Abstract
While mortality following ST-segment elevation myocardial infarction (STEMI) is on the decline, the number of patients developing heart failure due to prior myocardial infarction (MI) is on the rise. Apart from timely reperfusion by primary percutaneous coronary intervention (PPCI), there is currently no established therapy for reducing MI size. As such new cardioprotective therapies are required to improve clinical outcomes following STEMI. Cardiovascular magnetic resonance (CMR) has emerged as an important imaging modality for assessing the efficacy of novel therapies for reducing MI size and preventing subsequent adverse left ventricular remodeling. The recent availability of multi-parametric mapping CMR has provided new insights into the pathophysiology underlying myocardial edema, microvascular obstruction, intramyocardial hemorrhage, and changes in the remote myocardial interstitial space following STEMI. In this article, we provide an overview of the recent advances in CMR imaging in reperfused STEMI patients, discuss the controversies surrounding its use, and explore future applications of CMR in this setting.
Keywords: Cardiovascular magnetic resonance, ST elevation myocardial infarction, percutaneous coronary intervention, myocardial infarct size, area-at-risk, microvascular obstruction, intramyocardial hemorrhage, T1 mapping, T2 mapping, T2* mapping, extracellular volume fraction mapping, late gadolinium enhancement
Introduction
Improvements in the treatment of patients with acute ST-segment elevation myocardial infarction (STEMI) have led to a decline in mortality over the past 4 decades1, with 1-year cardiac mortality in STEMI patients plateauing at around 8%.2 However, morbidity due to post-myocardial infarction (MI) heart failure remains significant, and is on the rise.3 The process of reperfusion itself can paradoxically induce further myocardial injury and cardiomyocyte death, a phenomenon termed ‘myocardial reperfusion injury’.4 There is currently no effective therapy for reducing myocardial reperfusion injury, despite a wealth of research in this field. This has been partly attributed to undertaking clinical studies despite the lack of reproducible and robust preclinical data and the design of the clinical cardioprotection studies in terms of patient selection and inappropriate timing and mode of delivery of the cardioprotective agent.5 As such, the search continues for a novel and effective therapy for reducing MI size and preventing heart failure, which can be administered as an adjunct to PPCI following STEMI. Cardiovascular magnetic resonance (CMR) has emerged as an important imaging modality for assessing the cardioprotective efficacy of novel therapies for reducing MI size and prevent adverse left ventricle (LV) remodeling in reperfused STEMI patients.6, 7 It is currently the gold standard imaging modality for quantifying MI size,8 and is able to detect small subendocardial MI (as little as 1 gram)9 with good accuracy.10
With the recent availability of native T1, T2, T2* and post-contrast T1-mapping (to derive extracellular volume fraction [ECV] mapping)11, more in-depth insights can be obtained into the pathophysiological processes such as the evolution of myocardial edema in the first week post-STEMI12, 13, the chronic manifestation of intramyocardial hemorrhage, and its prognostic significance over microvascular obstruction14, 15 and changes in the remote myocardial interstitial space in those developing adverse LV remodeling16, 17 following STEMI. In this article, we provide an overview of some of the recent advances in CMR imaging in reperfused STEMI patients, discuss the recent controversies surrounding its use, and explore future applications of CMR in this setting.
Basic concepts
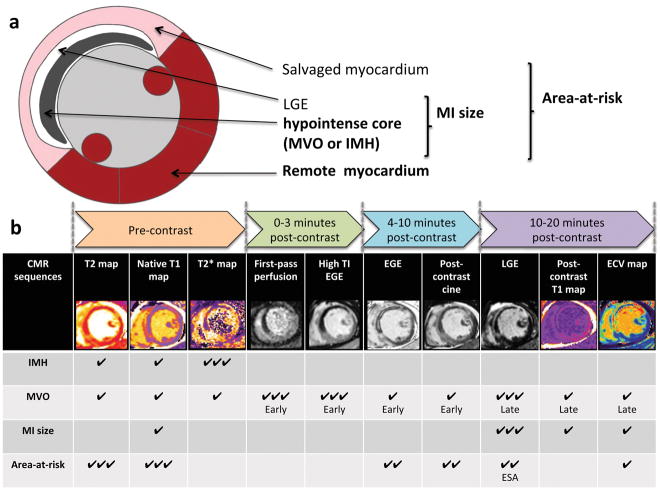
In this section, we provide a brief description of some of the basic concepts concerning the use of CMR in STEMI. Table 1 provides an explanation of the more technical terms used throughout this article. Figure 1a also provides an illustration of the various components of the myocardium that can be interrogated by CMR.
Table 1.
Technical descriptions of the various components of the myocardium assessed by CMR in STEMI
| Components | Technical description |
|---|---|
| Native T1, post-contrast T1 and ECV | This is the time constant to recover 63% of its longitudinal magnetization (spin–lattice relaxation) and is expressed in milliseconds. It is referred to as native T1 when performed prior to the administration of gadolinium chelate. 11 It is recommended that post-contrast T1 be performed at least 10 minutes following contrast administration.11 Once the native and post-contrast T1 of the blood and myocardium and the hematocrit are known, the ECV can be calculated using the formula below 11: |
| T2 | This refers to the constant representing the decay of transverse magnetization (spin-spin relaxation) to 37% of its initial equilibrium value and it is expressed in milliseconds. 11 |
| T2* | This is the time constant representing the decay of transverse magnetization in the presence of local magnetic field inhomogeneities and is expressed in milliseconds. 11 |
| Parametric mapping | A process where an anatomical map is generated with each pixel representing a specific magnetic tissue property (T1, T2, or T2* or ECV) derived from the spatially corresponding voxel of a set of co-registered magnetic resonance source images. 11 |
| MI size | This refers to the mass or volume of infarcted myocardium, and is conventionally quantified by late gadolinium enhancement. After 10 minutes, pseudo-equilibrium of gadolinium chelate concentration between blood and well-perfused myocardium develops. During this time, a higher concentration of contrast is distributed in areas of acute or chronic MI (significantly shortening T1) than in normal myocardium. Therefore, with appropriate nulling of the remote myocardium using inversion recovery T1-weighted sequences, the necrotic or scarred areas appear bright.6, 18 |
| Early microvascular obstruction | This can be identified as areas of dark core within the MI zone using first-pass perfusion or EGE imaging performed with a fixed, high TI (e.g. 440 to 500ms at 1.5T), acquired at 1–4 minutes post-contrast injection. |
| Late microvascular obstruction | This can be identified as a dark core within the areas of hyperenhancement on conventional late gadolinium enhancement sequences acquired >10 minutes post-contrast injection. |
| Intramyocardial hemorrhage | This can be identified using T2*-weighed imaging or T2*-mapping. From brain imaging data, the degradation of the extravasated erythrocytes to oxyhemoglobin, deoxyhemoglobin, and methemoglobin (strongly paramagnetic) is dynamic and exhibits different magnetic properties at various stages as previously described by Bradley and colleagues.19 Breakdown of the erythrocyte membrane eventually leads to ferritin and hemosiderin deposits within the macrophages and the iron-degradation products can be detected using T2* imaging. Most studies have used a cut-off value for T2*of < 20ms14, 20 to detect intramyocardial hemorrhage but a threshold-based method of 2 standard deviations below the mean remote myocardial T2* can also be used.21 In centers where T2* imaging is not available, many studies have also used T2-weighted imaging but its diagnostic performance is less robust than T2*-weighted imaging.22 |
| Area-at-risk | This refers to the territory supplied by the infarct-related artery and includes both the reversibility injured myocardial (the salvaged myocardium) and the infarcted myocardium. The area-at-risk can be indirectly assessed by CMR using late gadolinium enhancement imaging (to derive the endocardial surface area)23, T2-weighted imaging24, 25, T2-mapping26, 27, native T1-mapping27, 28, early gadolinium enhancement imaging29, 30, pre-contrast SSFP cine31, and post-contrast SSFP cine imaging32, 33 T1 and T2-mapping are currently considered the most robust of the CMR techniques as they have better contrast-to-noise ratio than the T2-weighted/ T1-weighted/cine imaging techniques; they are less prone to blood pool and motion artifacts; and they are less likely to suffer from signal drop-out due to surface coil inhomogeneities.26, 28, 34 |
| Myocardial Salvage and myocardial salvage index | Myocardial salvage can be calculated by subtracting the MI size from the area-at-risk. The myocardial salvage index refers to the ratio of the myocardial salvage to the area-at-risk. 35 |
| Remote myocardium | This is usually defined as a myocardial segment 180° from the infarcted territory. 36 |
ECV: extracellular volume fraction; EGE: early gadolinium enhancement; CMR: cardiovascular magnetic resonance; SSFP: steady state free precession
Figure 1. (a) Schematic representation of the different components of the myocardium in a patient with reperfused STEMI and; (b) methods for delineating the area-at-risk, MI size, microvascular obstruction and intramyocardial hemorrhage by CMR.
Figure 1a illustrates the various components of the myocardium in a reperfused STEMI patient that can be assessed by CMR, namely the area-at-risk, salvaged myocardium, the area of hyperenhancement and the hypointense core on late gadolinium enhancement images and the remote myocardium. The MI size includes both the areas of hyperenhancement and the hypointense core on late gadolinium enhancement images. The hypointense core on late gadolinium enhancement represents microvascular obstruction and a proportion of patients would also have intramyocardial hemorrhage within these areas (areas of low T2* on T2*-weighted images or mapping). The area-at-risk is a combination of both the MI size and the salvaged myocardium.
Figure 1b illustrates the CMR-based methods and their timing during CMR acquisition for delineating the area-at-risk, MI size, microvascular obstruction (early and late) and intramyocardial hemorrhage. The most robust method for demarcating the area-at-risk currently is T1 and T2-mapping. Late gadolinium enhancement is the gold standard for detecting MI size and late microvascular obstruction. T2*-mapping is currently the gold standard to detect intramyocardial hemorrhage. The table provides an indication of the abilities of each sequence to detect each components of an MI by STEMI and graded as: ✔✔✔: Robust, have been shown by several studies; ✔✔: Possible, have been shown by some studies; ✔: Theoretically possible and/or have only been shown by 1–2 studies
MVO: microvascular obstruction; IMH: intramyocardial hemorrhage; EGE: early gadolinium enhancement; LGE: late gadolinium enhancement; ESA: endocardial surface area
Myocardial Infarct Size
MI size refers to the infarcted myocardium and is conventionally quantified by late gadolinium enhancement (Table 1 and Figure 1b).37 The gadolinium chelate cannot cross the intact cell membranes.37 Following acute myocardial necrosis, the cell membranes are ruptured allowing the contrast agent to enter these cells. In the chronic setting, there is also expansion of the extracellular space due to the collagen deposits and the relatively low residual amount of intact cardiomyocytes in the region of the infarct. Therefore, following gadolinium chelate administration, the contrast redistributes itself from the vascular compartment to the interstitial space and at pseudo-equilibrium a higher concentration of contrast is distributed in areas of acute or chronic MI than in normal myocardium. 18 (Table 1).
Microvascular Obstruction and Intramyocardial Hemorrhage
Microvascular obstruction refers to the inability to reperfuse the coronary microcirculation in a previously ischemic region, despite opening the epicardial vessel.38 Microvascular obstruction can be identified as a dark hypo-intense core within the areas of hyper-enhancement on either early gadolinium enhancement (referred to as early microvascular obstruction) or conventional late gadolinium enhancement sequences (referred to as late microvascular obstruction) - defined in Table 1 and illustrated in Figure 1b.
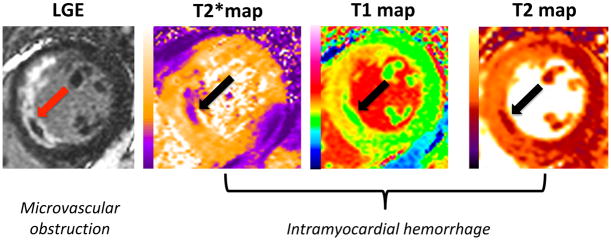
If the coronary microvasculature injury following STEMI is especially severe, and the integrity of the vessels are damaged, the extravasation of red blood cells into the myocardium can occur - termed intramyocardial hemorrhage.14 The breakdown products of hemoglobin within the myocardium can be detected as a hypo-intense core39 within the MI zone on T2*-imaging or T2*-mapping (Figures 1 and 2). Of the two approaches, T2*-mapping has been shown to have greater sensitivity for detecting intramyocardial hemorrhage following STEMI when compared to T2-mapping (Table 1).40,41
Figure 2. The infarct core of a patient with microvascular obstruction and intramyocardial hemorrhage.
This figure shows an example of a patient with an acute anterior STEMI treated by PPCI and the CMR images on day 4 showing the infarct core on the late gadolinium enhancement images representing microvascular obstruction and on the T2*, T1 and T2 maps representing intramyocardial hemorrhage (arrows).
LGE: late gadolinium enhancement
A small study previously showed that the hypointense core on the T1 or T2 maps could provide an alternative method to detect intramyocardial hemorrhage in cases where T2* is not available or not interpretable (Figures 1 and 2)39 and further studies are needed to confirm these findings.
Area-at-risk
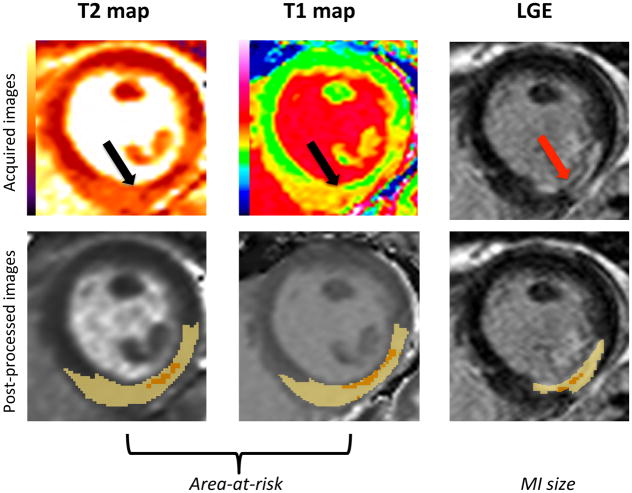
The area-at-risk refers to the territory supplied by the infarct-related artery that would have infarcted following STEMI, if reperfusion had not taken place to salvage viable myocardium. The area-at-risk includes both the reversibility injured myocardium (also referred to as the salvaged myocardium) and the infarcted myocardium. Figure 3 shows an example of the edema-based area-at-risk by T1 and T2-mapping and the corresponding MI size on late gadolinium enhancement in a patient presenting with an acute inferior STEMI treated by PPCI.
Figure 3. Delineation of the edema-based area-at-risk by T1 and T2-mapping, and MI size by late gadolinium enhancement that can be used to calculate myocardial salvage and myocardial salvage index.
The top panel shows an example of the edema-based area-at-risk by T1 and T2-mapping (indicated by black arrows) and the corresponding MI size on late gadolinium enhancement (red arrow) in a patient with an inferior STEMI treated by PPCI, with the CMR images acquired on day 3 post-reperfusion. The bottom panel shows the corresponding post-process images using a threshold-based method to delineate the area-at-risk (threshold of 2 standard deviations) and MI size (threshold of 5 standard deviations) as the highlighted areas with the areas of hypointense core included as part of the area-at-risk and MI size. The myocardial salvage (area-at-risk subtract the MI size) and myocardial salvage index (myocardial salvage as a ratio of the area-at-risk) can be retrospectively calculated from a single CMR scan.
LGE: late gadolinium enhancement
A number of CMR approaches to delineating the area-at-risk following STEMI have been described (see Table 1). However, they all have their limitations,42 and although each technique has been validated against histology in the preclinical setting24, 27, 30, 31, 33, there is currently no consensus on which CMR method should be used to quantify the area-at-risk in the clinical setting. So far, no studies have compared these techniques, head-to-head, in the same patient cohort. Once the area-at-risk and MI size are known, the myocardial salvage index can be calculated as described in Table 1.
The myocardial salvage index is considered a more sensitive measure for assessing the efficacy of novel cardioprotective therapies, when compared to MI size alone35, as it normalizes the MI size reduction to the area-at-risk, the latter of which varies from patients to patient. As such, the myocardial salvage index may help to reduce sample size for clinical cardioprotection studies in comparison to using MI size alone.36
Recent advances in the role of CMR in reperfused STEMI patients
In this section, first of all, we will summarize the advances in the use of T1 mapping to assess MI size. We will then elaborate how CMR has improved our understanding of the relationship between microvascular obstruction and intramyocardial hemorrhage; and the role of MI size, intramyocardial hemorrhage, residual myocardial iron and the remote myocardium in the development of adverse LV remodeling. Finally, we will explore the prognostic significance of these CMR-derived indices in the reperfused STEMI patients.
T1-mapping for the quantification of MI size
Post-contrast T1-mapping has recently emerged as a promising technique for the quantification of MI size, as an alternative to conventional late gadolinium enhancement imaging. Bulluck and colleagues43 recently showed that post-contrast T1-mapping can accurately quantify acute MI size in a small group of STEMI patients, obviating the need to perform late gadolinium enhancement imaging. Recent studies have also investigated whether native T1-mapping (pre-contrast) can assess MI size. Kali and colleagues44 showed that chronic MI (median of 13.6 years post-MI) could be detected using native T1-mapping at 3T in a small cohort of 25 patients. Liu and colleaguesl45 showed that native T1-mapping at 3T could also identify acute MI size. However, only 58 short-axis T1 maps without microvascular obstruction were analyzed in that study.45 These findings are of great interest as one could potentially perform a comprehensive CMR study in STEMI without the need of contrast agent and this approach would also significantly shorten the scan time and make CMR available to a wider range of patients.
Whether ECV on an acute CMR scan can estimate final MI size following STEMI has recently been investigated. Garg and colleagues46 showed that an ECV value of 0.46 or above could predict chronic MI size from the acute ECV maps. However, Bland-Altman analysis showed a bias of 1.9% and wide limits of agreement of ±10.5% and further studies are therefore needed to build on these findings.47 However, as it stands, ECV-mapping may complement late gadolinium enhancement imaging to assess MI size and predict wall motion recovery at follow-up, as recently shown by 2 small studies.16, 48
Despite the promises of T1-mapping in STEMI for measuring MI size, the published studies have been small, and from single-centers. Larger studies are therefore required to confirm these findings, using different field strengths, mapping sequences, and vendors, before they can be more widely adopted.
Relationship between microvascular obstruction and intramyocardial hemorrhage
Serial CMR imaging of STEMI patients in the first few days following PPCI has provided evidence that the incidence and extent of microvascular obstruction and intramyocardial hemorrhage vary with time. The extent of late microvascular obstruction has been reported to peak at 4–12 hours, to remain stable the first 2 days and to reduce in size by day 10 following STEMI.14 Furthermore, in those patients with microvascular obstruction persisting at one week, Orn and colleagues49 showed that they were more likely to develop adverse LV remodeling compared to those with microvascular obstruction at day 2 only. On the other hand, the detection of intramyocardial hemorrhage has been shown to peak at day 3, and to reduce in incidence by day 10 following PPCI.12
Hamirani and colleagues40 showed that intramyocardial hemorrhage (detected by either T2-weighted imaging or T2* imaging) was more strongly associated with late microvascular obstruction (R=0.89 to 0.93) than with early microvascular obstruction (R=0.30). Using the more robust method of T2*-mapping for the detection of intramyocardial hemorrhage, Carrick and colleagues14 in 286 patients showed that all patients with intramyocardial hemorrhage also had late microvascular obstruction.
Based on these recent studies14, 40, 50, 51, it appears that early microvascular obstruction occurs in around 60–65%; late microvascular obstruction in around 50–55%; and intramyocardial hemorrhage in around 35–40% of reperfused STEMI patients. However, the detection of microvascular obstruction and intramyocardial hemorrhage is dependent on the CMR techniques, the timing of imaging, and the definitions used.
Acute MI size and subsequent adverse LV remodeling
Conventional theory assumes that the larger the MI size, the higher the LV wall stress, and the LV therefore dilates to maintain the stroke volume as a compensatory mechanism (via the Frank-Starling principle). However, LV dilation, as described by the Laplace relationship, leads to further wall stress and begets more LV dilation, in the absence of compensatory LV hypertrophy. Westman and colleagues52 have recently proposed that there is an imperfect association between MI size and adverse LV remodeling. They showed in 122 STEMI patients, that 15% of those with MI size <18.5% developed adverse LV remodeling, and 40% of those with MI size ≥18.5% developed adverse LV remodeling. However, Westman and colleagues52 used a definition of >10ml/m2 increase in indexed LV end-diastolic volume in their study. A more appropriate definition for adverse LV remodeling post-STEMI by CMR may be a cut-off value of 12% change in LV end-diastolic volume.53 This study also showed an imperfect association between acute MI size and adverse LV remodeling, and between the presence of microvascular obstruction and adverse LV remodeling.53 Some patients with large MI size and microvascular obstruction developed reverse LV remodeling and whereas other patients with small MI size and no microvascular obstruction also developed adverse LV remodeling.53 As eluded to by Westman and colleagues52, the development of adverse LV remodeling is complex and multi-factorial and an excessive inflammatory response together with MI size may play an important role following STEMI.
Residual myocardial iron and adverse LV remodeling
There are emerging data that intramyocardial hemorrhage at the time of PPCI leads to residual myocardial iron during the convalescent phase, and it may be a source of prolonged inflammation and impact on adverse LV remodeling.54 Kali and colleagues54 showed in a small cohort of 15 STEMI patients and in 20 canines (with histological validation) that intramyocardial hemorrhage resulted in residual myocardial iron within the MI zone, and this provided a source of prolonged inflammatory burden in the chronic phase. These findings were recently confirmed in a canine model of MI, and the extent of residual iron was strongly correlated with markers of inflammation and adverse LV remodeling.55 Roghi and colleagues56 found higher levels of non-transferrin bound iron in 7 out of 15 STEMI patients with microvascular obstruction and postulated that intramyocardial hemorrhage could be a source of cardiotoxicity in these patients.
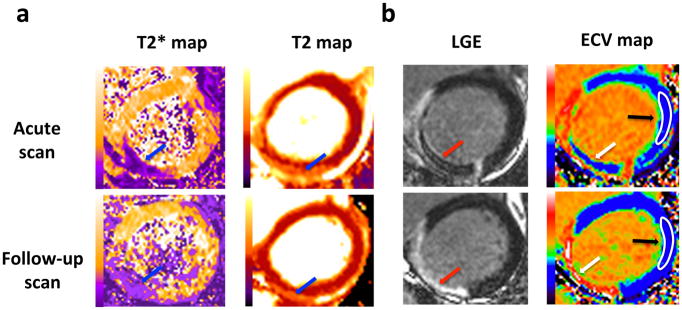
In a small cohort of STEMI patients, Bulluck and colleagues15 recently found that intramyocardial hemorrhage and subsequent residual myocardial iron at follow-up were associated with persistently elevated T2 values in the surrounding infarct tissue and with adverse LV remodeling.15 Carberry and colleagues57 have recently confirmed these findings in a cohort of 203 STEMI patients. They showed that 36% of their cohort had intramyocardial hemorrhage by T2*-mapping and 59% of those had residual myocardial iron at 6 months. Residual myocardial iron was associated with adverse LV remodeling at 6 months and worse clinical outcomes after a median follow-up of 4 years.57 Figure 4a shows an example of a patient with an acute inferior STEMI treated by PPCI, and with late microvascular obstruction and intramyocardial hemorrhage despite restoring normal flow in the epicardial coronary artery during PPCI. At follow-up, there was residual myocardial iron and persistently elevated T2 values in the areas surrounding the residual iron, which may represent persistent myocardial inflammation.
Figure 4. (a) Paired acute and follow-up T2* and T2 maps; (b) Paired acute and follow-up automated ECV maps of a patient with an inferior STEMI.
This figure shows an example of the paired acute and follow-up (6 months) late gadolinium enhancement and automated ECV maps, T2* and T2 maps of a patient with an inferior STEMI, treated by PPCI with restoration of normal flow in the infarct-related epicardial coronary artery. The arrows show the area of microvascular obstruction with intramyocardial hemorrhage. The corresponding ECV maps show the area of microvascular obstruction where contrast fails to penetrate the areas of microvascular obstruction and appears as pseudo-normal myocardium on the acute scan. At follow-up, the microvascular obstruction has resolved and the space previously occupied by the microvascular obstruction has very high ECV (white areas within the MI zone corresponding to the upper limit of the look-up color scale). The white regions of interest on the ECV maps (black arrows) are representative areas of the remote myocardium that can be used to assess changes in the extracellular matrix. There was residual myocardial iron at follow-up and persistently elevated T2 in the areas surrounding the residual iron (blue arrows on the follow-up images.
LGE: late gadolinium enhancement
The role of the remote myocardium and clinical outcomes
Whether changes in the extracellular matrix in the remote myocardium (Table 1) in STEMI patients treated by PPCI are associated with adverse LV remodeling, has been a topic of on-going research.16, 17, 58–60 Using automated ECV maps, Bulluck and colleagues16 have showed in a small cohort of 40 STEMI patients that ECV in the remote myocardium was acutely elevated, and this elevation in ECV persisted in those who developed adverse LV remodeling at 5 months, suggesting that remote compensatory changes in the extracellular matrix occurred in this subset of patients. The increase in acute ECV could have been due to an increase in the intravascular compartment as a compensatory mechanism. At follow-up, the increase in ECV likely represented diffused interstitial fibrosis, as part of the remodeling process. Garg and colleagues60 recently confirmed these findings in a cohort of 50 STEMI patients. Furthermore, they also showed that remote segments with ECV expansion were associated with impaired wall thickening. In a larger cohort of patients (n=131), Carberry and colleagues17 showed that the change in ECV of the remote myocardium was a multivariable associate of the change in LV end-diastolic volume at 6 months. Whether this change in ECV could independently predict those at risk of adverse events remains to be tested in future, adequately powered studies. Figure 4b shows an example of the paired acute and follow-up (6 months) late gadolinium enhancement and automated ECV maps with representative regions of interest of the remote myocardium of a patient with an anterior STEMI treated by PPCI.
Carrick and colleagues59 demonstrated that higher native T1 of the remote myocardium of 267 reperfused STEMI patients on the acute CMR scan was associated with changes in LV end-diastolic volume from baseline to 6 months, and it was independently associated with adverse cardiac events after a median follow-up of 845 days. Most recently, in a similar number of patients, Reinstadler and colleagues58 also showed that the remote native myocardial T1 was independently associated with adverse events, after adjusting for clinical risk factors and other CMR variables after a follow-up of 6 months.
Therefore, T1 mapping has the potential to completement clinical and other CMR-derived parameters to improve the risk-stratification of STEMI patients and warrants further investigation in a multi-center setting.
Acute MI size and clinical outcomes
Morbidity and mortality post-STEMI is closely related to acute MI size. A recent meta-analysis of 2,632 patients showed that MI size measured by CMR or SPECT within a month post-PPCI from 10 randomized controlled trials (RCTs) was strongly associated with 1-year hospitalization for heart failure and all-cause mortality.61 For every 5% increase in MI size, there was a 20% increase in the relative hazard ratio for 1-year hospitalization for heart failure and all-cause mortality. The event rate was 1.2% in those with MI size ≤ 8% of the LV and there was a stepwise increase to 2.5% in those with MI size >8% – ≤ 17.9% of the LV; 5.6% in those with MI size >17.9% – 29.8% of the LV; and 8.8% in those with MI size >29.8% of the LV. This study adds to the growing body of evidence that acute MI size is prognostic, and therefore remains a valid surrogate endpoint for clinical trials. However, this study also highlights the fact that one of the limitations of using CMR in RCTs is that there is an element of selection bias as only those patients fit enough to tolerate a CMR study would enter these RCTs and the overall event rates were 2.2% and 2.6% for of all-cause mortality and hospitalization for heart failure at 1 year, respectively, much lower than those reported from clinical registries. 2, 3
Microvascular obstruction and intramyocardial hemorrhage and clinical outcomes
Both microvascular obstruction and intramyocardial hemorrhage are associated with larger MI size, adverse LV remodeling, and worse clinical outcomes.40, 50 In a meta-analysis of more than 1000 patients, Van Kranenburg and colleagues50 showed that the presence of microvascular obstruction was an independent predictor of major adverse clinical outcome at 2 years in STEMI patients, whereas MI size was not independently associated with adverse events. There was a graded reduction in event-free survival in patients with MI size <25% of the LV, with and without microvascular obstruction, and those with MI size >25% of the LV, with and without microvascular obstruction. The prognostic value of microvascular obstruction over MI size for mortality and hospitalization for heart failure was recently confirmed by de Waha and colleagues51 in a pooled analysis of patient-level data from 7 randomized controlled trials (n=1688) at 1 year and by Symons and colleagues62 in a longitudinal study of 810 patients following a median follow-up of 5.5 years.
Early studies showed that intramyocardial hemorrhage, detected by T2-weighted images, was closely related to the development of adverse LV remodeling and worse clinical outcomes.40 Most recently, using T2*-mapping, Carrick and colleagues14 showed that intramyocardial hemorrhage was more closely associated with adverse clinical outcomes than microvascular obstruction.
Therefore, based on the current evidence discussed so far, the prognosis worsens with larger MI size. Patients with larger MI size are also more likely to have microvascular obstruction and intramyocardial hemorrhage. The prognosis is worse for STEMI patients with microvascular obstruction when compared to those without microvascular obstruction63 and is worst for those with microvascular obstruction and intramyocardial hemorrhage.14
Currently, there are no effective methods to detect those at risk of microvascular obstruction and intramyocardial hemorrhage at the time of PPCI. Amier and colleagues64 recently showed that anterior STEMI and use glycoprotein IIb/IIIa inhibitors were associated with the development of intramyocardial hemorrhage. However, this was a retrospective post-hoc analysis and intramyocardial hemorrhage was identified using T2-weighted imaging instead of the more robust T2*-weighted imaging and their findings need to be confirmed in future studies. Furthermore, there are no established therapies to prevent or minimize the burden of microvascular obstruction and intramyocardial hemorrhage in the clinical setting4, although promising results are emerging in the preclinical setting.65
Current controversies in CMR imaging of STEMI patients
Is myocardial edema confined to the infarct zone or does it extend into the salvaged myocardium?
In the 1980s, Higgins and colleagues66 were the first to study myocardial T2 CMR in a canine model of MI. Interestingly, both T2 and T1 were shown to change similarly in the setting of an acute MI. The observed changes were theoretically consistent with myocardial edema, and correlated with the measurements of myocardial water content estimated by wet-weight to dry-weight ratios. The changes in myocardial T2 have been attributed to a combination of an increase in absolute tissue water, movement of water from the extracellular to the intracellular compartment, and the conversion of protein-bound water to free water.67
Not all experimental and clinical studies have supported the concept that the region of hyper-intensity on T2-weighted images delineates the area-at-risk. Aletras and colleagues24 and Tilak and colleagues68 validated the edema-based area-at-risk using T2-weighted images against microsphere in reperfused and non-reperfused canine MI. Ubach and colleagues69 showed that the area-at-risk derived from T2-weighted imaging matched that obtained from single-photon emission computed tomography, both in animals with and without late gadolinium enhancement.
In contrast, Kim and colleagues70 reported that T2-weighted imaging did not depict the area-at-risk in anesthetized dogs that were subjected to different durations of coronary occlusion. MI size by late gadolinium enhancement was compared to the gold standard method of triphenyl tetrazolium chloride staining, and area-at-risk by T2-weighted imaging was compared to the gold-standard method of fluorescent microspheres, 4 days after reperfusion. The T2-weighted hyper-intensity correlated and matched in shape better with the MI size than with the area-at-risk, and these findings were confirmed in a small group of acute MI patients. Although the findings from the canine model of MI may be due to the presence of well-developed collaterals in that species, the T2-weighted sequence in that study was different to what has been used so far by other studies.24, 68 In the clinical setting, using hybrid positron emission tomography/CMR imaging in reperfused STEMI patients, Bulluck and colleagues71 recently showed that the area of reduced 18F-fluorodeoxyglucose uptake was significantly larger than the MI size and closely matched the area-at-risk delineated by T2-mapping on the acute scan, supporting the notion that T2-mapping does delineate both the reversibly and the irreversibly injured myocardium within the area-at-risk71. In addition, the areas of reduced 18F-fluorodeoxyglucose uptake within the salvaged myocardium within the area-at-risk were no longer present on a follow-up scan several months later.71 Hammer-Hansen and colleagues72 have provided further insights on this topic, using a canine model of MI. They showed that T2 values were elevated both in the MI zone and salvaged myocardium, and they were both significantly higher than the T2 values in the remote myocardium.
Therefore, the majority of the current literature supports the notion that edema, assessed by CMR using T2-mapping, occurs both in the irreversibly and reversibly injured myocardium during the first week of a reperfused STEMI in the clinical setting.26, 28, 71, 73
Dynamic changes in the edema-based area-at-risk during the first week in reperfused STEMI patients
It was initially believed that the extent of myocardial edema was stable in the first week in STEMI patients73, 74, although both these studies only performed T2-weighted imaging on days 1 and 7. Emerging evidence suggests that the extent of myocardial edema is dynamic. Hammer-Hansen and colleagues72 have shown in a canine model of reperfused MI, that at 2 hours, T2 values were higher in both the infarcted and salvaged myocardium when compared to those who had the scan at 48 hours, highlighting that the extent of myocardial edema within the first few days of an MI is dynamic.
Fernandez-Jimenez and colleagues75 studied the interplay between myocardial water content (using desiccation) and T2 relaxation times in a porcine model of reperfused MI. They reported a ‘bimodal’ pattern of edema, appearing at 2 hours, resolving by 24 hours and then reappearing on day 4 and peaking at day 7. The first wave of edema was attributed to myocardial reperfusion injury, and the second wave of edema was attributed to inflammation during the tissue-healing phase.76 However, they did not take into account the presence of microvascular obstruction and intramyocardial hemorrhage, which may have interfered with their T2 measurements, and they had a small sample size of 5 pigs at each time point. Furthermore, the same animals were not serially scanned. Most recently, Fernandez-Jimenez and colleagues13 showed that the bimodal edematous response of the infarct related territory was present in humans as well. However, they used the less robust T2-weighted imaging modality instead for both quantifying the extent of the edema-based area-at-risk and for detecting intramyocardial hemorrhage. It was not clear how many of their 14 patients had intramyocardial hemorrhage in that cohort. Furthermore, on a patient-level basis (their supplementary material figures 3 and 4), not all patients displayed a bimodal response for of the intensity and extent of edema in the infarct-related territory.
It was initially believed that edema is stable in the first week of an MI.73, 74 However, both these studies only performed T2-weighted imaging on days 1 and 7 and the peak in the edema-based area-at-risk shown by Carrick and colleagues12 on day 3 was therefore not identified by the previous 2 studies.73
In contrast to these studies, in a cohort of 30 patients with serial imaging, Carrick and colleagues12 found that the extent of myocardial edema was maximal at day 3, and decreased by day 10 following PPCI, suggesting a unimodal peak in myocardial edema in the first few days following reperfusion. Interestingly, in STEMI patients with intramyocardial hemorrhage, they did observe a bimodal pattern in T2 and T2* values within the MI core, whereas in patients without intramyocardial hemorrhage, only a unimodal pattern in T2 and T2* values was observed, suggesting that the presence of intramyocardial hemorrhage may have been responsible for the apparent bimodal edema pattern in T2 and T2* values.
Nordlund and colleagues32 recently provided some evidence that there was no bimodal pattern of edema in the clinical setting. They combined data from 3 studies involving 215 patients and showed there were no difference in size, quality of the T2-weighted images and the ability to detect the culprit territory when patients having a CMR study on Day 1 to Day 6 onwards were compared.,
Given the dynamic changes in the extent of edema and acute MI size over the first few days following STEMI, it is important that future clinical cardioprotection studies define the time-window for performing the acute CMR scan in order to optimize the quantification of both acute MI size and AAR. There is currently no consensus on the optimal timing for performing the acute CMR scan although 3–5 days6 and 4–7 days13 post-PPCI have been proposed.
Cardioprotective therapies and the edema-based area-at-risk in reperfused STEMI patients
The utility of T2-weighted imaging for quantifying the area-at-risk has been put into question because certain cardioprotective therapies have been shown to reduce not only MI size, but the extent of myocardial edema measured by T2-weighted and T2-mapping imaging. Ischemic postconditioning77 and remote ischemic conditioning using transient arm or leg ischemia and reperfusion) 78 have been shown to reduce both MI size and the extent of edema delineated by T2-mapping and T2-weighted imaging, leading to an underestimation of the area-at-risk by these techniques. However, these were small studies of reperfused STEMI patients and they were not adequately powered to assess that endpoint. Intuitively, the fact that a cardioprotective therapy can reduce MI size, it should be expected to also limit the extent of myocardial edema, which itself is the result of myocardial ischemia and reperfusion injury.
However, in a recently published large study of 696 STEMI patients by Eitel and colleagues79, ischemic postconditioning or a combination of remote ischemic conditioning and ischemic postconditioning did not reduce the extent of myocardial edema when compared to the control arm. Furthermore, drugs such as metoprolol80 and exenatide81 that were effective at increasing the myocardial salvage index did not reduce the extent of myocardial edema. One potential explanation for this discrepancy could be that those therapies that are potent enough to reduce MI size in reperfused STEMI patients, are capable to reduce the extent of myocardial edema. In contrast, those therapies that are less potent and do not reduce MI size, and only increase myocardial salvage, do not affect the extent of myocardial edema. Either way, these findings may question the use of edema-based area-at-risk measured by CMR to assess myocardial salvage in clinical cardioprotection studies.
The optimal timing of late gadolinium enhancement imaging for acute and chronic MI size quantification
Acute MI size has been shown to be dynamic and to decrease significantly in size between Day 1 and Day 7 post-STEMI.82 In a recent study by Carrick and colleagues12, MI size was shown to be similar between days 1 and 3, and reduced in size by day 10. Jablonowski and colleagues83 recently provided some mechanistic insights into this phenomenon in a porcine model of acute MI. At day 1, they found higher ECV values in the peri-infarct zone that could be due to severe edema or a mixture of infarcted and salvaged myocardium in that area that contributed to the overestimation of MI size. By 7 days, the ECV values in the peri-infarct zone decreased to the same level as the rest of the salvaged myocardium, such that MI size assessed by late gadolinium enhancement matched that by histology.
Acquiring late gadolinium enhancement too early (<8 minutes post–contrast administration) can result in an overestimation of MI size84, and acquiring late gadolinium enhancement at 25 minutes for acute MI size has been shown to be a better predictor of LV recovery.85 However waiting 25 minutes post-contrast administration may be difficult to apply in the clinical setting due to time constraints, and would affect workflow. As such, late gadolinium enhancement images are currently acquired 10–15 minutes post-contrast6 as a compromise.
Several studies have already shown a significant regression in MI size (by 30–35%) occurring between the acute CMR scan and the chronic phase.49, 86, 87 There are several factors responsible for this observation. First of all, the composition of the different tissues being identified by late gadolinium enhancement in the acute (myocardial necrosis compounded with edema, intramyocardial hemorrhage and microvascular obstruction) and chronic phase (focal replacement fibrosis) is different. The regression of MI size between the acute, sub-acute and chronic phases therefore represents the gradual resolution of edema, intramyocardial hemorrhage and microvascular obstruction and the gradual replacement of the necrotic cardiomyocytes with focal scars.73, 87
As it stands, the optimal timing of CMR for MI size is not well established but late gadolinium enhancement appears to stabilize by 10 days post the index event.12 In most centers, STEMI patients with an uncomplicated inpatient stay are discharged within the first week. Logistically, this would mean that patients would have to come back for the CMR scan and this may lead to a proportion of patients dropping out.6 Efforts are underway to attempt to standardize the acquisition of CMR for clinical cardioprotection studies.6
Future directions
The need to standardize the use of CMR in reperfused STEMI patients
Both acute and chronic MI sizes by CMR have been shown to be stronger predictors of clinical outcomes when compared to LV ejection fraction or LV volumes.88, 89 Therefore, due to the robustness and high reproducibility of CMR to quantify MI size10, 37,90,88, 89, both acute and chronic MI size are increasingly being used as surrogate endpoints for randomized control trials assessing the effectiveness of new cardioprotective therapies, and can reduce the sample size required.6 However, acute MI size is influenced by a number of factors including the timing of the CMR scan, the dose of contrast used, the time elapsed following contrast administration and acquisition of late gadolinium enhancement imaging and the method used for the quantification of MI size.6, 91 Therefore, there is an urgent need for the CMR community to come up with a consensus position to standardize the acquisition of late gadolinium enhancement imaging and the quantification of MI size in future studies.6 This would no doubt help to strengthen the robustness of this technique and would also facilitate prospective or retrospective collaborative research with merging of CMR data from different centers around the world.
Towards improving the tolerability and accessibility of CMR in reperfused STEMI patients
There have already been some advances in free-breathing and motion corrected T292 and T2*-mapping93 and late gadolinium enhancement imaging94 negating the need for breath-holding and helping to accelerate the acquisition of these sequences. Other developments in real-time cine imaging for LV ejection fraction and future refinements in CMR fingerprinting techniques (for robust and fast acquisition of simultaneous T1 and T2-mapping data per slice within one breath-hold) will further reduce scan time, and will be highly desirable in the acute STEMI setting. These developments would make CMR, which is currently confined to those fit enough to lie inside the scanner for at least 30–45 minutes, accessible to a wider number of patients. Furthermore, reducing the duration of the scan to 20–30 minutes could also potentially reduce the cost of a scan, and improve the participation of more centers in STEMI-related CMR research. However, most of these new developments are still in the validation phase and are not available to all CMR vendors and platforms.
The use of gadolinium chelates is not recommended in those with a glomerular filtration rate < 30 mL/min/1.73m2, to minimize the risk of nephrogenic systemic fibrosis, and constitutes a contraindication in CMR-based clinical cardioprotection studies. Furthermore, the Food and Drug Administration (FDA) has recently issued a new warning regarding gadolinium remaining in patients’ brainstem, for months to years after receiving these drugs. However, gadolinium retention has not been directly linked to adverse events in patients with normal renal function and the current benefits of using gadolinium chelates outweigh any potential risks. Therefore, the potential for non-contrast CMR imaging such as T1 mapping20 and T2* mapping14 to provide adequate prognostic information over post-contrast imaging modalities and that could be applied to all patients, irrespective of renal function, warrants further investigation.
CMR as a tool to risk-stratify and guide management in STEMI patients at risk of developing adverse LV remodeling and subsequent heart failure
A number of cardioprotective therapies have had promising results in the experimental setting for reducing MI size and preventing adverse LV remodeling, but have failed to be translated into the clinical setting.95 A more targeted approach using CMR to identify those most at risk (e.g. according to their acute MI size or according to the presence or absence of microvascular obstruction) may improve the translation of those promising experimental results in the clinical setting, to prevent adverse LV remodeling and reduce the onset of heart failure in these patients. Stiermaier and colleagues96 and Pontone and colleagues97 most recently proposed CMR risk scores to identify those at high risk of adverse events. Their approach would be ideal to identify high-risk patients but the CMR indices and cut-off values included in each study differed and further validation work is required to build on their findings. Furthermore, CMR could identify those with intramyocardial hemorrhage, and they could be targeted with anti-inflammatory agents or chelation therapy15, 98, to minimize the cardiotoxic effects of the residual iron (which has been shown to occur in two-third of patients with intramyocardial hemorrhage from a pooled analysis of 4 studies)57 during the convalescent phase of an acute STEMI and this warrants further investigation.
CMR as a tool for risk-stratification of STEMI patients at risk of arrhythmic events
The current guidelines recommend an implantable cardioverter defibrillator (ICD) for primary prevention in those with symptomatic heart failure and LVEF<35% 40 days following the index event.99 However, mortality within the first 30 days post-MI has been shown to be the highest 100 but is predominantly believed to be due to MI or myocardial rupture.101 Despite that, a quarter of those deaths are still due to ventricular arrhythmias 101 and currently there is no established tool to risk stratify these patients in the acute phase of an MI. Acute MI size (cut-off of 23.5g/m2 or 31% of the LV) in combination with LVEF (cut-off of 36%) by CMR within the first week on an MI has been shown to predict adverse arrhythmic cardiac events at 2 years.102 Other studies have shown that CMR in the chronic phase could also be used to predict sudden cardiac death using the peri-infarct zone extent 103 or chronic MI size 104 in combination with LVEF. Furthermore, residual myocardial iron as a consequence of intramyocardial hemorrhage has also been shown to be pro-arrhythmic.105 Although all these were relatively small and single-center studies, CMR has shown promise to improve the risk-stratification of STEMI patients at risk of ventricular arrhythmias. With the availability of inline, automated ECV-mapping106 and free-breathing T2*-mapping93, characterization of the infarct size and peri-infarct zone and infarct core may become more objective and CMR would play an invaluable role to identify those most likely to benefit from a primary prevention ICD in a near future.
Conclusion
Over the last few years, CMR scans performed in the acute phase of reperfused STEMI have improved our understanding of the changes occurring in the infarcted, salvaged, and remote myocardium, and have provided insights into the impact of MI size, microvascular obstruction and intramyocardial hemorrhage on clinical outcomes. There are ongoing controversies surrounding the use of CMR to delineate the area-at-risk, and the dynamic changes in myocardial edema in the first few days following PPCI. However, more work is ongoing and CMR holds promise to become accessible to more patients, and to be used as a tool to risk-stratify patients, guide treatment, and improve clinical outcomes post-PPCI in the near future.
Acknowledgments
Funding sources
This work was supported in part by grants from the British Heart Foundation (FS/10/039/28270), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School; the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021), and the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology Action to Prof Hausenloy; in part by grants from the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health to Dr Arai; in part by grants from extramural funding from National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL136578 and R01 HL133407) to Dr Dharmakumar; and in part by grants from the British Heart Foundation Centre of Research Excellence Award (RE/13/5/30177), BHF grants (FS/15/54/31639, PG/11/2/28474, PG/14/64/31043), Medical Research Scotland, and the Chief Scientist Office and a Senior Fellowship from the Scottish Funding Council to Professor Berry.
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen F, Butrymovich V, Kelbaek H, Wachtell K, Helqvist S, Kastrup J, Holmvang L, Clemmensen P, Engstrom T, Grande P, Saunamaki K, Jorgensen E. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64:2101–2108. doi: 10.1016/j.jacc.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 4.Bulluck H, Foin N, Tan JW, Low AF, Sezer M, Hausenloy DJ. Invasive Assessment of the Coronary Microcirculation in Reperfused ST-Segment-Elevation Myocardial Infarction Patients: Where Do We Stand? Circ Cardiovasc Interv. 2017;10:e004373. doi: 10.1161/CIRCINTERVENTIONS.116.004373. [DOI] [PubMed] [Google Scholar]
- 5.Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart. 2016;102:341–348. doi: 10.1136/heartjnl-2015-307855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulluck H, Hammond-Haley M, Weinmann S, Martinez-Macias R, Hausenloy DJ. Myocardial Infarct Size by CMR in Clinical Cardioprotection Studies: Insights From Randomized Controlled Trials. JACC Cardiovasc Imaging. 2017;10:230–240. doi: 10.1016/j.jcmg.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P Group ESCSD. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 8.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55:1–16. doi: 10.1016/j.jacc.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 11.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrick D, Haig C, Ahmed N, Rauhalammi S, Clerfond G, Carberry J, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Mahrous A, Welsh P, Sattar N, Ford I, Oldroyd KG, Radjenovic A, Berry C. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients After Acute ST-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J Am Heart Assoc. 2016;5:e002834. doi: 10.1161/JAHA.115.002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Jimenez R, Barreiro-Perez M, Martin-Garcia A, Sanchez-Gonzalez J, Aguero J, Galan-Arriola C, Garcia-Prieto J, Diaz-Pelaez E, Vara P, Martinez I, Zamarro I, Garde B, Sanz J, Fuster V, Sanchez PL, Ibanez B. Dynamic Edematous Response of the Human Heart to Myocardial Infarction: Implications for Assessing Myocardial Area at Risk and Salvage. Circulation. 2017;136:1288–1300. doi: 10.1161/CIRCULATIONAHA.116.025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, Mahrous A, Mordi I, Rauhalammi S, Sattar N, Welsh P, Radjenovic A, Ford I, Oldroyd KG, Berry C. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ Cardiovasc Imaging. 2016;9:e004148. doi: 10.1161/CIRCIMAGING.115.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, Fontana M, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Kellman P, Moon JC, Hausenloy DJ. Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ Cardiovasc Imaging. 2016;9:e004940. doi: 10.1161/CIRCIMAGING.116.004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, Fontana M, Gonzalez-Lopez E, Reant P, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Kellman P, Moon JC, Hausenloy DJ. Automated Extracellular Volume Fraction Mapping Provides Insights Into the Pathophysiology of Left Ventricular Remodeling Post-Reperfused ST-Elevation Myocardial Infarction. J Am Heart Assoc. 2016;5:e003555. doi: 10.1161/JAHA.116.003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carberry J, Carrick D, Haig C, Rauhalammi SM, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Davie A, Mahrous A, Ford I, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Remote Zone Extracellular Volume and Left Ventricular Remodeling in Survivors of ST-Elevation Myocardial Infarction. Hypertension. 2016;68:385–391. doi: 10.1161/HYPERTENSIONAHA.116.07222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular Magnetic Resonance in Patients With Myocardial Infarction. J Am Coll Cardiol. 2009;55:1–16. doi: 10.1016/j.jacc.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 19.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 20.Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Mahrous A, Ford I, Tzemos N, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044–1059. doi: 10.1093/eurheartj/ehv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Green JD, Sykes JM, Ephrat P, Carson JJ, Mitchell AJ, Wisenberg G, Friedrich MG. Detection and quantification of myocardial reperfusion hemorrhage using T2*-weighted CMR. JACC Cardiovasc Imaging. 2011;4:1274–1283. doi: 10.1016/j.jcmg.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Kali A, Tang RL, Kumar A, Min JK, Dharmakumar R. Detection of acute reperfusion myocardial hemorrhage with cardiac MR imaging: T2 versus T2. Radiology. 2013;269:387–395. doi: 10.1148/radiol.13122397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Perez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. doi: 10.1093/eurheartj/ehm212. [DOI] [PubMed] [Google Scholar]
- 24.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 25.Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu L-Y, Aletras AH, Arai AE. Magnetic Resonance Imaging Delineates the Ischemic Area at Risk and Myocardial Salvage in Patients With Acute Myocardial Infarction. Circulation-Cardiovascular Imaging. 2010;3:527–535. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–278. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulluck H, White SK, Rosmini S, Bhuva A, Treibel TA, Fontana M, Abdel-Gadir A, Herrey A, Manisty C, Wan SM, Groves A, Menezes L, Moon JC, Hausenloy DJ. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. J Cardiovasc Magn Reson. 2015;17:73. doi: 10.1186/s12968-015-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto H, Matsuda T, Miyamoto K, Shimada T, Mikuri M, Hiraoka Y. Peri-infarct zone on early contrast-enhanced CMR imaging in patients with acute myocardial infarction. JACC Cardiovasc Imaging. 2011;4:610–618. doi: 10.1016/j.jcmg.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Hammer-Hansen S, Leung SW, Hsu LY, Wilson JR, Taylor J, Greve AM, Thune JJ, Kober L, Kellman P, Arai AE. Early Gadolinium Enhancement for Determination of Area at Risk: A Preclinical Validation Study. JACC Cardiovasc Imaging. 2017;10:130–139. doi: 10.1016/j.jcmg.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Beohar N, Arumana JM, Larose E, Li D, Friedrich MG, Dharmakumar R. CMR imaging of edema in myocardial infarction using cine balanced steady-state free precession. JACC Cardiovasc Imaging. 2011;4:1265–1273. doi: 10.1016/j.jcmg.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordlund D, Klug G, Heiberg E, Koul S, Larsen TH, Hoffmann P, Metzler B, Erlinge D, Atar D, Aletras AH, Carlsson M, Engblom H, Arheden H. Multi-vendor, multicentre comparison of contrast-enhanced SSFP and T2-STIR CMR for determining myocardium at risk in ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2016;17:744–753. doi: 10.1093/ehjci/jew027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordlund D, Kanski M, Jablonowski R, Koul S, Erlinge D, Carlsson M, Engblom H, Aletras AH, Arheden H. Experimental validation of contrast-enhanced SSFP cine CMR for quantification of myocardium at risk in acute myocardial infarction. J Cardiovasc Magn Reson. 2017;19:12. doi: 10.1186/s12968-017-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dall’Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM, Cuculi F, Kharbanda RK, Banning AP, Choudhury RP, Karamitsos TD, Neubauer S. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14:15. doi: 10.1186/1532-429X-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botker HE, Kaltoft AK, Pedersen SF, Kim WY. Measuring myocardial salvage. Cardiovasc Res. 2012;94:266–275. doi: 10.1093/cvr/cvs081. [DOI] [PubMed] [Google Scholar]
- 36.Engblom H, Heiberg E, Erlinge D, Jensen SE, Nordrehaug JE, Dubois-Rande JL, Halvorsen S, Hoffmann P, Koul S, Carlsson M, Atar D, Arheden H. Sample Size in Clinical Cardioprotection Trials Using Myocardial Salvage Index, Infarct Size, or Biochemical Markers as Endpoint. J Am Heart Assoc. 2016;5:e002708. doi: 10.1161/JAHA.115.002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 38.Krug A, Du Mesnil de R, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ Res. 1966;19:57–62. doi: 10.1161/01.res.19.1.57. [DOI] [PubMed] [Google Scholar]
- 39.Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Gonzalez-Lopez E, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Moon JC, Hausenloy DJ. Diagnostic performance of T1 and T2 mapping to detect intramyocardial hemorrhage in reperfused ST-segment elevation myocardial infarction (STEMI) patients. J Magn Reson Imaging. 2017;46:877–886. doi: 10.1002/jmri.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940–952. doi: 10.1016/j.jcmg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kali A, Tang RLQ, Kumar A, Min JK, Dharmakumar R. Detection of acute reperfusion myocardial hemorrhage with cardiac MR imaging: T2 versus T2. Radiology. 2013;269:387–395. doi: 10.1148/radiol.13122397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arai AE. Magnetic resonance imaging for area at risk, myocardial infarction, and myocardial salvage. J Cardiovasc Pharmacol Ther. 2011;16:313–320. doi: 10.1177/1074248411412378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulluck H, Hammond-Haley M, Fontana M, Knight DS, Sirker A, Herrey AS, Manisty C, Kellman P, Moon JC, Hausenloy DJ. Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J Cardiovasc Magn Reson. 2017;19:57. doi: 10.1186/s12968-017-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kali A, Choi EY, Sharif B, Kim YJ, Bi X, Spottiswoode B, Cokic I, Yang HJ, Tighiouart M, Conte AH, Li D, Berman DS, Choi BW, Chang HJ, Dharmakumar R. Native T1 Mapping by 3-T CMR Imaging for Characterization of Chronic Myocardial Infarctions. JACC Cardiovasc Imaging. 2015;8:1019–1030. doi: 10.1016/j.jcmg.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Borlotti A, Viliani D, Jerosch-Herold M, Alkhalil M, De Maria GL, Fahrni G, Dawkins S, Wijesurendra R, Francis J, Ferreira V, Piechnik S, Robson MD, Banning A, Choudhury R, Neubauer S, Channon K, Kharbanda R, Dall’Armellina E. CMR Native T1 Mapping Allows Differentiation of Reversible Versus Irreversible Myocardial Damage in ST-Segment-Elevation Myocardial Infarction: An OxAMI Study (Oxford Acute Myocardial Infarction) Circ Cardiovasc Imaging. 2017;10:e005986. doi: 10.1161/CIRCIMAGING.116.005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg P, Broadbent D, Swoboda P, Foley JR, Fent GJ, Musa TA, Ripley DP, Erhayiem B, Dobson LE, McDiarmid A, Haaf P, Kidambi A, Geest RJVD, PGJ, Plein S. Acute Infarct Extracellular Volume Mapping to Quantify Myocardial Area at Risk and Chronic Infarct Size on Cardiovascular Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2017;10:e006182. doi: 10.1161/CIRCIMAGING.117.006182. [DOI] [PubMed] [Google Scholar]
- 47.Bulluck H, Hausenloy DJ. Mapping Myocardial Salvage Index by Extracellular Volume Fraction: Are We There Yet? Circ Cardiovasc Imaging. 2017;10:e006680. doi: 10.1161/CIRCIMAGING.117.006680. [DOI] [PubMed] [Google Scholar]
- 48.Kidambi A, Motwani M, Uddin A, Ripley DP, McDiarmid AK, Swoboda PP, Broadbent DA, Musa TA, Erhayiem B, Leader J, Croisille P, Clarysse P, Greenwood JP, Plein S. Myocardial Extracellular Volume Estimation by CMR Predicts Functional Recovery Following Acute MI. JACC Cardiovasc Imaging. 2017;10:989–999. doi: 10.1016/j.jcmg.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orn S, Manhenke C, Greve OJ, Larsen AI, Bonarjee VV, Edvardsen T, Dickstein K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J. 2009;30:1978–1985. doi: 10.1093/eurheartj/ehp219. [DOI] [PubMed] [Google Scholar]
- 50.van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930–939. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 51.de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben-Yehuda O, Jenkins P, Thiele H, Stone GW. Relationship between microvascular obstruction and adverse events following primary primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 2017;38:3502–3510. doi: 10.1093/eurheartj/ehx414. [DOI] [PubMed] [Google Scholar]
- 52.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol. 2016;67:2050–2060. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 53.Bulluck H, Go YY, Crimi G, Ludman AJ, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Pica S, Raineri C, Sirker A, Herrey AS, Manisty C, Groves A, Moon JC, Hausenloy DJ. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:26. doi: 10.1186/s12968-017-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kali A, Kumar A, Cokic I, Tang RL, Tsaftaris SA, Friedrich MG, Dharmakumar R. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: a cardiovascular magnetic resonance study with ex vivo validation. Circ Cardiovasc Imaging. 2013;6:218–228. doi: 10.1161/CIRCIMAGING.112.000133. [DOI] [PubMed] [Google Scholar]
- 55.Kali A, Cokic I, Tang R, Dohnalkova A, Kovarik L, Yang HJ, Kumar A, Prato FS, Wood JC, Underhill D, Marban E, Dharmakumar R. Persistent Microvascular Obstruction After Myocardial Infarction Culminates in the Confluence of Ferric Iron Oxide Crystals, Proinflammatory Burden, and Adverse Remodeling. Circ Cardiovasc Imaging. 2016;9:e004996. doi: 10.1161/CIRCIMAGING.115.004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roghi A, Poggiali E, Duca L, Mafrici A, Pedrotti P, Paccagnini S, Brenna S, Galli A, Consonni D, Cappellini MD. Role of Non-Transferrin-Bound Iron in the pathogenesis of cardiotoxicity in patients with ST-elevation myocardial infarction assessed by Cardiac Magnetic Resonance Imaging. Int J Cardiol. 2015;199:326–332. doi: 10.1016/j.ijcard.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 57.Carberry J, Carrick D, Haig C, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Davie A, Mahrous A, Ford I, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Persistent Iron Within the Infarct Core After ST-Segment Elevation Myocardial Infarction: Implications for Left Ventricular Remodeling and Health Outcomes. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.08.027. S1936–1878X(1917)30916-30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinstadler SJ, Stiermaier T, Liebetrau J, Fuernau G, Eitel C, de Waha S, Desch S, Reil JC, Poss J, Metzler B, Lucke C, Gutberlet M, Schuler G, Thiele H, Eitel I. Prognostic Significance of Remote Myocardium Alterations Assessed by Quantitative Noncontrast T1 Mapping in ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.03.015. pii: S1936–1878X(1917)30399-30396. [DOI] [PubMed] [Google Scholar]
- 59.Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Watkins S, Hood S, Davie A, Mahrous A, Sattar N, Welsh P, Tzemos N, Radjenovic A, Ford I, Oldroyd KG, Berry C. Pathophysiology of LV Remodeling in Survivors of STEMI: Inflammation, Remote Myocardium, and Prognosis. JACC Cardiovasc Imaging. 2015;8:779–789. doi: 10.1016/j.jcmg.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg P, Broadbent DA, Swoboda PP, Foley JRJ, Fent GJ, Musa TA, Ripley DP, Erhayiem B, Dobson LE, McDiarmid AK, Haaf P, Kidambi A, Crandon S, Chew PG, van der Geest RJ, Greenwood JP, Plein S. Extra-cellular expansion in the normal, non-infarcted myocardium is associated with worsening of regional myocardial function after acute myocardial infarction. J Cardiovasc Magn Reson. 2017;19:73. doi: 10.1186/s12968-017-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 62.Symons R, Pontone G, Schwitter J, Francone M, Iglesias JF, Barison A, Zalewski J, de Luca L, Degrauwe S, Claus P, Guglielmo M, Nessler J, Carbone I, Ferro G, Durak M, Magistrelli P, Lo Presti A, Aquaro GD, Eeckhout E, Roguelov C, Andreini D, Vogt P, Guaricci AI, Mushtaq S, Lorenzoni V, Muller O, Desmet W, Agati L, Janssens S, Bogaert J, Masci PG. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance After ST-Segment Elevation Myocardial Infarction: A Study of the Collaborative Registry on CMR in STEMI. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.05.023. S1936–1878X(1917)30620-30624. [DOI] [PubMed] [Google Scholar]
- 63.Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–1226. doi: 10.1016/j.jacc.2014.06.1194. [DOI] [PubMed] [Google Scholar]
- 64.Amier RP, Tijssen RYG, Teunissen PFA, Fernandez-Jimenez R, Pizarro G, Garcia-Lunar I, Bastante T, van de Ven PM, Beek AM, Smulders MW, Bekkers S, van Royen N, Ibanez B, Nijveldt R. Predictors of Intramyocardial Hemorrhage After Reperfused ST-Segment Elevation Myocardial Infarction. J Am Heart Assoc. 2017;6:e005651. doi: 10.1161/JAHA.117.005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marban L, Marban E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8:322–332. doi: 10.1161/CIRCHEARTFAILURE.114.001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, Crooks LE. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184–188. doi: 10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 67.Friedrich MG. Myocardial edema--a new clinical entity? Nat Rev Cardiol. 2010;7:292–296. doi: 10.1038/nrcardio.2010.28. [DOI] [PubMed] [Google Scholar]
- 68.Tilak GS, Hsu LY, Hoyt RF, Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 69.Ubachs JF, Engblom H, Koul S, Kanski M, Andersson P, van der Pals J, Carlsson M, Erlinge D, Arheden H. Myocardium at risk can be determined by ex vivo T2-weighted magnetic resonance imaging even in the presence of gadolinium: comparison to myocardial perfusion single photon emission computed tomography. Eur Heart J Cardiovasc Imaging. 2013;14:261–268. doi: 10.1093/ehjci/jes142. [DOI] [PubMed] [Google Scholar]
- 70.Kim HW, Van Assche L, Jennings RB, Wince WB, Jensen CJ, Rehwald WG, Wendell DC, Bhatti L, Spatz DM, Parker MA, Jenista ER, Klem I, Crowley AL, Chen EL, Judd RM, Kim RJ. Relationship of T2-Weighted MRI Myocardial Hyperintensity and the Ischemic Area-At-Risk. Circ Res. 2015;117:254–265. doi: 10.1161/CIRCRESAHA.117.305771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bulluck H, White SK, Frohlich GM, Casson SG, O’Meara C, Newton A, Nicholas J, Weale P, Wan SM, Sirker A, Moon JC, Yellon DM, Groves A, Menezes L, Hausenloy DJ. Quantifying the Area at Risk in Reperfused ST-Segment-Elevation Myocardial Infarction Patients Using Hybrid Cardiac Positron Emission Tomography-Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2016;9:e003900. doi: 10.1161/CIRCIMAGING.115.003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammer-Hansen S, Ugander M, Hsu LY, Taylor J, Thune JJ, Kober L, Kellman P, Arai AE. Distinction of salvaged and infarcted myocardium within the ischaemic area-at-risk with T2 mapping. Eur Heart J Cardiovasc Imaging. 2014;15:1048–1053. doi: 10.1093/ehjci/jeu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dall’Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, Kellman P, Francis JM, Forfar C, Prendergast BD, Banning AP, Channon KM, Kharbanda RK, Neubauer S, Choudhury RP. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228–236. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlsson M, Ubachs JF, Hedstrom E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–576. doi: 10.1016/j.jcmg.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Jimenez R, Sanchez-Gonzalez J, Aguero J, Garcia-Prieto J, Lopez-Martin GJ, Garcia-Ruiz JM, Molina-Iracheta A, Rossello X, Fernandez-Friera L, Pizarro G, Garcia-Alvarez A, Dall’Armellina E, Macaya C, Choudhury RP, Fuster V, Ibanez B. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol. 2015;65:315–323. doi: 10.1016/j.jacc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Jimenez R, Garcia-Prieto J, Sanchez-Gonzalez J, Aguero J, Lopez-Martin GJ, Galan-Arriola C, Molina-Iracheta A, Doohan R, Fuster V, Ibanez B. Pathophysiology Underlying the Bimodal Edema Phenomenon After Myocardial Ischemia/Reperfusion. J Am Coll Cardiol. 2015;66:816–828. doi: 10.1016/j.jacc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Thuny F, Lairez O, Roubille F, Mewton N, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Thibault H, Cung TT, Finet G, Argaud L, Revel D, Derumeaux G, Bonnefoy-Cudraz E, Elbaz M, Piot C, Ovize M, Croisille P. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 78.White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049–3057. doi: 10.1093/eurheartj/ehv463. [DOI] [PubMed] [Google Scholar]
- 80.Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de Prado A, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 81.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 82.Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schomig A, Schwaiger M. Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology. 2010;254:88–97. doi: 10.1148/radiol.09090660. [DOI] [PubMed] [Google Scholar]
- 83.Jablonowski R, Engblom H, Kanski M, Nordlund D, Koul S, van der Pals J, Englund E, Heiberg E, Erlinge D, Carlsson M, Arheden H. Contrast-Enhanced CMR Overestimates Early Myocardial Infarct Size: Mechanistic Insights Using ECV Measurements on Day 1 and Day 7. JACC Cardiovasc Imaging. 2015;8:1379–1389. doi: 10.1016/j.jcmg.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 84.Hammer-Hansen S, Bandettini WP, Hsu LY, Leung SW, Shanbhag S, Mancini C, Greve AM, Kober L, Thune JJ, Kellman P, Arai AE. Mechanisms for overestimating acute myocardial infarct size with gadolinium-enhanced cardiovascular magnetic resonance imaging in humans: a quantitative and kinetic study. Eur Heart J Cardiovasc Imaging. 2016;17:76–84. doi: 10.1093/ehjci/jev123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Palomares JF, Ortiz-Perez JT, Lee DC, Bucciarelli-Ducci C, Tejedor P, Bonow RO, Wu E. Time elapsed after contrast injection is crucial to determine infarct transmurality and myocardial functional recovery after an acute myocardial infarction. J Cardiovasc Magn Reson. 2015;17:43. doi: 10.1186/s12968-015-0139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Timing of cardiovascular MR imaging after acute myocardial infarction: effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology. 2011;261:116–126. doi: 10.1148/radiol.11110228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engblom H, Hedstrom E, Heiberg E, Wagner GS, Pahlm O, Arheden H. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri-infarction zone: one-year follow-up by MRI. Circ Cardiovasc Imaging. 2009;2:47–55. doi: 10.1161/CIRCIMAGING.108.802199. [DOI] [PubMed] [Google Scholar]
- 88.Lonborg J, Vejlstrup N, Kelbaek H, Holmvang L, Jorgensen E, Helqvist S, Saunamaki K, Ahtarovski KA, Botker HE, Kim WY, Clemmensen P, Engstrom T. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: an observational study. Eur Heart J Cardiovasc Imaging. 2013;14:387–395. doi: 10.1093/ehjci/jes271. [DOI] [PubMed] [Google Scholar]
- 89.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 90.Thiele H, Kappl MJ, Conradi S, Niebauer J, Hambrecht R, Schuler G. Reproducibility of chronic and acute infarct size measurement by delayed enhancement-magnetic resonance imaging. J Am Coll Cardiol. 2006;47:1641–1645. doi: 10.1016/j.jacc.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 91.Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Weinmann S, Sirker A, Herrey AS, Manisty C, Moon JC, Hausenloy DJ. Impact of microvascular obstruction on semiautomated techniques for quantifying acute and chronic myocardial infarction by cardiovascular magnetic resonance. Open Heart. 2016;3:e000535. doi: 10.1136/openhrt-2016-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang HJ, Sharif B, Pang J, Kali A, Bi X, Cokic I, Li D, Dharmakumar R. Free-breathing, motion-corrected, highly efficient whole heart T2 mapping at 3T with hybrid radial-cartesian trajectory. Magn Reson Med. 2016;75:126–136. doi: 10.1002/mrm.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kellman P, Xue H, Spottiswoode BS, Sandino CM, Hansen MS, Abdel-Gadir A, Treibel TA, Rosmini S, Mancini C, Bandettini WP, McGill LA, Gatehouse P, Moon JC, Pennell DJ, Arai AE. Free-breathing T2* mapping using respiratory motion corrected averaging. J Cardiovasc Magn Reson. 2015;17:3. doi: 10.1186/s12968-014-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kellman P, Arai AE. Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging. 2012;36:529–542. doi: 10.1002/jmri.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 96.Stiermaier T, Jobs A, de Waha S, Fuernau G, Poss J, Desch S, Thiele H, Eitel I. Optimized Prognosis Assessment in ST-Segment-Elevation Myocardial Infarction Using a Cardiac Magnetic Resonance Imaging Risk Score. Circ Cardiovasc Imaging. 2017;10:e006774. doi: 10.1161/CIRCIMAGING.117.006774. [DOI] [PubMed] [Google Scholar]
- 97.Pontone G, Guaricci AI, Andreini D, Ferro G, Guglielmo M, Baggiano A, Fusini L, Muscogiuri G, Lorenzoni V, Mushtaq S, Conte E, Annoni A, Formenti A, Mancini ME, Carita P, Verdecchia M, Pica S, Fazzari F, Cosentino N, Marenzi G, Rabbat MG, Agostoni P, Bartorelli AL, Pepi M, Masci PG. Prognostic Stratification of Patients With ST-Segment-Elevation Myocardial Infarction (PROSPECT): A Cardiac Magnetic Resonance Study. Circ Cardiovasc Imaging. 2017;10:e006428. doi: 10.1161/CIRCIMAGING.117.006428. [DOI] [PubMed] [Google Scholar]
- 98.Dharmakumar R. “Rusty Hearts”: Is It Time to Rethink Iron Chelation Therapies in Post-Myocardial-Infarction Setting? Circ Cardiovasc Imaging. 2016;9:e005541. doi: 10.1161/CIRCIMAGING.116.005541. [DOI] [PubMed] [Google Scholar]
- 99.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX American College of Cardiology F, American Heart Association Task Force on Practice G, American College of Emergency P, Society for Cardiovascular A and Interventions. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E1–27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- 100.Zaman S, Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation. 2014;129:2426–2435. doi: 10.1161/CIRCULATIONAHA.113.007497. [DOI] [PubMed] [Google Scholar]
- 101.Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov YN, Mareev V, Velazquez EJ, Rouleau JL, Maggioni AP, Kober L, Califf RM, McMurray JJ, Pfeffer MA, Solomon SD Investigators V. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 102.Izquierdo M, Ruiz-Granell R, Bonanad C, Chaustre F, Gomez C, Ferrero A, Lopez-Lereu P, Monmeneu JV, Nunez J, Chorro FJ, Bodi V. Value of early cardiovascular magnetic resonance for the prediction of adverse arrhythmic cardiac events after a first noncomplicated ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2013;6:755–761. doi: 10.1161/CIRCIMAGING.113.000702. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe E, Abbasi SA, Heydari B, Coelho-Filho OR, Shah R, Neilan TG, Murthy VL, Mongeon FP, Barbhaiya C, Jerosch-Herold M, Blankstein R, Hatabu H, van der Geest RJ, Stevenson WG, Kwong RY. Infarct tissue heterogeneity by contrast-enhanced magnetic resonance imaging is a novel predictor of mortality in patients with chronic coronary artery disease and left ventricular dysfunction. Circ Cardiovasc Imaging. 2014;7:887–894. doi: 10.1161/CIRCIMAGING.113.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cokic I, Kali A, Yang HJ, Yee R, Tang R, Tighiouart M, Wang X, Jackman WS, Chugh SS, White JA, Dharmakumar R. Iron-Sensitive Cardiac Magnetic Resonance Imaging for Prediction of Ventricular Arrhythmia Risk in Patients With Chronic Myocardial Infarction: Early Evidence. Circ Cardiovasc Imaging. 2015;8:e003642. doi: 10.1161/CIRCIMAGING.115.003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Treibel TA, Fontana M, Maestrini V, Castelletti S, Rosmini S, Simpson J, Nasis A, Bhuva AN, Bulluck H, Abdel-Gadir A, White SK, Manisty C, Spottiswoode BS, Wong TC, Piechnik SK, Kellman P, Robson MD, Schelbert EB, Moon JC. Automatic Measurement of the Myocardial Interstitium: Synthetic Extracellular Volume Quantification Without Hematocrit Sampling. JACC Cardiovasc Imaging. 2016;9:54–63. doi: 10.1016/j.jcmg.2015.11.008. [DOI] [PubMed] [Google Scholar]