Abstract
Ethylene is known to interact with auxin in regulating stem growth, and yet evidence for the role of ethylene in tropic responses is contradictory. Our analysis of four mutants of tomato (Lycopersicon esculentum) altered in their response to gravity, auxin, and/or ethylene revealed concentration-dependent modulation of shoot gravitropism by ethylene. Ethylene inhibitors reduce wild-type gravicurvature, and extremely low (0.0005–0.001 μL L−1) ethylene concentrations can restore the reduced gravitropic response of the auxin-resistant dgt (diageotropica) mutant to wild-type levels. Slightly higher concentrations of ethylene inhibit the gravitropic response of all but the ethylene-insensitive nr (never-ripe) mutant. The gravitropic responses of nr and the constitutive-response mutant epi (epinastic) are slightly and significantly delayed, respectively, but otherwise normal. The reversal of shoot gravicurvature by red light in the lz-2(lazy-2) mutant is not affected by ethylene. Taken together, these data indicate that, although ethylene does not play a primary role in the gravitropic response of tomato, low levels of ethylene are necessary for a full gravitropic response, and moderate levels of the hormone specifically inhibit gravicurvature in a manner different from ethylene inhibition of overall growth.
Gravity and light are important environmental cues that aid plants in orienting themselves optimally to access life-supporting resources such as water and light. The process by which plants orient their roots and shoots with respect to gravity, gravitropism, has been studied intensively for more than 100 years (Darwin, 1888). The Cholodny-Went theory (Went and Thimann, 1937), widely regarded as the leading hypothesis explaining gravitropism, postulates that the plant hormone auxin, which is synthesized in the shoot apex and transported basipetally down the shoot, is redistributed asymmetrically in response to gravistimulation. This lateral redistribution of auxin leads to higher concentrations of the hormone in the lower half of the stem, which triggers an increased growth response in that region and results in upward curvature of the plant.
The role of ethylene in the gravitropic response has been discussed extensively in the literature, with research results split between two opposing groups: those indicating that ethylene plays a role in the gravitropic response (Zobel, 1973; Kang and Burg, 1974; Wheeler and Salisbury, 1980, 1981; Clifford and Oxlade, 1989; Philosoph-Hadas et al., 1996) and those supporting the opposing view (Clifford et al., 1983; Kaufman et al., 1985; Harrison and Pickard, 1986; Woltering, 1991). It has also been reported that ethylene, at concentrations of 100 μL L−1, can redirect 7-d-old etiolated pea plants to grow downward rather than upward when gravistimulated (Burg and Kang, 1993).
Most auxin-resistant mutants exhibit an altered gravitropic phenotype, such as the Arabidopsis mutants aux1 (Bennett et al., 1996), axr1 (Lincoln et al., 1990), axr2 (Wilson et al., 1990), and axr3 (Leyser et al., 1996), as well as the tomato (Lycopersicon esculentum) mutant dgt (diageotropica; Kelly and Bradford, 1986; Hicks et al., 1989). These auxin-resistant mutants are also resistant to ethylene. However, small amounts of ethylene have been reported to restore a normal gravitropic response in dgt (Zobel, 1974), suggesting that ethylene may act downstream of auxin in gravitropic signal transduction. Elongation of dgt roots is less sensitive to application of ethylene than its isogenic parent cv VFN8 (Muday et al., 1995), whereas the sensitivity of dgt shoot elongation to ethylene is greatly increased when compared with the wild-type response (Shi and Cline, 1992). In contrast, it has been reported that the gravitropic behavior of light-grown wild-type tomato seedlings treated with ethylene and ethylene inhibitors is not altered (Harrison and Pickard, 1986). Although a transitory burst of ethylene has been observed in tomato seedlings within 2 min of horizontal placement (Harrison and Pickard, 1984), a subsequent study concluded that the lack of measurable changes in ethylene production during the first 3 h of the gravitropic response was evidence that ethylene does not play a role in the signal transduction cascade of graviresponses (Harrison and Pickard, 1986). Kaufman et al. (1985) reported a sharp increase in ethylene production between 6 and 24 h after gravistimulation in oats but concluded that this increase occurred too late to be a cause for gravitropism. An increase in the production of ethylene on the lower half of gravistimulated dandelion plants has been observed; however, this increase also occurred hours after the gravitropic response had been initiated (Clifford et al., 1983), which led to the conclusion that ethylene may modulate but not initiate the gravitropic response.
Applied ethylene is known to inhibit hypocotyl elongation growth in etiolated plants as part of the ”triple response” phenomenon (Goeschl and Kays, 1975; Ecker, 1995). Growth inhibition due to high ethylene leads to decreased tropic responses, which are by definition dependent on growth. There is evidence that the reduction in elongation caused by ethylene occurs via an interaction between ethylene and auxin. Whereas ethylene production is stimulated by auxin, ethylene can suppress polar (basipetal) transport of the auxin IAA (Schwark and Schierle, 1992) and can also influence asymmetric distribution of auxin (Schwark and Bopp, 1993). Ethylene mediates the formation and maintenance of the seedling apical hook via an unknown component downstream of CTR1, a protein kinase that is part of the ethylene signal transduction pathway (Peck et al., 1998), and it has been suggested that the Arabidopsis HLS1 gene controls differential cell growth during hook formation by regulating auxin activity via its N-acetyltransferase activity (Lehman et al., 1996). The acetylation process itself may also be modulated by ethylene. Recently, Luschnig et al. (1998) isolated the EIR1 gene from Arabidopsis. This gene shows homology to a bacterial membrane transporter and, if mutated, confers reduced sensitivity to ethylene and agravitropism to roots. Experimental evidence in yeast suggests that EIR1 may play a role in auxin transport (Luschnig et al., 1998).
Although growth inhibition by ethylene has been described mostly for etiolated plants, it has been reported that ethylene promotes cell growth and elongation in light-grown Arabidopsis seedlings maintained on nutrient-deficient medium (Smalle et al., 1997). Ethylene promotion of cell growth and elongation in the stem has also been reported for the aquatic plant Ranunculus sceleratus when submerged in water (Abeles et al., 1992), as well as for ethylene-treated etiolated rice coleoptiles (Satler and Kende, 1985). In the meadow grass Poa pratensis and oat, the ethylene-releasing compound ethephon has been implicated in the increase of tiller internode length (Abeles et al., 1992). Thus, it appears that ethylene can both inhibit and promote stem growth. Although it is intriguing to compare the multitude of different effects that ethylene has been reported to exert on the growth process, it is important to note that this information has been gathered from a large number of different species. Different species may respond to the same level of ethylene in different ways, as the literature clearly demonstrates.
Ethylene may also play a role in the integration of signals from light and gravity. In soybean, red light was found to reduce ethylene production by as much as 45% while promoting hypocotyl elongation (Samimy, 1978). Both effects were found to be reversible by FR, suggesting that phytochrome regulates hypocotyl growth via ethylene (Samimy, 1978). Arabidopsis seedlings, when grown under red light, lose their ability to reorient themselves to the gravity vector. This loss is also reversible by FR and has been shown to be controlled by both phytochrome A and phytochrome B (Poppe et al., 1996). In Arabidopsis, the plant hormone cytokinin, acting via ethylene, can restore gravitropism in seedlings that were rendered agravitropic by red light (Golan et al., 1996).
One approach to elucidating how auxin, ethylene, and light interact in shoot gravitropism is to study the response of mutants that are altered in their response to one or more of these factors. To this end, we used two tomato mutants with altered gravitropic responses: the auxin-resistant dgt mutant (Kelly and Bradford, 1986; Hicks et al., 1989), which exhibits a reduced gravitropic response (Lomax et al., 1993), and the lz-2 (lazy-2) mutant in which the direction of shoot gravitropism is reversed in a phytochrome-dependent manner (Gaiser and Lomax, 1993). We also investigated whether two tomato mutants altered in their ethylene physiology, the ethylene-overproducing epi (epinastic) mutant of tomato (Fujino et al., 1988) and the ethylene-insensitive nr (never-ripe) mutant (Wilkinson et al., 1995; Yen et al., 1995) exhibit alterations in their gravitropic response mechanism. Taken together, the results from these experiments lead us to propose that ethylene plays multiple, but not primary, roles in modulating the gravitropic response in tomato.
MATERIALS AND METHODS
Plant Material
Wild-type tomato (Lycopersicon esculentum Mill.) varieties Ailsa Craig (AC) and Pearson (P), as well as four mutants, epi, dgt, nr, and lz-2 were used. The dgt and lz-2 mutants were maintained in the AC background, whereas epi was in the VFN8 background. nr was used in AC, as well as the P isogenic parent line for curvature experiments. Seeds of lz-2, epi, and dgt, nr in AC, and AC were originally obtained from C.M. Rick (University of California, Davis). Seeds of nr in the P background, as well as wild-type P seeds, were kindly provided by Dr. Harry Klee (Univerisity of Florida, Gainesville). All lines were propagated by selfing at the Oregon State University Botany Farm (Corvallis).
Gravicurvature Experiments
For the gravicurvature measurements (Figs. 1 and 2), plants were grown in 10- × 10-cm pots filled with vermiculite and kept in darkness at 29°C for 4 to 5 d (epi plants were grown for 6–7 d). Seedlings were gravistimulated in their pots in red light (General Electric F40/Pl fluorescent lights filtered through a Roscolux Filter no. 27, 2.51 μmol m−2 s−1 measured from 640–680 nm, transmission maximum λ = 660 nm; Rosco, Hollywood, CA) in a plant incubation chamber (Hoffman, Albany, OR) at 29°C. At the indicated times, individual representative plants were excised at the vermiculite level and photocopied, and the curvature was determined with a protractor. The data presented were pooled from three experiments.
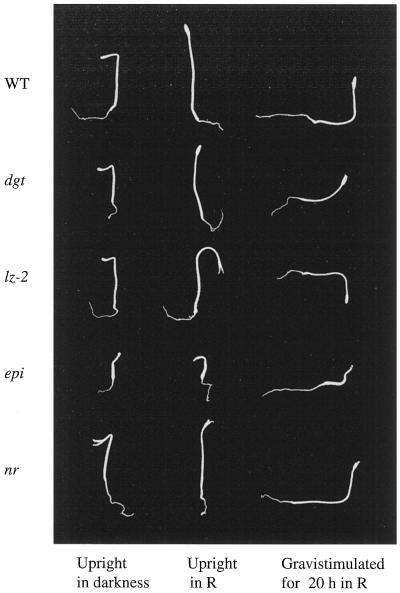
Figure 1.
Phenotype of wild type (WT, cv AC), dgt, lz-2, epi, and nr tomato seedlings. Seedlings were germinated in the dark for 4.5 d (epi for 6.5 d) and then oriented vertically in darkness (left), vertically in red light (R, center), or horizontally in red light (right) for 20 h. Representative seedlings were selected and photographed. Note that the lz-2 mutant in the upright or horizontal positions bends downward when exposed to red light.
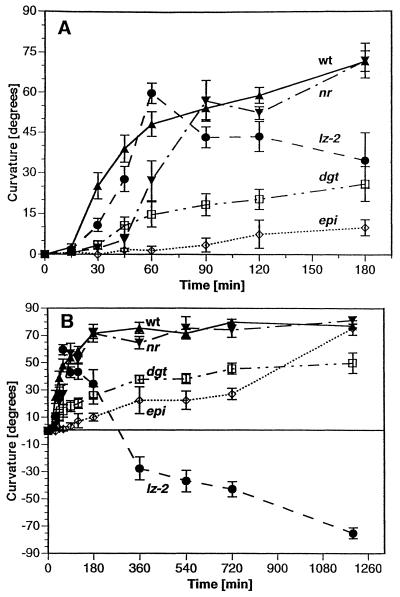
Figure 2.
The kinetics of gravitropic responses of etiolated auxin- and ethylene-response mutants of tomato in red light (R) at 29°C compared with wild type (WT). A, Short-term kinetics. B, Kinetics over 20 h from the same experiments. ▴, Wild type cv AC; □, dgt; •, lz-2; ▾, nr in AC background; and ⋄, epi. Plants were grown in darkness for 4.5 d (epi for 6.5 d) and subsequently transferred to an incubator equipped with fluorescent lights filtered through red Roscolux filters. At the times indicated, representative plants were cut at the vermiculite level and photocopied. The angle of gravicurvature was determined from the photocopies using a protractor. Data were pooled from three independent experiments and are mean values. Error bars reflect ±se, n = 6 to 21 (with n = 9.3 on average per time point).
Ethylene Evolution Measurements
A gas chromatograph (model GC-8A, Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and a 122-cm Poropak Q column (Waters) was used for all measurements of ethylene evolution. Approximately 20 seeds were germinated on 1 mL of 1% agar in 10-mL vials (Fisher Scientific) and grown in the dark for 4.5 d at 29°C. Prior to gravistimulation, the vials were capped with an airtight serum stopper (Fisher Scientific). Care was taken to use plants that were short enough not to reach the top of the vial or the serum stopper to prevent artifactual ethylene production resulting from seedling damage or stress response (Lehman et al., 1996). Upright controls or gravistimulated seedlings, which had been reoriented 90°, were either exposed to red light or kept in the dark. At the indicated times, a 1-mL headspace sample was withdrawn from the vial using a 1-mL tuberculin syringe with a 25-gauge needle (Becton Dickinson) and injected into the gas chromatograph. Ethylene concentrations were determined from a standard curve, and total ethylene evolution was normalized to the fresh weight of the seedlings.
Gravicurvature Response to Applied Ethylene
Plants used for measurements of gravicurvature in response to various ethylene concentrations (Fig. 5) were grown in 3-mL scintillation vials filled with vermiculite and kept in darkness at 29°C for 4 to 5 d (epi plants were grown for 6–7 d). Plants measuring approximately 1.5 to 2 cm from the root/shoot node to the hook were selected (epi plants were generally shorter and measured only 1–1.5 cm), and an interval 1 cm down the hypocotyl from the top of the hook was marked with black ink (Steig Products, Lakewood, NJ) to monitor elongation growth during gravistimulation. Subsequently, six to seven vials containing one seedling each were set in a holder with the cotyledons pointing up. The holders were transferred into 1-L Mason jars lined with Whatman 3MM paper, and the jars were sealed airtight. Prior to gravistimulation, the jars were injected with ethylene at various concentrations and then reoriented 90°. All plants were kept under red light (General Electric 40R red fluorescent tubes filtered through red acrylic, Shinkolite 102 [Argo Plastics Co., Los Angeles, CA] 0.95 μmol m−2 s−1 measured from 640–680 nm, transmission maximum λ = 642 nm) during gravistimulation. Fluence measurements were made with an LI-1800 spectroradiometer (LI-COR, Lincoln, NE). All manipulations of the plants were done under dim-green safelight. After gravistimulation, the plants were excised at the vermiculite level and photocopied. The length of the marked interval was measured, and curvature was determined with a protractor. Data were pooled from three to six independent experiments, and the se was calculated.
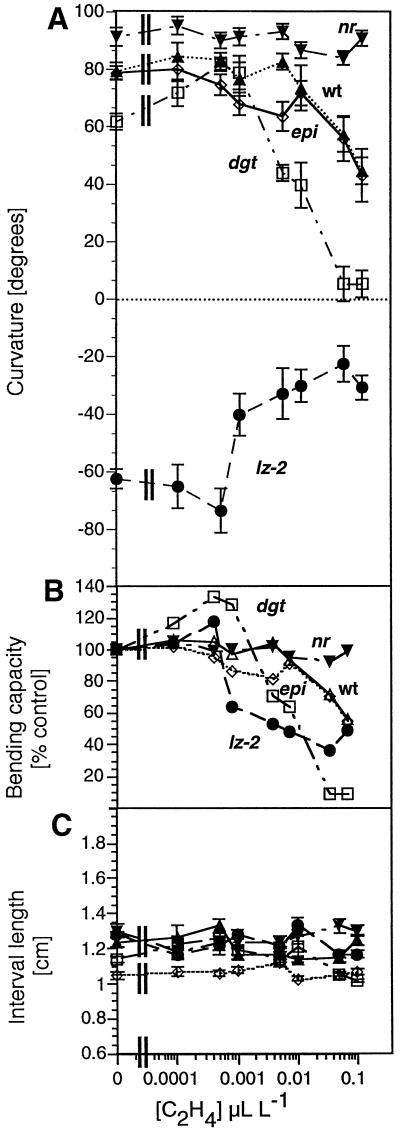
Figure 5.
Ethylene stimulation and inhibition of gravicurvature at concentrations that do not inhibit hypocotyl growth. Wild type (WT, ▴), lzy-2 (•), dgt (□), nr in AC (▾), and epi (⋄). Gravicurvature (A) and elongation (total length of marked interval after 20 h, C) were measured after 20 h of gravistimulation in red light. The bending capacity (B) was derived from the gravicurvature data in A as the percentage change to show differences of sensitivity in the mutants.
Ethylene Inhibitor Studies
For studies with ethylene inhibitors (Fig. 4), plants were grown and gravistimulated in sealed jars as described above for the gravicurvature experiments. NBD (Aldrich) was pipetted onto the filter paper just prior to gravistimulation and allowed to evaporate after the jars were sealed airtight. AVG (Sigma) was applied by watering upright plants with AVG solutions 5 h before gravistimulation to allow time for uptake of AVG through the root system.
Figure 4.
The ethylene action inhibitor NBD inhibits wild type (WT) gravicurvature but not elongation. The 4.5-d-old etiolated wild-type (AC) seedlings were marked with ink at the top and bottom of a 1cm interval extending from the top of the hook down the hypocotyl. Total hypocotyl length was approximately 1.5 cm. Seedlings in air-tight jars were treated with the indicated concentrations of NBD and reoriented with respect to gravity. After 20 h of gravistimulation, the increase in length of the marked hypocotyl region (% elongation increase of marked interval at 0 h; •) and hypocotyl curvature (▪) were measured. Data are representative of three experiments, n = 6 to 8 for each point. Results are means ± se.
Statistical Analysis
All statistical analyses were performed using Microsoft Excel software. P values reflect those of two-sided Student's t test analyses.
RESULTS
The dgt, lz-2, epi, and nr Mutants of Tomato Exhibit Altered Gravitropic Responses
If ethylene plays an important role in the gravitropic response of plants, then mutants that are altered in ethylene perception or response should exhibit altered gravitropism. Alternatively, analysis of the ethylene physiology of known gravitropic mutants may provide insight into the role of ethylene in plant responses to gravity. In Figure 1, the morphology of four tomato mutants, epi (an ethylene-overproducing mutant), nr (an ethylene-insensitive mutant that has been demonstrated to lack an ethylene receptor), dgt (an auxin-resistant mutant with a retarded gravitropic response), and lz-2 (a mutant that exhibits a phytochrome-regulated reversal of the shoot gravitropic response), is compared with that of wild-type seedlings. All seedlings were grown in ambient air and kept either upright in darkness (Fig. 1, left), upright in red light (Fig. 1, center), or gravistimulated in red light (Fig. 1, right). When vertically oriented, all of the seedlings maintained correct orientation away from the gravity vector with the exception of lz-2 seedlings which bent downward in red light. Gravistimulation, achieved by placing the plants horizontally in the presence of red light results in complete upward reorientation of wild-type, epi, and nr seedlings, incomplete upward curvature of dgt seedlings, and reversed (positive) curvature of lz-2 seedlings. Under all of the conditions, seedlings carrying the epi lesion display characteristics of wild-type seedlings treated with high ethylene concentrations, including shortened and thickened hypocotyls. It is interesting that epi seedlings achieved correct reorientation even with severely stunted hypocotyl growth.
Kinetic analysis of gravitropic curvature revealed additional alterations in the gravitropic responses of all four mutants (Fig. 2). The normal upward gravitropic response of dark-grown wild-type tomato seedlings was initiated within 15 to 30 min of gravistimulation and was completed by 4 h, whereas the auxin-resistant dgt mutant exhibited a slower and incomplete response to gravity, reaching only approximately 50° curvature after 20 h. The gravitropic response of the ethylene overproducer epi was also reduced during the first 12 h after gravistimulation but reached wild-type levels (75°–80° curvature) by 20 h. Under these experimental conditions, the lz-2 mutant curved upward during the first 3 h of gravistimulation before it reoriented and by 20 h had curved about 80° downward. Whereas the nr mutant in the AC background exhibited a nearly normal gravitropic response, the initiation of curvature was slightly delayed in nr plants when compared with the wild-type response. This delay was detected during the first 30 to 60 min after reorientation (Fig. 2A). However, curvature of nr plants reached wild-type levels within 90 min of gravistimulation. Similar results were observed with nr in the P background (data not shown).
Ethylene Evolution during the Gravitropic Response
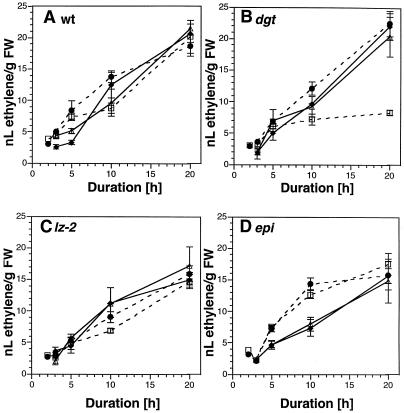
Because phytochrome mediates the reversal of gravicurvature in the lz-2 mutant and red light has been shown to regulate ethylene concentrations and hypocotyl elongation, we hypothesized that the lz-2 lesion altered ethylene synthesis or action. We tested this hypothesis by comparing the evolution of ethylene by wild-type, dgt, lz-2, and epi plants, which had either been gravistimulated or maintained upright in either darkness or red light. During a 20 h time course, we observed similar rates of ethylene evolution for wild-type, lz-2, and epi plants in both red light and darkness and dgt plants in darkness (Fig. 3). The exception was a sharp decrease at 20 h in ethylene evolution by dgt seedlings that were gravistimulated in red light. However, the kinetics of this reduction did not correlate with the reduction in the dgt gravitropic response observed as early as 30 min after gravistimulation (compare Fig. 2A and Fig. 3B). It was interesting to note that at 10 h gravistimulated wild-type and lz-2 plants, as well as both stimulated and unstimulated dgt plants, showed a plateau in their ethylene production. The rate of ethylene production was increased between 10 and 20 h in wild type, lz-2, and the ungravistimulated dgt mutant, which was in sharp contrast to the gravistimulated dgt mutant. It is interesting that with the epi mutant a linear increase in ethylene production was observed for ungravistimulated plants, whereas gravistimulated plants evolved approximately twice as much ethylene as the vertically oriented plants at 10 h. By 20 h, this difference in ethylene production between gravistimulated and vertical epi plants had disappeared (Fig. 3D).
Figure 3.
Ethylene evolution is not altered by gravistimulation and/or red light. Approximately 20 seeds were planted in each 10-mL vial containing 1 mL of 1% agar and incubated at 29°C in the dark for 4.5 d. Vials were capped with serum stoppers prior to treatment. A, Wild type (wt, AC); B, dgt; C, lz-2; D, epi. ▵, Upright plants in red light; •, gravistimulated plants in darkness (dashed line); □, gravistimulated plants in red light (dashed line); ♦, upright plants in darkness. se bars are shown where larger than the symbol, n = 5 to 20 vials per point. Data were pooled from six independent experiments. FW, Fresh weight.
Ethylene Inhibitors Specifically Inhibit the Gravitropic Response
If ethylene is required for a complete shoot gravitropic response, then inhibition of ethylene action or synthesis should inhibit gravitropism. Gravicurvature of wild-type plants exposed to varying concentrations of the ethylene action inhibitor NBD was inhibited approximately 50% at 0.87 mm (70 μL) NBD, with maximum inhibition (75%) achieved at 1.13 mm (90 μL; Fig. 4) NBD. The overall elongation of the uppermost 1 cm of the hypocotyl was not significantly affected by NBD at these concentrations (Fig. 4). Treatment with the ethylene synthesis inhibitor AVG yielded results similar to those observed with NBD. At the highest concentration tested (100 μm), AVG inhibited curvature 18% (data not shown), which was a statistically significant decrease (P = 0.01). As with NBD, elongation growth was not affected by concentrations of AVG, which significantly affected gravicurvature (data not shown).
Ethylene Has Concentration-Dependent Effects on Curvature
To further test the potential of ethylene to modify the response of plants to gravity, we measured gravicurvature in the presence of ethylene concentrations varying from 0.0001 to 0.1 μL L−1 (Fig. 5A). The gravitropic response of the ethylene-insensitive nr mutant was not altered at any ethylene concentrations tested. Seedlings of all other genotypes showed a sharp decrease in curvature in response to low concentrations of ethylene. Inhibition of the gravitropic response of wild-type and epi seedlings was detected at 0.01 μL L−1 with 50% inhibition at 0.1 μL L−1, whereas curvature of lz-2 and dgt seedlings was inhibited at even lower ethylene concentrations (significant inhibition was observed at 0.001 and 0.005 μL L−1, respectively; Fig. 5B). Treatment with 0.05 to 0.1 μL L−1 ethylene resulted in nearly complete inhibition of the dgt gravitropic response but only 50% inhibition for wild-type, epi, or lz-2 plants (Fig. 5B). Thus, all tomato genotypes that retain sensitivity to ethylene exhibit dose-dependent inhibition of their bending behavior in response to gravistimulation.
Those ethylene concentrations that significantly inhibited gravicurvature (0.001–0.1 μL L−1) produced no significant change in overall elongation growth in the 1-cm interval below the hook, which included the curvature zone (Fig. 5C). Statistically significant reduction of elongation growth was observed only for dgt and lz-2 at ethylene concentrations higher than 0.05 μL L−1 (P = 0.01). Those ethylene concentrations are 10- to 50-fold higher than those necessary to significantly inhibit gravicurvature (compare Fig. 5, B and C). Thus, inhibition of elongation growth and curvature apparently occur independently at the exogenous ethylene concentrations used here.
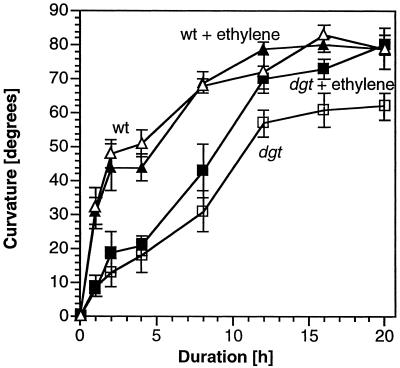
The reduced gravitropic response of the dgt mutant is restored by very low levels of ethylene. Fumigation of dgt seedlings with ethylene concentrations as low as 0.0005 μL L−1 for 20 h resulted in gravicurvature equal to that of wild-type plants (Fig. 5A). This is a 40% stimulation of bending capacity over untreated dgt plants (Fig. 5B). To determine whether ethylene restored the dgt mutant to a full, wild-type gravitropic response, we compared the kinetics of curvature of gravistimulated wild-type and dgt seedlings in the presence and absence of the optimal ethylene concentration, 0.0005 μL L−1 (Fig. 6). Whereas the wild-type response was similar in either ambient air or ethylene, the gravitropic response of the dgt mutant was accelerated by ethylene at this very low concentration. The kinetics of gravicurvature for dgt seedlings in the presence of ethylene were, however, not identical to the wild-type gravity response. Curvature of dgt seedlings in the presence of ethylene is still slower than that of wild-type seedlings and the acceleration of dgt bending by ethylene is not noticeable until 8 h after gravistimulation. By this time, reorientation of wild-type seedlings with respect to gravity was essentially complete. In comparison, the complete reorientation of dgt seedlings required 16 h even in the presence of optimal ethylene concentrations. It appears that ethylene enhances and sustains the long-term response of dgt hypocotyls to gravity but does not phenocopy the wild-type gravitropic response (Fig. 6).
Figure 6.
Low concentrations of ethylene (0.0005 μL L−1) restore the dgt gravitropic response but not with wild-type (WT) kinetics. Plants were grown and treated as for Figure 5. Wild type with ethylene (▴), WT without ethylene (▵), dgt with ethylene (▪), and dgt without ethylene (□). Results are means ± se, n = 10 to 25, pooled from three to six independent experiments.
DISCUSSION
If ethylene plays a primary role in shoot gravitropism, as has been proposed (Wheeler and Salisbury, 1980, 1981), then mutants that are altered in ethylene responsiveness should exhibit profound alterations in their gravitropic response. We found that seedlings of the tomato ethylene-response mutants nr and epi do exhibit a gravitropic phenotype (Fig. 2). The epi mutant was previously shown to overproduce ethylene (Fujino et al., 1988), whereas nr is an ethylene-insensitive mutant (Wilkinson et al., 1995). Curvature of both mutants in response to gravistimulation is delayed in comparison with wild-type seedlings, with the epi phenotype being much more severe than that of nr (Fig. 2). Both mutants can, however, achieve full reorientation with respect to gravity within 20 h. This relatively minor reduction in the gravitropic response of these ethylene mutants provides strong evidence that ethylene does not play an essential or primary role in the gravitropic response of tomato.
Seedlings carrying the dgt lesion have a slower gravitropic response and never achieve greater than 50o curvature in ambient air (Fig. 2). The dgt lesion was previously shown to confer greatly reduced auxin sensitivity in hypocotyls (Kelly and Bradford, 1986) and increased sensitivity to ethylene in shoots (Shi and Cline, 1992; Fig. 5), and it confers a different gravitropic phenotype than either epi or nr. The slow but complete gravitropic response of nr and epi also did not resemble that of the lz-2 mutant of tomato, which, under the red-light conditions used here, initially curved upward in a manner similar to wild-type seedlings and then reversed direction of growth, resulting in downward curvature (Figs. 1 and 2). The lz-2 mutant seems to exhibit a biphasic gravitropic response. Similarly, epi responds to gravistimulation initially only in an extremely delayed and reduced fashion but curves up rather rapidly after 12 h, reaching full curvature after 20 h of gravistimulation. This biphasic response in epi correlates with an increase in ethylene evolution at 10 h (Fig. 3D).
There are contradictory reports in the literature with regard to ethylene synthesis in response to gravistimulation (Kaufman et al., 1985; Harrison and Pickard, 1986). Treatment with ethylene was reported to restore normal gravitropic orientation to mature dgt plants (Zobel, 1973; Jackson, 1979), and application of ethylene can reverse the direction of the shoot gravitropic response in etiolated pea seedlings (Kang and Burg, 1974; Burg and Kang, 1993) and in mature tomato petioles (Kang and Burg, 1974). However, neither red-light treatment nor gravistimulation induced changes in the ethylene synthesized by etiolated seedlings of wild-type or any of the mutants tested (Fig. 3). These observations agree with previous reports showing no measurable differences in ethylene production within 3 h of gravistimulation using light-grown wild-type tomato seedlings (Harrison and Pickard, 1986). Overall levels of ethylene evolution by the epi and lz-2 mutants were slightly lower than those observed for wild-type or dgt plants. Red light, therefore, does not act through increased ethylene evolution to reverse the lz-2 gravitropic response.
Although the epi mutant has been characterized as an ethylene overproducer, ethylene synthesis by epi seedlings was similar or even reduced compared to wild-type seedlings. This finding is in agreement with observations that hypocotyls are the tissue least affected by the epi lesion and the only organ tested that does not overproduce ethylene (Fujino et al., 1988). The morphology of epi seedlings is strikingly similar to a constitutive ethylene response in many respects, including thicker and shorter hypocotyls (Fig. 1; Ursin, 1987). However, it has not been shown that epi cosegregates with the constitutive triple-response gene (ctr) isolated from Arabidopsis (Kieber et al., 1993). Although the reduced gravitropic phenotype of nr and epi seedlings indicated that normal ethylene responsiveness was necessary for a full gravitropic response, large changes in ethylene synthesis were not observed (Fig. 3).
One exception to the inability of red light or gravistimulation to produce changes in ethylene evolution measurable by GC was the reduction in ethylene synthesis by dgt seedlings that had been gravistimulated in the presence of red light (Fig. 3B). This suggested that the sluggish gravitropic response exhibited by dgt (Fig. 2) may be the result of diminished ethylene production. However, the observed difference in ethylene evolution was significant only after 20 h of treatment and thus cannot explain the reduction in the dgt gravitropic response, which was measurable as early as 30 min after gravistimulation (compare Fig. 2 with Fig. 3B). In addition, curvature of dgt seedlings gravistimulated in darkness for 20 h was not significantly different from that of dgt plants gravistimulated in red light (data not shown). Whereas red light was previously reported to cause a marked deceleration of ethylene production in pea (Goeschl et al., 1967) and soybean (Samimy, 1978), we observed no effect of either red light or gravistimulation alone on ethylene production in dgt or any other tomato genotype tested. The dgt lesion does, however, render ethylene synthesis sensitive to the simultaneous application of gravity stimulus and red light (Fig. 3B). The basis for this sensitivity remains to be elucidated. It is interesting to note that increased sensitivity occurred only in dgt shoots, which exhibit increased sensitivity to ethylene (Shi and Cline, 1992; Fig. 5) and resistance to auxin (Kelly and Bradford, 1986; Muday et al., 1995; Coenen and Lomax, 1998).
The epi mutant was reported to overproduce ethylene in all tissues except hypocotyls (Fujino et al., 1988). Our results confirm this finding for the most part. However, at 10 h of gravistimulation we observed significantly higher ethylene evolution by gravistimulated epi seedlings versus nongravistimulated epi plants. This increased evolution of ethylene approximately correlates with a somewhat increased rate of curvature at 10 h in Figure 2, indicating that ethylene may cause (or be the result of) a second phase of curvature leading to full 90° curvature after 20 h of gravistimulation. Although these correlations are intriguing, it is important to note that measurements of ethylene evolution by intact seedlings do not focus on the target tissue that is affected during gravitropic elongation growth. Therefore, graviresponsiveness or the lack thereof may not necessarily reflect the necessity for ethylene in a normal response in this experiment.
Evidence that ethylene can modulate the gravitropic response is provided by the ability of ethylene action and synthesis inhibitors to reduce gravicurvature in wild-type seedlings. Inhibition of curvature by NBD, an ethylene action inhibitor (Fig. 4), and AVG, an ethylene synthesis inhibitor (data not shown), occurred at concentrations that did not significantly inhibit the overall elongation of the marked region of the hypocotyl. This confirmed results of experiments conducted in cocklebur, which demonstrated a reduction in gravicurvature by ethylene action and synthesis inhibitors (Wheeler and Salisbury, 1980, 1981) and indicates that low levels of ethylene are necessary for a full gravitropic response. However, a basal gravitropic response still occurred even at high concentrations of NBD. AVG was less effective in inhibiting the gravitropic response than NBD, possibly because of inefficient uptake by the roots.
Further support for the ability of ethylene to stimulate the gravitropic response of tomato seedlings was revealed by the restoration of curvature in dgt seedlings to wild-type levels by treatment with extremely low levels of ethylene (Figs. 5 and 6). These ethylene concentrations are 5- to 10- fold lower that those reported to restore the wild-type gravitropic phenotype of mature dgt plants (Zobel, 1973, 1974; Jackson, 1979). However, even in the presence of 0.0005 μL L−1 ethylene, the initial curvature of dgt seedlings was much slower than that of wild-type seedlings. An increase in the curvature rate of ethylene-treated dgt seedlings after 12 to 20 h (Fig. 6) subsequently restored the mutant hypocotyls to full curvature. This divergence from wild-type kinetics suggests that low levels of ethylene can stimulate gravicurvature but not via direct repair of the dgt lesion (Fig. 6).
Ethylene was found to play additional concentration-dependent roles in modulating the gravitropic response of etiolated tomato seedlings. Whereas extremely low levels of ethylene appeared to be necessary for a full gravitropic response, intermediate ethylene concentrations (0.005–0.1 μL L−1) inhibited wild-type gravicurvature (Fig. 5, A and B). The gravitropic response of the dgt and lz-2 mutants exhibited increased sensitivity to inhibition by ethylene, whereas the nr gravitropic response was not inhibited by ethylene concentrations as high as 0.1 μL L−1. Inhibition of the reversed gravitropic curvature of lz-2 plants in red light occurred at 0.001 to 0.1 μL L−1 ethylene, concentrations that are 1000-fold lower than those reported to induce reversal of gravitropic orientation of etiolated pea seedlings (Burg and Kang, 1993). In the etiolated seedlings used here, lz-2 and wild-type seedlings exhibited severe triple-response symptoms (stunted growth, radial expansion, and exaggerated hook curvature) at concentrations greater than 1.0 μL L−1 (data not shown). Since neither red light nor gravistimulation altered ethylene evolution by lz-2 plants, and ethylene treatment inhibited gravitropism but did not repair the reversed-gravitropic lz-2 phenotype, we suggest that ethylene does not play a role in red-light regulation of the direction of growth in tomato.
Ethylene inhibits hypocotyl elongation in a variety of species, including etiolated Arabidopsis (Goeschl and Kays, 1975; Ecker, 1995; Peck et al., 1998) and yet accelerates hypocotyl growth in light-grown Arabidopsis (Smalle et al., 1997) and peanut (Goeschl and Kays, 1975). In this study we found no significant inhibition or stimulation of overall elongation within the marked region that included the gravitropic bending zone by ethylene concentrations that inhibited gravicurvature (Fig. 5C). The lack of correlation between inhibition of gravicurvature and inhibition of growth rates suggests that the differential growth involved in gravicurvature is not regulated in the same manner as overall stem elongation.
If ethylene plays a primary role in gravitropism (Wheeler and Salisbury, 1980, 1981; Clifford and Oxlade, 1989; Philosoph-Hadas et al., 1996), then an ethylene-insensitive mutant should be agravitropic. Surprisingly, the nr mutant, which is insensitive to ethylene in both seedling and mature stages (Fig. 5; Wilkinson et al., 1995), displayed only a slightly retarded gravitropic response (Fig. 2). Although it is possible that the nr mutation is leaky or that other members of the ethylene receptor gene family can compensate for the missing nr gene product, this does not seem likely because nr is completely insensitive to ethylene with respect to inhibition of the gravitropic response at the concentrations tested in Figure 5A. The fact that nr seedlings are insensitive to ethylene inhibition of curvature and can attain full reorientation with respect to gravity suggests that inhibition of curvature by ethylene does not play a prominent role in the generation of a normal gravitropic response. The ethylene-overproducing epi mutant could be expected to be either severely inhibited in its graviresponsiveness or enhanced, depending on which part of the ethylene dose-response bell curve is mimicked by the mutation. However, although exhibiting striking similarities to the constitutively ethylene-responding Arabidopsis ctr mutant, epi did not exhibit an opposite graviresponse to nr. It is possible that higher ethylene concentrations within the tissue lead to a condition that is supraoptimal for the gravitropic response and therefore inhibit or retard the process. However, until the gene products of dgt, epi, and lz-2 are identified, these questions cannot be answered.
The gravitropic response of the auxin-resistant dgt mutant is more severely delayed and reduced than that of nr. Therefore, sensitivity to auxin appears to play a more important role in the gravitropic response mechanism than ethylene sensitivity or synthesis. Application of very low levels of ethylene can, however, compensate for reduced auxin responsiveness by enhancing and sustaining the slower dgt response (Fig. 6). The Cholodny-Went hypothesis suggests that tropic curvatures are the result of increased auxin concentrations on one side of a stem (Went and Thimann, 1937). Other studies have provided evidence that gravistimulation results in alterations in auxin sensitivity (MacDonald and Hart, 1987; Rorabaugh and Salisbury, 1989). Our results suggest that ethylene may amplify a signal that either stimulates the asymmetric redistribution of auxin or increases the auxin sensitivity of the cells in the lower half of a gravistimulated hypocotyl. It has been proposed that ethylene modulates lateral auxin transport, especially in the apical hook of etiolated seedlings (Schwark and Bopp, 1993; Lehman et al., 1996; Peck et al., 1998). Ethylene may compensate for the reduced auxin responsiveness of the dgt mutant by enhancing lateral transport of auxin to target cells in the hypocotyl epidermis. In mutants that have reduced ethylene sensitivity, such as nr, the stimulation of auxin transport by ethylene may be attenuated, leading to a partial or delayed gravitropic response but not eliminating the basal rate of lateral transport of auxin. This possibility is also supported by the recent finding that eir-1, a root-specific, agravitropic, ethylene-insensitive mutant of Arabidopsis, likely owes its phenotype to a dysfunctional auxin transporter (Luschnig et al., 1998). However, it is possible that stimuli other than ethylene also enhance lateral auxin transport and thus compensate for the inability of nr to respond to ethylene normally. The same argument can also be applied to ethylene stimulation of auxin responsiveness in the dgt mutant.
Our genetic analysis has revealed that, although ethylene does not play a primary role in the gravitropic response of etiolated seedlings, it can act as a modulator of gravitropism by either stimulating or inhibiting curvature. The mechanism by which ethylene influences gravitropism remains to be elucidated. However, these studies indicate that ethylene is part of a complex feedback mechanism in the gravitropic response similar to that demonstrated for the role of ethylene in elongation growth. Ethylene may play an important role in the initiation or maintenance of differential growth responses that help emerging seedlings detect obstructive objects and adjust growth rates and direction accordingly. Alternatively, ethylene modulation of the gravitropic response may be a residual interaction resulting from the mechanisms governing hook formation and maintenance. These studies provide testable hypotheses that can be used to elucidate the interaction between auxin and ethylene not only in regulating gravitropism but also in integrating that information with other environmental cues.
ACKNOWLEDGMENTS
We thank Dr. Harry Klee for the generous gift of mutant nr and P seeds, Dr. Daniel Arp for use of the gas chromatograph, and TJ White for critical reading of the manuscript.
Abbreviations:
- AVG
aminovinylglycine
- FR
far red light
- NBD
norbornadiene
Footnotes
This research was supported by a doctoral fellowship from the Deutsche Studienstiftung (to A.M.), a National Aeronautics Space Administration (NASA) Space Biology Research Associate award (to F.J.B.), and grants from the NASA Gravitational Biology and Ecology Program and the National Science Foundation Integrative Plant Biology Program to T.L.L.
LITERATURE CITED
- Abeles FB, Morgan PW, Mikal E, Saltveit J. Ethylene in Plant Biology. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Wall AR, Schulz B, Feldman KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Burg SP, Kang BG. Gravity dependent ethylene action. In: Pech JC, Latache A, Balague C, editors. Cellular and Molecular Aspects of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 335–341. [Google Scholar]
- Clifford PE, Oxlade EL. Ethylene production, georesponse, and extension growth in dandelion peduncles. Can J Bot. 1989;67:1927–1929. [Google Scholar]
- Clifford PE, Reid DM, Pharis RP. Endogenous ethylene does not initiate but may modify geobending: a role for ethylene autotropism. Plant Cell Environ. 1983;6:433–436. [Google Scholar]
- Coenen C, Lomax TL. The Diageotropica gene differentially affects auxin and cytokinin responses throughout development in tomato. Plant Physiol. 1998;117:63–72. doi: 10.1104/pp.117.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1888) The Power of Movement in Plants. D. Appleton and Co., New York
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Fujino DW, Burger DW, Yang SF, Bradford KJ. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. cultivar VFN8) Plant Physiol. 1988;88:774–779. doi: 10.1104/pp.88.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiser CJ, Lomax TL. The altered gravitropic response of the lazy-2 mutant of tomato is phytochrome regulated. Plant Physiol. 1993;102:339–344. doi: 10.1104/pp.102.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Kays SJ. Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean, and cotton seedlings. Plant Physiol. 1975;55:670–677. doi: 10.1104/pp.55.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Pratt HK, Bonner BA. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol. 1967;42:1077–1080. doi: 10.1104/pp.42.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan A, Tepper M, Soudry E, Horwitz B, Gepstein S. Cytokinin acting through ethylene, restores gravitropism to Arabidopsis seedlings grown under red light. Plant Physiol. 1996;112:901–904. doi: 10.1104/pp.112.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M, Pickard BG. Burst of ethylene upon horizontal placement of tomato seedlings. Plant Physiol. 1984;75:1167–1169. doi: 10.1104/pp.75.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MA, Pickard BG. Evaluation of ethylene as a mediator of gravitropism by tomato hypocotyls. Plant Physiol. 1986;80:592–595. doi: 10.1104/pp.80.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Rayle DL, Lomax TL. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989;245:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Is the diageotropic tomato ethylene deficient? Physiol Plant. 1979;46:347–351. [Google Scholar]
- Kang BG, Burg SP (1974) Ethylene action on lateral auxin transport in tropic responses, leaf epinasty, and horizontal mutation. In NG Kaigi, ed, Plant Growth Substances 1973. Hirokowa Publishing, Tokyo, pp 1090–1094
- Kaufman P, Pharis RP, Reid DM, Beall FD. Investigations into the possible regulation of negative gravitropic curvature in intact Avena sativa plants and in isolated stem segments by ethylene and gibberellins. Physiol Plant. 1985;65:237–244. doi: 10.1111/j.1399-3054.1985.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986;82:713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Picket FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Coenen C, Gaiser JC, Hopkins R, Rayle DL, Rice MS (1993) Auxin perception and the regulation of tomato growth and development. In J Yoder, ed, Molecular Biology of Tomato. Technomic Publishing AG, Lancaster, PA, pp 129–139
- Luschnig C, Gaxialo RA, Grisafi P, Fink GR. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IR, Hart JW. Tropisms as indicators of hormone-mediated growth phenomena. In: Hoad GV, Lenton JR, Jackson MB, Atkin RK, editors. Hormone Action in Plant Development: A Critical Approach. London: Butterworths; 1987. pp. 231–249. [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Peck SC, Pawlowsky K, Kende H. Asymmetric responsiveness to ethylene mediates cell elongation in the apical hook of peas. Plant Cell. 1998;10:713–719. [Google Scholar]
- Philosoph-Hadas S, Meir S, Rosenberger I, Halevy AH. Regulation of the gravitropic response and ethylene biosynthesis in gravistimulated snapdragon spikes by calcium chelators and ethylene inhibitors. Plant Physiol. 1996;110:301–310. doi: 10.1104/pp.110.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Hangarter RP, Sharrock RA, Nagy F, Schäfer E. The light-induced reduction of the gravitropic growth-orientation of seedlings of Arabidopsis thaliana (L.) is a photomorphogenic response mediated synergistically by the far-red-absorbing forms of phytochromes A and B. Planta. 1996;199:511–514. doi: 10.1007/BF00195180. [DOI] [PubMed] [Google Scholar]
- Rorabaugh PA, Salisbury FB. Gravitropism in higher plant shoots. Plant Physiol. 1989;91:1329–1338. doi: 10.1104/pp.91.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimy C. Effect of light on ethylene production and hypocotyl growth of soybean seedlings. Plant Physiol. 1978;61:772–774. doi: 10.1104/pp.61.5.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler SO, Kende H. Ethylene and the growth of rice seedlings. Plant Physiol. 1985;79:194–198. doi: 10.1104/pp.79.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwark A, Bopp M. Interaction of ethylene and auxin in the regulation of hook growth. II. The role of ethylene in different growing regions of the hypocotyl of Phaseolus vulgaris. J Plant Physiol. 1993;142:585–592. [Google Scholar]
- Schwark A, Schierle J. Interaction of ethylene and auxin in the regulation of hook growth. I. The role of auxin in different growing regions of the hypocotyl hook of Phaseolus vulgaris. J Plant Physiol. 1992;140:562–570. [Google Scholar]
- Shi L, Cline M. Shoot inversion-induced ethylene production in the Diageotropica tomato mutant. Ann Bot. 1992;69:119–122. [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin VM (1987) Morphogenetic and physiological analyses of two developmental mutants of tomato, epinastic and diageotropica. PhD thesis. University of California, Davis
- Went FW, Thimann KV (1937) Phytohormones. The Macmillan Co., New York
- Wheeler RM, Salisbury FB. Gravitropism in plant stems may require ethylene. Science. 1980;209:1126–1127. doi: 10.1126/science.209.4461.1126. [DOI] [PubMed] [Google Scholar]
- Wheeler RM, Salisbury FB. Gravitropism in higher plant shoots. Plant Physiol. 1981;67:686–690. doi: 10.1104/pp.67.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1808. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Woltering EJ. Regulation of ethylene biosynthesis in gravistimulated Kniphofia (hybrid) flower stalks. Plant Physiol. 1991;138:443–449. [Google Scholar]
- Yen H-C, Lee S, Tanksley SD, Lanahan MB, Klee HJ, Giovannoni JJ. The tomato Never-ripe locus regulates ethylene-inducible gene expression and is linked to a homolog of the Arabidopsis ETR1 gene. Plant Physiol. 1995;107:1343–1353. doi: 10.1104/pp.107.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol. 1973;52:385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Control of morphogenesis in the ethylene-requiring tomato mutant, diageotropica. Can J Bot. 1974;52:735–741. [Google Scholar]