit peptide by truncation and heterologous expression of the corresponding cDNA yielded a fully active, “pseudomature” form of the enzyme, which is consistent with the concept of plastidial targeting and subsequent proteolytic processing (Williams et al., 1998).
Because of the rigorous conditions required to disrupt oil gland cells of mint, classical subcellular fractionation studies to locate the operationally soluble enzyme in an intact organelle have not been possible. In this paper, we confirm the leucoplastidial localization of limonene synthase by immunogold cytochemical studies and by in vitro synthesis of the labeled preprotein coupled to plastidial import and processing experiments, thereby demonstrating that this committed step of monoterpene biosynthesis in mint occurs exclusively at this subcellular site.
MATERIALS AND METHODS
Materials
Peppermint (Mentha × piperita L. cv Black Mitcham) plants were propagated and grown under controlled conditions, as described previously (Alonso et al., 1992). Young, folded leaves approximately 6 to 10 mm in length (about 2 weeks old) were used in all experiments. Pea (Pisum sativum var Little Marvel) seeds were obtained from the Olds Seed Company (Madison, WI) and were grown as described previously (Bruce et al., 1994).
Polyclonal antibodies were generated in rabbits against the SDS-denatured limonene synthase from spearmint (Mentha spicata) as previously described (Alonso et al., 1993). Gold-conjugated goat anti-rabbit secondary antibodies were obtained from Goldmark Biologicals (Phillipsburg, NJ). Percoll and ATP were purchased from Sigma, l-[35S]Met from DuPont/NEN, and nuclease-treated rabbit reticulocyte lysate from Promega.
Tissue Preparation
Several different fixation protocols were used that varied in the quality of tissue preservation and retention of immunogenicity but that gave similar overall patterns of labeling. Some specimens were fixed for 3 h at 4°C with 1% (v/v) glutaraldehyde in 50 mm Pipes buffer, pH 7.2, prior to dehydration in a graded ethanol series to 100% ethanol. The samples were then slowly infiltrated with LR White resin through a graded series (resin:ethanol 1:3, 1:1, and 3:1) for 12 h each, followed by three 12-h incubations in pure resin, prior to polymerization at 60°C. These specimens retained good immunogenicity but cellular preservation was relatively poor. Other specimens were fixed with 0.75% (v/v) glutaraldehyde, 1% (v/v) paraformaldehyde, and approximately 0.02% (w/v) osmium tetraoxide in 50 mm Pipes buffer, pH 7.3, for 2 h at 4°C. These specimens were then transferred to an identical solution lacking the osmium tetraoxide, and fixed for an additional 12 h at 4°C prior to dehydration and embedding as before. These samples showed good immunogenicity, low levels of background staining, and adequate tissue preservation.
Additionally, some specimens were rapidly fixed and dehydrated with a microwave tissue-processing system (model 3450, Pelco, Clovis, CA; Gibberson et al., 1997) prior to embedding. These samples were fixed for 2.5 min at 37°C in buffered 1% (v/v) glutaraldehyde, followed by rapid dehydration in a graded ethanol series (10% increments, 80 s for each step at 40°C). This procedure gave superior tissue preservation (especially for postsecretory stage glands), but less immunogenicity was retained.
Immunolabeling
Thin sections (70–90 nm), collected on uncoated, 200-mesh nickel grids, were first incubated for 1 h in a blocking solution of TBS (10 mm Tris/NaOH, pH 7.8, with 0.5 m NaCl) containing 0.1% (v/v) Tween 20 and 1% (w/v) BSA (TBST-BSA). For some trials, 0.2% (w/v) PVP (Mr 10,000, Sigma) was added to the blocking solutions to reduce nonspecific labeling of phenolic compounds. Sections were incubated for 6 h in the TBST-BSA solution containing either a 1:30 dilution of rabbit polyclonal antibodies raised against spearmint limonene synthase (Alonso et al., 1993), or a comparable dilution of preimmune serum. After washing with TBST-BSA, all sections were incubated for 1 h in TBST-BSA containing a 1:50 dilution of gold (10 or 20 nm) labeled goat anti-rabbit polyclonal antibodies. Sections were then rinsed in TBST-BSA, then TBST, and then water. Sections were stained for 15 min in a 1:4 mixture of 1% (w/v) potassium permanganate and 1% (w/v) aqueous uranyl acetate prior to viewing with an electron microscope (model JEM 1200EX, JEOL). Specimens were photographed with electron microscopy film (Kodak).
Import Experiments
Intact chloroplasts were isolated from 8- to 12-d-old pea seedlings and purified over a Percoll gradient as previously described (Bruce et al., 1994). Intact chloroplasts were re-isolated and resuspended in import buffer (50 mm Hepes/KOH, pH 8.0, containing 330 mm sorbitol) at a concentration of 1 mg chlorophyll/mL, and stored in the dark on ice prior to use in import experiments.
The plasmid pLC 5.2 encoding the presumptive limonene synthase preprotein (Colby et al., 1993) was linearized and transcribed with T3 RNA polymerase. Limonene synthase was then translated in the presence of [35S]Met using a nuclease-treated rabbit reticulocyte lysate system according to the manufacturer's protocol (Promega). The plasmid encoding the precursor to SSU (Olsen and Keegstra, 1992) was linearized with PstI, transcribed with SP6 RNA polymerase, and translated using a wheat germ system and [35S]Met, as previously described (Bruce et al., 1994). After translation, residual nucleotides were removed by gel filtration (Olsen et al., 1989).
Each binding or import reaction (adapted from Bruce et al., 1994) contained the following: 106 dpm of either [35S]limonene synthase or [35S]SSU as a control; either 0.1 mm ATP for binding or 4 mm ATP for translocation (final concentration); and intact chloroplasts corresponding to 25 μg of chlorophyll in a final volume of 150 μL. Binding and translocation reactions were run at room temperature for the times indicated in the figure legends. Reactions were terminated by recovering intact chloroplasts via sedimentation through a 40% Percoll cushion. Recovered chloroplasts were then separated into a crude membrane and a soluble fraction according to the method of Bruce et al. (1994). In a similar fashion, protease protection assays were performed on recovered chloroplasts according to the method of Cline et al. (1984). All samples were solubilized in 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE (Laemmli, 1970), followed by fluorography and documentation by exposure to film (Bruce et al., 1994).
RESULTS AND DISCUSSION
Limonene Synthase Is Localized to Leucoplasts of Secretory Cells
(−)-4S-Limonene synthase appears to originate as a preprotein bearing an amino-terminal plastid-targeting sequence (Colby et al., 1993), which is consistent with considerable circumstantial evidence indicating that at least the early steps of monoterpene biosynthesis are localized to this organelle (Gershenzon and Croteau, 1990; McGarvey and Croteau, 1995; Wise and Croteau, 1999). The operationally soluble native enzyme cannot be directly localized by classical subcellular fractionation techniques because of experimental limitations imposed by the walls and cuticle surrounding the oil gland secretory cells (Gershenzon et al., 1991, 1992). This means that approaches to enzyme localization are limited to immunocytochemical and in vitro methods.
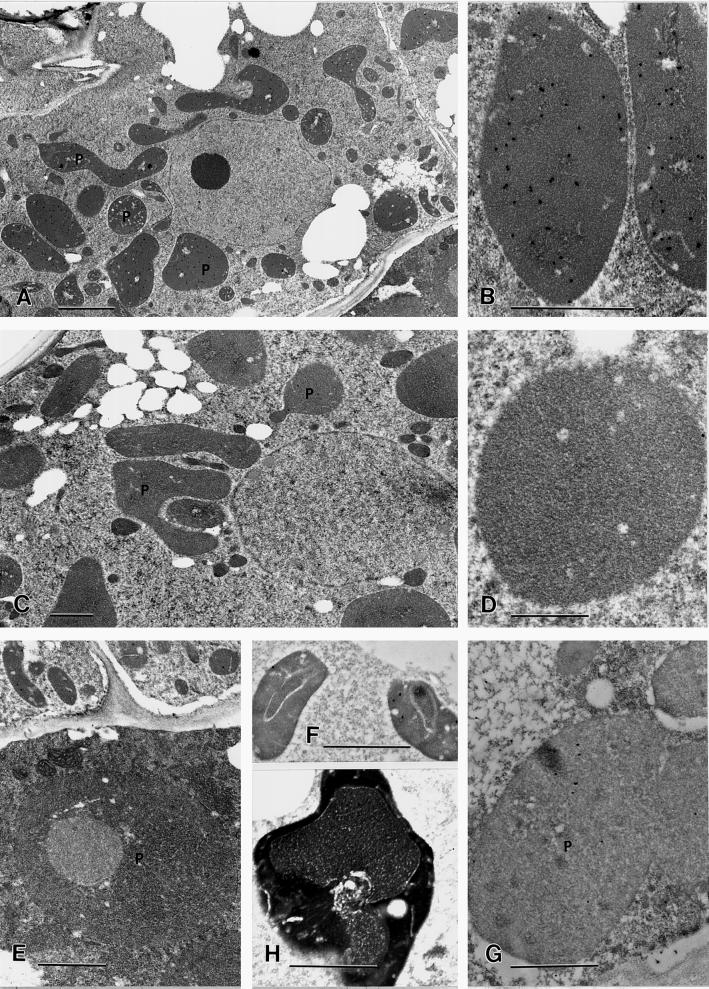
Immunoblotting analysis revealed that the polyclonal antibodies previously prepared against the spearmint limonene synthase were very specific for denatured limonene synthase from all Mentha sp., but these antibodies failed to recognize the native protein by immunoprecipitation or Ouchterlony double-diffusion assay (Alonso et al., 1993). However, preliminary immunohistochemistry indicated the presence of an antigen target in only the glandular (not the nonglandular) cells. Further investigations showed dense, specific labeling with the anti-limonene synthase antibodies to the stroma of leucoplasts of the disc cells from secretory-phase peltate glandular trichomes (Fig. 3, A and B), which was absent from the corresponding leucoplasts of the preimmune serum controls (Fig. 3, C and D).
Figure 3.
A, Immunogold labeling against limonene synthase. Colloidal gold particles strongly label leucoplasts (P) of a secretory stage glandular cap cell, while cytoplasm and other organelles are essentially unlabeled. Bar = 2 μm. B, High magnification of a leucoplast from a secretory stage glandular cap cell showing immunolabeling against limonene synthase. Bar = 1 μm. C, Preimmune serum control. Leucoplasts (P) of this secretory stage glandular cap cell are unlabeled after treatment with preimmune serum and colloidal gold labeled secondary antibodies. Bar = 1 μm. D, High magnification of a leucoplast from a secretory stage glandular cap cell showing no labeling when treated with preimmune serum and colloidal gold labeled secondary antibodies. Bar = 500 nm. E, High magnification of a plastid (P) from a stalk cell of a secretory stage peltate gland immunolabeled against limonene synthase showing very little labeling. Bar = 1 μm. F, Leucoplasts from a presecretory stage glandular cap cell immunolabeled against limonene synthase showing relatively little labeling. Bar = 1 μm. G, Leucoplasts (P) from a microwave-fixed, postsecretory-stage glandular cap cell immunolabeled against limonene synthase showing relatively little labeling. (Generally, microwave-fixed specimens showed less labeling than specimens prepared by other methods.) Bar = 1 μm. H, Plastid from a capitate glandular trichome immunolabeled against limonene synthase showing relatively little labeling. Bar = 1 μm.
Immunogold labeling was absent from mesophyll chloroplasts, from basal cell plastids, and from stalk cell plastids of secretory phase peltate glands (Fig. 3E). Intense immunogold labeling was observed in the stroma of leucoplasts only during the relatively short (approximately 20 h) secretory phase of gland development, in which filling of the subcuticular storage cavity with the essential oil occurs. Plastids of pre-secretory peltate gland cells (Fig. 3F), cells of postsecretory glands (Fig. 3G), and cells of capitate trichomes (Fig. 3H) exhibited little labeling above background levels. However, substantial labeling was observed to occur along cell walls, especially secondary walls of tracheary elements, but this labeling represented a nonspecific affinity for wall material, since both the anti-limonene synthase antibodies and the preimmune serum controls exhibited cell wall labeling of equal intensity. These observations indicate that limonene synthase accumulates in the stroma of secretory cell leucoplasts and only during the secretory phase of oil gland filling.
Limonene Synthase Is Imported into Chloroplasts and Is Localized to the Stroma
Most chloroplastic proteins are nuclear encoded, synthesized on cytosolic ribosomes, and imported posttranslationally across the two membranes of the chloroplast envelope (Chua and Schmidt, 1978; Highfield and Ellis, 1978). An amino-terminal extension called a transit peptide targets these precursor proteins to chloroplasts (Schmidt et al., 1979). During or after translocation, the transit peptide is cleaved by the stromal processing peptidase (Oblong and Lamppa, 1992; VanderVere et al., 1995; Richter and Lamppa, 1998). The newly imported proteins are then folded or further directed to the thylakoid membrane.
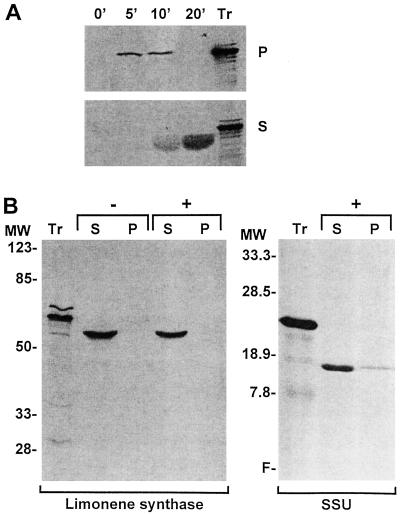
The limonene synthase cDNA encodes a predicted protein with a molecular mass of 72.4 kD, and analysis of the amino acid sequence suggests the presence of a potential transit peptide at the amino terminus (Colby et al., 1993). To determine if limonene synthase can be targeted to and subsequently imported into chloroplasts, in vitro import assays with the in vitro-translated preprotein were performed using isolated pea chloroplasts. Figure 4A demonstrates that limonene synthase binds to and is imported into pea chloroplasts in a time-dependent manner. Five minutes after the initiation of the assay, the radiolabeled full-length preprotein (72.4 kD) was associated entirely with the chloroplast pellet, the membrane fraction. However, at 10 min, a smaller product (65 kD) began to appear in the supernatant, and by 20 min only this mature protein was observed exclusively in the supernatant. These changes are consistent with initial binding of the preprotein to the surface of the chloroplast, followed by transport across the envelope membranes, processing to remove the amino-terminal transit peptide, and eventual release of the mature protein into the stroma.
Figure 4.
Analysis of import and localization of limonene synthase. A, Time course of import of 35S-labeled limonene synthase into pea chloroplasts. Following confirmation of binding at 0°C to 4°C in the dark in the presence of 0.1 mm Mg-ATP (data not shown), import assays were performed at room temperature in the presence of 4 mm Mg-ATP. Aliquots of the chloroplast preparation were removed at 0, 5, 10, and 20 min, sedimented through a 40% Percoll cushion, lysed, and then separated into a crude pellet (P) and a supernatant (S) fraction. All samples were analyzed by SDS-PAGE and fluorography. Tr, Unmodified limonene synthase translation product at about 72 kD. The protein band at about 65 kD is diffuse, likely due to imprecise proteolytic processing to mature forms of limonene synthase (Williams et al., 1998). B, Protease treatment and fractionation of imported limonene synthase. Limonene synthase was imported into pea chloroplasts for 20 min at room temperature as above and the intact chloroplasts, recovered by sedimentation through a 40% Percoll cushion, were incubated in the absence (−) or presence (+) of thermolysin for 30 min on ice in the dark. Proteolysis was terminated by the addition of EDTA, and the intact chloroplasts were recovered by sedimentation through a 40% Percoll cushion containing 5 mm EDTA. Chloroplasts were lysed and separated into a crude membrane fraction (P) and a soluble fraction (S). MW, Molecular-mass standards (in kilodaltons); Tr, limonene synthase translation product; SSU, positive control experiment conducted with the precursor to the SSU.
The localization of limonene synthase within the chloroplasts was investigated by employing import assays with the protease thermolysin added at a concentration that would degrade any externally exposed proteins but would not cause lysis of the chloroplast (Cline et al., 1984). Thermolysin treatment did not affect the appearance of the mature limonene synthase in the supernatant (Fig. 4B), indicating that the processed protein must reside in the stroma, where it is protected from protease action. A stromal location is consistent with the soluble nature of the native limonene synthase activity observed in cell-free extracts of peppermint (Alonso et al., 1992; Rajaonarivony et al., 1992).
Also depicted in Figure 4B are the results of an import assay conducted with an aliquot from the same chloroplast preparation using the SSU, a nuclear-encoded protein known to be targeted to the plastid (Keegstra et al., 1989). The protease-protected import of SSU into the stroma and its concurrent reduction in size shows that these chloroplasts were fully competent in protein uptake and processing. Taken together, the plastid-import experiments and immunocytochemical studies demonstrate that the amino-terminal region of the limonene synthase preprotein can serve as a targeting sequence, and that this transit peptide is sufficient to direct the import of limonene synthase into plastids.
Within the peppermint secretory cell leucoplasts, limonene synthase appeared to be distributed throughout the stroma and was not associated with any particular region of the stromal compartment. Other plastid-localized enzymes of terpenoid biosynthesis have been reported to have more restricted distributions within this organelle. For instance, in developing chromoplasts of pepper fruits, the prenyltransferase geranylgeranyl diphosphate synthase is concentrated at certain sites in the stroma in association with plastoglobuli (Cheniclet et al., 1992). In chloroplasts, some steps of isoprenoid formation are associated with the envelope membrane (Biggs et al., 1990; Joyard et al., 1991) or the thylakoids (Linden et al., 1993).
The successful import of limonene synthase into pea chloroplasts not only confirms the plastidial location of this enzyme, but also attests to the basic similarities between the protein import machinery of chloroplasts and that of secretory cell leucoplasts. Early work on plastid protein import suggested that various transit peptides could be recognized by all types of plastids (Halpin et al., 1989; Klosgen et al., 1989). However, more recently, two isoforms of plastid pyruvate kinase have been described whose import characteristics with leucoplasts and chloroplasts differ as a consequence of differences in their transit peptides (Wan et al., 1995). The present study also hints that differences may exist between chloroplast and leucoplast import, because the broad, indistinct nature of the mature protein band shown in Figure 4A (panel S, 10- and 20-min lanes) indicates that processing of leucoplast-targeted limonene synthase by pea chloroplasts is imprecise. However, the processing of limonene synthase in planta by peppermint secretory cell leucoplasts is also inexact, as MS analysis of the purified native enzyme has revealed a heterogeneous population of modified forms (Williams et al., 1998).
Several lines of evidence suggest that, in addition to limonene synthase, all of the preceding steps of monoterpene biosynthesis in peppermint are localized in the leucoplasts of the glandular trichome secretory cells. First, the isotopic labeling pattern of monoterpenes derived from applied [13C]Glc (Eisenreich et al., 1997) indicates that these metabolites originate from isopentenyl diphosphate via the glyceraldehyde-3-P/pyruvate pathway, a mevalonate-independent sequence of reactions that is plastid localized in higher plants (Lichtenthaler et al., 1997). Second, two specific monoterpene biosynthetic enzymes, deoxyxylulose-5-P synthase, which catalyzes the first step of the glyceraldehyde-P/pyruvate pathway (Lange et al., 1998), and geranyl diphosphate synthase, which mediates the condensation of isopentenyl diphosphate and dimethylallyl diphosphate to geranyl diphosphate (M. Wildung, C. Burke, J. Gershenzon, and R. Croteau, unpublished results) (Fig. 2), both possess plastid-targeting peptides. However, the limonene hydroxylases responsible for the conversion of limonene to (−)-trans-isopiperitenol and to (−)-trans-carveol (Fig. 2) do not bear plastidial transit peptides, but rather amino-terminal membrane insertion sequences more typical of this class of Cyt P450 monooxygenases (Lupien et al., 1995). Additionally, these Cyt P450 hydroxylases have been localized to the microsomal fraction of oil gland cell homogenates (Karp et al., 1990), further suggesting that these enzymes reside in the extensive smooth ER characteristic of these secretory cells.
Preliminary evidence (B.M. Lange and R. Croteau, unpublished results) indicates that the catalysts responsible for the redox metabolism of trans-isopiperitenol and trans-carveol are cytosolic, suggesting that enzymes from multiple subcellular compartments participate in monoterpene biosynthesis in mint. Additional immunocytochemical studies are in progress to examine pathway organization in greater detail.
Plastids have long been recognized as a major subcellular site of terpenoid metabolism, a fact underscored by the recent discovery that this organelle possesses a novel mevalonate-independent pathway (Lichtenthaler et al., 1997) for the production of the universal terpenoid precursor isopentenyl diphosphate (McCaskill and Croteau, 1999). The biosynthesis of hemiterpenes (isoprene), diterpenes, tetraterpenes (carotenoids), and a variety of prenylated quinones occurs in plastids (Gray, 1987; Kleinig, 1989; Wildermuth and Fall, 1996). The localization of limonene synthase described in the present study directly confirms that monoterpenes are also of plastidial origin. Several branches or segments of terpenoid metabolism are found outside the plastids in the cytosol, ER, and mitochondria (Gray, 1987; Kleinig, 1989; Disch et al., 1998). For example, sesquiterpenes and triterpenes are synthesized in the cytosol/ER compartment (Chappell, 1995), and microsomal Cyt P450 oxygenases and cytosolic redox enzymes are largely responsible for synthesizing derivatives of the various terpenoid structural types of plastidial origin (Gershenzon and Croteau, 1993; McGarvey and Croteau, 1995; Wise and Croteau, 1999). The organization of terpenoid metabolism involving several different subcellular compartments may be important in regulating the production of this large and diverse group of natural products.
ACKNOWLEDGMENTS
We thank John Crock and Vincent Franceschi for helpful discussions, Thom Koehler for raising the plants, and Joyce Tamura for typing the manuscript.
Abbreviation:
- SSU
small subunit of Rubisco
Footnotes
This work was supported in part by the U.S. Department of Energy, Division of Energy Biosciences, the National Science Foundation Cell Biology Program, the Mint Industry Research Council, and Project 0268 from the Agricultural Research Center, Washington State University.
LITERATURE CITED
- Adam K-P, Theil R, Zapp J, Becker H. Involvement of the mevalonic acid pathway and the glyceraldehyde-pyruvate pathway in terpenoid biosynthesis of the liverworts Ricciocarpos natans, and Conocephalum conicum. Arch Biochem Biophys. 1998;354:181–187. doi: 10.1006/abbi.1998.0666. [DOI] [PubMed] [Google Scholar]
- Alonso WR, Crock JE, Croteau R. Production and characterization of polyclonal antibodies in rabbits to 4S-limonene synthase from spearmint (Mentha spicata) Arch Biochem Biophys. 1993;301:58–63. doi: 10.1006/abbi.1993.1114. [DOI] [PubMed] [Google Scholar]
- Alonso WR, Rajaonarivony JIM, Gershenzon J, Croteau R. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) and spearmint (M. spicata) J Biol Chem. 1992;267:7582–7587. [PubMed] [Google Scholar]
- Amelunxen F. Electromikroskopishe Untersuchungen an den Drüsenschuppen von Mentha piperita L. Planta Med. 1965;13:457–473. [Google Scholar]
- Amelunxen F, Wahlig T, Arbeiter H. Über den Nachweis des atherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Z Pflanzenphysiol. 1969;61:68–72. [Google Scholar]
- Ascensao L, Marques N, Pais MS. Peltate glandular trichomes of Leonotis leonurus leaves: ultrastructure and histochemical characterization of secretions. Int J Plant Sci. 1997;158:249–258. [Google Scholar]
- Biggs DR, Welle R, Grisebach H. Intracellular localization of prenyltransferases of isoflavonoid phytoalexin biosynthesis in bean and soybean. Planta. 1990;181:244–248. doi: 10.1007/BF02411546. [DOI] [PubMed] [Google Scholar]
- Bosabalidis A, Tsekos I. Glandular scale development and essential oil secretion in Origanum dictamnus. Planta. 1982;156:496–504. doi: 10.1007/BF00392771. [DOI] [PubMed] [Google Scholar]
- Bourett TM, Howard RJ, O'Keefe DP, Hallahan DL. Gland development on leaf surfaces of Nepeta racemosa. Int J Plant Sci. 1994;155:623–632. [Google Scholar]
- Bruce BD, Perry S, Froehlich J, Keegstra K. In vitro import of protein into chloroplasts. In: Gelvin SB, Schilperoort RB, editors. Plant Molecular Biology Manual. Boston: Kluwer Academic Publishers; 1994. pp. 1–15. [Google Scholar]
- Carde J-P. Leucoplasts: a distinct kind of organelles lacking typical 70S ribosomes and free thylakoids. Eur J Cell Biol. 1984;34:18–26. [PubMed] [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:521–547. [Google Scholar]
- Cheniclet C, Carde J-P. Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Isr J Bot. 1985;34:219–238. [Google Scholar]
- Cheniclet C, Rafia F, Saint-Guily A, Verna A, Carde J-P. Localization of the enzyme geranylgeranylpyrophosphate synthase in Capsicum fruits by immunogold cytochemistry after conventional chemical fixation or quick-freezing followed by freeze-substitution. Labelling evolution during fruit ripening. Biol Cell. 1992;75:145–154. [Google Scholar]
- Chua NH, Schmidt GW. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci USA. 1978;75:6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984;75:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Alonso WR, Katahira EJ, McGarvey DJ, Croteau R. 4S-Limonene synthase from the oil glands of spearmint (Mentha spicata): cDNA isolation, characterization and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem. 1993;268:23016–23024. [PubMed] [Google Scholar]
- Croteau R (1991) Metabolism of monoterpenes in mint (Mentha) species. Planta Med 57 (Suppl): 10–14 [DOI] [PubMed]
- Croteau R (1993) Biosynthesis of limonene in Mentha species. In P Schreier, P Winterhalter, eds, Progress in Flavor Precursor Studies: Analysis, Generation, Biotechnology. Allured Publishing Co, Wheaton, IL, pp 113–122
- Croteau R, Gershenzon J (1994) Genetic control of monoterpene biosynthesis in mints (Mentha:Lamiaceae). In BE Ellis, G Kuroki, HA Stafford, eds, Genetic Engineering of Plant Secondary Metabolism. Plenum Press, New York, pp 193–229
- Dell B, McComb AJ. Plant resins: their formation, secretion, and possible functions. Adv Bot Res. 1978;6:277–316. [Google Scholar]
- Disch A, Hemmerlin A, Bach TJ, Rohmer M (1998) Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochem J 331 (part 2): 615–621 [DOI] [PMC free article] [PubMed]
- Eisenreich W, Sagner S, Zenk MH, Bacher A. Monoterpenoid essential oils are not of mevalonoid origin. Tetrahedron Lett. 1997;38:3889–3892. [Google Scholar]
- Fahn A (1979) Secretory Tissues in Plants. Academic Press, London, pp 162–164
- Fahn A. Secretory tissues in vascular plants. New Phytol. 1988;108:229–257. doi: 10.1111/j.1469-8137.1988.tb04159.x. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Croteau R (1990) Regulation of monoterpene biosynthesis in higher plants. In GHN Towers, HA Stafford, eds, Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Plenum Press, New York, pp 99–160
- Gershenzon J, Croteau R (1993) Terpenoid biosynthesis: the basic pathway and formation of monoterpenes, sesquiterpenes and diterpenes. In TS Moore Jr, ed, Lipid Metabolism in Plants. CRC Press, Boca Raton, FL, pp 340–388
- Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, Croteau R (1991) Biosynthetic methods for plant natural products: new procedures for the study of glandular trichome constituents. In NH Fischer, MB Isman, HA Stafford, eds, Modern Phytochemical Methods. Plenum Press, New York, pp 347–370
- Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, Croteau R. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal Biochem. 1992;200:130–138. doi: 10.1016/0003-2697(92)90288-i. [DOI] [PubMed] [Google Scholar]
- Gibberson RT, Demaree RS, Jr, Nordhausen RW. Four-hour processing of clinical/diagnostic specimens for electron microscopy using microwave techniques. J Vet Diagn Invest. 1997;9:61–67. doi: 10.1177/104063879700900111. [DOI] [PubMed] [Google Scholar]
- Gleizes M, Pauly G, Carde J-P, Marpeau A, Bernard-Dagan C. Monoterpene hydrocarbon biosynthesis by isolated leucoplasts of Citrofortunella mitis. Planta. 1983;159:373–381. doi: 10.1007/BF00393177. [DOI] [PubMed] [Google Scholar]
- Gray JC. Control of isoprenoid biosynthesis in higher plants. Adv Bot Res. 1987;14:25–91. [Google Scholar]
- Halpin C, Musgrove JE, Lord JM, Robinson C. Import processing of proteins by castor bean leucoplasts. FEBS Letts. 1989;258:32–34. [Google Scholar]
- Highfield PE, Ellis RJ. Synthesis and transport of the small subunit of chloroplastic ribulose bisphosphate carboxylase. Nature. 1978;271:420–424. [Google Scholar]
- Joyard J, Block MA, Douce R. Molecular aspects of plastid envelope biochemistry. Eur J Biochem. 1991;199:489–509. doi: 10.1111/j.1432-1033.1991.tb16148.x. [DOI] [PubMed] [Google Scholar]
- Karp F, Mihaliak CA, Harris JL, Croteau R. Monoterpene biosynthesis: specificity of the hydroxylations of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata), and perilla (Perilla frutescens) leaves. Arch Biochem Biophys. 1990;276:219–226. doi: 10.1016/0003-9861(90)90029-x. [DOI] [PubMed] [Google Scholar]
- Keegstra K, Olsen LJ, Theg SM. Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:471–501. [Google Scholar]
- Kleinig H. The role of plastids in isoprenoid biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:39–59. [Google Scholar]
- Klosgen RB, Saedler H, Weil J-H. The amyloplast-targeting transit peptide of the waxy protein of maize also mediates protein transport in vitro into chloroplasts. Mol Gen Genet. 1989;217:155–161. doi: 10.1007/BF00330955. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, McCaskill DG, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Rohmer M, Schwender J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol Plant. 1997;101:643–652. [Google Scholar]
- Linden H, Lucas MM, Rosario de Felipe M, Sandmann G. Immunogold localization of phytoene desaturase in higher plant chloroplasts. Physiol Plant. 1993;88:229–236. [Google Scholar]
- Lupien S, Karp F, Ponnamperuma K, Wildung M, Croteau R. Cytochrome P450 limonene hydroxylases of Mentha species. Drug Metab Drug Interact. 1995;12:245–260. doi: 10.1515/dmdi.1995.12.3-4.245. [DOI] [PubMed] [Google Scholar]
- McCaskill DG, Croteau R. Isopentenyl diphosphate is the terminal product of the deoxyxylulose-5-phosphate pathway for terpenoid biosynthesis in plants. Tetrahedron Lett. 1999;40:653–656. [Google Scholar]
- McCaskill DG, Gershenzon J, Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- McGarvey D, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettal U, Boland W, Beyer P, Kleinig H. Biosynthesis of monoterpene hydrocarbons by isolated chromoplasts from daffodil flowers. Eur J Biochem. 1988;170:613–616. doi: 10.1111/j.1432-1033.1988.tb13741.x. [DOI] [PubMed] [Google Scholar]
- Oblong JE, Lamppa GK. Identification of two structurally related proteins involved in proteolytic processing of precursors targeted to the chloroplast. EMBO J. 1992;11:4401–4409. doi: 10.1002/j.1460-2075.1992.tb05540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen LJ, Keegstra K. The binding of precursor proteins to chloroplast requires nucleotide triphosphates in the intermembrane space. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- Olsen LJ, Theg SM, Selman BR, Keegstra K. ATP is required for the binding of precursor proteins to chloroplasts. J Biol Chem. 1989;264:6724–6729. [PubMed] [Google Scholar]
- Pauly G, Belingheri L, Marpeau A, Gleizes M. Monoterpene formation by leucoplasts of Citrofortunella mitis and Citrus unshiu: steps and conditions of biosynthesis. Plant Cell Rep. 1986;5:19–22. doi: 10.1007/BF00269709. [DOI] [PubMed] [Google Scholar]
- Pérez LM, Pauly G, Carde J-P, Berlingheri L, Gleizes M. Biosynthesis of limonene by isolated chromoplasts from Citrus sinensis fruits. Plant Physiol Biochem. 1990;28:221–229. [Google Scholar]
- Rajaonarivony JIM, Gershenzon J, Croteau R. Characterization and mechanism of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) Arch Biochem Biophys. 1992;296:49–57. doi: 10.1016/0003-9861(92)90543-6. [DOI] [PubMed] [Google Scholar]
- Richter S, Lamppa GK. A chloroplastic processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA. 1998;95:7463–7468. doi: 10.1073/pnas.95.13.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GW, Devillers-Thiery A, Desruisseaux H, Blobel G, Chua NH. NH2-terminal amino acid sequences of precursor and mature forms of the ribulose-1,5-bisphosphate carboxylase small subunit from Chlamydomonas reinhardtii. J Cell Biol. 1979;83:615–622. doi: 10.1083/jcb.83.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E. Gland cells. In: Robards AW, editor. Dynamic Aspects of Plant Ultrastructure. Berkshire, UK: McGraw-Hill; 1974. pp. 331–357. [Google Scholar]
- Soler E, Feron G, Clastre M, Dargent R, Gleizes M, Ambid C. Evidence for a geranyl-diphosphate synthase located within plastids of Vitis vinifera L. cultivated in vitro. Planta. 1992;187:171–175. doi: 10.1007/BF00201934. [DOI] [PubMed] [Google Scholar]
- VanderVere PS, Bennett TM, Oblong JE, Lamppa GK. A chloroplast processing enzyme involved in precursor maturation shares a zinc-binding motif with a recently recognized family of metalloendopeptidases. Proc Natl Acad Sci USA. 1995;92:7177–7181. doi: 10.1073/pnas.92.16.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Blakeley SD, Dennis DT, Ko K. Import characteristics of a leucoplast pyruvate kinase are influenced by a 19-amino-acid domain within the protein. J Biol Chem. 1995;270:16731–16739. doi: 10.1074/jbc.270.28.16731. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Fall R. Light-dependent isoprene emission. Characterization of a thylakoid-bound isoprene synthase in Salix discolor chloroplasts. Plant Physiol. 1996;112:171–182. doi: 10.1104/pp.112.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DC, McGarvey DJ, Katahira EJ, Croteau R. Truncation of limonene synthase preprotein provides a fully active 'pseudomature' form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry. 1998;37:12213–12220. doi: 10.1021/bi980854k. [DOI] [PubMed] [Google Scholar]
- Wise ML, Croteau R. Monoterpene biosynthesis. In: Cane DE, editor. Comprehensive Natural Products Chemistry: Isoprenoids. Oxford, UK: Elsevier Science; 1999. pp. 97–153. [Google Scholar]