Abstract
Hypocotyls of kidney beans (Phaseolus vulgaris L.) accumulated ascorbate after preincubation with a number of possible precursors, mainly l-galactono-γ-lactone (l-GL) and l-gulono-γ-lactone. The increase in the intracellular ascorbate concentration was parallel to the high stimulation of the l-GL dehydrogenase (l-GLD) activity measured in vitro using l-GL as a substrate and cytochrome c as an electron acceptor. Cell fractionation using a continuous linear Percoll gradient demonstrated that l-GLD is associated with mitochondria; therefore, pure mitochondria were isolated and subjected to detergent treatment to separate soluble from membrane-linked proteins. l-GLD activity was mainly associated with the detergent phase, suggesting that a membrane-intrinsic protein is responsible for the ascorbic acid biosynthetic activity. Subfractionation of mitochondria demonstrated that l-GLD is located at the inner membrane.
All higher plants contain high levels of vitamin C or ASC distributed in many different cell compartments, such as the cytosol, mitochondria, chloroplast, and apoplast. The reducing activity of ASC is responsible for many of its roles in plant physiology. Although the function of ASC in plants is not completely understood, it has been demonstrated to play a role in the defense mechanism as a free-radical scavenger (Foyer et al., 1991; Córdoba and González-Reyes, 1994). ASC appears to be a growth-regulating agent in plant cells (Arrigoni, 1994; Córdoba-Pedregosa et al., 1996).
Despite these important functions of ASC in plant physiology, the metabolic pathway leading to ASC biosynthesis is only partially known (Smirnoff, 1996). In animals ASC is synthesized by a microsomal l-GUL oxidase (EC 1.1.3.8) (Kiuchi et al., 1982). However, two biosynthetic routes have been suggested for plants. One involves l-GL as the last precursor, which is oxidized by a dehydrogenase reaction in which Cyt c is used as an electron acceptor (Ôba et al., 1994; Wheeler et al., 1998). This reaction is catalyzed by l-GLD (EC 1.3.2.3). The other alternative pathway involves d-Glc, d-glucosone, and l-sorbosone as intermediate precursors of ASC biosynthesis (Loewus, 1988; Loewus et al., 1990; Saito et al., 1990). For yeast and plants, a third pathway that is catalyzed by l-GL oxidase (EC 1.1.3.24), involves l-GL and dioxygen as electron acceptors, and yields ASC and hydrogen peroxide has been suggested (Baig et al., 1970; Bleeg and Christensen, 1982).
Recently, l-GLD has been purified from potato (Ôba et al., 1995) and cauliflower (Østergaard et al., 1997) mitochondria. The enzyme was also expressed in yeast, and the physiological relevance of l-GL as the last precursor of the ASC biosynthesis pathway in plants was emphasized. Both purified enzymes have the same molecular mass (56 kD), although some other properties are different (e.g. substrate specificity and inhibitor sensitivity).
In this paper we provide evidence (for the first time to our knowledge) that l-GLD is a membrane-intrinsic protein linked to the inner membrane of mitochondria.
MATERIALS AND METHODS
Growth Conditions
Kidney bean (Phaseolus vulgaris L.) seeds were purchased from Semillas Fitó (Barcelona, Spain), washed for 10 min in tap water, soaked in distilled water overnight, and grown for 6 to 8 d in the dark on filter paper moistened with water. Roots were then discarded, and the hypocotyls were cut off in 2- to 4-mm fragments. From this material mitochondria were isolated as described below. Where indicated, seeds were grown in the presence of 2 mm l-GL, l-GUL, d-Glc, or d-Gal.
Isolation and Purification of Mitochondria
All procedures were performed at 0°C to 4°C. Hypocotyl fragments were mixed with ice-cold homogenization buffer (0.25 m Suc, 2 mm EGTA, 30 mm mercaptoethanol, 0.35 m d-mannitol, 0.1% BSA, and 30 mm Hepes, pH 7.6) in a ratio of 1 g fresh weight per milliliter of buffer and immediately homogenized in a blender using two 5-s strokes with an interval of 30 s. The homogenate was filtered on cheesecloth and centrifuged at 1,500g for 15 min to eliminate cell debris. The supernatant was again centrifuged at 14,000g for 20 min. The resulting supernatant consisted of the microsomal fraction and the pellet consisted of the crude mitochondrial fraction, which was resuspended in the homogenization buffer at pH 7.2 without mercaptoethanol.
Crude mitochondria were then subjected to a further purification by fractionation in a linear gradient of Percoll (Goldstein et al., 1980). The crude mitochondrial fraction (1.5–3 mL at 12–24 mg of protein) was added to 12 mL of a preformed 2% to 60% linear Percoll gradient plus 0.25 m Suc, 2 mm EGTA, and 30 mm Hepes, pH 7.2, and centrifuged at 37,000g for 30 min. One-milliliter aliquots were then analyzed by marker enzymes and electron microscopy to identify cell organelles. Where indicated, homogenate was also fractionated directly by a linear gradient of bxPercoll. The following marker enzymes were used: Cyt c oxidase for mitochondrial inner membrane (Storrie and Madden, 1990), catalase for microbodies (Aebi, 1983), NADPH-Cyt c oxidoreductase for ER (Storrie and Madden, 1990), pyrophosphatase for tonoplast (Chanson, 1990), and NADH-Cyt c oxidoreductase for mitochondrial outer membrane (Moore and Proudlove, 1983).
Subfractionation of Pure Mitochondria
Procedures were according to the method of Greenawalt (1979) with minor modifications. Pure mitochondria were mixed with isolation buffer at 10 mg protein mL−1 buffer. Isolation buffer consisted of 70 mm Suc, 220 mm mannitol, 0.05% BSA, and 2 mm Hepes, pH 7.2. One milliliter of digitonin was immediately added and the mixture was incubated for 15 min. Eight milliliters of isolation buffer was then added, and the suspension was centrifuged in Eppendorf tubes at 12,000g for 10 min. The pellet consisted of mitoplasts and the supernatant contained outer membrane and intermembrane proteins. While the supernatant was stored ice-cold, the pellet was diluted in 5 mL of isolation buffer plus 5 mg mL−1 Lubrol PX and centrifuged at 144,000g for 60 min to obtain the inner membrane vesicles (pellet) and the mitochondrial matrix (supernatant). The stored supernatant was also centrifuged at 144,000g for 60 min to obtain a pellet containing outer membranes and a supernatant containing intermembrane space proteins.
Separation of Integral and Peripheral Membrane Proteins
Peripheral and integral proteins were separated as described by Bordier (1981). Basically, the method consists of several incubations of the mitochondrial membranes (1 mg of protein per milliliter) with Triton X-114 (1%, w/v) at different temperatures (0°C and 37°C). After 3 min of centrifugation at 300g, two fractions were obtained. The upper, so-called “aqueous” phase contained peripheral (extrinsic) proteins, while an oily droplet was also obtained at the bottom of the tube. The “detergent” phase contained integral (intrinsic) proteins of amphiphilic structure (Bordier, 1981).
l-GLD Assay
l-GLD activity was determined according to the method of Ôba et al. (1994) with minor modifications. Triton X-100 (0.03%) was included in the assay medium. In its absence, no l-GLD activity could be detected. The activity was quantified as the Cyt c reduction rate at 550 nm (extinction coefficient 29.5 mm−1). Cyanide was included to prevent reoxidation of Cyt c by Cyt c oxidase present in mitochondrial fractions. No inhibition of l-GLD by cyanide had been detected previously (Ôba et al., 1994).
ASC Determination
Intracellular ASC concentration was determined in hypocotyls subjected to different experimental conditions by the bipyrydyl method described by Knörzer et al. (1996). Previously, an ASC standard calibration curve was run and an extinction coefficient of 12.3 mm−1 was obtained.
Protein Determination
Protein was determined using Coomassie Blue and the Bradford (1976) method as modified by Stoscheck (1990) for membrane proteins, except in the case of detergent-included samples, which were analyzed by the bicinchoninic acid method according to the method of Smith et al. (1985). Bovine γ-globulin was used as a standard.
RESULTS
In Vivo Correlation between ASC Concentration and l-GLD Activity
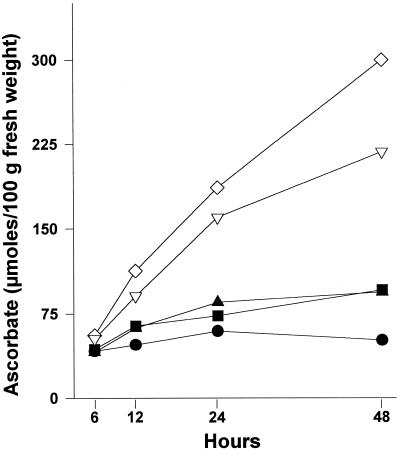
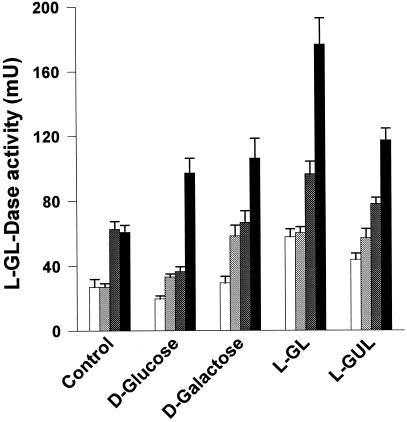
Hypocotyls accumulated ASC at a rate that depended on the precursor used in the incubation medium. After 48 h of treatment, ASC concentration from l-GL-preincubated hypocotyls increased about 6-fold with respect to control hypocotyls incubated in distilled water. Preincubation with l-GUL also increased the intracellular ASC concentration up to about 4 times that of control. Other compounds, such as d-Glc or d-Gal, led to ASC increases up to 1.3 times that of control hypocotyls (Fig. 1). l-GLD activity also increased at a rate depending on the precursor used in the incubation media. As expected, the highest activities were obtained after preincubation with l-GL (Fig. 2).
Figure 1.
Accumulation of l-ASC in hypocotyls under different growth conditions. Bean seeds were washed and allowed to growth for 6 to 8 d. Plantlets were then transferred to hydroponic cultures in distilled water (control, •) or in 2 mm d-Glc (▪), d-Gal (▴), l-GUL (▿), or l-GAL (⋄). At the indicated times, hypocotyls were cut off, homogenized, and used to measure intracellular ASC concentration.
Figure 2.
Stimulation of l-GLD activity in hypocotyls under different growth conditions. Hypocotyls grown as explained in Figure 1 were cut off at 6 h (white bars), 12 h (light gray bars), 24 h (dark gray bars), or 48 h (black bars). After homogenization, l-GLD activity was determined in vitro using l-GL and Cyt c as substrates.
When l-GL was substituted in the in vitro enzyme assay by other substrates such as l-GUL or l-manono-γ-lactone, Cyt c reduction was significantly lower (23% and 14%, respectively) than that of the l-GL-dependent reaction. Other possible electron acceptors, such as Fe3+-EDTA, NAD(P)+, or 2,6-dichlorophenolindophenol, used in place of Cyt c did not serve as a substrate for the reaction, since enzyme activity was undetectable. In other experimental series, hypocotyl homogenates were incubated with l-GL under continuous shaking but in the absence of Cyt c to determine whether oxygen alone would sustain the reaction. However, the results were negative since neither ASC (measured at a maximum absortion wavelength of 265 nm) nor hydrogen peroxide (measured by using guaiacol and a peroxidase-coupled reaction) were produced.
l-GLD Is an Intrinsic Protein Located at the Inner Mitochondrial Membrane
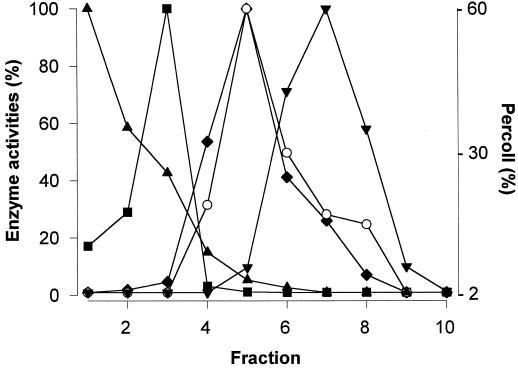
A linear Percoll gradient previously calibrated using density markers was used to separate cell organelles from homogenates obtained from kidney bean hypocotyls. Subcellular fractions were clearly separated using this procedure. The l-GLD activity profile was parallel to that of Cyt c oxidase activity, which was used as mitochondrial enzyme marker. No l-GLD activity was detected in those fractions corresponding to the ER, tonoplast, or peroxisomes (Fig. 3). Therefore, our results suggest that l-GLD activity is associated with mitochondria.
Figure 3.
Subcellular fractionation of hypocotyl homogenates. Eight-day-old hypocotyls were homogenized as indicated in “Materials and Methods,” and fractionated on a linear Percoll gradient. Fractions (1 mL) were then analyzed for marker enzymes: catalase (▴), pyrophosphatase (▪), Cyt c oxidase (♦), and NADPH-Cyt c oxidoreductase (▾). l-GLD activity (○) was also determined in all fractions.
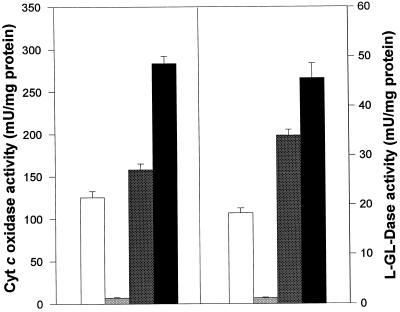
To verify these results, mitochondria were highly purified by a procedure that included the separation of a crude mitochondrial fraction from cell endomembranes and plasma membrane vesicles (microsomal fraction). Centrifugation of the crude mitochondrial fraction in a linear gradient of Percoll resulted in a highly purified mitochondrial fraction, as deduced from enzyme marker analysis and electron microscopy studies (not shown). When distribution of Cyt c oxidase (a mitochondrial marker) and l-GLD activities were investigated in these subcellular fractions, a very similar distribution of both enzyme activities was obtained (Fig. 4).
Figure 4.
Distribution of Cyt c oxidase and l-GLD in the subcellular fractions. Cyt c oxidase and l-GLD activities were assayed in homogenate (white bars), the microsomal fraction (light gray bars), the crude mitochondria (dark gray bars), and the pure mitochondrial fraction (black bars). Data represent specific activities for each analyzed fraction.
Afterward, pure mitochondria were subjected to Triton X-114 solubilization according to the method of Bordier (1981), and the results are shown in Table I. Interestingly, the distribution of l-GLD was nearly identical to that of Cyt c oxidase, a well-known intrinsic protein, strongly suggesting that the biosynthetic ASC activity is due to a protein directly linked to a mitochondrial membrane.
Table I.
Effects of Triton X-114 on l-GLD activity distribution
| Enzyme | Aqueous Phasea | Detergent Phaseb | Ratio |
|---|---|---|---|
| nmol min−1 | % | ||
| l-GLD | 4.5 ± 0.28 | 19 ± 0.23 | 80.9 |
| Cyt c oxidase | 10.22 ± 0.19 | 180 ± 5.6 | 94.6 |
| NADH-Cyt c oxidoreductase | 6.4 ± 0.3 | 173.59 ± 14.39 | 96.4 |
Pure mitochondrial fractions were subjected to Triton X-114 treatment, and l-GLD activity was determined in the aqueous and detergent phases. For comparative purposes, Cyt c oxidase and NADH-Cyt c oxidoreductase activities were also determined. Activities represent means ± sd from three independent experiments. Ratio is defined as the proportion of activity at the detergent phase with respect to the total (aqueous plus detergent phases).
Total protein, 0.7 mg.
Total protein, 0.98 mg.
Finally, after subfractionation of pure mitochondria, the distribution of l-GLD activity was highly parallel to that of Cyt c oxidase (Table II), demonstrating that l-GLD activity is associated with the inner mitochondrial membrane.
Table II.
Distribution of l-GLD activity in submitochondrial fractions
| Enzyme | MMa | IMb | OMc | ISd |
|---|---|---|---|---|
| nmol min−1 | ||||
| l-GLD | 3.74 ± 0.3 | 39.9 ± 1.59 | 3.7 ± 1 | NDe |
| Cyt c oxidase | 192.4 ± 30 | 1305 ± 28 | 84.3 ± 1.7 | ND |
| NADH-Cyt c oxidoreductase | 12.1 ± 0.3 | 17.5 ± 0.4 | 59.4 ± 1.1 | 4.02 ± 0.02 |
Submitochondrial fractions were isolated from pure mitochondria. l-GLD, Cyt c oxidase (as a marker of inner membrane), and NADH-Cyt c oxidoreductase (as a marker of outer membrane) activities were determined. Data represent means ± sd from three independent experiments.
MM, Mitochondrial matrix (1.4 mg total protein).
IM, Inner membrane (1.75 mg total protein).
OM, Outer membrane (0.36 mg total protein).
IS, Intermembrane space (0.9 mg total protein).
ND, Not detected.
DISCUSSION
ASC has been shown to play a relevant role in higher plant physiology. However, details on its biosynthesis have not been thoroughly investigated, despite the fact that plants are the main nutritional source of vitamin C for human beings.
In this paper, we provide evidence supporting the idea that ASC is mainly synthesized in vivo and in vitro from l-GL or l-GUL, although l-GL is a better substrate than l-GUL. The addition of possible immediate ASC precursors to incubation media rapidly increased intracellular ASC levels, and GLD activity was simultaneously enhanced, particularly after incubation with l-GL. l-GUL, d-Gal, and d-Glc were also able to stimulate the enzyme activity after 48 h of preincubation. In a similar manner, Loewus et al. (1990), Baig et al. (1970), and De Gara et al. (1994) showed correlations between l-GL or l-GUL additions to the culture media and increased intracellular ASC.
Previous results and those reported here indicate that all tested metabolites are easily taken up by cells and may be interconnected by different metabolic pathways (Wheeler et al., 1998); however, l-GL appears to be the last common immediate precursor of ASC biosynthesis. Cyt c could not be replaced with other electron acceptors such as Fe3+-EDTA, NAD(P)+, or 2,6-dichlorophenolindophenol. Moreover, dioxygen did not oxidize l-GL, nor was hydrogen peroxide produced during the course of the reaction, discounting the possibility that, like yeast enzyme (Bleeg and Christensen, 1982), hypocotyl enzyme may act as an oxidase.
Previous data from other authors have indicated that the protein responsible for l-GLD activity is located at the mitochondrial fraction of plant cells (Ôba et al., 1994, 1995; Mutsuda et al., 1995; Østergaard et al., 1997). However, its exact localization has not as yet been investigated. Ôba et al. (1994) reported the subcellular distribution of GLD in potato tubers after linear Suc density gradient centrifugation, and found that the enzyme was mainly associated with mitochondria (but also with the ER and peroxisomes). Mutsuda et al. (1995) indicated that the enzyme was associated with mitochondria of spinach leaves after linear Suc density or discontinuous Percoll gradient centrifugation. Nevertheless, these authors only investigated the enzyme distribution in intact mitochondria and chloroplasts. Our results using a self-generated Percoll linear gradient showed that l-GLD in exclusively asssociated with mitochondria; no activity was detected in membrane fractions derived from microsomes, peroxisomes, or tonoplast.
In the GLD-purification protocol used by Ôba et al. (1995), the enzyme was firstly solubilized by sonication. Arrigoni et al. (1996) used Tween 20 to solubilize the enzyme before in vitro assays, and Mutsuda et al. (1995) solubilized the enzyme using a variety of detergents, such as Triton X-100, deoxycholate, or Chaps. We used 0.03% Triton X-100 in the in vitro assay to detect the GLD activity. In the absence of the detergent the activity was negligible, suggesting the possible association of l-GLD with membranes. To test this possibility, pure mitochondria were subjected to Triton X-114 treatments according to the method of Bordier (1981). This experimental procedure allowed us to separate integral from peripheral membrane proteins. Our results indicate that l-GLD activity is found mainly in the detergent phase. This was similar to Cyt c oxidase and NADH-Cyt c oxidoreductase activities, which were used as marker enzymes for integral inner and outer mitochondrial membranes, respectively. Thus, our results clearly indicate that l-GLD activity is due to a membrane-linked protein.
To ascertain the exact localization of the enzyme, pure mitochondria were subfractionated into those from the matrix, outer and inner membranes, and intermembrane space. Cyt c oxidase and NADH-Cyt c oxidoreductase were again used as membrane markers. Results show that l-GLD is found mainly in the inner membrane of mitochondria. As far as we know, this is the first time that l-GLD has been reported to be intimately linked to the inner membrane of mitochondria. Although at present it is speculative to suggest a reaction mechanism for the enzyme, the possibility exists that l-GL and Cyt c are natural substrates for the last step in the ASC-biosynthesis pathway. However, further investigations are needed to determine the topology of the enzyme and its mechanism of action.
Abbreviations:
- ASC
ascorbic acid or ascorbate
- GL
galactono-γ-lactone
- GLD
galactono-γ-lactone dehydrogenase
- GUL
gulono-γ-lactone
Footnotes
This research was partially supported by the Spanish Dirección General de Enseñanza Superior (project no. PB95-0560).
LITERATURE CITED
- Aebi HE (1983) Catalase. In J Bergmeyer, M Grassl, eds, Methods of Enzymatic Analysis. Vol III, Enzymes: Oxidoreductases, Transferases. Verlag Chemie, Weinheim, Germany, pp 273–286
- Arrigoni O. Ascorbate system in plant development. J Bioenerg Biomembr. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, Paciolla C, De Gara L. Inhibition of galactonolactone dehydrogenase activity by lycorine. Boll Soc Ital Biol Sper. 1996;72:37–43. [PubMed] [Google Scholar]
- Baig MM, Kelly S, Loewus F. l-Ascorbic acid biosynthesis in higher plants from l-gulono-1,4-lactone and l-galactono-1,4-lactone. Plant Physiol. 1970;46:277–280. doi: 10.1104/pp.46.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeg HS, Christensen F. Biosynthesis of ascorbate in yeast: purification of l-galactono-1,4-lactone oxidase with properties different from mammalian l-gulonolactone oxidase. Eur J Biochem. 1982;127:391–396. doi: 10.1111/j.1432-1033.1982.tb06884.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chanson A. Use of pyrophosphatase activity as a reliable tonoplast marker in maize roots. Plant Sci. 1990;71:199–207. [Google Scholar]
- Córdoba F, González-Reyes JA. Ascorbate and plant cell growth. J Bioenerg Biomembr. 1994;26:399–405. doi: 10.1007/BF00762781. [DOI] [PubMed] [Google Scholar]
- Córdoba-Pedregosa MC, González-Reyes JA, Cañadillas MS, Navas P, Córdoba F. Role of apoplastic and cell-wall peroxidases on the stimulation of root elongation by ascorbate. Plant Physiol. 1996;112:1119–1125. doi: 10.1104/pp.112.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gara L, Paciolla C, Tommasi F, Liso R, Arrigoni O. In vivo inhibition of galactono-γ-lactone conversion to ascorbate by lycorine. J Plant Physiol. 1994;144:649–653. [Google Scholar]
- Foyer CH, Lelandais M, Edwards EA, Mullineaux PM (1991) The role of ascorbate in plants, interactions with photosynthesis, and regulatory significance. In E Pell, K Steffens, eds, The Active Oxigen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 131–144
- Goldstein AH, Anderson JO, McDaniel RG. Cyanide-insensitive and cyanide-sensitive O2 uptake in wheat. Plant Physiol. 1980;66:488–495. doi: 10.1104/pp.66.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt JW. Survey and update of outer and inner mitochondrial membrane separation. Methods Enzymol. 1979;55:88–98. doi: 10.1016/0076-6879(79)55012-5. [DOI] [PubMed] [Google Scholar]
- Kiuchi K, Nishikimi M, Yagi K. Purification and characterization of l-gulonolactone oxidase from chicken kidney microsomes. Biochemistry. 1982;21:5076–5082. doi: 10.1021/bi00263a035. [DOI] [PubMed] [Google Scholar]
- Knözer OC, Durner J, Böger P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant. 1996;97:388–396. [Google Scholar]
- Loewus FA (1988) Ascorbic acid and its metabolic products. In J Preiss, ed, The Biochemistry of Plants, Vol 14. Academic Press, New York, pp 85–107
- Loewus MW, Bedgar DL, Saito K, Loewus FA. Conversion of l-sorbosone to l-ascorbic acid by a NADP-dependent dehydrogenase in bean and spinach leaf. Plant Physiol. 1990;94:1492–1495. doi: 10.1104/pp.94.3.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Proudlove MO. Mitochondria and sub-mitochondrial particles. In: Hall JL, Moore AL, editors. Isolation of Membranes and Organelles from Plant Cells. London: Academic Press; 1983. pp. 153–184. [Google Scholar]
- Mutsuda M, Ishikawa T, Takeda T, Shigeoka S. Subcellular localization and properties of l-galactono-γ-lactone dehydrogenase in spinach leaves. Biosci Biotech Biochem. 1995;59:1983–1984. [Google Scholar]
- Ôba K, Fukui M, Imai Y, Iriyama S, Nogami K. l-Galactono-γ-lactone dehydrogenase: partial characterization, induction of activity and role in the synthesis of ascorbic acid in wounded white potato tuber tissue. Plant Cell Physiol. 1994;35:473–478. [Google Scholar]
- Ôba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T. Purification and properties of l-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem. 1995;117:120–124. doi: 10.1093/oxfordjournals.jbchem.a124697. [DOI] [PubMed] [Google Scholar]
- Østergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M. Isolation of a cDNA coding for l-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. J Biol Chem. 1997;272:30009–30016. doi: 10.1074/jbc.272.48.30009. [DOI] [PubMed] [Google Scholar]
- Saito K, Nick JA, Loewus FA. d-Glucosone and l-sorbosone, putative intermediates of l-ascorbic acid synthesis in detached bean and spinach leaves. Plant Physiol. 1990;94:1496–1500. doi: 10.1104/pp.94.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Storrie B, Madden EA. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–235. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]