Abstract
Objective
The present study applied the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement to observational studies published in prestigious occupational medicine and health journals.
Results
A total of 60 articles was evaluated. All sub-items were reported in 63.74% (95% confidence interval [CI], 56.24–71.24%), not reported in 29.70% (95% CI, 20.2–39.2%), and not applicable in 6.56% (95% CI, 4.86–8.26%) of the studies. Of the 45 sub-items investigated in this survey, eight were reported 100% of the time, 13 were addressed in more than 90% of the articles, 22 were included in more than 75% of the studies, and 27 sub-items were applied in more than 50% of the articles published in the journals included in this study.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3367-9) contains supplementary material, which is available to authorized users.
Keywords: STROBE statement, Observational studies, Occupational medicine and health, Authors, Reviewers, Editors, Journals
Introduction
Observational studies have an important role in researching the benefits and harms of medical interventions [1]. The results of these studies should be reported as transparently as possible “so that readers can follow what was planned, what was done, what was found, and what conclusions were drawn” [2]. The credibility of a research depends on a critical assessment by others, in the study design, conduct, and analysis of it [3]. In order to assess the strengths and weaknesses of the evidence from the observational studies von Elm et al. designed in 2007 a 22-item checklist to assist with clear reporting of observational studies called: the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [2]. This checklist includes a description of methodological items and instructions on how to use them to transparently report observational studies. Several extensions of these statements have been published with additional recommendations for specialized fields of research—for example, the Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection (STROBE-NI) [4] and Strengthening the Reporting of Observational Studies in Epidemiology—nutritional epidemiology (STROBE-nut) [5].
To our knowledge, the quality of reporting in occupational observational studies has not been assessed. Observational study designs are often used in studies published in occupational medicine and health journals. Therefore, the present study investigated the quality of occupational observational studies reporting post-STROBE statements. Application of these recommendations by upcoming observational studies in occupational health and medical journals, will increase the value of new data and avoid wasted research.
Main text
Methods
Journal identification
We conducted a cross-sectional study and selected four top occupational medicine and health journals with high-impact factors (IFs) based on the Information Sciences Institute (ISI) in the Web of Knowledge (http://www.webofknowledge.com, date of access 14 Aug 2017) as of 2016, including journals in the first quartile in category (Q1) of scientific journal ranking (http://www.scimagojr.com, date of access 14 Aug 2017) among the most prestigious and impactful occupational medicine and health journals indexed in international databases. In addition, we checked the `Instruction for authors’ section of the websites for each of the included journals to determine if they contained the STROBE statement for authors or endorsed it as a guideline for reporting observational research articles (Table 1).
Table 1.
Top four occupational medicine and health journals and impact factor and STROBE endorsement
| Scientific journal ranking | Full journal title (NLM title abbreviation) | Impact factor in 2016 | Endorse STROBE? |
|---|---|---|---|
| (Q1) | Scandinavian Journal of Work, Environment and Health (Scand J Work Environ Health) | 4.071 | Yes |
| (Q1) | Occupational and Environmental Medicine (Occup Environ Med) | 3.912 | Yes |
| (Q1) | Journal of Occupational and Environmental Medicine (J Occup Environ Med) | 1.861 | No |
| (Q1) | American Journal of Industrial Medicine (Am J Ind Med) | 1.732 | No |
Study search and selection
We first evaluated the archives from January 1 until July 19, 2017, to retrieve observational studies (cohort, case–control, and cross-sectional studies) published in each of the selected journals. During this period, we identified enough cohort and cross sectional studies, but did not find enough case–control studies; thus, only for this type of study, we expanded the search range from January 1, 2016, to July 19, 2017. The search revealed 188 observational articles.
Second, the types of articles were classified according to the journal in which they were published. Next, a specific number was assigned to each selected article and, based on random numbers table, we randomly selected 15 observational (five each cohort, case–control, and cross sectional) studies published in each of the four prestigious occupational medicine and health journals. Accordingly, we enrolled 60 observational articles in our study, which were randomly assigned to two reviewers without blinding the name of the journal or the authors of the articles. The reviewers independently made decisions regarding the number of items from the STROBE check-list which were addressed in the selected studies.
Reliability and validity adherence
To check the judgment of two reviewers regarding the quality of observational studies, we conducted a pilot study as follows: first, among the articles that were previously retrieved, an article was selected randomly and the strengths and weaknesses of its evidence were assessed based on the predetermined checklist of items. Any disagreement over the items was discussed to reach the same interpretation of the checklist in order to increase the reliability between the two reviewers.
Second, two articles were selected randomly from among all the selected studies for the two reviewers to evaluate the checklist items based on three choices for each sub-item (reported/not reported/not applicable). This work was done in order to calculate an indicator of the reliability between two reviewers, the KAPPA statistic, in evaluating the items of the STROBE checklist in the selected articles. The KAPPA statistic was calculated according to the formula below:
Landis and Koch suggested that a Kappa greater than 0.75 represents excellent agreement beyond chance, a Kappa below 0.40 represents poor agreement and a Kappa of 0.40–0.75 represents intermediate to good agreement [6]. Based on Additional file 1: Table S1 and Additional file 2: Table S2, the Kappa statistic between reviewers A and B in this study was 53%
In order to assess the validity of the reviewers’ judgments in this cross-sectional study, reviewers A (J. Ah) and B (K. Mo) read and scored the included observational articles and independently made decisions regarding the quality of reporting in each observational study. Any disagreements were resolved by adjudication with the third (I. Mo) and fourth authors (B. Ma).
Data analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp; Armonk, NY, USA). The absolute frequencies and percentages of each sub-item in the selected articles were addressed. The total percentage for all sub-items (1–22) was reported (Table 2).
Table 2.
Percentage of items in the STROBE checklist which were addressed in cohort, case control, and cross-sectional studies published in four top scientific occupational journals in 2017
| Item | Recommendation | Reported n (%) | Not reported n (%) | Not applicable n (%) |
|---|---|---|---|---|
| Title and abstract | ||||
| 1a | Indicate the study’s design with a commonly used term in the title or the abstract | 39 (65.0) | 21 (35.0) | 0 (0.0) |
| 1b | Provide in the abstract an informative and balanced summary of what was done and what was found | 45 (75.0) | 15 (25.0) | 0 (0.0) |
| Introduction | ||||
| 2 | Explain the scientific background and rationale for the investigation being reported | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 3 | State specific objectives, including any pre-specified hypotheses | 53 (88.3) | 7 (11.7) | 0 (0.0) |
| Methods | ||||
| 4 | Present key elements of study design early in the paper | 31 (51.7) | 29 (48.3) | 0 (0.0) |
| 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 54 (90.0) | 6 (10.0) | 0 (0.0) |
| 6a | Give the eligibility criteria | 51 (85.0) | 7 (11.7) | 2 (3.3) |
| 6b | Describe methods of follow-up | 56 (93.3) | 4 (6.7) | 0 (0.0) |
| 6c | Give matching criteria | 7 (11.7) | 3 (5) | 50 (83.3) |
| 6d | Give number of exposed and unexposed in matched studies | 4 (6.7) | 4 (6.7) | 52 (86.7) |
| 7a | Clearly, define all outcomes | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 7b | Clearly, define all exposures | 53 (88.3) | 6 (10.0) | 1 (1.7) |
| 7c | Clearly, define all predictors | 55 (91.7) | 3 (5.0) | 2 (3.3) |
| 7d | Clearly, define all potential confounders | 41 (68.3) | 19 (31.7) | 0 (0.0) |
| 7e | Clearly, define all effect modifiers | 25 (41.7) | 35 (58.3) | 0 (0.0) |
| 8a | give sources of data | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 8b | details of methods of assessment (measurement) | 52 (86.7) | 8 (13.3) | 0 (0.0) |
| 9 | Describe any efforts to address potential sources of bias | 20 (33.3) | 40 (66.7) | 0 (0.0) |
| 10 | Explain how the study size was arrived at | 21 (35.0) | 22 (36.7) | 17 (28.3) |
| 11 | If applicable, describe which groupings were chosen and why | 46 (76.7) | 9 (15.0) | 5 (8.3) |
| 12a | Describe all statistical methods, including those used to control for confounding | 60 (100) | 0 (0.0) | 0 (0.0) |
| 12b | Describe all statistical software | 38 (63.3) | 22 (36.7) | 0 (0.0) |
| 12c | Describe any methods used to examine subgroups and interactions | 13 (21.7) | 45 (75.0) | 2 (3.3) |
| 12d | Explain how missing data were addressed | 29 (48.3) | 29 (48.3) | 2 (3.3) |
| 12e | If applicable, explain how loss to follow-up was addressed | 23 (38.3) | 24 (40) | 13 (21.7) |
| 12f | Describe any sensitivity analyses | 5 (8.3) | 55 (91.7) | 0 (0.0) |
| Results | ||||
| 13a | Report numbers of individuals at each stage of study | 28 (46.7) | 26 (43.3) | 6 (10.0) |
| 13b | Give reasons for non-participation at each stage | 4 (6.7) | 56 (93.3) | 0 (0.0) |
| 13c | Consider use of a flow diagram | 28 (46.7) | 32 (53.3) | 0 (0.0) |
| 14a | Give characteristics of study participants | 44 (73.3) | 16 (26.7) | 0 (0.0) |
| 14b | Indicate number of participants with missing data for each variable of interest | 8 (13.3) | 48 (80.0) | 4 (6.7) |
| 14c | Summarise follow-up time (e.g., average and total amount) | 21 (35.0) | 38 (63.3) | 1 (1.7) |
| 15 | Report numbers of outcome events or summary measures | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 16a | Give unadjusted estimates | 36 (60.0) | 24 (40.0) | 0 (0.0) |
| 16b | Give confounder-adjusted estimates | 26 (43.3) | 34 (56.7) | 0 (0.0) |
| 16c | Give estimates precision/confidence interval | 54 (90.0) | 6 (10.0) | 0 (0.0) |
| 16d | Report category boundaries when continuous variables were categorized | 48 (80.0) | 4 (6.7) | 8 (13.3) |
| 16e | If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | 14 (23.3) | 34 (56.7) | 12 (20.0) |
| 17 | Report other analyses done—e.g. analyses of subgroups and interactions and sensitivity analyses | 14 (23.3) | 46 (76) | 0 (0.0) |
| Discussion | ||||
| 18 | Summarise key results with reference to study objectives | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 53 (88.3) | 7 (11.7) | 0 (0.0) |
| 20a | Give a cautious interpretation of results considering objectives | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 20b | Explain results from similar studies | 60 (100.0) | 0 (0.0) | 0 (0.0) |
| 21 | Discuss the generalisability (external validity) of the study results | 46 (76.7) | 14 (23.3) | 0 (0.0) |
| Other information | ||||
| 22 | Give the source of funding and the role of the funders for the present study | 56 (93.3) | 4 (6.7) | 0 (0.0) |
| 1–22 | Total | 63.74 | 29.70 | 6.56 |
The STROBE statement is the guidelines for reporting observational studies, defined as Strengthening the Reporting of Observational Studies in Epidemiology
Results
Figure 1 shows the flow diagram of the search process and the numbers of observational articles included at each stage of the study published in prestigious scientific occupational medicine and health journals in 2016–2017. During the search process, of 281 original articles, 93 were not observational studies and were excluded from the study. Finally, we selected 60 observational studies from four prestigious scientific occupational medicine and health journals, including Scand J Work Environ Health, Occup Environ Med, J Occup Environ Med, and Am J Ind Med.
Fig. 1.
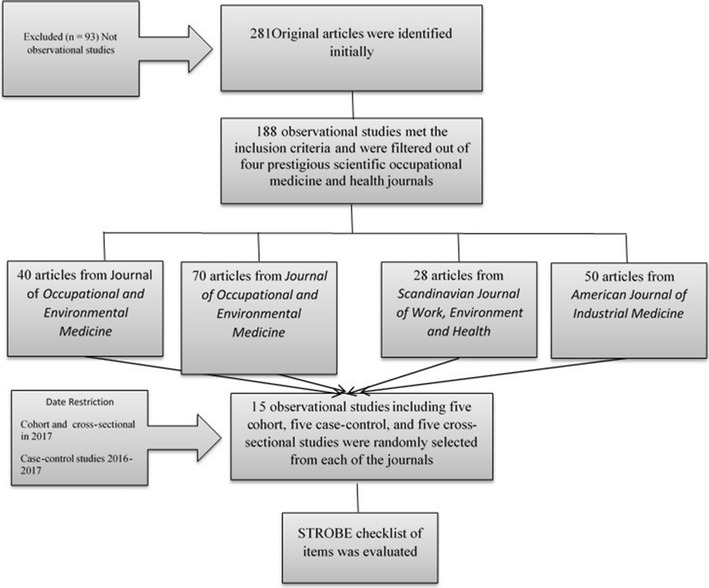
The overview of study design. STROBE checklist of items was addressed in prestigious scientific occupational medicine and health journals, 2016–2017
The absolute frequencies and percentages of items and sub-items addressed by these studies are summarized in Table 2. The sub-items were reported in 63.74% (95% confidence interval[CI], 56.24–71.24%), not reported in 29.70% (95% CI, 20.2–39.2%), and not applicable in 6.56% (95% CI, 4.86–8.26%) of the cohort, case–control, and cross sectional studies. This table also shows that, of the 45 sub-items investigated in this survey, collectively, eight sub-items were reported 100% of the time, 13 sub-items were addressed in more than 90% of the articles, 22 sub-items were included in more than 75% of the studies, and 27 sub-items were applied in more than 50% of the articles assessed from the journals included in this study.
Additional file 3: Figure S3 shows the percentages of adequately reported STROBE sub-items in articles published in the top four occupational medicine and health journals. In this figure, sub-items including 2-Introduction, 7a-Outcomes, 8a-Sources of data, 12a-Control for confounding, 15-N of outcome events, 18-Key results, 20a-Interpretation, and 20b-Similar studies were all reported in the articles published in the four prestigious journals included in this study.
Additional file 4: Figure S4 shows the percentages of STROBE sub-items not reported in articles published in the top four occupational medicine and health journals. Collectively, the five sub-items with the lowest reporting in the articles were 12f-Sensitivity analyses, 13b-Non-participation, 17-Other analyses, 14b-N of participants with missing data, and 12c-Interactions.
Additional file 5: Figure S5 shows that among 45 sub-items in the STROBE statement, those such as 12e-Loss to follow-up, 6d-N of exposed and unexposed, and 6c-matching criteria were the most common not applicable items in the articles included in the present study.
Discussion
The STROBE statement provides valuable recommendations for all authors when reporting the results of analytical observational studies, supports editors and reviewers when considering these articles for publication, and helped readers to critically appraise published articles [2]. In total, approximately 63.47% of the items and sub-items in the STROBE checklist were reported in cohort, case–control, and cross-sectional studies published in the four journals of occupational medicine and health included in the present study, 11 years after the dissemination of the STROBE statement. However, numerous observational studies are published in less fastidious peer-reviewed occupational medicine and health journals. Thus, it is expected that the quality of reporting of such studies is poorer than that reported in the present survey, although the results of the present study indicate that even this quality is not sufficient. To our knowledge, this study is the first to assess the quality of reporting of observational studies in occupational medicine and health literature; however, similar work has been performed in other disciplines; for example, Hendriksma et al. [7]. in 2016 con-ducted a similar study to evaluate the quality of reporting of observational studies in otorhinolaryngology based on the STROBE statement. They reported that the articles in the top five general medical journals reported a mean of 69.2% (95% CI 65.8 ± 72.7%) of items, compared to 51.4% (95% CI 47.7 ± 55.0%) in the top five ear, nose, and throat journals. These results are similar to the mean of 63.74% (95% CI 56.24–71.24%) obtained in our study. Jeelani et al. [8]. conducted a similar study in 2014 to assess the quality of reporting of cross-sectional studies published in the Indian Journal of Community Medicine by evaluating the extent to which they adhered to the STROBE statement. In this study, the most frequently reported checklist items included the summary of what was done and what was found in the abstract, background/rationale, objectives, setting, outcome data, key results in the discussion, and interpretation of results. In our study (Additional file 2: Table S2) the most frequently reported STROBE checklist items were the explanation of the scientific background and rationale for the investigation being reported; clearly defining all outcomes; providing sources of data; and describing all statistical methods, including those used to control for confounding; reporting the numbers of outcome events or summary measures; summarizing key results with reference to study objectives; providing a cautious interpretation of results considering the objectives, and explaining the results of similar studies. The findings in our study are consistent with those reported in the previous study [8, 9].
In 2016, Agha et al. [9] assessed the compliance of observational studies in plastic surgery using the STROBE statement checklist. The average STROBE score in his study was 12.4 (range 2–20.1) with a standard deviation of 3.36. This mean in plastic surgery articles is not satisfactory and lower than the average obtained from the occupational medicine and health journals evaluated in the present study.
The results of this study reveal that the quality reporting of observational studies published in the most prestigious occupational medicine and health journals is yet not clear and desirable enough. Thus, this issue should be the focus of the both authors’ and editors’ special attention when reporting and/or reviewing the reports of observational studies.
Limitations
First, the random selection of cohort, case–control, and cross sectional studies from prestigious occupational medicine and health journals may result in a selection bias in the results.
Second, the limited number of articles selected and evaluated in this survey, may in-crease the possibility of random error.
Third, the quality and accuracy of the STROBE sub-items addressed in the selected articles depended primarily on the judgment of the reviewer, which increased the probability of information bias in the results.
Additional files
Additional file 1: Table S1. Percent agreement between reviewers A and B.
Additional file 2: Table S2. Percent agreement between reviewers A and B expected by chance alone.
Additional file 3: Figure S1. Percentages of adequately reported STROBE sub-items in articles published in the top four occupational medicine and health journals.
Additional file 4: Figure S2. Percentages of STROBE items not adequately reported in articles published in the top four occupational medicine and health journals.
Additional file 5: Figure S5. Percentages of not applicable STROBE items in articles published in the top four occupational medicine and health journals.
Authors’ contributions
Conceived, development and design of methodology in the study: IM and JA. Helped to data collection: JA-A, KM, JA and BM. Analyzed the data: JA-A, KM, JA and BM. Wrote the paper: IM and JA. All authors read and approved the final manuscript.
Acknowledgements
We thank Urmia University Medical Sciences for supporting this study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated during the current study available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This study was supported by a Grant (2183) and funding from the Urmia University Medical Sciences. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- STROBE
The Strengthening the Reporting of Observational Studies in Epidemiology
- IFs
impact factors
- ISI
The Information Sciences Institute
- Q1
in the first quartile in category
- Scand J Work Environ Health
Scandinavian Journal of Work, Environment and Health
- Occup Environ Med
Occupational and Environmental Medicine
- J Occup Environ Med
Journal of Occupational and Environmental Medicine
- Am J Ind Med
American Journal of Industrial Medicine
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3367-9) contains supplementary material, which is available to authorized users.
Contributor Information
Javad Aghazadeh-Attari, Email: mirza-aghazadeh-attari@tbzmed.ac.ir.
Kazhal Mobaraki, Email: mobaraki.kazhal@yahoo.com.
Jamal Ahmadzadeh, Email: ahmadzadeh.j@umsu.ac.ir.
Behnam Mansorian, Email: mansorian.b@umsu.ac.ir.
Iraj Mohebbi, Phone: +98-32231930, Email: irajmohebbi@umsu.ac.ir.
References
- 1.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ Br Med J. 1996;312(7040):1215. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ Br Med J. 2001;323(7303):42. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitchett EJ, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, Agarwal R, Ayede AI, Bhutta ZA, Black R. Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16(10):e202–e213. doi: 10.1016/S1473-3099(16)30082-2. [DOI] [PubMed] [Google Scholar]
- 5.Lachat C, Hawwash D, Ocké MC, Berg C, Forsum E, Hörnell A, Sonestedt E, Wirfält E, Åkesson A, Kolsteren P. Strengthening the Reporting of Observational Studies in Epidemiology–nutritional epidemiology (STROBE-nut): an extension of the STROBE statement. Nutrition bulletin. 2016;41(3):240–251. doi: 10.1111/nbu.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidotti LG. Epidemiology. 4. Philadelphia: Saunders; 2008. [Google Scholar]
- 7.Hendriksma M, Joosten MH, Peters JP, Grolman W, Stegeman I. Evaluation of the Quality of Reporting of Observational Studies in Otorhinolaryngology-Based on the STROBE Statement. PLoS ONE. 2017;12(1):e0169316. doi: 10.1371/journal.pone.0169316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeelani A, Malik W, Haq I, Aleem S, Mujtaba M, Syed N. Cross sectional studies published in Indian journal of community medicine: evaluation of adherence to the strengthening the reporting of observational studies in epidemiology statement. Ann Med Health Sci Res. 2014;4(6):875–878. doi: 10.4103/2141-9248.144889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agha RA, Lee S-Y, Jeong KJL, Fowler AJ, Orgill DP. Reporting quality of observational studies in plastic surgery needs improvement: a systematic review. Ann Plast Surg. 2016;76(5):585–589. doi: 10.1097/SAP.0000000000000419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Percent agreement between reviewers A and B.
Additional file 2: Table S2. Percent agreement between reviewers A and B expected by chance alone.
Additional file 3: Figure S1. Percentages of adequately reported STROBE sub-items in articles published in the top four occupational medicine and health journals.
Additional file 4: Figure S2. Percentages of STROBE items not adequately reported in articles published in the top four occupational medicine and health journals.
Additional file 5: Figure S5. Percentages of not applicable STROBE items in articles published in the top four occupational medicine and health journals.
Data Availability Statement
The datasets generated during the current study available from the corresponding author on reasonable request.


