Abstract
Ultraviolet radiation (UVR) is a known genotoxic agent. Although its effects on DNA have been well-documented, its impact on RNA and RNA modifications is less studied. By using Escherichia coli tRNA (tRNA) as a model system, we identify the UVA (370 nm) susceptible chemical groups and bonds in a large variety of modified nucleosides. We use liquid chromatography tandem mass spectrometry to identify specific nucleoside photoproducts under in vitro and in vivo conditions, which were then verified by employing stable-isotope labeled tRNAs. These studies suggest that the -amino or -oxy groups of modified nucleosides, in addition to sulfur, are labile in the oxidative environment generated by UVA exposure. Further, these studies document a range of RNA photoproducts and post-transcriptional modifications that arise because of UVR-induced cellular stress.
Graphical abstract
RNA contains >140 different naturally occurring nucleoside modifications,1 and recent reports find that modified nucleosides in tRNA (tRNA) can vary in response to cellular stress conditions.2–7 We have an interest in understanding modification variations under particular oxidative stress conditions such as ultraviolet radiation (UVR) exposure. The incident UVR on the earth’s surface comprises 95% UVA (315–400 nm) and 5% UVB.8 The high incidence of UVA (20-fold higher than UVB), its oxidative potential through cellular photosensitizers,9–11 and its ability to reach melanocytes and dividing stem cells12 could deliver multiple potential impacts to RNA.
While earlier work has shown that sulfur-containing modifications (e.g., 2-thiouridine) are degraded and converted to 4-pyrimidinone upon H2O2-induced oxidative stress,13 affecting the structure and function of tRNAs,14 a comprehensive picture of UVA-induced changes in the nucleoside modifications of tRNAs is lacking. Such information is important as sublethal oxidative damage to coding and noncoding RNA is shown to contribute to neuronal cell death and neurodegenerative diseases such as epilepsy, atherosclerosis, and Alzheimer’s and Parkinson’s disease.15 We have conducted investigations to identify the affected RNA modifications and the photoproducts formed upon UVA exposure by liquid chromatography tandem mass spectrometry (LC-MS/MS). We report the identification of modifications beyond thiolations that are affected by UVA-induced stress under in vitro and in vivo conditions and postulate the sites of attack in these modifications.
To understand the oxidative environment generated by UVA exposure, we exposed E. coli total tRNA with or without riboflavin (RF, a photosensitizer) for 20 min and measured purine oxidation using LC-MS/MS following complete hydrolysis of tRNA. Without RF, UVA induced a small but reproducible increase of the two-electron oxidation product, 8-oxoguanosine (8-oxo-rG; Supporting Information Figure S1). The addition of 10 μM RF generated the four-electron oxidation product, 5-guanidinohydantoin-ribonucleoside (Gh), whose level increased at 100 μM RF but then was constant at higher concentrations of RF. Time-dependent UVA exposure of tRNA in the presence of 100 μM RF revealed formation of 8-oxo-rG at 2 min and Gh after 2 min. These studies indicate that longer UVA exposure and higher RF concentrations yield primarily the four-electron product, Gh. A small percentage of adenosine oxidation was observed in the presence of RF following 2 min of exposure. The subsequent oxidation product, 5-formamidopyrimidine (Fapy-A), was detected after 10 min of exposure. Generation of Gh and Fapy-A were confirmed by employing 15N-labeled tRNA, where molecular ions at m/z 295.0943 corresponding to Gh (C9O6 15N5H16, calculated m/z 295.0952, 3 ppm error) and m/z 291.0994 corresponding to Fapy-rA (C10O515N5H16, calculated m/z 291.0999, 2 ppm error) were detected (Supporting Information Figure S2). These data suggest that RF and longer exposure to UVA generate conditions that could easily oxidize purines under in vitro conditions. These conditions did not yield any oxidation products of pyrimidines (data not shown).
After understanding the impact of UVA and RF on canonical nucleosides, we probed their effects on post-transcriptional modifications. The effects detected were characteristically different depending on the chemical composition of the modified nucleoside. UVA alone caused significant decreases in the abundances of mostly sulfur-containing modifications (2-thiocytidine-s2C, 4-thiouridine-s4U, uridine 5-oxyacetic acid-cmo5U, 5-methylaminomethyl-2-thiouridine-mnm5s2U, 5-carboxymethylaminomethyl-2-thiouridine-cmnm5s2U, N6-isopentenyladenosine-i6A, and 2-methylthio-N6-isopentenyladenosine-ms2i6A; Figure 1, panel A; Supporting Information Figure S3, panel A). Similarly, RF alone adversely affected the integrity of i6A, cmo5U, ms2i6A, and mnm5s2U, and UVA exposure accelerated their degradation (Figure 1, panel B; Supporting Information Figure S3, panel B). The UVA and RF combination led to significant decreases in 2-lysidine-k2C, N6-methyl-N6-threonylcarbamoyladenosine-m6t6A, N6-methyladenosine-m6A, N6-threonylcarbamoyladenosine-t6A, 5-carboxymethylaminomethyl-2′-O-methyluridine-cmnm5Um, 1-methylguanosine-m1G, and epoxyqueuosine/queuosine-oQ/Q under in vitro conditions (Figure 1). The impact on sulfur containing modifications is consistent with the previously reported reactive behavior with hydrogen peroxide.16 These modifications are known to maintain tRNA structural stability, as well as the accurate and efficient decoding of mRNA.17–22
Figure 1.
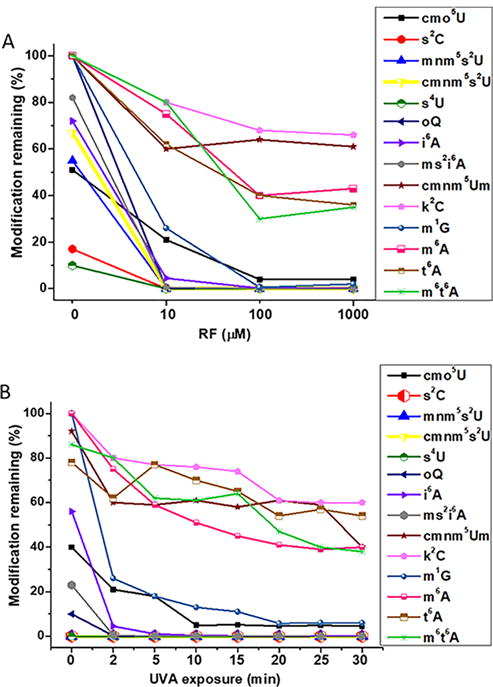
Changes in levels of post-transcriptional modifications of E. coli tRNA following UVA exposure. (A) Five micrograms of tRNA was exposed to UVA (0.752 mJ cm−2) for 20 min at 0, 10, 100, and 1000 μM added riboflavin and analyzed by LC-MS for percent changes in levels of various modifications upon UVA exposure following normalization with the corresponding canonical nucleosides. (B) Effect of UVA exposure period on modifications. Five micrograms of tRNA was exposed to UVA (0.752 mJ/cm2) for 0–30 min in the presence of 100 μM riboflavin, and analyzed for changes in modification levels by LC-MS. For the sake of clarity, the representative data of the mean of five replicates is shown without the error bars.
After identifying modified nucleosides affected by UVA and RF, we probed for potential modification photoproducts. We observed a progressive increase in 5-methylaminomethyluridine-mnm5U, 5-aminomethyluridine-nm5U, and 5-hydroxyuridine-ho5U in the UVA exposed sample. Although a small amount of mnm5U is detected in the unexposed sample, its abundance increased significantly upon UVA exposure (Supporting Information Figure S4). After 10 min, a decrease in mnm5U was accompanied by an increase of nm5U (Figure 2, panel A). The presence of these photoproducts is further supported by the data obtained with the 15N-labeled tRNA (Supporting Information Figure S5). Although these studies do not reveal the exact origin of these photoproducts, the dynamic changes in levels of mnm5U and nm5U over a period of increased UVA exposure suggest that nm5U could have originated from mnm5U, which itself is an oxidative product of mnm5s2U. Thus, nm5U might form through a series of chemical reactions involving dethiolation,14 oxidation, and demethylation (Figure 2, panel B).23 Similarly, mo5U and ho5U were detected in the UVA exposed RNA samples. Although their source is unclear from this experiment, their detection in significant amounts in the UVR-exposed cmo5U standard (Supporting Information Figure S6) suggests that cmo5U could be their potential precursor.
Figure 2.
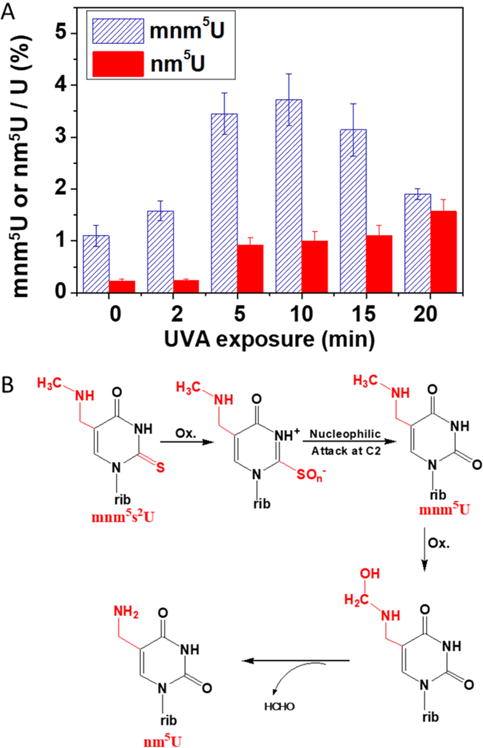
Time-dependent UVA photoproduct generation. (A) Comparative analysis of the levels of mnm5U and nm5U with increasing UVR exposure. The percentage changes in levels of mnm5U and nm5U as a fraction of uridine were computed and plotted against each UVA exposure period. (B) Proposed mechanism for the conversion of mnm5s2U into mnm5U and nm5U involving dethiolation, oxidation, and demethylation is shown.
To better understand the oxidative effects on post-transcriptional modifications observed under intracellular conditions, mid log phase E. coli K-12 cell culture was exposed to UVA for 1 and 2 h. Subsequent nucleoside analysis of isolated tRNA by LC-MS/MS revealed 8-oxo-rG as the predominant purine oxidation product. The four-electron oxidation product, Gh, was not detected under these conditions presumably due to a weaker oxidative environment inside UVA exposed cells compared to the in vitro conditions. The lack of any oxidized adenosine in the data also supports this conclusion.
Sulfur-containing modifications significantly decreased upon exposure of cells to UVA. Similarly, nonsulfur modifications such as cmo5U, i6A, Q, cmnm5Um, k2C, ac4C (N4-acetylcytidine), t6A, and m6t6A significantly decreased following UVA exposure (Figure 3, panel A). All these modifications were adversely affected by in vitro exposure except ac4C, which surprisingly did not change in abundance for the in vitro studies. In general, longer exposure resulted in increased adverse effect on modification levels. Two methylated nucleosides, m1G and m6A, which were degraded under in vitro conditions, were unaffected even after 2 h of exposure under in vivo conditions. These observations indicate that although there is some utility in extrapolating in vitro studies to in vivo conditions, no generalizations about modified nucleoside behavior can be made. Interestingly, the level of the photo-oxidative product nm5U increased in abundance following cellular exposure to UVA (Figure 3, panel A), which is consistent with the in vitro observations (Figure 2, panel A).
Figure 3.
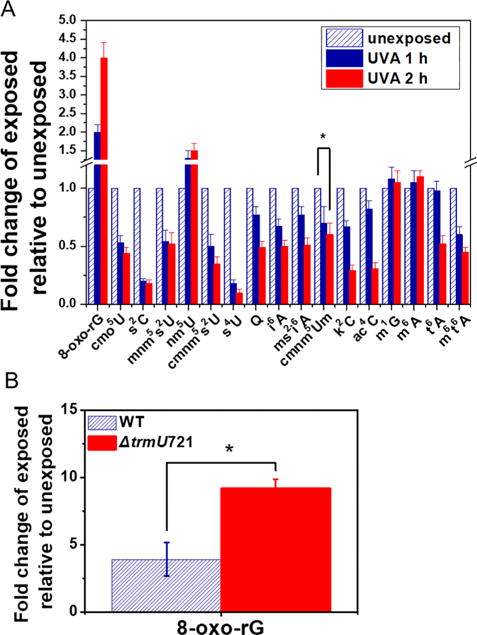
LC-MS analysis of UVA-induced effects on tRNA modifications under in vivo conditions. (A) Changes in tRNA modification levels following UVA exposure of E. coli cells. Each modification is expressed as a ratio of the corresponding signal in the exposed versus unexposed sample. The P values for each modified nucleoside (saving m1G and m6A) as determined by Student’s t test varied by 0.008547 (cmnm5Um, indicated by asterisk) or less. (B) Altered levels of guanosine oxidation following UVA exposure of mnm5s2U-deficient E. coli strain. Fold change in 8-oxo-rG level in tRNA isolated from UVA (3 mJ cm−2, 2 h) exposed cells over unexposed cells is plotted for wild-type (striped bar) and mutant ΔtrmU (white bar). The signal for 8-oxo-rG was normalized to its canonical G in each sample. Data represent mean ± SD values of five biological replicates. Asterisk denotes the P value (0.004137) as determined by Student’s t test.
Because sulfur-containing modifications were adversely affected by UVA consistently under in vivo and in vitro conditions, we explored the possibility of their association with oxidative stress effects. To test this, we exposed a mid log phase cell culture of E. coli strain ΔtrmU (deficient in the enzyme responsible for mnm5s2U biosynthesis)24 to UVA. The tRNAs isolated from the UVA-exposed mutant strain exhibited higher levels of 8-oxo-rG compared to the K-12 wild type strain (Figure 3, panel B). These data suggest a protective role for this sulfur-containing modification against UVA-induced oxidative stress, although additional studies are required to confirm this possibility. The mutant strain also exhibited reduced viability as revealed by serial dilution studies compared to wild type following UVR exposure (Supporting Information Figure S7).
An evaluation of the affected modifications (Figure 4) indicate that -amino or -oxy groups, in addition to sulfur, are labile in the oxidative environment generated by UVA exposure. Detection of ho5U from cmo5U and progressively increasing levels of nm5U with a corresponding increase in UVA exposure are consistent with this interpretation of the data. However, an accurate determination of all the degradation products requires investigations with modified nucleoside standards and comparison against synthetic intermediates.
Figure 4.
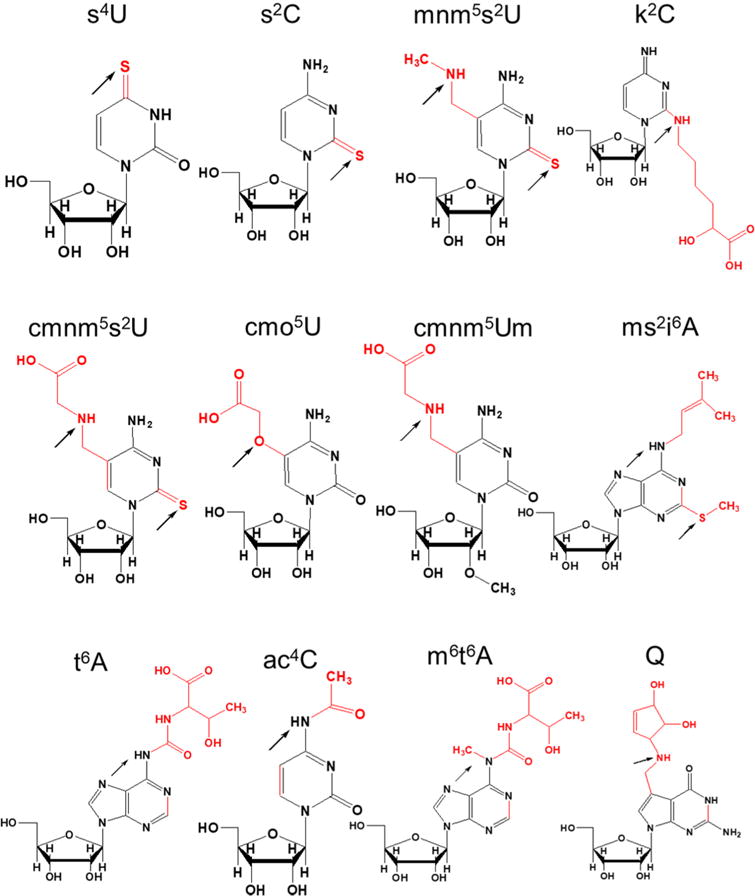
Post-transcriptional RNA modifications affected by UVA stress. Potential sites of oxidative attack on each nucleoside modification1 are shown by an arrow.
These studies open the opportunity to evaluate the impacts of UVA on tRNA structure, function, and global effects on cellular translation. Previous reports indicate that dethiolation of s2C at position 32 of tRNA(ArgICG) could lead to the removal of restrictions on translation of multiple codons, CGU, CGC, and CGA.25 RNA duplexes containing an mnm5U-A base pair are less stable in the helical structure than those containing the mnm5s2U-A base pair.14 Similarly, the loss of other anticodon loop modifications, cmnm5s2U, cmo5U, cmnm5Um, or k2C (at position 34) and ms2i6A, i6A, t6A, and m6t6A (at position 37) of various tRNAs would severely impair the decoding function. Likewise, the loss of oQ can lead to read-through of the stop codon by tRNA(Tyr),26 besides reducing the decoding efficiency of CAU (histidine) codons.27
Studies using the mutant E. coli strain ΔtrmU reveal a greater abundance of oxidative damage products, implying a protective effect for sulfur containing modifications.20,21 Loss of a similar U34 modification mcm5s2U (5-methoxycarbonylmethyl-2-thiouridine)28 and variants of ELP229 and ELP330 proteins (responsible for U34 thiolation) have been implicated in recessive neurodegenerative disease in humans. Although the data from E. coli and humans are coincidental, further studies will determine whether the unavailability of sulfur-containing modifications could lead to a loss of protective function and likely intensification of the recessive phenotypic effects by oxidative stress. This notion could be supported by the observation that the loss of thiolation at U34 of tRNAs leads to decreased viability upon nutrient starvation, oxidative stress, or exposure to higher temperatures in yeast and nematodes.31,32
In summary, many post-transcriptional modifications are found to be vulnerable to degradation upon UVA-induced oxidative stress. Stable-isotope (15N) labeled E. coli K-12 MG1655 tRNA was used to confirm the generation of some oxidative photoproducts. These studies identified-amino and -oxy groups, beyond thiolations, that are labile after UVA exposure under both in vitro and in vivo conditions. These studies also reveal that many structural modifications (such as m7G, Gm, acp3U, etc.) are insensitive to UVA exposure, suggesting tRNA tertiary interactions may be preserved while functional fine-tuning may suffer. This work provides new insights into the cellular lability of modified nucleosides, and future work will be able to expand these studies into eukaryotic and mammalian systems to determine whether cellular antioxidants in those organisms mitigate the effects found here with a simple bacterial system.
METHODS
Escherichia coli tRNA was procured from Roche Diagnostics and 15NH4Cl from Martek isotopes. Nuclease P1, Trireagent, and nucleoside test mix were obtained from Sigma-Aldrich. Riboflavin and all other chemicals were procured from Fisher Scientific.
E. coli Cell Culture
K-12 MG1655 and JW1119-1 (E. coli Genetic Stock Center) strains of E. coli were cultured in Luria–Bertani medium until mid log phase (OD600 ∼ 0.5). The JW1119-1 strain harbors a deletion of the trmU gene, which is deficient in the modified nucleoside, mnm5s2U, found at position 34 of E. coli tRNA(Lys).24
Isotope Labeled tRNA
15N-labeled tRNAs were prepared by growing E. coli (K-12) in M9 minimal medium containing 15NH4Cl (>99.7% purity). Cells were harvested at mid log phase (OD600 ∼ 0.5) and either exposed to UVA or used for purification of 15N-labeled tRNA33
UVA Exposure
E. coli tRNA (5 μg; 0.5 μg μL−1) or nucleoside standards (gift from Prof. McCloskey) were irradiated with UVA (370 nm peak wavelength, 360–400 nm range, 0.752 mJ cm−2) in the presence of 0–1000 μM riboflavin (RF) for 0–30 min at RT (25 °C). The UVA source was made in-house by using Nichia STS-DAI-2749D LEDs (# 161031). Control or unexposed RNA samples were incubated in darkness in the presence of riboflavin for identical periods of time. For UVA exposure of tRNA in vivo, 2 mL of resuspended cells were exposed in a home-built unit designed to hold Petri plates. The LEDs (LED Engin catalog # LZ1–00UV00) used in this unit exhibited a 370 nm peak wavelength generating 3 mJ cm−2.
Nucleoside Analysis
The UVA-exposed and unexposed tRNAs were hydrolyzed to ribonucleosides34 and subjected to high resolution liquid chromatography coupled with mass spectrometry (HR-LC-MS) using a Vanquish UHPLC system coupled to an Orbitrap Fusion Lumos (Thermo Scientific) mass spectrometer. Nucleosides are identified based on their relative retention time, accurate mass, and tandem mass spectral (MS/MS) behavior following collision-induced dissociation (CID).34
Supplementary Material
Acknowledgments
Financial support of this work was provided by the National Institutes of Health (NIGMS R01 058843 and OD OD018485 to P.A.L.) and the University of Cincinnati.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.7b00898.
LC-MS details and supplemental figures (PDF)
ORCID
Balasubrahmanyam Addepalli: 0000-0001-6257-7700
Notes
The authors declare no competing financial interest.
References
- 1.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, Helm M, Bujnicki JM, Grosjean H. MODOMICS: a database of RNA modification pathways– 2013 update. Nucleic Acids Res. 2012;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng W, Babu IR, Su D, Yin S, Begley TJ, Dedon PC. Trm9-Catalyzed tRNA Modifications Regulate Global Protein Expression by Codon-Biased Translation. PLoS Genet. 2015;11:e1005706. doi: 10.1371/journal.pgen.1005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duechler M, Leszczynska G, Sochacka E, Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell Mol Life Sci. 2016;73:3075–3095. doi: 10.1007/s00018-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Delft P, Akay A, Huber SM, Bueschl C, Rudolph KLM, Di Domenico T, Schuhmacher R, Miska EA, Balasubramanian S. The Profile and Dynamics of RNA Modifications in Animals. ChemBioChem. 2017;18:979–984. doi: 10.1002/cbic.201700093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, Kellner S, Hölter SM, Garrett L, Wurst W, Becker L, Klopstock T, Fuchs H, Gailus-Durner V, Hrabě De Angelis M, Káradõttir RT, Helm M, Ule J, Gleeson JG, Odom DT, Frye M. Aberrant methylation of tRNAs links cellular stress to neurodevelopmental disorders. EMBO J. 2014;33:2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popis MC, Blanco S, Frye M. Post-transcriptional methylation of transfer and ribosomal RNA in stress response pathways, cell differentiation, and cancer. Curr Opin Oncol. 2016;28:65–71. doi: 10.1097/CCO.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aucamp PJ, Bjorn LO, Lucas R. Questions and answers about the environmental effects of ozone depletion and its interactions with climate change: 2010 assessment. Photochem Photobiol Sci. 2011;10:301–316. doi: 10.1039/c0pp90045a. [DOI] [PubMed] [Google Scholar]
- 9.Cadet J, Douki T, Ravanat JL, Di Mascio P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem Photobiol Sci. 2009;8:903–911. doi: 10.1039/b905343n. [DOI] [PubMed] [Google Scholar]
- 10.Yagura T, Schuch AP, Garcia CCM, Rocha CRR, Moreno NC, Angeli JPF, Mendes D, Severino D, Bianchini Sanchez A, Di Mascio P, de Medeiros MHG, Menck CFM. Direct participation of DNA in the formation of singlet oxygen and base damage under UVA irradiation. Free Radical Biol Med. 2017;108:86–93. doi: 10.1016/j.freeradbiomed.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Sage E, Girard PM, Francesconi S. Unravelling UVA-induced mutagenesis. Photochem Photobiol Sci. 2012;11:74–80. doi: 10.1039/c1pp05219e. [DOI] [PubMed] [Google Scholar]
- 12.Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radical Biol Med. 2017;107:110–124. doi: 10.1016/j.freeradbiomed.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Sochacka E, Kraszewska K, Sochacki M, Sobczak M, Janicka M, Nawrot B. The 2-thiouridine unit in the RNA strand is desulfured predominantly to 4-pyrimidinone nucleoside under in vitro oxidative stress conditions. Chem Commun (Cambridge, U K) 2011;47:4914–4916. doi: 10.1039/c1cc10973a. [DOI] [PubMed] [Google Scholar]
- 14.Nawrot B, Sochacka E, Duchler M. tRNA structural and functional changes induced by oxidative stress. Cell Mol Life Sci. 2011;68:4023–4032. doi: 10.1007/s00018-011-0773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunomura A, Moreira PI, Castellani RJ, Lee HG, Zhu X, Smith MA, Perry G. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotoxic Res. 2012;22:231–248. doi: 10.1007/s12640-012-9331-x. [DOI] [PubMed] [Google Scholar]
- 16.Sochacka E, Bartos P, Kraszewska K, Nawrot B. Desulfuration of 2-thiouridine with hydrogen peroxide in the physiological pH range 6.6–7.6 is pH-dependent and results in two distinct products. Bioorg Med Chem Lett. 2013;23:5803–5805. doi: 10.1016/j.bmcl.2013.08.114. [DOI] [PubMed] [Google Scholar]
- 17.Helm M, Alfonzo JD. Posttranscriptional RNA modifications: Playing metabolic games in a cell’s chemical legoland. Chem Biol. 2014;21:174–185. doi: 10.1016/j.chembiol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motorin Y, Helm M. TRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Ibba M. Bacterial transfer RNAs. FEMS Microbiol Rev. 2015;39:280–300. doi: 10.1093/femsre/fuv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björk G, Hagervall T. Transfer RNA Modification: Presence, Synthesis, and Function. EcoSal Plus. 2014;6 doi: 10.1128/ecosalplus.ESP-0007-2013. [DOI] [PubMed] [Google Scholar]
- 21.Čavužić M, Liu Y. Biosynthesis of Sulfur-Containing tRNA Modifications: A Comparison of Bacterial, Archaeal, and Eukaryotic Pathways. Biomolecules. 2017;7:27. doi: 10.3390/biom7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agris PF, Narendran A, Sarachan K, Vare VYP, Eruysal E. The importance of being modified: the role of RNA modifications in translational fidelity. Enzymes. 2017;41:1–50. doi: 10.1016/bs.enz.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh JDW, Sun L, Lau LZM, Tan J, Low JJA, Tang CWQ, Cheong EJY, Tan MJH, Chen Y, Hong W, Gao YG, Woon ECY. A strategy based on nucleotide specificity leads to a subfamily-selective and cell-active inhibitor of N6-methyladenosine demethylase FTO. Chem Sci. 2015;6:112–122. doi: 10.1039/c4sc02554g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan MA, Cannon JF, Webb FH, Bock RM. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985;161:368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantara WA, Bilbille Y, Kim J, Kaiser R, Leszczynska G, Malkiewicz A, Agris PF. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNA-(Arg1,2) J Mol Biol. 2012;416:579–597. doi: 10.1016/j.jmb.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 26.Vinayak M, Pathak C. Queuosine modification of tRNA: its divergent role in cellular machinery. Biosci Rep. 2009;30:135. doi: 10.1042/BSR20090057. [DOI] [PubMed] [Google Scholar]
- 27.Meier F, Suter B, Grosjean H, Keith G, Kubli E. Queuosine modification of the wobble base in tRNAHis influences ‘in vivo’ decoding properties. EMBO J. 1985;4:823–827. doi: 10.1002/j.1460-2075.1985.tb03704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsborn T, Tukenmez H, Chen C, Bystrom AS. Familial dysautonomia (FD) patients have reduced levels of the modified wobble nucleoside mcm(5)s(2)U in tRNA. Biochem Biophys Res Commun. 2014;454:441–445. doi: 10.1016/j.bbrc.2014.10.116. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JS, Srivastava S, Farwell KD, Lu HM, Zeng W, Lu H, Chao EC, Fatemi A. ELP2 is a novel gene implicated in neurodevelopmental disabilities. Am J Med Genet, Part A. 2015;167:1391–1395. doi: 10.1002/ajmg.a.36935. [DOI] [PubMed] [Google Scholar]
- 30.Simpson CL, Lemmens R, Miskiewicz K, Broom WJ, Hansen VK, van Vught PW, Landers JE, Sapp P, Van Den Bosch L, Knight J, Neale BM, Turner MR, Veldink JH, Ophoff RA, Tripathi VB, Beleza A, Shah MN, Proitsi P, Van Hoecke A, Carmeliet P, Horvitz HR, Leigh PN, Shaw CE, van den Berg LH, Sham PC, Powell JF, Verstreken P, Brown RH, Jr, Robberecht W, Al-Chalabi A. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum Mol Genet. 2009;18:472–481. doi: 10.1093/hmg/ddn375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewez M, Bauer F, Dieu M, Raes M, Vandenhaute J, Hermand D. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci U S A. 2008;105:5459–5464. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, Schmidt A, Aebersold R, Boone C, Hofmann K, Peter M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 33.Addepalli B, Limbach PA. Mass spectrometry-based quantification of pseudouridine in RNA. J Am Soc Mass Spectrom. 2011;22:1363–1372. doi: 10.1007/s13361-011-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell SP, Limbach PA. Evaluating the reproducibility of quantifying modified nucleosides from ribonucleic acids by LC-UV-MS. J Chromatogr B: Anal Technol Biomed Life Sci. 2013;923–924:74–82. doi: 10.1016/j.jchromb.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


