Abstract
Recently, research on the processing of raw functional materials with the aim of improving various physiological activities has been conducted. In this study, we investigated the antioxidant activity of royal jelly (RJ) hydrolysates obtained from three commercial proteases. Enzyme-treated royal jelly (ERJ), in which the RJ hydrolysates were converted into easy-to-absorb shorter chain monomers through the removal of two known allergen proteins, showed no difference in the content of (E)-10-hydroxydec-2-enoicacid (10-HDA) or the freshness parameter and showed a significant increase in total free amino acid content. The antioxidant activity of ERJ was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and chemical assays. The ERJ showed about 80% DPPH-radical scavenging activity at same concentration of ascorbic acid. The antioxidant effect of ERJ was confirmed to be due to reduction of intracellular reactive oxidative species (ROS) and nitric oxide (NO) production in LPS-treated macrophages. Moreover, ERJ significantly increased the activity of the antioxidant enzyme superoxide dismutase (SOD) and the level of the antioxidant glutathione (GSH) in a dose-dependent manner. Interestingly, these antioxidant activities of ERJ were stronger than those of non-treated RJ. These findings indicate that ERJ has high potential as an antioxidant agent for use in human and animal diets.
Keywords: antioxidation, enzyme, hydrolysates, royal jelly
Introduction
Researches have been conducted to develop functional raw materials to improve physiological activity. For example, enzymatic treatments have been proven to be effective for improving the quality of certain existing raw materials by modifying their structure or redistributing their composition (Lebesi and Tzia 2012; Liu et al., 2017; Ma and Mu 2016; Santala et al., 2014). It has been proven that reduction in raw material particle size not only alters structural characteristics, but also improves technological properties when using a raw material in food development (Chen et al., 2013; Raghavendra et al., 2006). Enzymatic hydrolysates possess many biological activities as potential physiological modulators. They usually contain various amino acid residues, and the activities are based on amino acid composition. Recently, studies have reported their possible utility as alternative antioxidants (Saiga et al., 2003).
Antioxidant is a broad term defined as any substance that significantly delays or inhibits oxidation of a substrate. Antioxidant agents protect living organisms from oxidative damage, resulting in the prevention of various diseases, such as cancer, cardiovascular diseases, and diabetes (Napolitano et al., 2006). Therefore, the existence of protective agents against free radicals and other oxidants in living cells is very important. It is becoming important to identify antioxidants from natural sources by modify technique.
RJ is a traditional product commonly used as a supplement to protect against diseases. Royal jelly is made by young worker bees and is the sole food for young larvae and queen bee. Royal jelly is composed of important compounds with biological activities, such as free amino acids, proteins, sugars, fatty acids, minerals, and vitamins. Also it contains physiologically active substances such as 10-hydroxy-2-decenoic acid (10-HAD) (Suguru Fujiwara et al., 1990). It has been reported to have functional effects, such as activation of the autonomic nervous system, antibacterial activity, prevention and alleviation of circulatory diseases, anti-inflammatory and anti-cancer effects (Guo et al., 2007; Sver et al., 1996). Above all, RJ’s antioxidant functions have been suggested to have an excellent effect (Nagaia et al., 2001). However, RJ contains two proteins, one is 47 kDa and the other is 55 kDa, which are considered to be major allergens (Rosmilah et al., 2008). The patients with sensitivity to RJ showed high serum IgE level. Therefore, many studies have been conducted to solve these problems and to make RJ easier to ingest (Kim et al., 2013; Yoon et al., 2014).
In this study, RJ was converted into an easy-to-absorb shorter chain monomer using three commercial protease enzymes. The objective of this study was to prepare ERJ hydrolysates using protease enzymes and to evaluate the antioxidant effect of ERJ on peritoneal macrophages obtained from mice.
Materials and Methods
Preparation of enzyme-treated RJ
Freeze-dried RJ powder was supplied from Zhejiang Jiangshan Bee Enterprise CO., LTD (Jiangshan, China). Proteolytic enzymes, including endo-protease obtained from cultures of Bacillus licheniformis, and endo- and exo-protease obtained from cultures of Aspergilus oryzae, were purchased from Vision Biochem Co., Ltd (Gyeongsan, Korea). RJ powder was mixed with purified water at a ratio of 1:10. The mixture was added endo- and exo-proteases, and mixed well in 380 mmHg for respective 3 h at 55-58℃, 45-50℃, 50-55℃ and for 18 h at 55℃ without measuring the pH value. Finally, it was heat-treated at 85-95℃ for 15 min to inactivate the enzymes, followed by drying to effect pulverization.
The test solution of 10-HDA, a standard component of RJ, was prepared as follows. 1 g of ERJ powder was added to 50 mL of distilled water, reacted at 50℃ for 1 h. And then the mixture was added 350 mL of methanol, mixed using ultrasonic waves for 30 min. The supernatant obtained by centrifugation (12,000 rpm, 10 min) with addition of 100 mL of methanol was filtered through a 0.2 μm syringe filter (Adventec, Tokyo, Japan) and used as a test solution. 10-HDA standards were diluted with methanol to 100, 10, or 1 μg/ml, respectively, and the same amount was injected to prepare a standard curve. HPLC (Dionex Ultimate 3000, Thermo Scientific, Milan, Italy) analysis was carried out on a Zorbax Extend-C18 Column.
For measuring total free amino acid content, 1 g of ERJ powder was diluted 10-fold in distilled water, treated in a water bath at 100℃ for 1 h, centrifuged to extract the supernatant and used as a test solution. Standards were prepared by diluting 17 different kinds of amino acid solutions (Pickering Laboratories, Inc., Mountain View, CA, USA) with 0.1 N HCl. The same amount of each standard solution was injected to prepare a standard curve. HPLC (Dionex Ultimate 3000, Thermo Scientific, Milan, Italy) analysis was carried out using a cation ion exchange column.
SDS-PAGE & Coomassie blue staining
After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), coomassie blue staining (Wang et al., 2006) was carried out for observing changes in allergic proteins. Quantification of the total protein was performed with BCA protein assay reagents (Bio-Rad Laboratories, Hercules, CA, USA). After resolving the proteins with SDS-PAGE (10% acrylamide gels), the gel was stained with Coomassie brilliant blue G-250 (Sigma, St. Louis, MO, USA).
DPPH assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay was performed as previously reported (Fan et al., 2014). Each sample was diluted to 1 mg/ml, and ascorbic acid was used as a positive control. The absorbance was measured at 517 nm with a microplate reader (BioTek, Winooski, VT, USA).
Cell viability assay
A cell viability assay was performed with peritoneal macrophages obtained from BALB/c mice to investigate the influence of ERJ on macrophages. The macrophages were plated in 96-well plates for 4 h at 37℃ in a 5% CO2 incubator (Sanyo Electric Co., Osaka, Japan). The macrophages was treated with various concentrations of ERJ (25, 50, or 100 μ g/mL) or RJ (100 μg/mL) for 24 h, without or with LPS (1 μg/mL) co-treatment. Cell viability was determined using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay kit from Sigma according to the manufacturer’s instructions.
Antioxidant analysis
To investigate the antioxidant activity of ERJ, macrophages was treated with ERJ or RJ, followed by LPS (1 μg/mL) for 24 h. The level of Reactive oxidative species (ROS) was quantified by fluorescence using 2′, 7′-dichlordihydrofluorescin diacetate (H2DCF-DA) (Invitrogen, CA, USA) according to manufacturer’s instructions. Superoxide dismutase (SOD) and Glutathione (GSH) levels were measured using a commercial SOD/GSH Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), and Nitric oxide (NO) level was measured using the Griess Reagent System (Promega, Madison, WI, USA). All experiments were conducted according to the manufacturer's instructions.
Statistical analysis
All values were expressed as mean ± SEM. Statistical differences among experimental groups were assessed with Student`s t-test for two groups. One-way analysis of variance (ANOVA) was used for comparison among more than two groups. Differences were considered statistically significant when p<0.05 (Andrikopoulos et al., 2008).
Results and Discussion
Confirmation of allergen protein removal and ingredient content change
The water-soluble proteins of RJ have been studied in terms of their characteristics and physiological functions according to size in a total of nine fractions (Tamura et al., 2009). Among them, water-soluble protein 1 (molecular weight (MW): 55 kDa) and water-soluble protein 2 (MW: 47 kDa) have been reported as major proteins causing allergies (Yoko et al., 2011). Water-soluble proteins 1 and 2 have been reported to stimulate the secretion of TNF-α, a pro-inflammatory cytokine, in macrophages in mice (imúth et al., 2004) and to cause acute bronchial asthma, skin hypersensitivity, and colitis. Therefore, in this study, we examined whether these allergen-induced proteins were removed and the level of 10-HAD was changed by enzyme treatment.
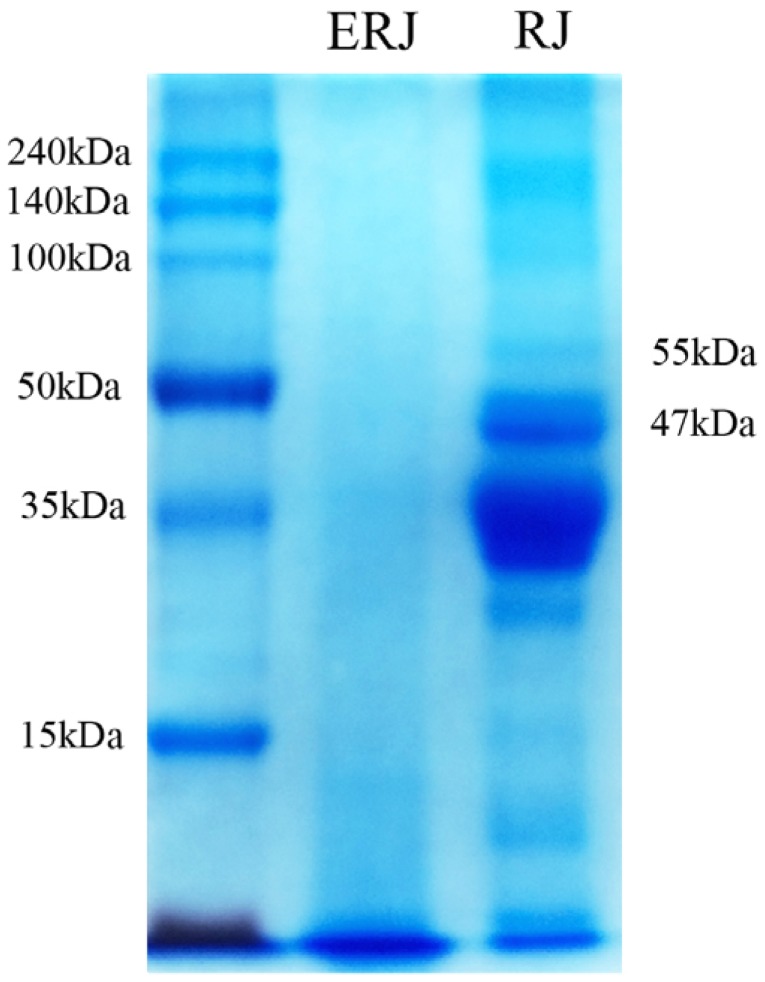
The electrophoretic patterns of the RJ hydrolysates obtained from the enzyme treatments are shown in Fig. 1. Compared with RJ, ERJ did not show expression of either of the two proteins (MW: 47, 55kDa) causing allergic reaction. The enzyme treatment also resulted in an increase in the level of total free amino acids due to the decomposition of small molecules of polymer (Table 1). Nevertheless, the level of 10-HDA was not changed by the enzyme treatment (Table 1). Therefore, with ERJ, there is no need to worry about the risk of allergies when ingested, and the enzymatic processing of RJ is expected to improve the absorption rate.
Fig. 1. The removal of two allergen proteins by enzyme treatment.
ERJ: enzyme-treated royal jelly, RJ: non-enzyme treated royal jelly. Data were expressed as the mean ± SEM (n=3).
Table 1. Changes in 10-HDA and total free amino-acid contents in ERJ.
| RJ | ERJ | |
|---|---|---|
| 10-HDA | 50.0 ± 0.0 | 51.0 ± 0.1 |
| Total free amino acid content | 5.72 g/mg | 83.89 ± 2.44 g/mg |
Cell protective effects of ERJ
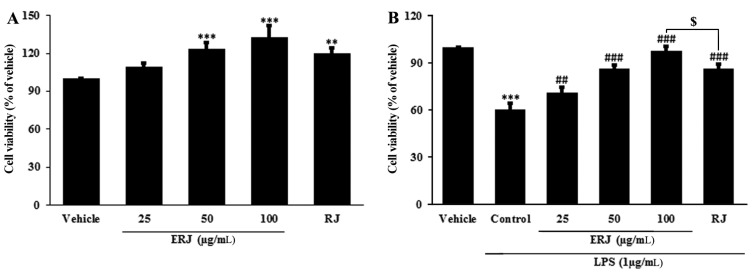
To assess the cytotoxicity of ERJ in peritoneal macrophages, MTT assays were conducted (Fig. 2A). The cell viability of macrophages treated with ERJ increased in a dose-dependent manner. To investigate the cell protective effect of ERJ, MTT assays were performed in LPS-treated peritoneal macrophages (Fig. 2B). The cell viability after 1 μg/mL LPS treatment for 24 hours was reduced to 60% of the normal group. Thus, 1 μg/mL LPS was used in all subsequent experiments. The viabilities of ERJ or RJ, co-treated with LPS for 24 h, were significantly increased compared to cells treated with LPS alone. ERJ resulted in a more effect on proliferation compared to treatment with the same concentration (100 μg/mL) of RJ. This result shows that ERJ had the protective effect against LPS-treatment-induced cell stress.
Fig. 2. Protective effect of ERJ in LPS-treated macrophages.
(A) Cell viability of macrophage cells treated with ERJ for 24 h. (B) Cell viability of macrophage cells pretreated with ERJ or RJ and followed by LPS treatment for 24 h. ERJ: enzyme-treated royal jelly, RJ: non-enzyme treated royal jelly. Data were expressed as the mean ± SEM (n=3). **p<0.01, ***p<0.001, compared with Vehicle. ##p<0.01, ###p<0.001, compared with Control. $p<0.05, compared with RJ.
Antioxidant effect of ERJ
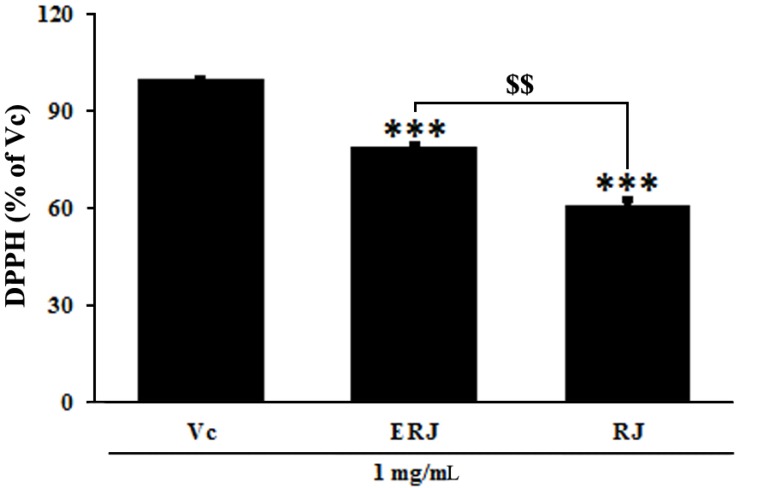
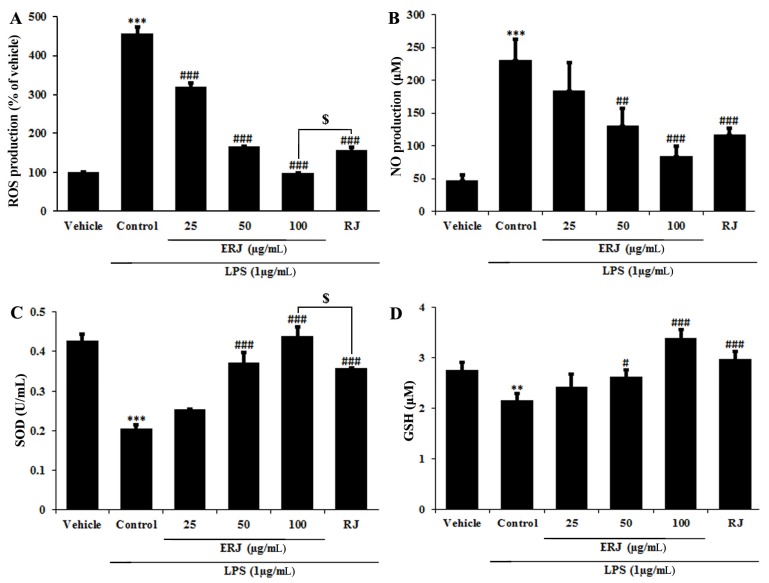
RJ exerts its positive effects on the human body due to its natural pharmacological activities, such as its antioxidative, immunomodulating, hypotensive and blood regulatory, and anti-inflammatory effects (Lebesi and Tzia 2012; Viuda-Martos et al., 2008). The antioxidant activity of ERJ was confirmed by DPPH assay, ROS and NO productions, and SOD and GSH densities. A DPPH assay was used to assess antioxidant activity by measuring the free radical scavenging ability of ERJ. At the same concentration of 1 mg/mL, ERJ showed 20% higher DPPH free radical scavenging activity than RJ (Fig. 3). ROS produced during the metabolic process causes oxidative stress in the body, resulting in an increase in NO production and an acceleration of cell damage caused by the inflammatory reaction. In addition, SOD and GSH are produced as part of a protective mechanism (Freeman et al., 1983) that occurs as a response to oxidative damage (Creppy et al., 1989). The experimental results showed that ROS and NO production after treatment with LPS alone significantly increased compared with non-treated cells. However, ROS and NO production in ERJ and LPS co-treated cells significantly decreased in a dose-dependent manner. Especially, compared to treatment with the same concentration (100 μg/mL) of RJ, treatment with ERJ resulted in a decrease in ROS production (Fig. 4A, B). Antioxidant enzymes that act as a defense mechanism against ROS include SOD, GSH, and catalase (CAT), all of which are present in various organelles, the cytoplasm, and nucleus (Lopes et al., 2001). SOD converts the superoxide anion to hydrogen peroxide. The resulting hydrogen peroxide is degraded by CAT and glutathione peroxidase (GSH-Px). The excess hydrogen peroxide, which has not been degraded, reacts with Fe2+ radicals and damages cellular proteins, cell membranes, and DNA, leading to cell aging and necrosis (Emerit et al., 2001; Kim et al., 2008). In this study, SOD and GSH production in cells treated with LPS alone were significantly decreased, whereas ERJ significantly increased their production in a dose-dependent manner. Compared to treatment with the same concentration (100 μg/mL) of RJ, treatment with ERJ resulted in higher SOD and GSH production (Fig. 4C, D).
Fig. 3. DPPH radical scavenging activity of ERJ.
Vc: ascorbic acid, ERJ: enzyme-treated royal jelly, RJ: non-enzyme treated royal jelly. Data were expressed as the mean ± SEM (n=3). *** p<0.001, compared with Vc. $$p<0.01, compared with RJ.
Fig. 4. Antioxidant effects of ERJ in LPS-treated macrophages.
(A) ROS assay, (B) NO assay, (C) SOD assay, (D) GSH assay. ERJ: enzyme-treated royal jelly, RJ: non-enzyme treated royal jelly. Data were expressed as the mean ± SEM (n=3). **p<0.01, ***p<0.001, compared with Vehicle. #p<0.05, ##p<0.01, ###p<0.001, compared with Control. $p<0.05, compared with RJ.
Conclusions
The results obtained in this study demonstrate that ERJ is a bioactive compound based on its excellent antioxidant activities. In this study, when two allergen-inducing proteins were removed from ERJ, there was the increase in total amino acid content compared to non-treated RJ. In addition, no difference in the content of 10-HDA was observed in ERJ. Also, the evaluated antioxidant activities of ERJ were stronger than those of RJ. These results suggest that ERJ could be used as a new material in the development of medicinal foods with a potential antioxidant capacity.
Acknowledgments
This work (C0453904) was supported by the Business for Cooperative R&D between Industry, Academy, and Research Institute funded the Korea Small and Medium Business Administration in 2017.
References
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Aurora N, Lanzuise S, Ruocco M, Arlotti G, Ranieri R, Knutsen SH, Lorito M, Fogliano V. Treatment of Cereal Products with a Tailored Preparation of Trichoderma Enzymes Increases the Amount of Soluble Dietary Fiber. J Agric Food Chem. 2006;54:7863–7869. doi: 10.1021/jf0612777. [DOI] [PubMed] [Google Scholar]
- Celira CS, Marcucci MC. Quantitative determination of trans-10-Hydroxy-2-Decenoic Acid (10-HDA) in Brazilian royal jelly and commercial products containing royal jelly. J Apic Res. 2015;46:149–153. [Google Scholar]
- Chen J, Gao D, Yang L, Gao Y. Effect of microfluidization process on the functional properties of insoluble dietary fiber. J Food Res. 2013;54:1821–1827. doi: 10.1016/j.foodres.2013.09.025. [DOI] [Google Scholar]
- Creppy EE, Chakor K, Fisher MJ, Dirheimer G. The mycotoxin ochratoxin A is a substrate for phenylalanine hydroxylase in isolated rat hepatocytes and in vivo. Arch Toxicol. 1989;64:279–284. doi: 10.1007/BF01972987. [DOI] [PubMed] [Google Scholar]
- Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury'. Biomed Pharmacother. 2001;55:333–339. doi: 10.1016/S0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- Fan YJ, Gittis AH, Juge F, Qiu C, Xu YZ, Rabinow L. Multifunctional RNA processing protein SRm160 induces apoptosis and regulates eye and genital development in Drosophila. Genetics. 2014;197:1251–1265. doi: 10.1534/genetics.114.164434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA, Young SL, Crapo J.D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983;258:12534–12542. [PubMed] [Google Scholar]
- Guo H, Saiga A, Sato M, Miyazawz I, Shibata M, Takahata Y, Morimatsu F. Royal jelly supplementation improves lipoprotein metabolism in human. J Nutr Sci Vitaminol. 2007;53:345–348. doi: 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- Iwao O, Taniguchi Y, Kunikata T, Kohno K, Iwaki K, Ikeda M, Kurimoto M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003;73:2029–2045. doi: 10.1016/S0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- Jozef , Bíliková K, Kováčová E, Kuzmová Z, Schroder W. Immunochemical Approach to Detection of Adulteration in Honey: Physiologically Active Royal Jelly Protein Stimulating TNF-r Release Is a Regular Component of Honey. J Agric Food Chem. 2004;52:2154–2158. doi: 10.1021/jf034777y. [DOI] [PubMed] [Google Scholar]
- Keizo K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M, Kurimoto M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci Biotechnol Biochem. 2004;68:138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son IS, Kim JS, Kim KH, Kwon CS. Lipase-Inhibitory and Anti-Oxidative Activity of the Methanol Extract and the Powder of Phellinus linteus. J Korean Soc Food Sci Nutr. 2008;37:154–161. doi: 10.3746/jkfn.2008.37.2.154. [DOI] [Google Scholar]
- Kim JM, Han SM, Cho ML, Lee KG, Lee ML, Lee MY, Woo SO, Hong IP, Sim HS, Choi YS. Characterization of Water Soluble Royal Jelly Removed Allergenic protein. J Apic. 2013;28:19–23. [Google Scholar]
- Lebesi DM, Tzia C. Use of endoxylanase treated cereal brans for development of dietary fiber enriched cakes. J Innovative Food Sci Emerg Technol. 2012;13:207–214. doi: 10.1016/j.ifset.2011.08.001. [DOI] [Google Scholar]
- Liu L, Wen W, Zhang R, Wei Z, Deng Y, Xiao J, Zhang M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017;214:1–8. doi: 10.1016/j.foodchem.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Lopes PA, Pinheiro T, Santos MC, Mathias ML, Collares-Pereira MJ, Viegas-Crespo M. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. The Sci Total Environ. 2001;280:53–163. doi: 10.1016/s0048-9697(01)00822-1. [DOI] [PubMed] [Google Scholar]
- Ma M, Mu T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr Polym. 2016;136:87–94. doi: 10.1016/j.carbpol.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Outi S, Kiran A, Sozer N, Poutanen K, Nordlund E. Enzymatic modification and particle size reduction of wheat bran improves the mechanical properties and structure of bran-enriched expanded extrudates. J Cereal Sci. 2014;60:448–456. doi: 10.1016/j.jcs.2014.04.003. [DOI] [Google Scholar]
- Raghavendra SN, Ramachandra Swamy SR, Rastogi NK, Raghavarao KSMS, Kumar S, Tharanathan RN. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J Food Eng. 2006;72:281–286. doi: 10.1016/j.jfoodeng.2004.12.008. [DOI] [Google Scholar]
- Rosmilah M, Shahnaz M, Patel G, Lock J, Rahman D, Masita A, Noormalin A. Characterization of major allergens of royal jelly Apis mellifera. Trop Biomed. 2008;25:243–251. [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant Activity of Peptides Obtained from Porcine Myofibrillar Proteins by Protease Treatment. J Agric Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Sangnarka A, Noomhorm A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003;80:221–229. doi: 10.1016/S0308-8146(02)00257-1. [DOI] [Google Scholar]
- Suguru F, Jiro I, Mineko F, Tomoko Y, Takuji K, Kumpei K. A Potent Antibacterial Protein in Royal Jelly. J Biol Chem. 1990;265:11333–11337. [PubMed] [Google Scholar]
- Sver L, Orsolic N, Tadic Z, Njari B, Valpotic I, Basic I. A Royal Jelly as a New Potential Immunomodulator in Rats and Mice. Comp Immunol Microbiol Infect Dis. 1996;19:31–38. doi: 10.1016/0147-9571(95)00020-8. [DOI] [PubMed] [Google Scholar]
- Takeshi N, Sakaia M, Inoue R, Inoue H, Suzuki N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001;75:237–240. doi: 10.1016/S0308-8146(01)00193-5. [DOI] [Google Scholar]
- Tamura S, Toru K, Chika H, Kikuji Y, Takanori M. Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem. 2009;114:1491–1497. doi: 10.1016/j.foodchem.2008.11.058. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, Perez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Gao P, Shi D, Liu H, Gao H. Changes in the structural properties and rate of hydrolysis of cotton fibers during extended enzymatic hydrolysis. Biotechnol Bioeng. 2006;93:443–456. doi: 10.1002/bit.20730. [DOI] [PubMed] [Google Scholar]
- Yoko M, Yoshinao S, Toshiya T, Takahiko T, Tatsuya M, Mariko S. Major royal jelly protein 3 as a possible allergen in royal jelly-induced anaphylaxis. J Dermatol. 2011;38:1079–1081. doi: 10.1111/j.1346-8138.2010.01179.x. [DOI] [PubMed] [Google Scholar]
- Yoon JM, Kang HJ, Choi ES, Moon MS, Kim KS, Kim YH. Development of Preparation Method of Enzyme- Treated Royal Jelly. J Apic. 2014;29:161–166. [Google Scholar]