Abstract
Chemical compositions, trypsin inhibitory activity, and gelling properties of albumen from duck egg during salting of 30 days were studied. As the salting time increased, moisture content decreased, the salt content and surface hydrophobicity increased (p<0.05). Trypsin inhibitory activity and specific activity were continuously decreased throughout the salting time of 30 days (p<0.05). This coincided with the decrease in band intensity of inhibitor with molecular weight of 44 kDa as examined by inhibitory activity staining. Nevertheless, no differences in protein patterns were observed in albumen during the salting of 30 days. Based on texture profile analysis, hardness, springiness, gumminess, chewiness, and resilience of albumen gel decreased with increasing salting time. Conversely, salted albumen gels exhibited higher cohesiveness and adhesiveness, compared to those of fresh albumen. Scanning electron microscopic study revealed that gel of salted albumen showed the larger voids and less compactness. In general, salting lowered trypsin inhibitory activity and gelling property of albumen from duck egg to some extent. Nevertheless, the salted albumen with the remaining inhibitor could be an alternative additive for surimi or other meat products to prevent proteolysis.
Keywords: albumen, duck egg, gelling property, inhibitory activity, salting, trypsin inhibitor
Introduction
Salted egg is a popular traditional product in some Asian countries. To manufacture salted eggs, fresh eggs are soaked in brine solution or coated with salt ash or charcoal (Chi and Tseng, 1998; Yang et al., 2016). Commonly, customers prefer salted duck egg rather than hen counterpart, since its characteristics are better with richer flavor. In general, salted egg yolk is of higher demand than salted albumen, which is commonly discarded. Albumen of salted duck egg contains 4-7% sodium chloride, which is not suitable to apply in food products (Kaewmanee et al., 2011b; Mmadi et al., 2014). To conquer such a problem, desalination process and electrodialysis were used to remove salt in albumen (Huang et al., 1999; Mmadi et al., 2014). Furthermore, fresh duck egg yolk was separated from albumen and directly salted. As a consequence, no salt is contaminated in egg albumen and it can be used as raw material for food products or other applications (Wang, 2016).
Albumen is wellknown with diversity of bioactive compositions and nutrients. Lysozyme has been functionally prescribed as N-acetylmuramoyl hydrolase (Ren et al., 2010). Ovomucoid, ovoinhibitor, and cystatin are considered as protease inhibitors; ovotransferrin, ovoflavoprotein, and avidin are functionally clarified as mineral and vitamin binding agents (Rossi et al., 2013). In general, physicochemical properties of albumen in duck egg underwent changes during salting. Moisture content decreased gradually, whereas salt, ash, protein and lipid contents increased. Moreover, albumen obtained from cooked salted whole duck egg had lower hardness as the salting time increased (Kaewmanee et al., 2011a, 2011b).
Albumen from salted duck egg, considered as waste, could be exploited as food additives, particularly in muscle food gel, in which salt is required for solubilization of proteins. Furthermore, the protease inhibitors remaining in albumen could also serve as additive to prevent proteolysis associated with gel softening (Benjakul and Visessanguan, 2000). Additionally, salted albumen with gel forming ability could co-gel with major myofibrillar proteins during gelation of processed muscle foods. This could help strengthen the resulting gel. Thus, salted albumen could be employed as the additives in surimi or muscle food industry. However, a little information regarding trypsin inhibitor, protein pattern, and gelling properties of liquid duck egg albumen as affected by salting time exists. Therefore, the objective of this study was to investigate the changes of chemical compositions, trypsin inhibitory activity and gelling properties of duck egg albumen separated from salted whole egg during salting of 30 days.
Materials and Methods
Chemicals
All chemicals used in this study were of analytical grade. Na-Benzoyl-DL-arginine-ρ-nitroanilide (BAPNA), trypsin from bovine pancreas (Type I, ~10,000 BAEE units/mg protein), and bovine serum albumin (BSA) were obtained from Sigma Chemical Co. (USA), and high and low molecular protein markers were purchased from GE healthcare UK Limited (UK). Sodium dodecyl sulfate (SDS), β-mercaptoethanol (β-ME), 8-anilino-1-naphthalenesulfonic acid (ANS), glutaraldehyde, ethanol and Coomassie blue R-250 were obtained from Merck (Germany).
Preparation of salted duck egg
Fresh duck eggs within 24 h after laying were collected from a farm in Kantang, Trang province, Thailand. After cleaning with tap water, the eggs were immersed in the brine solution (25%, w/v) using 1 egg/100 mL brine (Kaewmanee et al., 2011b). Brining was performed at room temperature (28-30°C). Ten eggs were randomly taken every 5 days. The samples were broken and albumen was separated from egg yolk manually. Albumen was subsequently subjected to analyses.
Changes in chemical compositions of albumen
Determination of moisture, salt, and protein contents
Moisture and protein contents of albumen samples were determined using oven method (AOAC, 2000) and the Biuret method (Robinson and Hogden, 1940), respectively. Salt content in albumen samples was measured by the AOAC method described as Kaewmanee et al. (2011b).
Determination of surface hydrophobicity
Surface hydrophobicity of albumen samples was determined according to the method of Kaewmanee et al. (2011a) using 8-anilo-1-naphthalenesulfonic acid (ANS) as a probe. Surface hydrophobicity was calculated from the initial slope of the plot of fluorescence intensity against protein concentration determined by the Biuret method using a linear regression analysis. The initial slope was referred to as surface hydrophobicity (S0ANS).
Determination of trypsin inhibitory activity
Trypsin inhibitory activity was measured as per the method of Benjakul et al. (2001). Albumen samples with an appropriate dilution (200 µL) were incubated with 200 µL of porcine pancreas trypsin (0.05 mg/mL) at 37°C for 15 min. Then, 1000 µL of reaction buffer (50 mM Tris-HCl containing 20 mM CaCl2, pH 8.2) were added. Thereafter, 200 µL of BAPNA (2 mg/mL) were added and the mixture was incubated at 37°C for 15 min. To terminate the reaction, 200 µL of 30% acetic acid (v/v) was added. The release of ρ-nitroaniline was monitored by measuring the absorbance at 410 nm using a spectrophotometer (UV-16001, SHIMADZU, Japan). One unit of trypsin activity was defined as the enzyme causing an increase of 0.01 absorbance unit/min under the assay condition. One unit of trypsin inhibitory activity was defined as the amount of inhibitor, which reduced trypsin activity by one unit. At the sampling times, trypsin inhibitor was expressed as kunits/mg solids. Specific activity was also calculated and reported as kunits/mg protein.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE)
Protein patterns of albumen samples were determined by SDS-PAGE according to the method of Laemmli (1970). To prepare protein sample, 2 mL of albumen were mixed with 12 mL of 5% SDS. The prepared sample (15 µg protein) was loaded onto the gel (12% running gel and 4% stacking gel) and subjected to electrophoresis at constant current of 15 mA/gel using electrophoresis unit (Mini-protein II; Bio-Rad Laboratories, USA). The gels were stained with Coomassie Brilliant Blue R-125 (0.125%) in 25% methanol and 10% acetic acid. Destaining was performed using 40% methanol and 10% acetic acid. Molecular weight (MW) of protein bands was estimated from the plot of MW standards and Rf.
Inhibitory activity staining
Inhibitory activity staining was conducted according to the method of Benjakul and Visessanguan (2000). Albumen samples were mixed with the sample buffer without β-ME. Samples without heat treatment (15 µg) were applied onto two identical gels. The proteins were separated via electrophoresis as previously described. To reduce the heat generated, the electrophoresis unit was embedded in crushed ice. One gel was fixed and stained for total proteins with Coomassie Blue R-250 and used as the control gel. Another gel was washed in 2.5% Triton X-100 for 15 min to remove SDS and then washed in distilled water. The gel was immersed in 50 mL of 0.2 mg/mL trypsin in 50 mM Tris buffer, pH 8.2, containing 20 mM CaCl2. The gel was incubated for 30 min at 0-4°C and 60 min at room temperature to allow the trypsin to diffuse into the gels. Gel was washed with distilled water and incubated for 90 min at 37°C in 10 mg/mL casein solution prepared in 50 mM Tris buffer, pH 8.2. The gels were rinsed with distilled water, fixed and stained with Coomassie Blue R-250 to develop inhibitory zones, detected as dark bands on a clear background. The apparent molecular weights of trypsin inhibitors were estimated from the control gels by comparing migration rates with those of protein standards.
Determination of gel properties
Preparation of gel
Gels of albumen samples were prepared following the method of Mmadi et al. (2014) with a slight modification. Albumen solutions (10% solid) were prepared using distilled water as diluent. The mixture was stirred gently. Then, the solution was poured into a casing (diameter of 25 mm). Both ends were sealed tightly and heated at 90°C for 30 min. Thereafter, the gels were cooled immediately at 4°C and kept overnight. Finally, gel samples were cut into cylinders (diameter 25 mm, height 30 mm) prior to analyses.
Texture profile analysis
Texture profile analysis of gels was performed using a texture analyzer (Model TA-XT2i, Stable Micro System, England). The samples (diameter 25 mm, height 30 mm) were compressed twice to 40% of their original height with a compression cylindrical aluminum probe (50 mm diameter). Force-distance deformation curve was recorded at a cross head speed of 3 mm/s and the recording speed was 3 mm/s. Hardness, adhesiveness, springiness, cohesiveness, resilience, chewiness, and gumminess were evaluated using the MicroStable software version 6 (England).
Determination of color
The color of gel samples was determined by a colorimeter (ColorFlex, Hunter Lab Reston, USA) and reported in CIE system. L*, a*, b* and ΔE*, representing lightness, redness/greenness, yellowness/blueness and total difference of color, respectively, were recorded.
The whiteness of gels was calculated (Kaewmanee et al., 2011b) using the following equation:
The ΔE* was also calculated by the following formula:
where ΔL*, Δa*, Δb* are the difference between color parameters of the samples and those of the white standard (L* = 92.82, a* = −1.24, b* = 0.50).
Determination of microstructure
Microstructure of albumen gels with the salting time of 0, 15, and 30 days was examined by a scanning electron microscopy as described by Kaewmanee et al. (2011b). Albumen gels were cut into small pieces ((1x1x1 mm3). Samples were fixed with 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 12 h. Then, fixed samples were rinsed with distilled water for 1 h. Subsequently, samples were dehydrated sequentially using ethanol with various concentrations (25, 50, 70, 80, 90, and 100%) for 15 min at each concentration. The dehydrated samples were subjected to critical point drying (CPD). The samples were coated with 100% gold (sputter coater SPI-Module, USA). The gel microstructure was visualized by a scanning electron microscope (JEOL JSM-5800LV, Japan).
Statistical analysis
All the experiments were conducted in triplicate using three lots of samples. Data were presented as mean value with standard deviation. One way variance of analysis (ANOVA) was performed. The Duncan’s multiple range test was carried out to determine the significant difference between samples at p<0.05 level using the statistical program (SPSS 11.0 for Windows, SPSS Inc., USA).
Results and Discussion
Effect of salting time on chemical compositions of duck egg albumen
Moisture and salt contents
Moisture and salt contents of albumen from duck egg during salting of 30 days are shown in Table 1. Moisture content decreased continuously during salting (p<0.05). After 30 days, the moisture content was decreased to 81.20%. The decrease in moisture content was coincidental with the increase in salt content of albumen. Salt content generally increased as salting time increased (p<0.05) (Table 1). The salt content of freshly laid duck egg albumen increased dramatically from 0.31% up to 6.19% after 30 days of salting. This indicated the migration of salt into albumen during salting. The loss of water from albumen through shell membrane and pore to the outside took place, mainly caused by the osmosis process. This was mediated by a difference in osmotic pressure between albumen and brine. Chi and Tseng (1998) reported that rate of water migration from albumen was also governed by pore sizes and structure of the shell. Although high salt content in albumen might induce water migration from egg yolk, the amount of water loss from albumen to outside was higher (Kaewmanee et al., 2011b). As a consequence, the decrease in moisture content in albumen was noticeable.
Table 1. Changes in moisture and salt contents, color, and whiteness of albumen gels from duck egg during salting of 30 days.
| Salting time (days) | Moisture content (%) | Salt content (%) | CIE L* | CIE a* | CIE b* | △E* | Whiteness |
|---|---|---|---|---|---|---|---|
| 0 | 87.93 ± 0.18*a | 0.31 ± 0.07f | 110.47 ± 4.52a | −3.44 ± 0.02e | −3.40 ± 0.16e | 19.90 ± 1.96a | 86.23 ± 2.14c |
| 5 | 86.68 ± 0.45b | 2.07 ± 0.08e | 94.62 ± 0.14b | −1.63 ± 0.04d | −0.36 ± 0.04d | 2.03 ± 0.10b | 94.36 ± 0.10a |
| 10 | 85.55 ± 0.10c | 3.42 ± 0.01d | 93.87 ± 0.14bc | −1.54 ± 0.02c | 0.16 ± 0.01c | 1.19 ± 0.12bc | 93.65 ± 0.16ab |
| 15 | 84.61 ± 0.04d | 5.10 ± 0.08c | 93.71 ± 0.20bc | −1.08 ± 0.04b | 0.25 ± 0.02c | 0.95 ± 0.06bc | 93.61 ± 0.20ab |
| 20 | 83.64 ± 0.11e | 6.19 ± 0.00b | 93.44 ± 0.21bc | −1.04 ± 0.06b | 0.55 ± 0.01b | 0.72 ± 0.07c | 93.36 ± 0.11ab |
| 25 | 82.36 ± 0.22f | 7.78 ± 0.01a | 92.62 ± 0.18c | −1.00 ± 0.11a | 0.81 ± 0.02a | 0.45 ± 0.02c | 92.63 ± 0.13b |
| 30 | 81.20 ± 0.26g | 8.06 ± 0.09a | 92.56 ± 0.02c | −1.01 ± 0.43a | 0.80 ± 0.01a | 0.46 ± 0.01c | 92.45 ± 0.02b |
*Mean ± SD (n=3).
Different superscripts in the same column indicate significant differences during salting time (p<0.05).
Surface hydrophobicity
Surface hydrophobicity of duck albumen during 30 days of salting was monitored. The gradual increase in surface hydrophobicity was observed within the first 20 days of salting, in which S0ANS increased from 735.04 to 821.45 at day 20 (data not shown). Surface hydrophobicity of a protein is useful in understanding and predicting the effects of manipulation of the sequence of structural or functional protein domains. The number and the relative size of hydrophobic sites on a protein's surface usually dictates its solubility and propensity to aggregate under physiological conditions of pH, temperature, and ionic strength (Cardamone and Puri, 1992). Ji et al. (2013) also found that the surface hydrophobicity of duck albumen was increased after 4 days of salting. The increase in S0ANS indicated protein conformational change induced by salting. Hydrophobic domains originally buried in the protein core were exposed, thus more readily available for binding with ANS (Huang et al., 1999; Ji et al., 2013). Nevertheless, the decrease in S0ANS was noticeable at day 30 (538.84). At high salt content with lower moisture content, the interaction of hydrophobic domains might be enhanced. Consequently, those hydrophobic residues were embedded inside the aggregates (Kaewmanee et al., 2011a) as shown by the lowered S0ANS.
Trypsin inhibitory activity
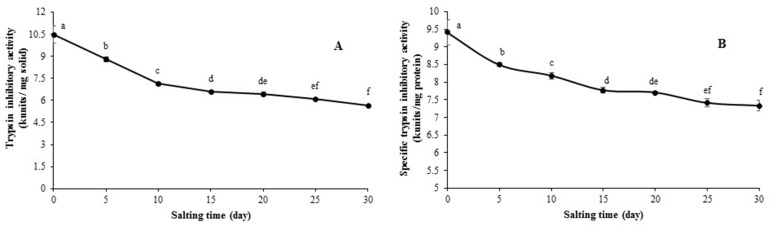
The changes in trypsin inhibitory activity and specific trypsin inhibitory activity in albumen from duck egg as a function of salting time are depicted in Fig. 1. Trypsin inhibitory activity in freshly laid duck egg decreased continuously within the first 15 days of salting, in which 63% of activity was retained (Fig. 1(A)). The lowest inhibitory activity was found at 30 days of salting (5.68 kunits/mg solid). The similar result was also observed for specific trypsin inhibitory activity, which was decreased with increasing salting time (p<0.05) (Fig. 1(B)). After 30 days of salting, the specific inhibitory activity was reduced to 7.77 kunits/mg protein from 9.41 kunits/mg protein detected in freshly laid egg. The decrease in trypsin inhibitory activity was coincidental with the increase in salt content in salted albumen. During salting, salt at high concentration in duck egg albumen more likely altered the conformation of proteins including trypsin inhibitors (Huang et al., 1999). Thus, the denaturation of trypsin inhibitors might be associated with the loss of their bioactivity. It can be postulated that the reduction in antiprotease activity correlates with the deterioration of some pivotal proteins such as ovomucoid and ovoinhibitor. Those proteins might undergo denaturation, thus having the lower antiprotease activity under the high salt environment (Qiu et al., 2012). However, the remaining trypsin inhibitor in salted albumen could play a role in controlling proteolysis in surimi or other meat mediated by endogenous protease.
Fig. 1. Trypsin inhibitory (A) and specific inhibitory (B) activities of albumen during salting of 30 days. Bars represent the standard deviation (n=3). Different lowercase letters on the bars indicate significant differences (p<0.05).
Protein patterns
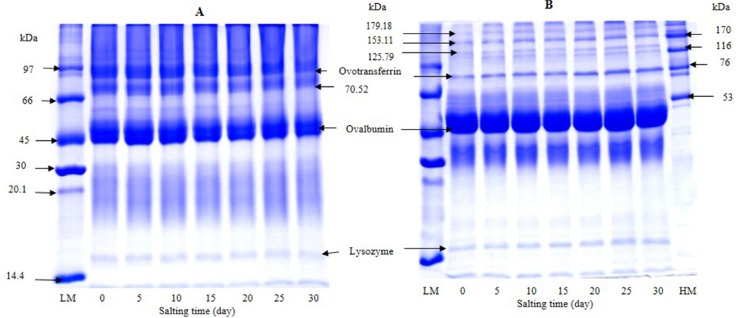
SDS-PAGE patterns of albumen from duck egg under reducing and non-reducing condition during salting of 30 days are illustrated in Fig. 2. The most dominant protein was ovalbumin with MW of 44 kDa. Under non-reducing condition, proteins with MW of 81, 70, and 16 kDa were observed. Proteins with MW of 81 and 16 kDa were more likely ovotransferrin and lysozyme, respectively. Ovalbumin (54%) and ovotransferrin (12%) are considered as the main proteins found in egg white (Abeyrathne et al., 2013). Hu et al. (2016) found that a large number of the identified proteins from duck albumen were belonging to the ovalbumin family using 2-DE gel and MALDI-TOF MS/MS analysis. Those included ovalbumin (OVA), ovalbumin-related protein Y (OVAY), and ovalbumin related protein X (OVAX). Under reducing condition, proteins with MW of 50, 72, and 15 kDa were dominant and considered as ovalbumin, ovotransferrin and lysozyme, respectively. Nevertheless, some bands were also observed. Those having MW of 179, 153, 125 kDa might be the subunits of egg albumen proteins stabilized by disulfide bonds. In general, no change in protein patterns of albumen was noticeable as the salting time increased up to day 15. In accordance with the results of Ji et al. (2013) and Kaewmanee et al. (2009), similar protein patterns were found in salted duck egg albumen during 2 weeks of salting. It was reported that the amount of precipitated protein increased with salting time (Huang et al., 1999). Those aggregates might be stabilized by weak bonds such as ionic interaction, etc., which could be destroyed by SDS used for electrophoresis. As a consequence, no differences in protein patterns were observed.
Fig. 2. SDS-PAGE patterns of albumen from duck egg during salting of 30 days under non-reducing (A) and reducing (B) conditions. LM: low molecular weight standard; HW: high molecular weight standard.
Inhibitory activity staining
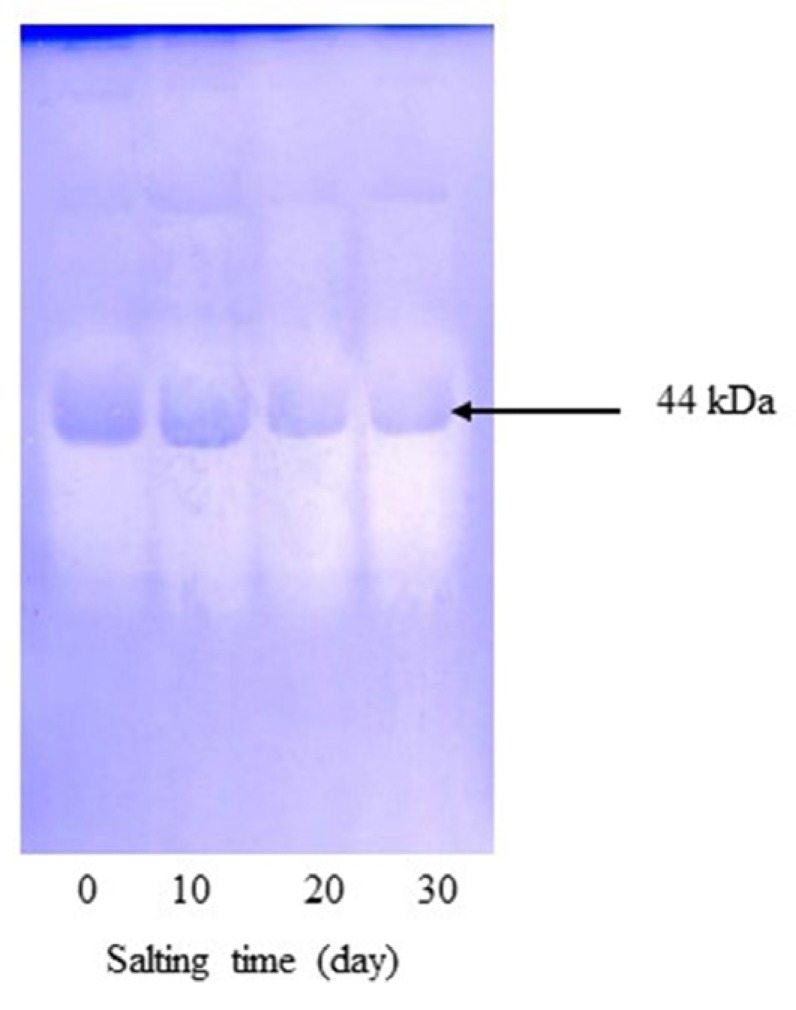
Based on inhibitory activity staining, only one band with MW of 44 kDa was retained (Fig. 3). This protein was plausibly ovalbumin or other proteins showing the similar MW to ovalbumin, which had inhibitory activity toward trypsin. Ovalbumin is known as a member of serpin family and shares sequence homology with α1-protease inhibitor, antithrombin III and angiotensinogen (Saxena and Tayyab, 1997). Takenawa et al. (2015) also showed that ovalbumin had ability to inhibit casein degradation induced by trypsin. It was noted that band of lysozyme disappeared after being subjected to inhibitory activity staining. The result indicated that lysozyme was hydrolyzed by trypsin completely. Lysozyme has been reported to exhibit an antibacterial activity against a number of food spoilage bacteria and pathogen (Cegielska-Radziejewska et al., 2008). Hu et al. (2016) also reported that ovoinhibitor, ovomucoid, clusterin, ex-FABP, and PG D2 synthase were not detected from duck albumen by 2-dimensional polyacrylamide gel electrophoresis (2-DE). Ovomucoid and ovoinhibitor are found in hen albumen, which are well known as protease inhibitors and are considered to be the main food allergen in egg albumen (Abeyrathne et al., 2015). It was noted that ovomucoid and ovoinhibitor were not detected by inhibitory activity staining. Those inhibitors might lose their inhibitor activity in the presence of SDS used for electrophoresis. As a result, the bands were not retained after trypsin digestion. After 20 days of salting, the band of protein with MW of 44 kDa became less intense than that of freshly laid egg and 10-day salting. It was suggested that protein with inhibitory activity lost in some activity with extended salting time. This might be due to the conformational change of inhibitory proteins induced by high salt concentration. This result was in agreement with the decrease in trypsin inhibitory activity of duck albumen during salting time (Fig. 1). Thus, salting time had the marked impact on the reduction of trypsin inhibitory activity of duck egg albumen.
Fig. 3. Trypsin inhibitory activity staining of salted duck egg albumen during salting under non-reducing condition. The numbers denot the salting time (day).
Effect of salting time on gelling properties of albumen
Texture profile analysis
Texture profile of albumen gel from duck egg during salting of 30 days is presented in Table 2. Hardness of albumen gel decreased with increasing salting time (p<0.05). The highest hardness was found in the gel from freshly laid egg albumen (19.08 N), whereas the lowest value (3.77 N) was obtained in the sample after 30 days of salting (p<0.05). After 5 days of salting, hardness of gel was reduced by 50%, in which the value of 9.26 N was attained. Hardness is related to the strength of gel structure under compression and is the peak force during the first compression cycle (Chandra and Shamasundar, 2015). The increased salt content of albumen plausibly caused the aggregation of albumen proteins, which was associated with a coarser structure and a weakened gel network (Huang et al., 1999). Salts tend to enhance aggregation of egg white proteins, resulting in weaker gels (Woodward, 1990).
Table 2. Changes in texture profile of albumen gels from duck egg during salting of 30 days.
| Salting time (day) | Hardness (N) | Cohesiveness | Adhesiveness (N.s) | Springiness (cm) | Gumminess (N) | Chewiness (N.cm) | Resilience |
|---|---|---|---|---|---|---|---|
| 0 | 19.08 ± 0.54*a | 0.72 ± 0.01d | −0.66 ± 0.01e | 0.92 ± 0.01a | 13.67 ± 0.29a | 12.64 ± 0.45a | 0.41 ± 0.00a |
| 5 | 9.26 ± 0.21b | 0.74 ± 0.00b | −0.61 ± 0.01d | 0.89 ± 0.01b | 6.64 ± 0.13b | 6.10 ± 0.11b | 0.39 ± 0.00b |
| 10 | 6.02 ± 0.20c | 0.75 ± 0.00a | −0.58 ± 0.02c | 0.87 ± 0.01c | 4.48 ± 0.10c | 3.93 ± 0.09c | 0.38 ± 0.00c |
| 15 | 4.74 ± 0.13d | 0.75 ± 0.00a | −0.47 ± 0.02b | 0.86 ± 0.01d | 3.54 ± 0.09d | 3.06 ± 0.09d | 0.38 ± 0.00d |
| 20 | 4.01 ± 0.05e | 0.73 ± 0.01c | −0.46 ± 0.01b | 0.85 ± 0.00d | 2.88 ± 0.06e | 2.49 ± 0.04e | 0.36 ± 0.00e |
| 25 | 3.81 ± 0.09e | 0.73 ± 0.00c | −0.37 ± 0.04a | 0.85 ± 0.01d | 2.79 ± 0.08e | 2.36 ± 0.06e | 0.36 ± 0.00e |
| 30 | 3.77 ± 0.10f | 0.72 ± 0.00c | −0.34 ± 0.02a | 0.82 ± 0.01e | 2.48 ± 0.07f | 2.23 ± 0.05f | 0.36 ± 0.00f |
*Mean ± SD (n=3).
Different superscripts in the same column indicate significant differences (p<0.05).
Cohesiveness of salted albumen gel increased gradually up to 15 days of salting (p<0.05) (Table 2) and reached the highest value of 0.75. The decrease in cohesiveness was observed for the rest of salting time (p<0.05). Cohesiveness is a parameter to measure the difficulty level in breaking down the internal structure of gel (Lau et al., 2000). Springiness of albumen gel decreased as salting time increased (p<0.05). Springiness is related to the height by which gel recovers during the end of the first bite and the start of the second bite. If springiness is high, it requires more mastication energy in the mouth (Chandra and Shamasundar, 2015). The decreased springiness was concomitant with the decreases in hardness. For adhesiveness of albumen gels, it increased drastically during salting (p<0.05). The freshly laid egg gel had the value of -0.66 and reached the highest value of -0.34 after 30 days of salting. Adhesiveness is defined as the negative force area for the first bite and represents the work required to overcome the attractive forces between the surface of a food and the surface of other materials (Chandra and Shamasundar, 2015).
Gumminess, chewiness, and resilience of gels from duck egg during salting are shown in Table 2. In general, these values decreased continuously as a function of salting time (p<0.05). The changes in gumminess, chewiness, and resilience were in correlation with those of hardness. Gumminess and chewiness were calculated based on hardness, which suggests resistance to compression force (Yilmaz et al., 2012). Resilience is a parameter of how the sample recovers from deformation both in terms of speed and force (Chandra and Shamasundar, 2015). Those changes were mainly mediated by the increasing salt environment. Therefore, gels of albumen had the change in textural characteristics as induced by salting. The change was more pronounced with increasing salting time.
Color
Changes in color of albumen gel from duck eggs during salting of 30 days are shown in Table 1. The highest lightness (L*- value) of gels was found in freshly laid duck egg albumen. L*- value was decreased after salting (p<0.05). There was no difference in L*- value between gels from albumen obtained from duck egg salted for 10-30 days (p>0.05). The decrease in lightness was probably caused by the formation of large coagulate with less surface area, resulting in the decreased light scattering (Kaewmanee et al., 2011b). The whiteness of all albumen gels obtained from salted eggs was higher than that of fresh egg. The increases in both a*- value and b*- value of albumen gel were observed throughout the salting period of 30 days (p<0.05). The values of a* and b* indicate redness/greenness and yellowness/blueness, respectively. Kaewmanee et al. (2011b) reported that the increase in redness and yellowness of salted albumen gel with increasing salting time was due to pigments located at the outer layer of yolk, which might be contaminated into egg white to some extent. Salting process might soften yolk membrane, thereby facilitating the migration of pigment from yolk to albumen. For ΔE*, the decrease in value was obtained for albumen gel from salted egg. The decrease in ΔE* was coincidental with the increase in whiteness throughout the salting period studied. Thus, salting also affected the color of albumen gel to some degree, apart from textural property (Table 2).
Microstructure
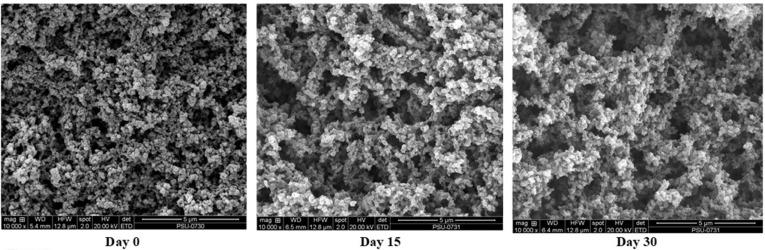
Scanning electron micrographs of albumen gel from duck eggs after salting for 15 and 30 days, in comparison with gel from fresh egg are illustrated in Fig. 4. Albumen gel from freshly laid duck egg gels had the denser network with the smaller voids, compared with those of salted duck egg. For salted albumen gel, the larger voids were observed. After 15 days of salting, the gel network became coarser with less uniformity. With increasing salting time up to 30 days, no marked difference was noted in comparison with that of 15-day salted egg. However, due to the lower protein concentration and higher salt content in salted albumen, especially after 30 days of salting, the interconnection of proteins became less. This was concomitant with the lower strength of protein network (Table 2). Egg white protein at high concentration was easily gelled by heat treatment (Iwashita et al., 2015). The lower hardness of albumen from egg salted for 30 days might be reflected by the coarser network with less protein content. The lower protein content in the salted egg, especially for longer salting time, was mostly associated with the dilution effect by salt and the lower moisture content. In the presence of sodium chloride, the coagulation was induced. During heating, those coagulums underwent interconnection, leading to the formation of coagulum type gel (Kaewmanee et al., 2011b). The result revealed that the salting time had the significant impact on gel structure of albumen from salted duck egg.
Fig. 4. Scanning electron microscopic photograph of albumen gel from fresh duck egg and egg salted for 15 and 30 days. Magnification: 10000X. Scale bar = 5 µm.
Conclusions
Gelling property and chemical composition of albumen from duck egg, especially protease inhibitor, were affected by salting time. Salting could reduce trypsin inhibitory activity in duck albumen to some degree. Protein with MW of 44 kDa acted as trypsin inhibitor in duck egg albumen. Gel became weaker with the increased whiteness as salting time increased. Due to the remaining gelling property and trypsin inhibitors of albumen from salted duck egg, the salted albumen could be applied in surimi or other meat products to improve their properties via strengthening the gel and inhibiting the proteolysis.
Acknowledgments
This work was supported by the Higher Education Research Promotion and the Thailand’s Education Hub for Southern Region of ASEAN Countries Project Office of the Higher Education Commission scholarship. The TRF Distinguished Research Professor Grant was also acknowledged.
References
- Abeyrathne E, Lee H, Jo C, Suh J, Ahn D. Enzymatic hydrolysis of ovomucoid and the functional properties of its hydrolysates. Poultry Sci. 2015;94:2280–2287. doi: 10.3382/ps/pev196. [DOI] [PubMed] [Google Scholar]
- Abeyrathne EDNS, Lee HY, Ahn DU. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Poultry Sci. 2013;92:3292–3299. doi: 10.3382/ps.2013-03391. [DOI] [PubMed] [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International. Gaithersburg, MD, USA: 2000. [Google Scholar]
- Benjakul S, Visessanguan W. Pig plasma protein: potential use as proteinase inhibitor for surimi manufacture; inhibitory activity and the active components. J Sci Food Agric. 2000;80:1351–1356. doi: 10.1002/1097-0010(200007)80:9<1351::AID-JSFA647>3.0.CO;2-I. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Srivilai C. Porcine plasma protein as proteinase inhibitor in bigeye snapper (Priacanthus Tayenus) muscle and surimi. J Sci Food Agric. 2001;81:1039–1046. doi: 10.1002/jsfa.887. [DOI] [Google Scholar]
- Cardamone M, Puri N. Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem J. 1992;282:589–593. doi: 10.1042/bj2820589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska-Radziejewska R, Lesnierowski G, Kijowski J. Properties and application of egg white lysozyme and its modified preparations-a review. Pol J Food Nutr. 2008;58:5–10. [Google Scholar]
- Chandra M, Shamasundar B. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int J Food Prop. 2015;18:572–584. doi: 10.1080/10942912.2013.845787. [DOI] [Google Scholar]
- Chi SP, Tseng KH. Physicochemical properties of salted pickled yolks from duck and chicken eggs. J Food Sci. 1998;63:27–30. doi: 10.1111/j.1365-2621.1998.tb15668.x. [DOI] [Google Scholar]
- Hu S, Qiu N, Liu Y, Zhao H, Gao D, Song R, Ma M. Identification and comparative proteomic study of quail and duck egg white protein using 2-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry analysis. Poultry Sci. 2016;95:1137–1144. doi: 10.3382/ps/pew033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JJ, Tsai JS, Pan BS. Pickling time and electrodialysis affects functional properties of salted duck egg white. J Food Biochem. 1999;23:607–618. doi: 10.1111/j.1745-4514.1999.tb00589.x. [DOI] [Google Scholar]
- Iwashita K, Inoue N, Handa A, , Shiraki K. Thermal aggregation of hen egg white proteins in the presence of salts. Protein J. 2015;34:212–219. doi: 10.1007/s10930-015-9612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Liu H, Cao C, Liu P, Wang H, Wang H. Chemical and structural changes in preserved white egg during pickled by vacuum technology. Food Sci Technol Int. 2013;19:123–131. doi: 10.1177/1082013212442186. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem. 2009;112:560–569. doi: 10.1016/j.foodchem.2008.06.011. [DOI] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Effect of NaCl on thermal aggregation of egg white proteins from duck egg. Food Chem. 2011a;125:706–712. doi: 10.1016/j.foodchem.2010.09.072. [DOI] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Effects of salting processes and time on the chemical composition, textural properties, and microstructure of cooked duck egg. J Food Sci. 2011b;76:139–147. doi: 10.1111/j.1750-3841.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau M, Tang J, Paulson A. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res Int. 2000;33:665–671. doi: 10.1016/S0963-9969(00)00111-3. [DOI] [Google Scholar]
- Mmadi M, Amza T, Wang YC, Zhang M. Effect of desalination on physicochemical and functional properties of duck (Anas Plotyrhyncus) egg whites. Adv J Food Sci Technol. 2014;6:784–791. doi: 10.19026/ajfst.6.111. [DOI] [Google Scholar]
- Qiu N, Ma M, Zhao L, Liu W, Li Y, Mine Y. Comparative proteomic analysis of egg white proteins under various storage temperatures. J Agric Food Chem. 2012;60:7746–7753. doi: 10.1021/jf302100m. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wu J, Renema R. Nutritional and health attributes of eggs In: Guerrero-Legarreta I., editor. Handbook of Poultry Science and Technology. John Wiley & Sons Inc; Hoboken, New Jersey: 2010. pp. 533–578. [Google Scholar]
- Robinson HW, Hogden CG. The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J Biol Chem. 1940;135:707–725. [Google Scholar]
- Rossi M, Nys Y, Anton M, Bain M, De Ketelaere B, De Reu K, Dunn I, Gautron J, Hammershøj M, Hidalgo A. Developments in understanding and assessment of egg and egg product quality over the last century. World Poultry Sci J. 2013;69:414–429. doi: 10.1017/S0043933913000408. [DOI] [Google Scholar]
- Saxena I, Tayyab S. Protein proteinase inhibitors from avian egg whites. Cell Mol Life Sci. 1997;53:13–23. doi: 10.1007/PL00000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Takahashi K, Le-Chang S, Okazaki E, Osako K. The effect of ovalbumin on the protease activity; Int. Symposium Aqua. Product Process; Bogor, Indonesia. 2015. pp. 39–41. [Google Scholar]
- Wang TH. Salting yolks directly using fresh duck egg yolks with salt and maltodextrin. J Poult Sci. 2016;54:97–102. doi: 10.2141/jpsa.0160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SA. Egg protein gels In: Harris P., editor. Food gels. Springer; Netherlands: 1990. pp. 175–199. [Google Scholar]
- Yang N, Jin Y, Xu Y, Bin Y, Xu X. Effect of pressure cooking on physicochemical properties of salted eggs. RSC Adv. 2016;6:97089–97095. doi: 10.1039/C6RA18737D. [DOI] [Google Scholar]
- Yilmaz MT, Karaman S, Dogan M, Yetim H, Kayacier A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis (TPA) parameters. J Food Eng. 2012;108:327–336. doi: 10.1016/j.jfoodeng.2011.08.005. [DOI] [Google Scholar]