Abstract
Mucormycosis is a rare but often fatal fungal infection caused by a group of fungus known as the Mucorales. This fungus can cause varieties of infections in human beings, especially in an immunocompromised condition. According to various studies, the mortality rate ranges from 10% to 100% depending on the location and site of infection accompanied by underlying diseases. Rhinomaxillary involvement is the most common form of mucormycosis predominantly occurring in patients with uncontrolled diabetes. Necrosis of the maxilla in patients with rhinomaxillary form is less evident as the maxilla is richly vascular, but in case of immunocompromised status, it becomes a common clinical finding. Due to the necrosis of the maxilla, maggots have been found in the nasal and oral cavity which adds to the deteriorating clinical condition. This case report describes a combined medical, surgical, psychological, and prosthetic approach in effectively managing one such case of rhinomaxillary mucormycosis.
Keywords: Amphotericin B, immunocompromised, interim obturator, mucormycosis, myiasis
Introduction
Mucormycosis, also known as zygomycosis or phycomycosis, was first described by “Paltauf” in 1885,[1] who also coined the term “mycosis mucorina”. Diabetes mellitus is a multifactorial systemic disorder affecting worldwide population and is an important predisposing factor for variety of opportunistic infections. Diabetic patients are predisposed to mucormycosis because of the decreased ability of their neutrophils to phagocytose and adhere to the endothelial cells. Furthermore, the acidosis and hyperglycemia provide a favorable environment for the fungus to grow. Mucormycosis and diabetes in combination create a lethal and fatal combination.[2]
Myiasis is an infection of tissues or organs of animals by larvae. In humans, myiasis occurs in unhealthy, immunocompromised individuals. Poor hygiene, low socioeconomic status, hot and humid climate, and presence of preexisting suppurative conditions are known to be associated with increased risk of developing myiasis.[3,4,5] We present a case of recurrent rhinomaxillary mucormycosis with nasal myiasis and its effective and systemic management involving medical, surgical, prosthetic, and psychological rehabilitation leading to favorable prognosis and outcome.
Case Report
An elderly female patient reported to the department of general medicine with the chief complaint of high fever, chills, severe right-side progressive headache, and pain in the right eye with swelling of the midface and predominantly on the right side for 10 days. The patient gave a history of 3–4 episodes of vomiting with abdominal pain, one bout of epistaxis, bleeding in the oral cavity associated with foul odor, and breathlessness. Medical history showed that the patient was suffering from type II diabetes mellitus for 10 years and was receiving oral hypoglycemic medication.
The patient's dental history revealed that she had developed severe pain in the left upper jaw region 6 months back for which she sought treatment in a local dental hospital wherein the maxillary central incisors, left lateral incisor, and canine were extracted. A removable prosthesis was fabricated and delivered for the missing dentition within a week. Within few days, the patient reported back to the dental hospital when her symptoms recurred. On evaluation, it was noted that the socket wound was unhealed and was associated with foul odor. Thereafter, the patient was referred to the ear, nose, and throat department where she was advised to undergo computed tomography (CT) paranasal sinus (PNS) which revealed multifocal erosive lesions with oroantral and oronasal communication. It was diagnosed as chronic sinusitis involving maxillary and sphenoidal sinuses. Biopsy was done, and histopathological examination reported as mucormycosis. The patient was hospitalized and amphotericin B (AmB) deoxycholate was administered intravenously for 14 days.
She was apparently normal for 6 months when she reported to our institution with recurrence of previous symptoms probably due to poor glycemic control. On general physical examination, the patient appeared disoriented, confused, and irritable with increased respiratory rate and tachycardia. Local examination revealed necrosed anterior maxilla with black discoloration of hard palate, oroantral and oronasal communication, and halitosis [Figure 1].
Figure 1.
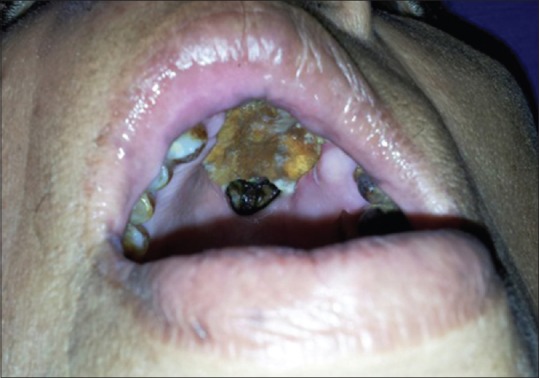
Intraoral photograph showing black discoloration of the palate and necrosis of the maxilla
Complete blood count showed increased erythrocyte sedimentation rate and white blood cell count. Random blood glucose levels were very high (383 mg/dl) with deranged electrolytes, and urine examination revealed plenty of pus cells. CT PNS was done and erosion of the nasal septum, turbinates, medial walls of the maxillary sinus, superior alveolar ridge, ethmoidal trabeculae, and left sphenoidal walls was evident. No orbital or intracranial extension was noted [Figure 2]. Maggots started emerging out of nasal cavity on the 2nd day of admission.
Figure 2.
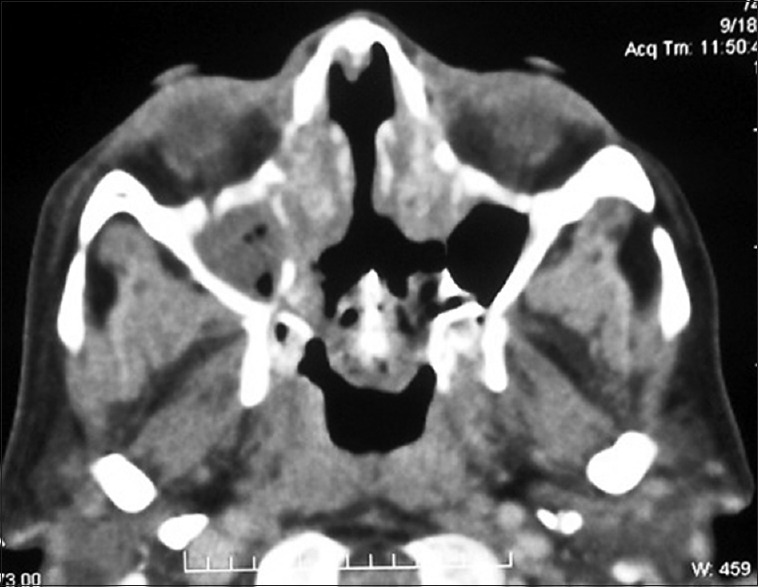
Computed tomography paranasal sinus showing erosion of the maxillary alveolar ridge, ethmoidal trabeculae, left sphenoidal wall, and no cranial or orbital involvement
The treatment included a combination of medical, surgical, and psychological approach followed by prosthetic rehabilitation. The patient was started on injection AmB deoxycholate, piperacillin, tazobactam, and metrogyl. The first dose of AmB deoxycholate given was 1 mg/kg/day over 3 h after hydrating the patient with normal saline. Infusion of antifungal continued for 1 week with close monitoring of renal parameters every 2nd day. However, elevation of serum creatinine to 1.7 was noted after 1 week, and hence, the dose of antifungal agent was reduced to 0.5 mg/kg/day. Simultaneously, glycemic levels were maintained within normal limits with human insulin injection.
Maggots were managed with turpentine oil, and by the end of 9 days, the patient was better and free of symptoms. This was followed by anterior maxillectomy with extensive surgical debridement. Intraoral facial degloving incision from maxillary left canine extending up to the right 2nd molar was given; subperiosteal dissection was done to expose the anterior necrosed maxilla. Anterior maxillary osteotomy was carried out using a sharp chisel and mallet. Aggressive surgical debridement was carried out to remove all necrotic tissue and adequate normal saline, and betadine irrigation with hydrogen peroxide was done. No immediate prosthesis was planned due to immunocompromised condition of the patient which would hinder healing. The patient was fed on soft and liquid diet at 3 L/day through a nasogastric tube for 10 days.
Necrosed maxilla was sent to histopathologic examination. It showed thick-walled ribbon-like aseptate hyphae branching at right angles confirming mucormycosis. Postsurgery, follow-up was done, blood glucose levels were well controlled, and the patient was continued on injection AmB 0.5 mg/kg/day for 30 days; the patient recovered well with resolution of signs and symptoms. After achieving optimum blood glucose levels, favorable healing in the midpalatal and posterior palatal area was observed. Interim obturator was fabricated and delivered to the patient [Figure 3]. Regular follow-up for 3 weeks was done. Psychological counseling was done to help the patient cope better with the handicap. She was asked to observe good oral hygiene practices and her family was educated about the importance of maintaining proper sanitation in the surroundings. Her psychological upliftment helped the patient in maintaining good glycemic control and better oral hygiene on follow-up.
Figure 3.
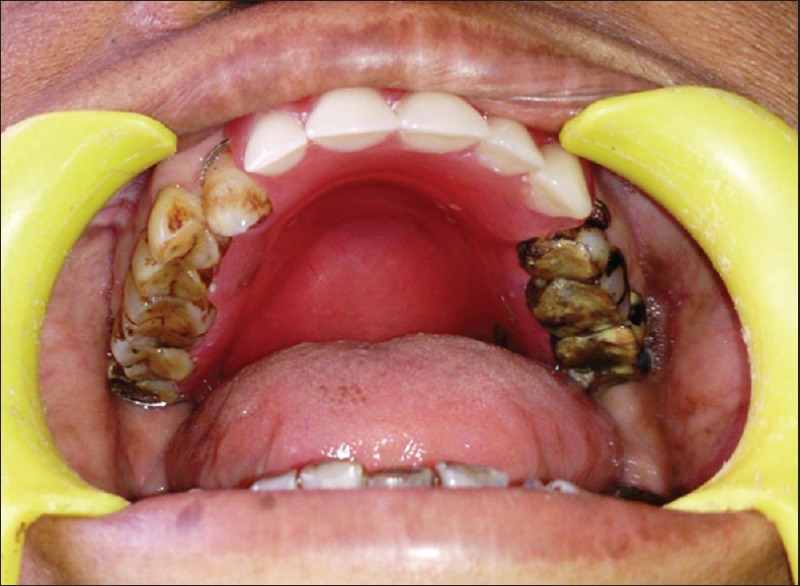
Intraoral view of the patient with obturator in place
The patient appeared normal on 6-month and 1-year follow-up with well-maintained blood glucose levels. An interdisciplinary approach involving medical, surgical, and prosthetic technique helped the patient recover well.
Discussion
Upper airway mucormycosis was first described in 1885 by Paltauf,[1] who coined the term “mycosis mucorina,” which subsequently became mucormycosis. With increasing recognition of the typical features of mucormycosis and development of AmB in 1956, the universal mortality of the disease has been reduced dramatically.[6] Mucormycosis is an acute opportunistic infection caused by saprophytic fungus found in soil, bread mold, decaying vegetation, and animal manure. The patterns of infection can be divided in to rhino-orbital-cerebral, pulmonary, gastrointestinal, cutaneous, renal mucormycosis, isolated central nervous system involvement, or disseminated disease.[2] The involvement of oral cavity is usually secondary to infection of PNSs or nasal cavity and usually presents as palatal necrosis or ulceration.[7]
Myiasis is an infection of tissues or organs of animals by fly larvae. Many species of flies have been reported to cause myiasis in humans. Nasal myiasis is rare in the world, but in the developing and tropical countries like India, it is quite common due to hot and humid climate, poor sanitation, and inadequate hygiene practices. Abundant cases have been reported from India pertaining to this condition.[2,4,5,8,9,10]
Flies are attracted by the foul-smelling nasal discharge emanating from the nose of mucormycosis patients. The female flies oviposit in the wound which within 8–24 h hatch into larvae. In this stage of development, the larvae feed voraciously causing extensive destruction of surrounding tissues resulting in pain and secondary bacterial infection.[11]
The clinical presentation of the patient with myiasis varies. In the initial 3–4 days, maggots produce intense irritation, sneezing, lacrimation, and headache with blood-stained discharge oozing from nostrils. Patients usually present with the complaint of epistaxis, foul-smelling nasal discharge, passage of worms from the nose, and pain. Maggots cause extensive tissue destruction in the nose, sinuses, and soft tissues of face, palate, and orbit. Death may occur due to meningitis.[11,12,13]
The task of correctly identifying the causative species is best done by a trained entomologist. The larvae need to be preserved in 70%–95% ethanol after extracting them from the patient. The specimen is then sent to the laboratory for analysis by the expert.[11] In our case, the maggots could not be identified due to unavailability of the required resources.
Turpentine oil and liquid paraffin nasal drops are said to be effective in managing myiasis.[13,14] Maggots are photophobic and they move into darker crevices making their extraction difficult. Turpentine irritates the maggots and forces them to crawl out while liquid paraffin cuts of the oxygen supply and maggots die of suffocation. The other drugs that can be used to treat mucormycosis are Ivermectin and Tiabendazole.[11]
Asphyxiating agents such as mineral oil and chloroform have been found effective in managing the maggots. The treatment of wound myiasis is usually based on the primary cause of ulceration.
A review of mucormycosis cases noted that diabetes was the most common risk factor, found in 36% of cases, followed by hematological malignancies 17%, solid organ transplantation 7%, deferoxamine therapy 6%, injection drug use 5%, bone marrow transplantation 5%, renal failure 5%, low birth weight infant 3%, diarrhea and malnutrition 3%, HIV infection 2%, and others 1%.[2] Jones et al. reported that mucormycosis can occur in patients with well-controlled diabetes with no underlying immunosuppressant risk factor, and these patients have high survival rate compared to immunocompromised patients.[15]
The mainstay of therapy for mucormycosis is reversal of the source of immunocompromised condition, systemic high dose of antifungal agents, surgical debridement of nonviable tissues, and psychological counseling with prosthetic rehabilitation.
Although AmB deoxycholate remains the classical treatment, the first-line antifungal therapy is now based on lipid formulation of AmB. This liposomal AmB (LAmB) formulation has better penetration into cerebral tissues than any other agents and very effective in rhinocerebral involvement. Furthermore, it is less nephrotoxic than AmB deoxycholate.
Posaconazole may also be effective in mucormycosis patients who are refractory or intolerant to LAmB, including kidney-impaired patients.[16]
Bone grafting was not suggested for the patient for concern of recurrent infection and also for the difficulty associated with differentiating graft failure and/or recurred infection during follow-up period.
Rhino-orbital-maxillary-cerebral mucormycosis has a higher survival rate compared to disseminated pattern. It can be readily diagnosed, and prognosis has been good. However, larval infestation secondary to mucormycosis can socially handicap a patient. Thus, educating the masses regarding importance of public and personal hygiene can save lives. A multidisciplinary management offers a better approach to treat this life-threatening condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr. Jagdish Hosmani, Reader, Department of Oral Pathology, MMDC, Belgaum, and Dr. Vikram Simha Reddy, Consultant Prosthodontist, Bangalore, are gratefully acknowledged.
References
- 1.Paltauf A. Mucormycosis mucorina. Virchows Arch Pathol Anat. 1885;102:543–64. [Google Scholar]
- 2.Ghafur A, Shareek PS, Senthur NP, Vidyalakshmi PR, Ramasubramanian V, Parameswaran A, et al. Mucormycosis in patients without cancer: A case series from A tertiary care hospital in South India. J Assoc Physicians India. 2013;61:305–8. [PubMed] [Google Scholar]
- 3.Caumes E, Carrière J, Guermonprez G, Bricaire F, Danis M, Gentilini M, et al. Dermatoses associated with travel to tropical countries: A prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin Infect Dis. 1995;20:542–8. doi: 10.1093/clinids/20.3.542. [DOI] [PubMed] [Google Scholar]
- 4.Yadav S, Tyagi S, Kumar P, Puri N. Oral myiasis involving palatal mucosa of a young female. J Nat Sci Biol Med. 2014;5:194–7. doi: 10.4103/0976-9668.127327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biradar S, Wankhede P, Munde A, Shaikh S. Extensive myiasis infestation associated with oral squamous cell carcinoma: Report of two cases. Dent Res J (Isfahan) 2015;12:100–5. doi: 10.4103/1735-3327.150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33:349–65. doi: 10.1016/s0030-6665(00)80010-9. [DOI] [PubMed] [Google Scholar]
- 7.Muzyka BC, Epifanio RN. Update on oral fungal infections. Dent Clin North Am. 2013;57:561–81. doi: 10.1016/j.cden.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Pereira T, Tamgadge AP, Chande MS, Bhalerao S, Tamgadge S. Oral myiasis. Contemp Clin Dent. 2010;1:275–6. doi: 10.4103/0976-237X.76401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saravanan T, Mohan MA, Thinakaran M, Ahammed S. Oral myiasis. Indian J Palliat Care. 2015;21:92–4. doi: 10.4103/0973-1075.150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar GV, Sowmya G, Shivananda S. Chrysomya bezziana oral myiasis. J Glob Infect Dis. 2011;3:393–5. doi: 10.4103/0974-777X.91066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79–105. doi: 10.1128/CMR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuruvilla G, Albert RR, Job A, Ranjith VT, Selvakumar P. Pneumocephalus: A rare complication of nasal myiasis. Am J Otolaryngol Head Neck Med Surg. 2006;27:133–5. doi: 10.1016/j.amjoto.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Sinha V, Shah S, Ninama M, Gupta D, Prajapati B, More Y. Nasal Myiasis. J Rhinol. 2006;13:120–3. [Google Scholar]
- 14.Rao GS, Chatra L, Prashanth SK. Oral myiasis: A rare entity. J Maxillofac Oral Surg. 2009;8:398–400. doi: 10.1007/s12663-009-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993;75:455–60. doi: 10.1016/0030-4220(93)90170-9. [DOI] [PubMed] [Google Scholar]
- 16.Rammaert B, Lanternier F, Poirée S, Kania R, Lortholary O. Diabetes and mucormycosis: A complex interplay. Diabetes Metab. 2012;38:193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]


